Novel Antischistosomal Drug Targets: Identification of Alkaloid Inhibitors of SmTGR via Integrated In Silico Methods
Abstract
1. Introduction
2. Materials and Methods
2.1. Dataset Treatment and Balancing (AlcSmTGR)
2.2. Calculation and Selection of Molecular Descriptors
2.3. Machine Learning
2.4. Cross-Validation (CV)
2.5. External Validation (Ts)
2.6. Model Evaluation
2.7. Applicability Domain (APD)
2.8. Construction of a Natural Product Alkaloid Database (AlcVS) for VS
2.9. Ligand-Based Virtual Screening (LBVS)
2.10. Consensus Analysis
2.11. Activity Forecast Interpretation
2.12. Molecular Docking and Structure-Based Virtual Screening (SBVS)
2.13. Normalization of Score Values
2.14. Combined Approach of LBVS and SBVS
3. Results and Discussion
3.1. Ligand-Based Virtual Screening (LBVS)
3.2. Consensus Analysis
3.3. Interpretation of Descriptors with the Greatest Contributions to Alkaloid Inhibition Activity
3.4. Structure-Based Virtual Screening (SBVS)
3.5. The Combined Approach
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PZQ | Praziquantel |
| SmTGR | Thioredoxin Glutathione Reductase from Schistosoma mansoni |
| VS | Virtual Screening |
| EROS | Oxygen-reactive species |
| ROC | Receiver Operating Characteristic Curve |
| SBVS | Structure-Based Virtual Screening |
| LBVS | Ligand-Based Virtual Screening |
| Trx | Thioredoxin |
| TrxR | Thioredoxin Reductase |
| Grx | Glutaredoxin |
| GR | GSH Reductase |
| GSH | Glutathione |
| QSAR | Quantitative Structure-Activity Relationship |
| RF | Randon Forest |
| TSs | External test |
| CV | Cross-validation |
| Tr | Train |
| Patv | Activity probability percentage |
| ASP | Astex Statistical Potential |
| APD | Applicability Domain |
References
- Spangenberg, T. Alternatives to Praziquantel for the Prevention and Control of Schistosomiasis. ACS Infect. Dis. 2021, 7, 939–942. [Google Scholar] [CrossRef] [PubMed]
- Abdelghani, E.; Zerpa, R.; Iliescu, G.; Escalante, C.P. Schistosomiasis and Liver Disease: Learning from the Past to Understand the Present. Clin. Case Rep. 2020, 8, 1522–1526. [Google Scholar] [CrossRef] [PubMed]
- Braun, L.; Grimes, J.E.T.; Templeton, M.R. The Effectiveness of Water Treatment Processes against Schistosome Cercariae: A Systematic Review. PLoS Negl. Trop. Dis. 2018, 12, e0006364. [Google Scholar] [CrossRef]
- Magero, V.O.; Kisara, S.; Suleman, M.A.; Wade, C.M. Distribution of the Schistosome Intermediate Snail Host Biomphalaria Pfeifferi in East Africa’s River Systems and the Prevalence of Schistosoma mansoni Infection. Trans. R. Soc. Trop. Med. Hyg. 2025, 119, 253–265. [Google Scholar] [CrossRef]
- Lago, E.M.; Xavier, R.P.; Teixeira, T.R.; Silva, L.M.; Da Silva Filho, A.A.; De Moraes, J. Antischistosomal Agents: State of Art and Perspectives. Future Med. Chem. 2018, 10, 89–120. [Google Scholar] [CrossRef]
- McManus, D.P.; Dunne, D.W.; Sacko, M.; Utzinger, J.; Vennervald, B.J.; Zhou, X.N. Schistosomiasis. Nat. Rev. Dis. Prim. 2018, 4, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Bergquist, R.; Utzinger, J.; Keiser, J. Controlling Schistosomiasis with Praziquantel: How Much Longer without a Viable Alternative? Infect. Dis. Poverty 2017, 6, 74. [Google Scholar] [CrossRef]
- De Menezes, R.P.B.; De Viana, J.O.; Muratov, E.; Scotti, L.; Scotti, M.T. Computer-Assisted Discovery of Alkaloids with Schistosomicidal Activity. Curr. Issues Mol. Biol. 2022, 44, 383–408. [Google Scholar] [CrossRef]
- Rodrigues, M.L.; dos Gois, A.R.S.; Domingues, A.L.C.; Silva, R.O.; Lopes, E.P. Metabolomics Assays Applied to Schistosomiasis Studies: A Scoping Review. BMC Infect. Dis. 2025, 25, 211. [Google Scholar] [CrossRef]
- Fernández-Soto, P.; Gandasegui, J.; Rodríguez, C.C.; Pérez-Arellano, J.L.; Crego-Vicente, B.; Diego, J.G.B.; López-Abán, J.; Vicente, B.; Muro, A. Detection of Schistosoma mansoni-Derived DNA in Human Urine Samples by Loop-Mediated Isothermal Amplification (LAMP). PLoS ONE 2019, 14, e0214125. [Google Scholar] [CrossRef]
- de Galvão, R.L.F.; Meneses, G.C.; Pinheiro, M.C.C.; Martins, A.M.C.; Daher, E.D.F.; Bezerra, F.S.M. Kidney Injury Biomarkers and Parasitic Loads of Schistosoma mansoni in a Highly Endemic Area in Northeastern Brazil. Acta Trop. 2022, 228, 106311. [Google Scholar] [CrossRef] [PubMed]
- Moreira-Filho, J.T.; Dantas, R.F.; Senger, M.R.; Silva, A.C.; Campos, D.M.B.; Muratov, E.; Silva-Junior, F.P.; Andrade, C.H.; Neves, B.J. Shortcuts to Schistosomiasis Drug Discovery: The State-of-the-Art. Annu. Rep. Med. Chem. 2019, 53, 139–180. [Google Scholar] [CrossRef]
- Mäder, P.; Rennar, G.A.; Ventura, A.M.P.; Grevelding, C.G.; Schlitzer, M. Chemotherapy for Fighting Schistosomiasis: Past, Present and Future. ChemMedChem 2018, 13, 2374–2389. [Google Scholar] [CrossRef]
- El-Refaiy, A.I.; Amer, N.S.; Alhejely, A.; Qahl, S.H.; Shaban, A.M.; Mohamed, A.E.; Saleh, A.A.; Badawy, A.A.; El-Magd, M.A. Impact of Dandelion (Taraxacum Officinale) Leaf Aqueous Extract on Immunological Response of Mice after Schistosoma mansoni Infection. Mol. Biochem. Parasitol. 2025, 262, 111673. [Google Scholar] [CrossRef] [PubMed]
- Neves, B.J.; Dantas, R.F.; Senger, M.R.; Melo-Filho, C.C.; Valente, W.C.G.; De Almeida, A.C.M.; Rezende-Neto, J.M.; Lima, E.F.C.; Paveley, R.; Furnham, N.; et al. Discovery of New Anti-Schistosomal Hits by Integration of QSAR-Based Virtual Screening and High Content Screening. J. Med. Chem. 2016, 59, 7075–7088. [Google Scholar] [CrossRef] [PubMed]
