The Effects of Low Concentrations of Nisin on Biofilm Formation by Staphylococcus aureus Isolated from Dairy Cattle †
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagent Preparation for Biofilm, Gene Expression and SEM Analysis
- -
- nisin: 39, 19, and 9 IU/mL
- -
- tetracycline 0.06 μg/mL
- -
- mixture of nisin and tetracycline: 39 IU/mL + 0.06 μg/mL, 18 IU/mL + 0.06 μg/mL, and 9 IU/mL + 0.06 μg/mL.
2.2. Strains
2.3. Minimum Inhibitory Concentration Analysis for Nisin
2.4. Biofilm Formation Assay
2.5. Gene Expression of the icaD Gene
2.6. Scanning Electron Microscopy
2.7. Statistical Analysis
3. Results
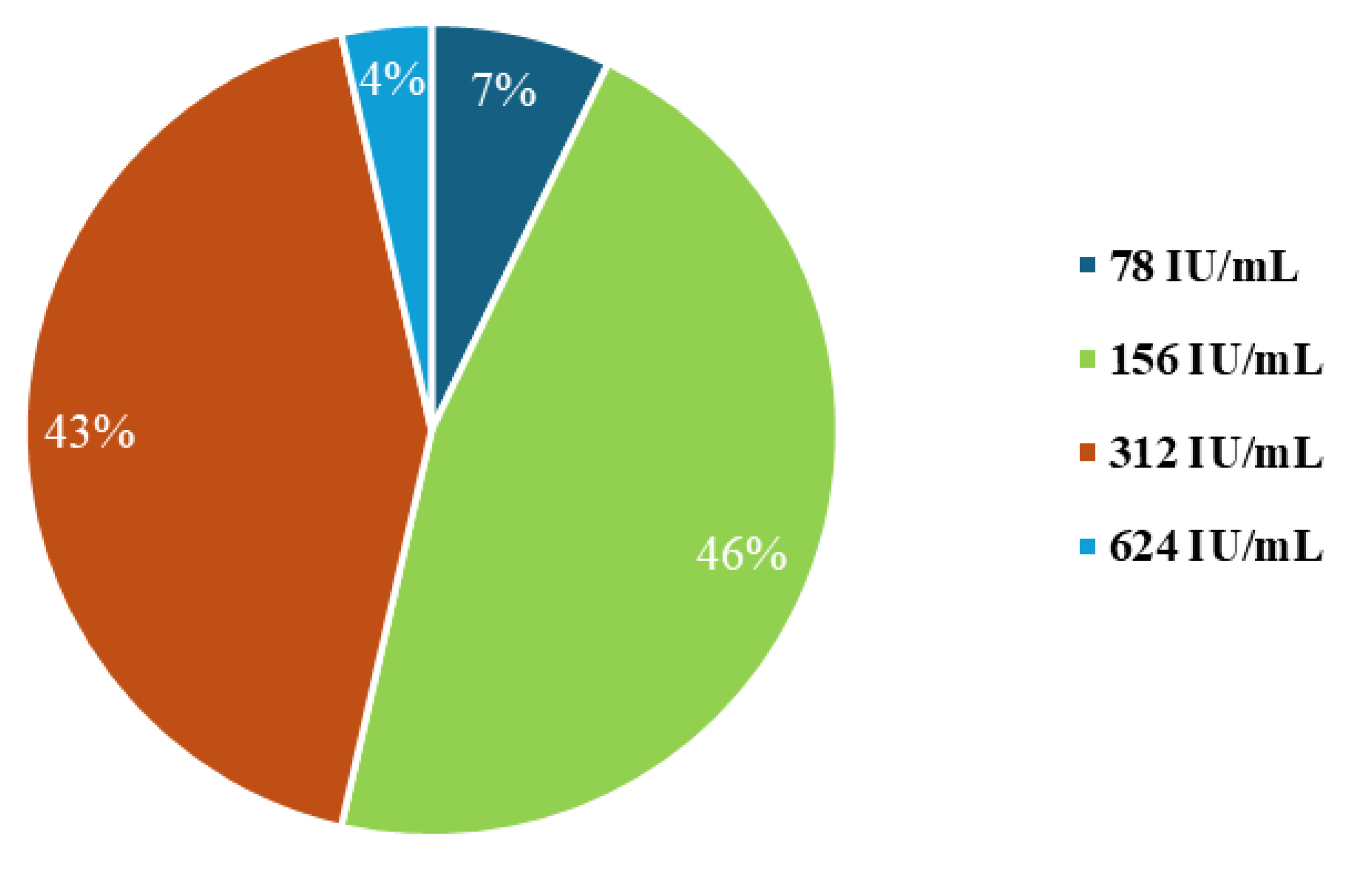
3.1. Minimum Inhibitory Concentration Analysis for Nisin
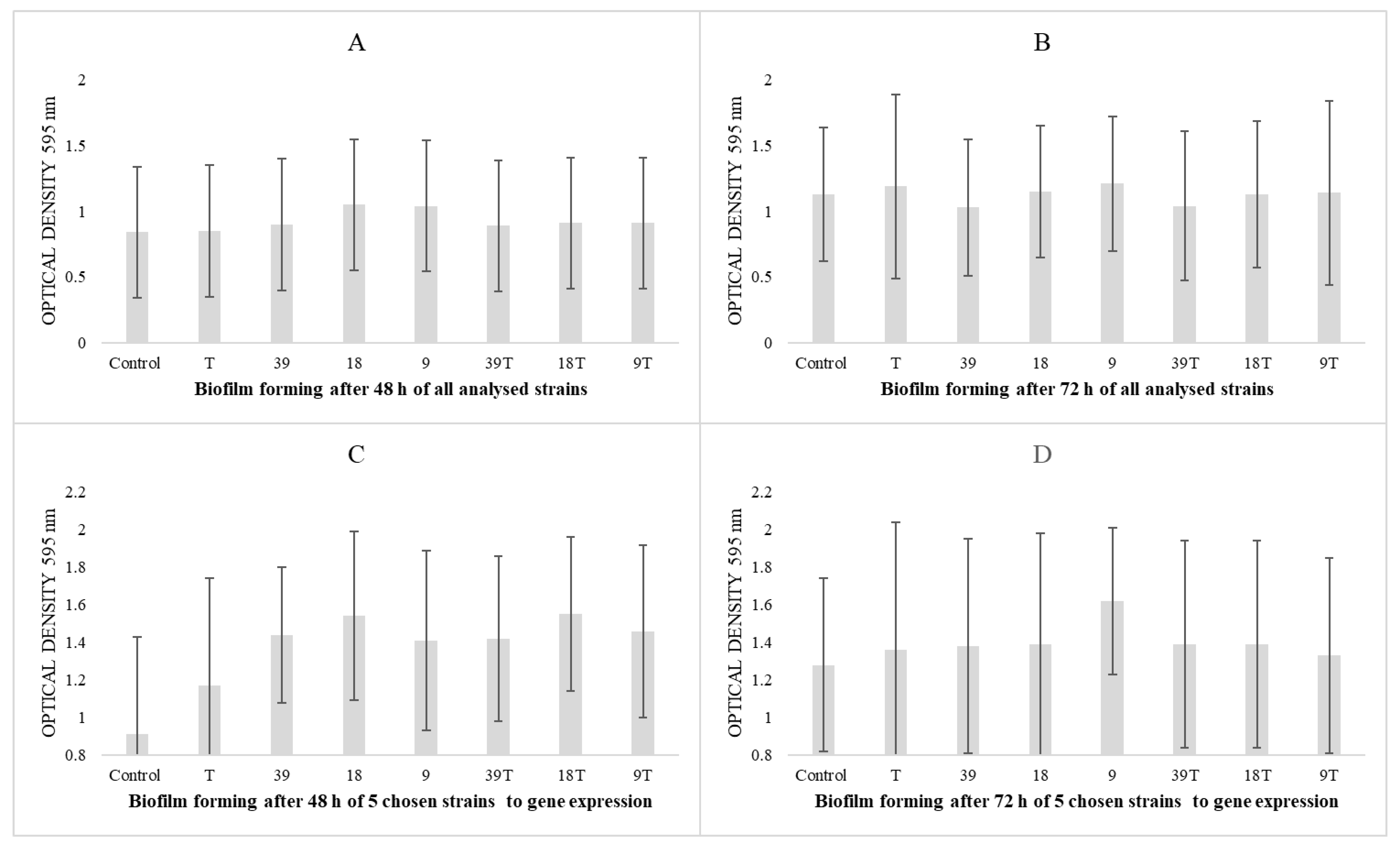
3.2. Results of Biofilm-Forming Ability
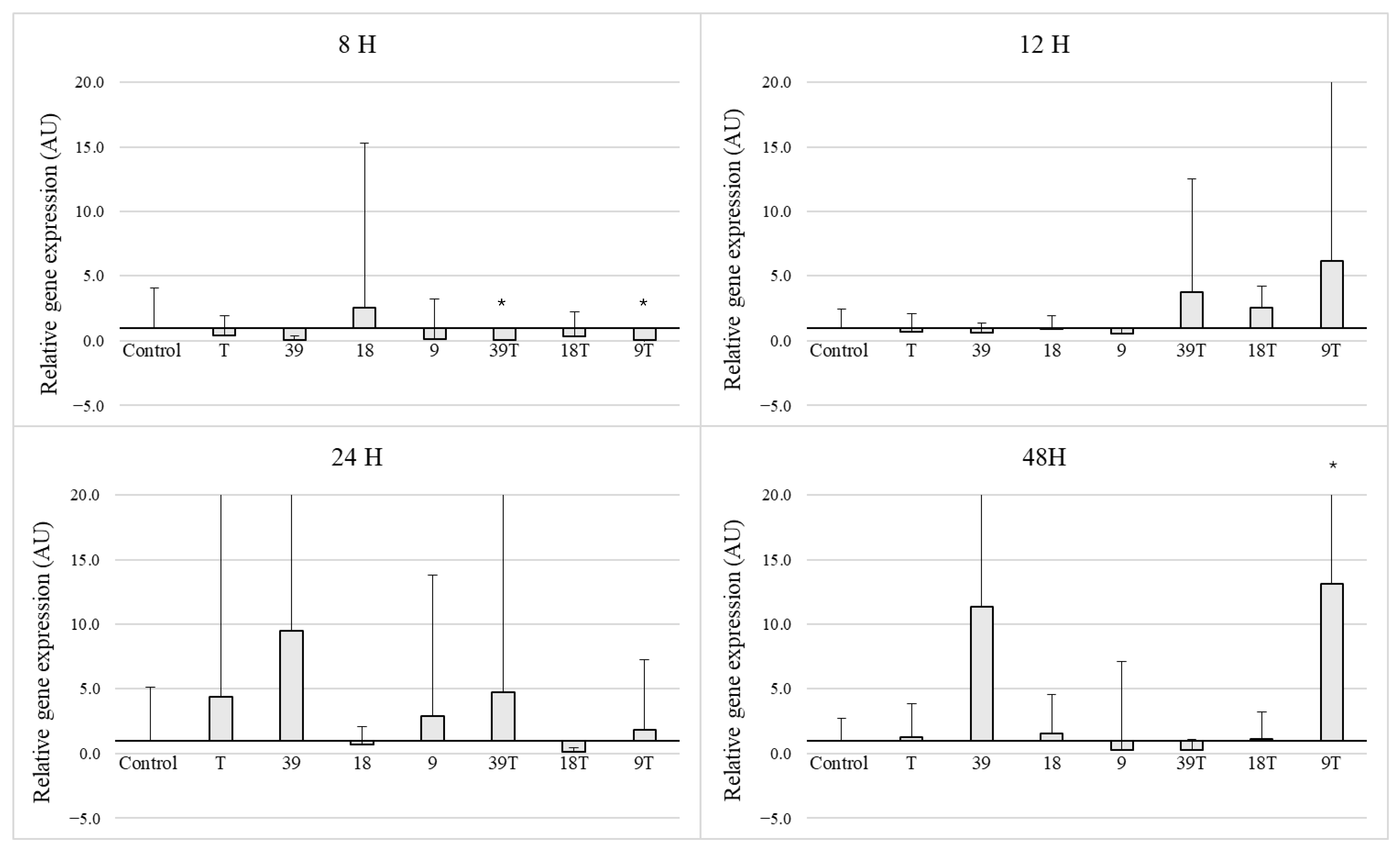