- de Souza Neto, L.R.; Montoya, B.O.; Brandão-Neto, J.; Verma, A.; Bowyer, S.; Moreira-Filho, J.T.; Dantas, R.F.; Neves, B.J.; Andrade, C.H.; von Delft, F.; et al. Fragment Library Screening by X-Ray Crystallography and Binding Site Analysis on Thioredoxin Glutathione Reductase of Schistosoma mansoni. Sci. Rep. 2024, 14, 1582. [Google Scholar] [CrossRef]
- Eweas, A.F.; Allam, G. Targeting Thioredoxin Glutathione Reductase as a Potential Antischistosomal Drug Target. Mol. Biochem. Parasitol. 2018, 225, 94–102. [Google Scholar] [CrossRef]
- Angelucci, F.; Miele, A.E.; Boumis, G.; Dimastrogiovanni, D.; Brunori, M.; Bellelli, A. Glutathione Reductase and Thioredoxin Reductase at the Crossroad: The Structure of Schistosoma mansoni Thioredoxin Glutathione Reductase. Proteins Struct. Funct. Genet. 2008, 72, 936–945. [Google Scholar] [CrossRef]
- Caroli, A.; Simeoni, S.; Lepore, R.; Tramontano, A.; Via, A. Investigation of a Potential Mechanism for the Inhibition of SmTGR by Auranofin and Its Implications for Plasmodium Falciparum Inhibition. Biochem. Biophys. Res. Commun. 2012, 417, 576–581. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Neves, B.J.; Andrade, C.H.; Cravo, P.V.L. Natural Products as Leads in Schistosome Drug Discovery. Molecules 2015, 20, 1872–1903. [Google Scholar] [CrossRef] [PubMed]
- Mtemeli, F.L.; Ndlovu, J.; Mugumbate, G.; Makwikwi, T.; Shoko, R. Advances in Schistosomiasis Drug Discovery Based on Natural Products. All Life 2022, 15, 608–622. [Google Scholar] [CrossRef]
- Guimarães, M.A.; De Oliveira, R.N.; De Almeida, R.L.; Mafud, A.C.; Sarkis, A.L.V.; Ganassin, R.; Da Silva, M.P.; Roquini, D.B.; Veras, L.M.; Sawada, T.C.H.; et al. Epiisopilosine Alkaloid Has Activity against Schistosoma mansoni in Mice without Acute Toxicity. PLoS ONE 2018, 13, e0196667. [Google Scholar] [CrossRef] [PubMed]
- Rocha, J.A.; Andrade, I.M.; Véras, L.M.C.; Quelemes, P.V.; Lima, D.F.; Soares, M.J.S.; Pinto, P.L.S.; Mayo, S.J.; Ivanova, G.; Rangel, M.; et al. Anthelmintic, Antibacterial and Cytotoxicity Activity of Imidazole Alkaloids from Pilocarpus Microphyllus Leaves. Phyther. Res. 2017, 31, 624–630. [Google Scholar] [CrossRef]
- de Souza Silva, M.S.; dos Santos, M.L.M.F.; da Silva, A.M.; França, W.W.M.; Araújo, S.B.; da Silva, R.L.; do Nascimento, W.R.C.; da Silva Santos, N.P.; da Cruz Filho, I.J.; de Azevedo Albuquerque, M.C.P.; et al. Sanguinarine: An Alkaloid with Promising in Vitro and in Vivo Antiparasitic Activity against Different Developmental Stages of Schistosoma mansoni and in Silico Pharmacokinetic Properties (ADMET). Parasitol. Res. 2024, 123, 143. [Google Scholar] [CrossRef] [PubMed]
- Rocha, J.A.; Rego, N.C.S.; Carvalho, B.T.S.; Silva, F.I.; Sousa, J.A.; Ramos, R.M.; Passos, I.N.G.; De Moraes, J.; Leite, J.R.S.A.; Lima, F.C.A. Computational Quantum Chemistry, Molecular Docking, and ADMET Predictions of Imidazole Alkaloids of Pilocarpus Microphyllus with Schistosomicidal Properties. PLoS ONE 2018, 13, e0198476. [Google Scholar] [CrossRef]
- Tempone, A.G.; Pieper, P.; Borborema, S.E.T.; Thevenard, F.; Lago, J.H.G.; Croft, S.L.; Anderson, E.A. Marine Alkaloids as Bioactive Agents against Protozoal Neglected Tropical Diseases and Malaria. Nat. Prod. Rep. 2021, 38, 2214–2235. [Google Scholar] [CrossRef]
- Alves, V.M.; Braga, R.C.; Muratov, E.N.; Andrade, C.H. Cheminformatics: An Introduction. Quim. Nova 2018, 41, 202–212. [Google Scholar] [CrossRef]
- Muratov, E.N.; Bajorath, J.; Sheridan, R.P.; Tetko, I.V.; Filimonov, D.; Poroikov, V.; Oprea, T.I.; Baskin, I.I.; Varnek, A.; Roitberg, A.; et al. QSAR without Borders. Chem. Soc. Rev. 2020, 49, 3525–3564. [Google Scholar] [CrossRef]
- Neves, B.J.; Braga, R.C.; Melo-Filho, C.C.; Moreira-Filho, J.T.; Muratov, E.N.; Andrade, C.H. QSAR-Based Virtual Screening: Advances and Applications in Drug Discovery. Front. Pharmacol. 2018, 9, 1275. [Google Scholar] [CrossRef]
- Naqvi, A.A.T.; Mohammad, T.; Hasan, G.M.; Hassan, M.I. Advancements in Docking and Molecular Dynamics Simulations Towards Ligand-Receptor Interactions and Structure-Function Relationships. Curr. Top. Med. Chem. 2018, 18, 1755–1768. [Google Scholar] [CrossRef] [PubMed]
- Ballante, F.; Kooistra, A.J.; Kampen, S.; de Graaf, C.; Carlsson, J. Structure-Based Virtual Screening for Ligands of G Protein–Coupled Receptors: What Can Molecular Docking Do for You?S. Pharmacol. Rev. 2021, 73, 1698–1736. [Google Scholar] [CrossRef] [PubMed]
- Macip, G.; Garcia-Segura, P.; Mestres-Truyol, J.; Saldivar-Espinoza, B.; Ojeda-Montes, M.J.; Gimeno, A.; Cereto-Massagué, A.; Garcia-Vallvé, S.; Pujadas, G. Haste Makes Waste: A Critical Review of Docking-Based Virtual Screening in Drug Repurposing for SARS-CoV-2 Main Protease (M-pro) Inhibition. Med. Res. Rev. 2022, 42, 744–769. [Google Scholar] [CrossRef] [PubMed]
- Acevedo, C.H.; Scotti, L.; Scotti, M.T. In Silico Studies Designed to Select Sesquiterpene Lactones with Potential Antichagasic Activity from an In-House Asteraceae Database. ChemMedChem 2018, 13, 634–645. [Google Scholar] [CrossRef]
- University of Konstanz. Knime Analitycs Platform, v. 4.0.2. Available online: https://www.knime.com/downloads (accessed on 1 May 2020).
- PubChem. National Center for Biotechnology Information. Available online: https://pubchem.ncbi.nlm.nih.gov/bioassay/485364 (accessed on 9 March 2021).
- Rodrigues, R.P.; Mantoani, S.P.; De Almeida, J.R.; Pinsetta, F.R.; Semighini, E.P.; Da Silva, V.B.; Da Silva, C.H.P. Virtual Screening Strategies in Drug Design. Rev. Virtual Quim. 2012, 4, 739–776. [Google Scholar] [CrossRef]
- Mauri, A. AlvaDesc: A Tool to Calculate and Analyze Molecular Descriptors and Fingerprints. In Ecotoxicological QSARs, 1st ed.; Humana Press Inc.: Totowa, NJ, USA, 2020; pp. 801–820. [Google Scholar] [CrossRef]
- Greg Landrum. RDKit: Open-Source Cheminformatics Software, V.2021.03.1. Available online: https://www.rdkit.org (accessed on 21 March 2021).
- Yap, C.W. PaDEL-Descriptor: An Open Source Software to Calculate Molecular Descriptors and Fingerprints. J. Comput. Chem. 2010, 32, 1466–1474. [Google Scholar] [CrossRef]
- Hall, M.; Frank, E.; Holmes, G.; Pfahringer, B.; Reutemann, P.; Witten, I.H. The WEKA Data Mining Software. ACM SIGKDD Explor. Newsl. 2009, 11, 10–18. [Google Scholar] [CrossRef]
- Hesping, E.; Chua, M.J.; Pflieger, M.; Qian, Y.; Dong, L.; Bachu, P.; Liu, L.; Kurz, T.; Fisher, G.M.; Skinner-Adams, T.S.; et al. QSAR Classification Models for Prediction of Hydroxamate Histone Deacetylase Inhibitor Activity against Malaria Parasites. ACS Infect. Dis. 2022, 8, 106–117. [Google Scholar] [CrossRef]
- Cohen, J. A Coefficient of Agreement for Nominal Scales. Educ. Psychol. Meas. 1960, 20, 37–46. [Google Scholar] [CrossRef]
- Roy, K.; Kar, S.; Das, R.N. Understanding the Basics of QSAR for Applications in Pharmaceutical Sciences and Risk Assessment, 1st ed.; Validation of QSAR Models, cap. 7; Academic Press: New York, NY, USA, 2015; pp. 231–286. [Google Scholar] [CrossRef]
- Melagraki, G.; Afantitis, A.; Sarimveis, H.; Igglessi-Markopoulou, O.; Koutentis, P.A.; Kollias, G. In Silico Exploration for Identifying Structure-Activity Relationship of MEK Inhibition and Oral Bioavailability for Isothiazole Derivatives. Chem. Biol. Drug Des. 2010, 76, 397–406. [Google Scholar] [CrossRef]
- Afantitis, A.; Melagraki, G.; Koutentis, P.A.; Sarimveis, H.; Kollias, G. Ligand-Based Virtual Screening Procedure for the Prediction and the Identification of Novel β-Amyloid Aggregation Inhibitors Using Kohonen Maps and Counterpropagation Artificial Neural Networks. Eur. J. Med. Chem. 2011, 46, 497–508. [Google Scholar] [CrossRef] [PubMed]
- ChemAxon Ltd. MarvinSketch, v 19.22. Available online: https://chemaxon.com/products/marvin (accessed on 21 March 2021).
- Rodríguez-Pérez, R.; Bajorath, J. Interpretation of Machine Learning Models Using Shapley Values: Application to Compound Potency and Multi-Target Activity Predictions. J. Comput. Aided. Mol. Des. 2020, 34, 1013–1026. [Google Scholar] [CrossRef]
- Berman, H.M.; Battistuz, T.; Bhat, T.N.; Bluhm, W.F.; Bourne, P.E.; Burkhardt, K.; Feng, Z.; Gilliland, G.L.; Iype, L.; Jain, S.; et al. The Protein Data Bank. Biol. Crystallogr. 2002, 58, 899–907. [Google Scholar] [CrossRef]
- The Cambridge Crystallographic Data Centre. GOLD, v. 2022.1.0. Available online: https://www.ccdc.cam.ac.uk (accessed on 21 March 2022).
- Ramírez, D.; Caballero, J. Is It Reliable to Take the Molecular Docking Top Scoring Position as the Best Solution without Considering Available Structural Data? Molecules 2018, 23, 1038. [Google Scholar] [CrossRef] [PubMed]
- Schrödinger, LLC. Pymol, v. 2.5. Available online: https://pymol.org/2/ (accessed on 29 July 2023).
- Gupta, A.; Chaudhary, N.; Kakularam, K.R.; Pallu, R.; Polamarasetty, A. The Augmenting Effects of Desolvation and Conformational Energy Terms on the Predictions of Docking Programs against MPGES-1. PLoS ONE 2015, 10, e0134472. [Google Scholar] [CrossRef]
- Sirous, H.; Campiani, G.; Brogi, S.; Calderone, V.; Chemi, G. Computer-Driven Development of an in Silico Tool for Finding Selective Histone Deacetylase 1 Inhibitors. Molecules 2020, 25, 1952. [Google Scholar] [CrossRef] [PubMed]
- Young, R.J.; Green, D.V.S.; Luscombe, C.N.; Hill, A.P. Getting Physical in Drug Discovery II: The Impact of Chromatographic Hydrophobicity Measurements and Aromaticity. Drug Discov. Today 2011, 16, 822–830. [Google Scholar] [CrossRef]
- Taylor, R.D.; Maccoss, M.; Lawson, A.D.G. Rings in Drugs. J. Med. Chem. 2014, 57, 5845–5859. [Google Scholar] [CrossRef]
- Hill, A.P.; Young, R.J. Getting Physical in Drug Discovery: A Contemporary Perspective on Solubility and Hydrophobicity. Drug Discov. Today 2010, 15, 648–655. [Google Scholar] [CrossRef]
- Balaban, A.T. On Pyrylium Cations, Molecular Graphs, Topological Indices for QSAR, and Various Other Structural Problems. Struct. Chem. 2019, 30, 1129–1139. [Google Scholar] [CrossRef]
- Deng, B.; Ye, C.; Liang, W.; Li, Y.; Su, X. Several Asymptotic Bounds on the Balaban Indices of Trees. Math. Probl. Eng. 2020, 2020, 2081241. [Google Scholar] [CrossRef]
- Kang, S.M.; Siddiqui, M.K.; Rehman, N.A.; Naeem, M.; Muhammad, M.H. Topological Properties of 2-Dimensional Silicon-Carbons. IEEE Access 2018, 6, 59362–59373. [Google Scholar] [CrossRef]
- Sharma, B.K.; Singh, P.; Shekhawat, M.; Pilania, P. A Rationale for the Activity Profile of Benzenesulfonamide Derivatives as Cyclooxygenase (COX) Inhibitors. Eur. J. Med. Chem. 2010, 45, 2389–2395. [Google Scholar] [CrossRef]
- He, Q.; Eisner, F.D.; Pearce, D.; Hodsden, T.; Rezasoltani, E.; Medranda, D.; Fei, Z.; Nelson, J.; Heeney, M. Ring Fusion in Tetrathienylethene Cored Perylene Diimide Tetramers Affords Acceptors with Strong and Broad Absorption in the Near-UV to Visible Region. J. Mater. Chem. C 2020, 8, 17237–17244. [Google Scholar] [CrossRef]
- Mukherjee, R.K.; Kumar, V.; Roy, K. Ecotoxicological QSTR and QSTTR Modeling for the Prediction of Acute Oral Toxicity of Pesticides against Multiple Avian Species. Environ. Sci. Technol. 2022, 56, 335–348. [Google Scholar] [CrossRef]
- Lyu, H.; Petukhov, P.A.; Banta, P.R.; Jadhav, A.; Lea, W.A.; Cheng, Q.; Arnér, E.S.J.; Simeonov, A.; Thatcher, G.R.J.; Angelucci, F.; et al. Characterization of Lead Compounds Targeting the Selenoprotein Thioredoxin Glutathione Reductase for Treatment of Schistosomiasis. ACS Infect. Dis. 2020, 6, 393–405. [Google Scholar] [CrossRef]
- Arthur, D.E.; Uzairu, A.; Mamza, P.; Abechi, S.E.; Shallangwa, G. Insilico Modelling of Quantitative Structure–Activity Relationship of PGI50 Anticancer Compounds on K-562 Cell Line. Cogent. Chem. 2018, 4, 1432520. [Google Scholar] [CrossRef]
- Studziński, W.; Przybyłek, M.; Gackowska, A. Application of Gas Chromatographic Data and 2D Molecular Descriptors for Accurate Global Mobility Potential Prediction. Environ. Pollut. 2023, 317, 120816. [Google Scholar] [CrossRef]
- Silvestri, I.; Lyu, H.; Fata, F.; Boumis, G.; Miele, A.E.; Ardini, M.; Ippoliti, R.; Bellelli, A.; Jadhav, A.; Lea, W.A.; et al. Fragment-Based Discovery of a Regulatory Site in Thioredoxin Glutathione Reductase Acting as “Doorstop” for NADPH Entry. ACS Chem. Biol. 2018, 13, 2190–2202. [Google Scholar] [CrossRef]
- Fokoue, H.H.; Pinheiro, P.S.M.; Fraga, C.A.M.; Sant’Anna, C.M.R. Is There Anything New about the Molecular Recognition Applied to Medicinal Chemistry? Quim. Nova 2020, 43, 78–89. [Google Scholar] [CrossRef]
- Murebwayire, S.; Ingkaninan, K.; Changwijit, K.; Frédérich, M.; Duez, P. Triclisia Sacleuxii (Pierre) Diels (Menispermaceae), a Potential Source of Acetylcholinesterase Inhibitors. J. Pharm. Pharmacol. 2008, 61, 103–107. [Google Scholar] [CrossRef]
- Osmakov, D.I.; Koshelev, S.G.; Palikov, V.A.; Palikova, Y.A.; Shaykhutdinova, E.R.; Dyachenko, I.A.; Andreev, Y.A.; Kozlov, S.A. Alkaloid Lindoldhamine Inhibits Acid-Sensing Ion Channel 1a and Reveals Anti-Inflammatory Properties. Toxins 2019, 11, 542. [Google Scholar] [CrossRef]
- Prast-Nielsen, S.; Huang, H.H.; Williams, D.L. Thioredoxin Glutathione Reductase: Its Role in Redox Biology and Potential as a Target for Drugs against Neglected Diseases. Biochim. Biophys. Acta-Gen. Subj. 2011, 1810, 1262–1271. [Google Scholar] [CrossRef]
- Bai, R.; Yao, C.; Zhong, Z.; Ge, J.; Bai, Z.; Ye, X.; Xie, T.; Xie, Y. Discovery of Natural Anti-Inflammatory Alkaloids: Potential Leads for the Drug Discovery for the Treatment of Inflammation. Eur. J. Med. Chem. 2021, 213, 113165. [Google Scholar] [CrossRef]
- Wong, S.L.; Chang, H.S.; Wang, G.J.; Chiang, M.Y.; Huang, H.Y.; Chen, C.H.; Tsai, S.C.; Lin, C.H.; Chen, I.S. Secondary Metabolites from the Roots of Neolitsea Daibuensis and Their Anti-Inflammatory Activity. J. Nat. Prod. 2011, 74, 2489–2496. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.A.; Junior, E.S.A.; Lopes, G.B.P.; Pinheiro, M.L.B.; Costa, E.V.; Bezerra, D.P.; Oliveira, K. Structural, Vibrational, UV–Vis, Quantum-Chemical Properties, Molecular Docking and Anti-Cancer Activity Study of Annomontine and N-Hydroxyannomontine β-Carboline Alkaloids: A Combined Experimental and DFT Approach. J. Mol. Struct. 2018, 1171, 682–695. [Google Scholar] [CrossRef]
- Costa, E.V.; Pinheiro, M.L.B.; Xavier, C.M.; Silva, J.R.A.; Amaral, A.C.F.; Souza, A.D.L.; Barison, A.; Campos, F.R.; Ferreira, A.G.; Machado, G.M.C.; et al. A Pyrimidine-β-Carboline and Other Alkaloids from Annona Foetida with Antileishmanial Activity. J. Nat. Prod. 2006, 69, 292–294. [Google Scholar] [CrossRef]
- Abreu Miranda, M.; Lemos, M.; Alves Cowart, K.; Rodenburg, D.; McChesney, J.D.; Radwan, M.M.; Furtado, N.A.J.C.; Kenupp Bastos, J. Gastroprotective Activity of the Hydroethanolic Extract and Isolated Compounds from the Leaves of Solanum Cernuum Vell. J. Ethnopharmacol. 2015, 172, 421–429. [Google Scholar] [CrossRef]
- Marinho, A.F.; De Jesus Oliveira, E.; Tavares, J.F.; Filho, R.B.; Barbosa-Filho, J.M. 1H and 13C NMR Assignments of Two New Isomeric Bisbenzylisoquinoline Alkaloids from Cissampelos Sympodialis Eichl. (Menispermaceae). Magn. Reson. Chem. 2013, 51, 312–315. [Google Scholar] [CrossRef]
- Alves, A.F.; Vieira, G.C.; Gadelha, F.A.A.F.; Cavalcante-Silva, L.H.A.; Martins, M.A.; Barbosa-Filho, J.M.; Piuvezam, M.R. Milonine, an Alkaloid of Cissampelos Sympodialis Eichl. (Menispermaceae) Inhibits Histamine Release of Activated Mast Cells. Inflammation 2017, 40, 2118–2128. [Google Scholar] [CrossRef] [PubMed]
- Cava, M.P.; BehforouZ, M.; Mitchell, M.J. Ocotea Alkaloids: Variabiline, a Novel Aminoaporphine. Tetrahedron. Lett. 1972, 13, 4647–4649. [Google Scholar] [CrossRef]
- Custódio, D.L.; Florêncio Da Veiga Junior, V. Lauraceae Alkaloids. RSC Adv. 2014, 4, 21864–21890. [Google Scholar] [CrossRef]
- Pettit, G.R.; Melody, N.; Chapuis, J.C. Antineoplastic Agents. 607. Emetine Auristatins. J. Nat. Prod. 2020, 83, 1571–1576. [Google Scholar] [CrossRef]
- POWO. Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. 2025. Available online: https://powo.science.kew.org/ (accessed on 3 June 2025).
- WFO. Carapichea Ipecacuanha (Brot.) L.Andersson. 2025. Available online: http://www.worldfloraonline.org/taxon/wfo-0000336046 (accessed on 3 June 2025).
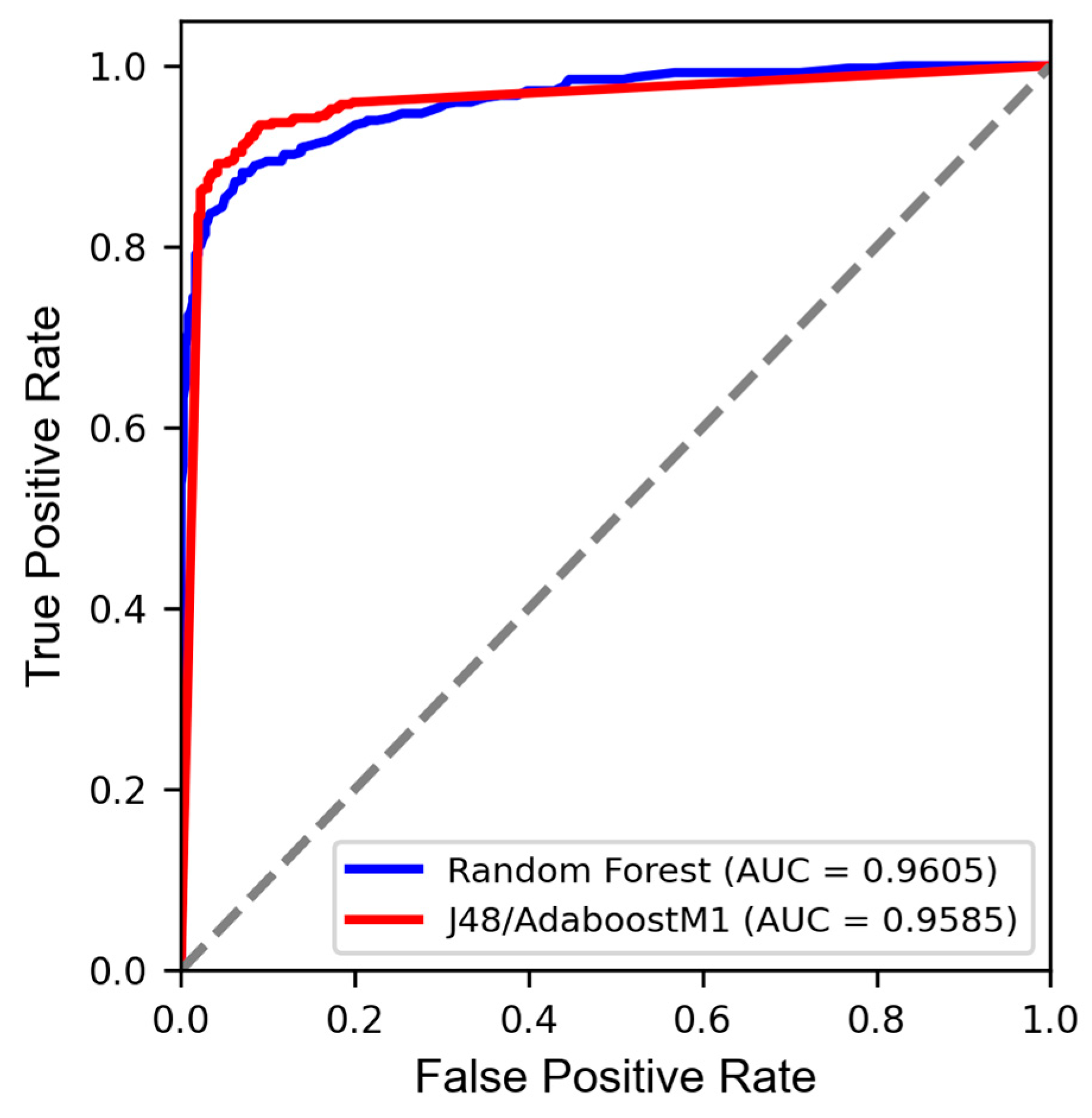
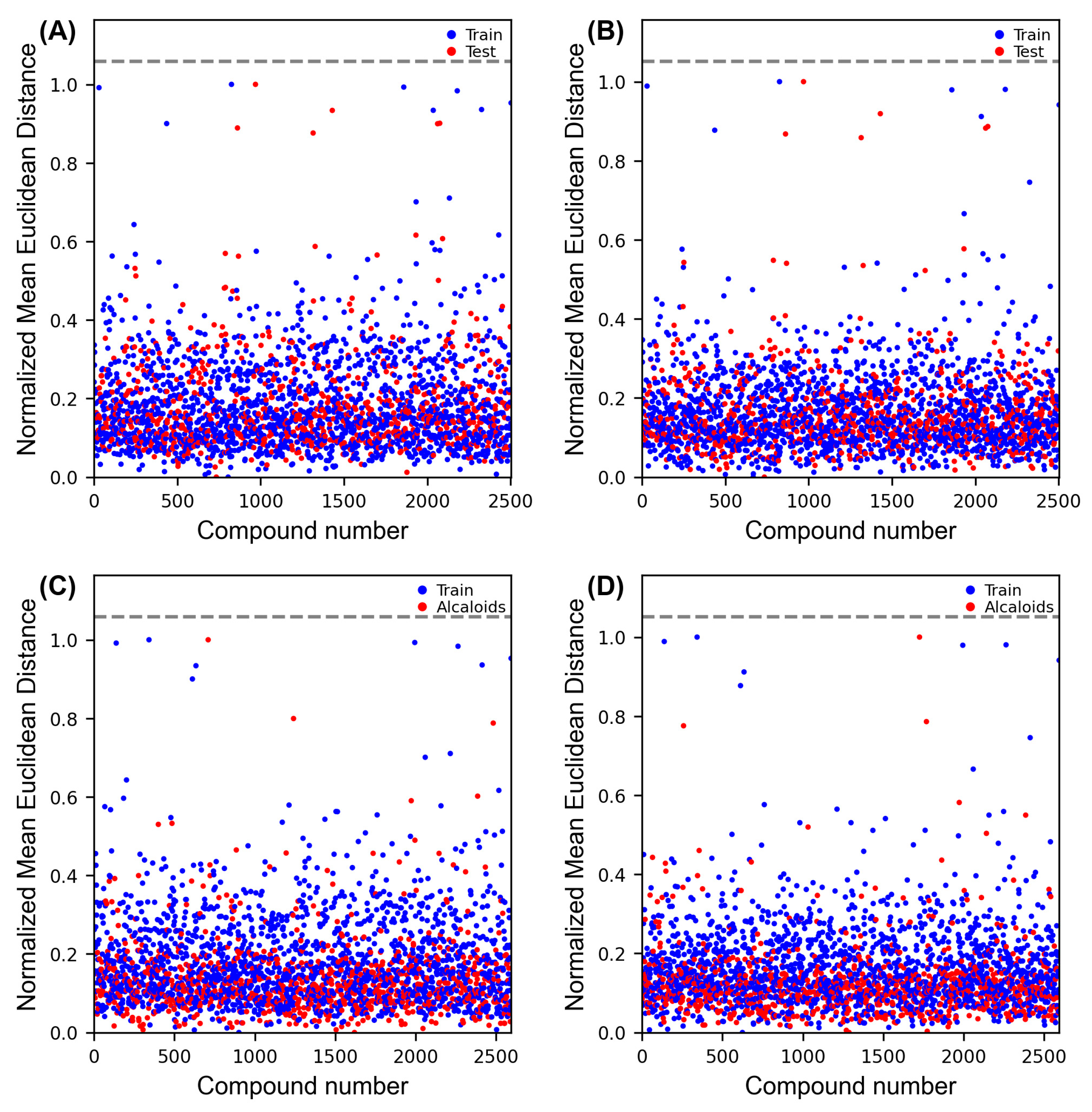
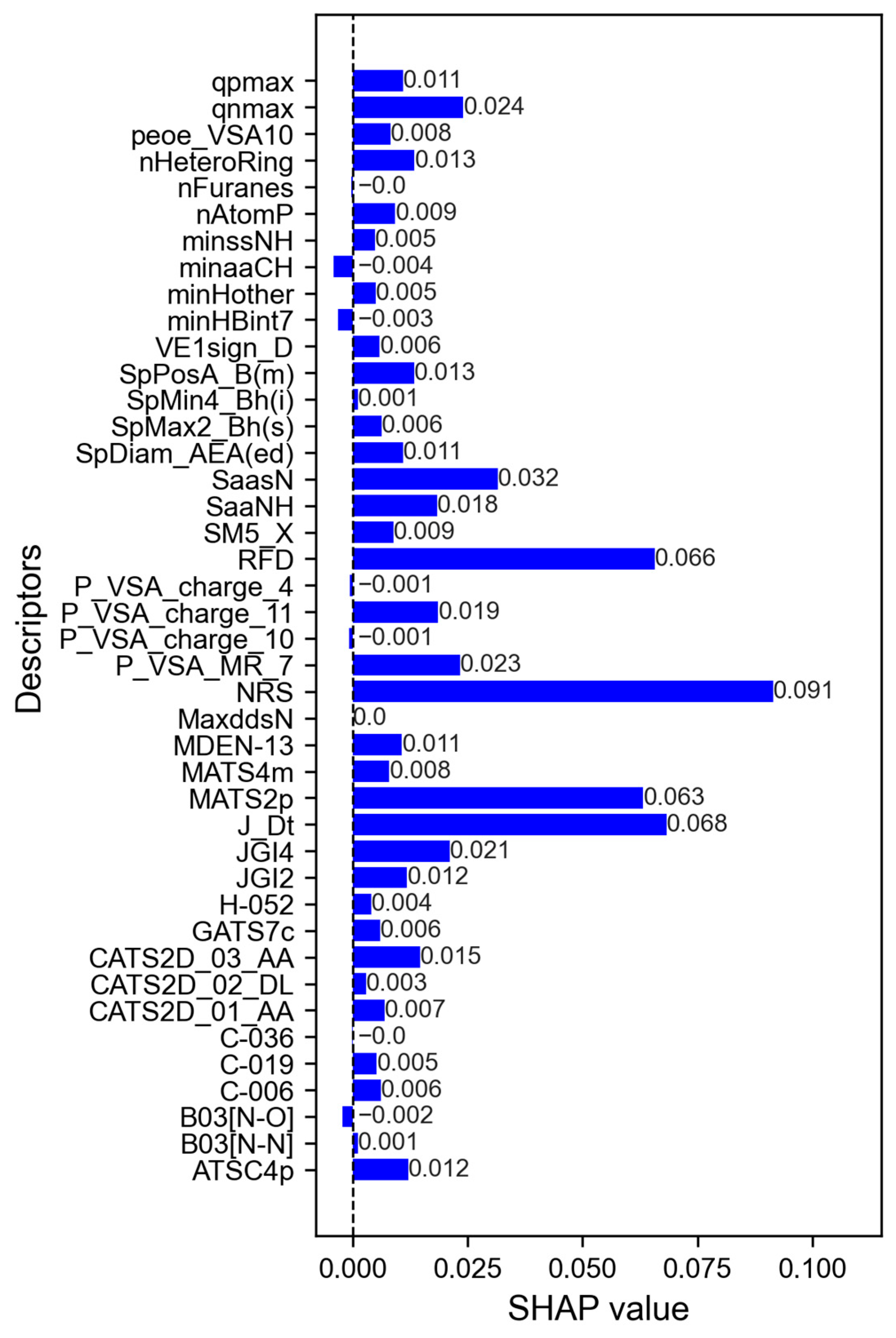
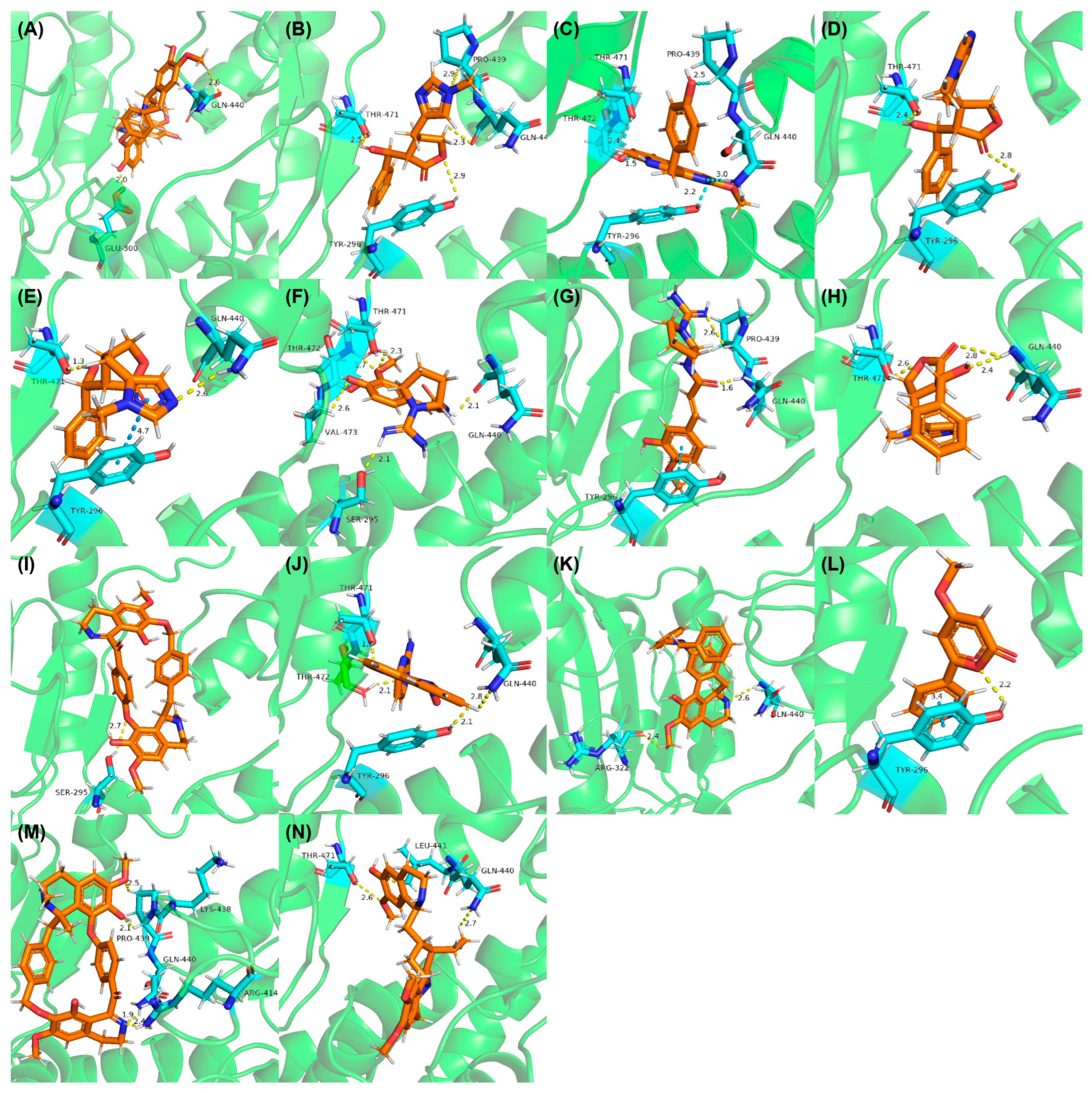
| Models | Ac (%) | κ | ROC Curve | SE | SP | Coverage (%) | |
|---|---|---|---|---|---|---|---|
| RF | Tr | 100 | 1 | 1 | 1 | 1 | |
| CV | 90.4 | 0.81 | 0.96 | 0.88 | 0.94 | ||
| Ts | 90.3 | 0.81 | 0.96 | 0.87 | 0.94 | 100% | |
| J48/AdaboostM1 | Tr | 100 | 1 | 1 | 1 | 1 | |
| CV | 91.9 | 0.84 | 0.96 | 0.89 | 0.95 | ||
| Ts | 91.8 | 0.84 | 0.96 | 0.91 | 0.93 | 100% |
| Alkaloids | Pcm (%) | Alkaloids | Pcm (%) |
|---|---|---|---|
| Episiopiloturine | 89.2 | Lindoldhamine | 77.5 |
| N-Hydroxyannomontine | 87.2 | Catuabine I | 72.8 |
| Epiisopilosine | 86.8 | 7α-hydroxycatuabine H | 69.1 |
| Isopilosine | 86.8 | 7β-hydroxycatuabine H | 69.1 |
| Pilosine | 86.8 | Cis-N-Oxycodamine | 68.3 |
| Daibucarboline A | 83.5 | Cephaeline | 67.9 |
| Anibine | 82.7 | Emetine | 67.9 |
| Des-7-O-methylroraimine | 81.5 | Vaccinine B | 67.5 |
| Epi-des-7-O-methylroraimine | 81.5 | Siamine | 65.5 |
| Cernumidine | 79.9 | Tueiaoine | 63.1 |
| Isocernumidine | 79.5 | Alstomicine | 56.4 |
| Variabiline | 77.5 |
| Structures | Alkaloids | Pcm (%) | Ps (%) | Pc (%) |
|---|---|---|---|---|
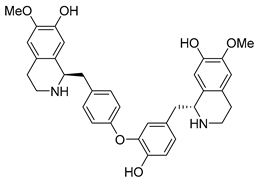 | Lindoldhamine | 77.52 | 100 | 85.18 |
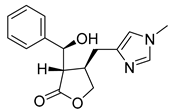 | Episiopiloturine | 89.17 | 51.26 | 76.25 |
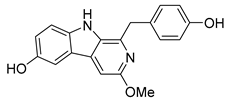 | Daibucarboline A | 83.55 | 60.85 | 75.81 |
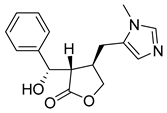 | Epiisopilosine | 86.76 | 50.16 | 74.29 |
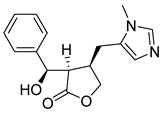 | Pilosine | 86.76 | 44.96 | 72.52 |
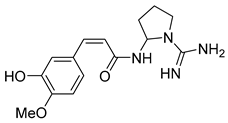 | Isocernumidine | 79.53 | 50.06 | 69.49 |
 | Cernumidine | 79.94 | 46.16 | 68.43 |
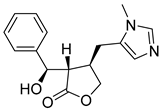 | Isopilosine | 86.76 | 31.76 | 68.02 |
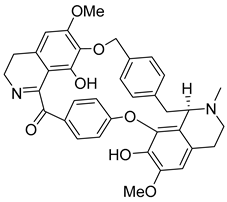 | Epi-des-7-O-methylroraimine | 81.54 | 39.86 | 67.34 |
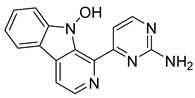 | N-Hydroxyannomontine | 87.16 | 18.66 | 63.82 |
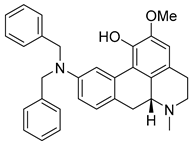 | Variabiline | 77.53 | 36.15 | 63.43 |
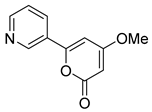 | Anibine | 82.74 | 25.86 | 63.36 |
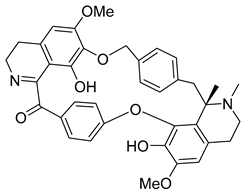 | Des-7-O-methylroraimine | 81.54 | 26.15 | 62.67 |
 | Emetine | 48.39 | 25.86 | 61.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paixão, V.V.M.; Santos, Y.J.A.; Fernandes, A.O.; Conceição, E.S.; Rodrigues, R.P.; Chagas-Paula, D.A.; Dolabella, S.S.; Oliveira, T.B. Novel Antischistosomal Drug Targets: Identification of Alkaloid Inhibitors of SmTGR via Integrated In Silico Methods. Pathogens 2025, 14, 591. https://doi.org/10.3390/pathogens14060591
Paixão VVM, Santos YJA, Fernandes AO, Conceição ES, Rodrigues RP, Chagas-Paula DA, Dolabella SS, Oliveira TB. Novel Antischistosomal Drug Targets: Identification of Alkaloid Inhibitors of SmTGR via Integrated In Silico Methods. Pathogens. 2025; 14(6):591. https://doi.org/10.3390/pathogens14060591
Chicago/Turabian StylePaixão, Valéria V. M., Yria J. A. Santos, Adriana O. Fernandes, Elaine S. Conceição, Ricardo P. Rodrigues, Daniela A. Chagas-Paula, Silvio S. Dolabella, and Tiago B. Oliveira. 2025. "Novel Antischistosomal Drug Targets: Identification of Alkaloid Inhibitors of SmTGR via Integrated In Silico Methods" Pathogens 14, no. 6: 591. https://doi.org/10.3390/pathogens14060591
APA StylePaixão, V. V. M., Santos, Y. J. A., Fernandes, A. O., Conceição, E. S., Rodrigues, R. P., Chagas-Paula, D. A., Dolabella, S. S., & Oliveira, T. B. (2025). Novel Antischistosomal Drug Targets: Identification of Alkaloid Inhibitors of SmTGR via Integrated In Silico Methods. Pathogens, 14(6), 591. https://doi.org/10.3390/pathogens14060591