3.3. Expression of the icaD Gene in 5 Selected Strains
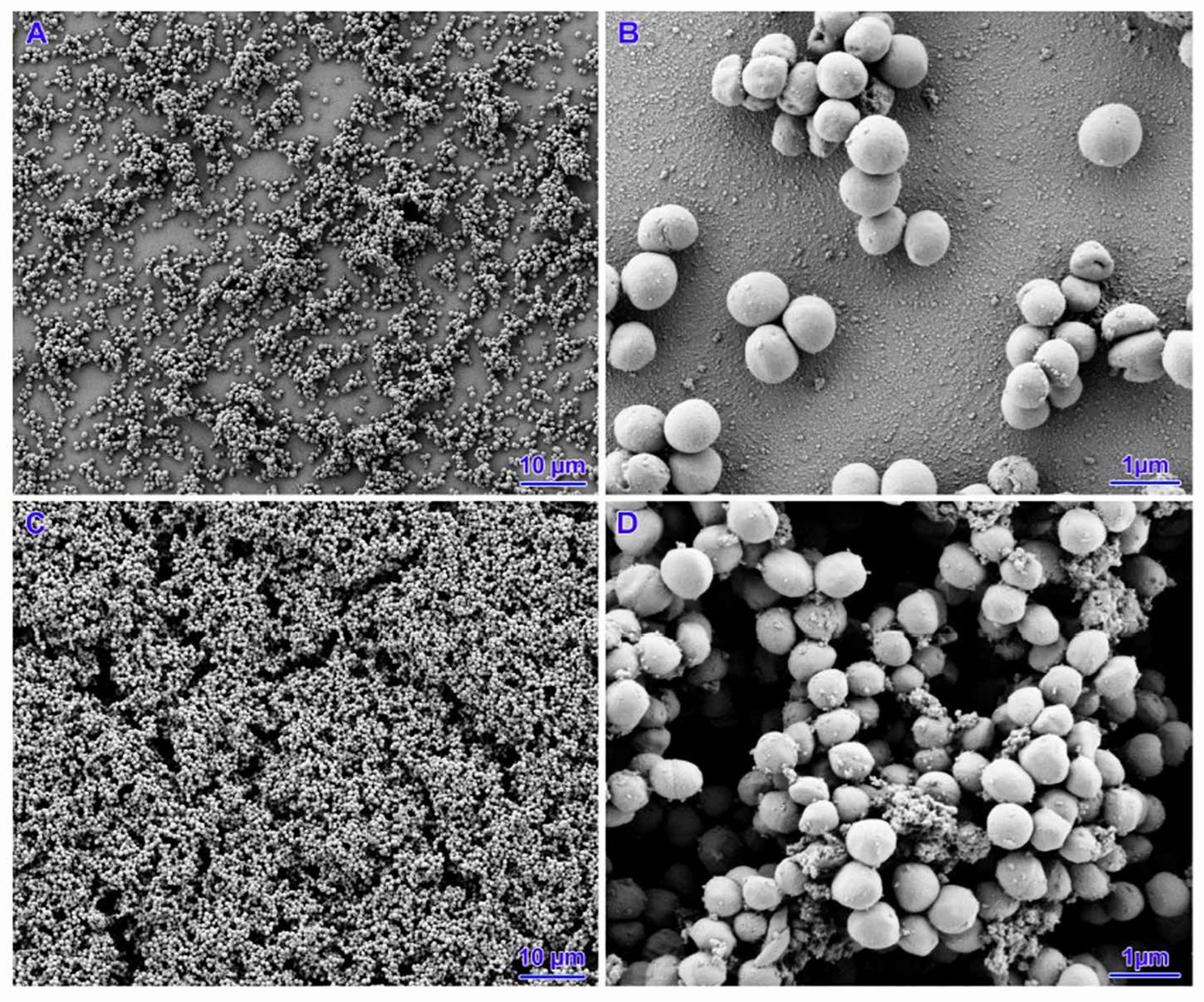
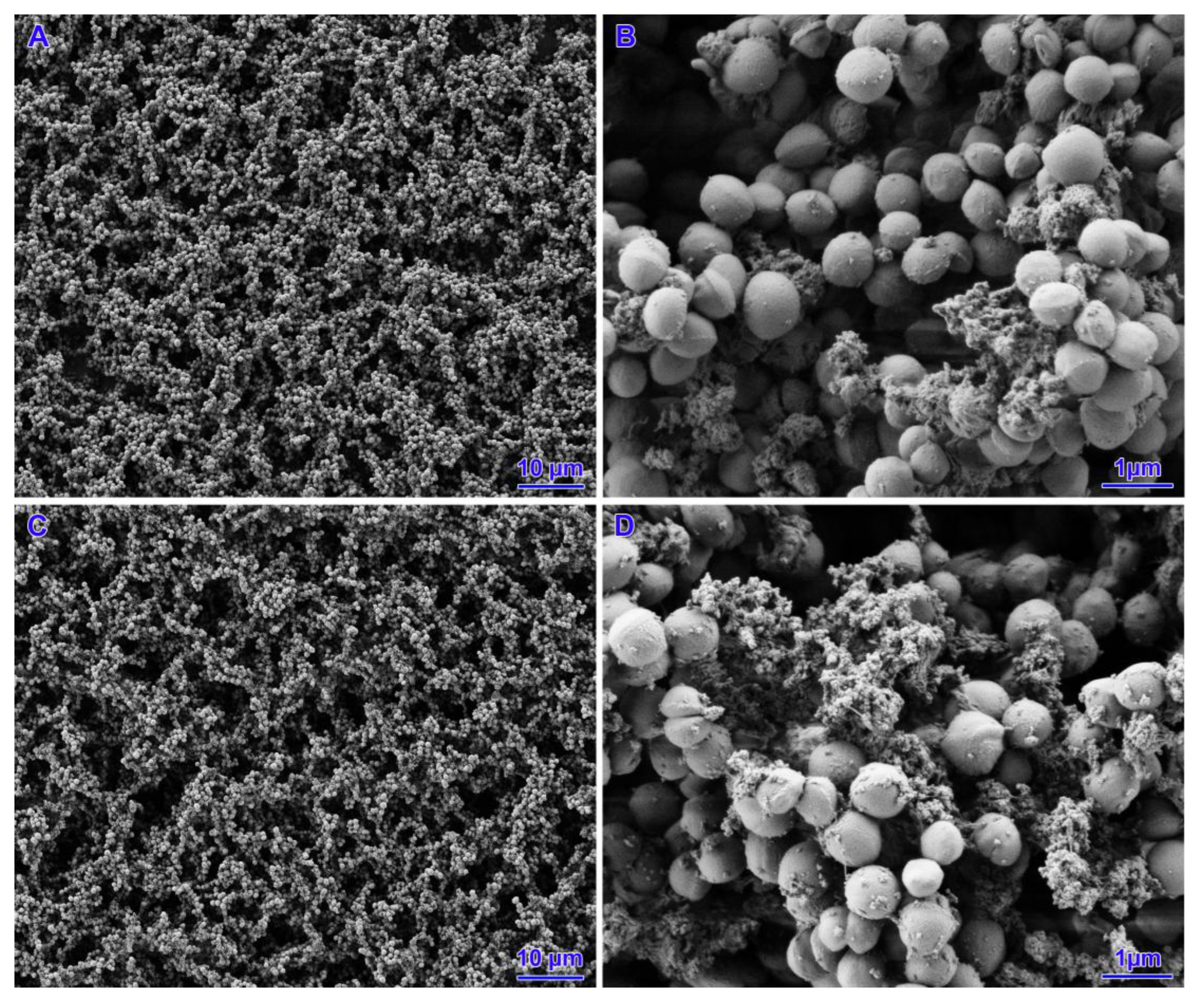
3.4. Scanning Electron Microscopy
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMR | antimicrobial resistance |
| AU | arbituary units |
| CAMHB | Cation Adjusted Mueller Hinton Broth |
| MIC | minimum inhibitory concentration |
| PBS | phosphate-buffered saline |
| EPS | exopolysaccharides |
| TSB | tryptic soy broth |
| kV | kilovolt |
| SEM | scanning electron microscopy |
| EPS | exopolysaccharide |
References
- Peton, V.; Le Loir, Y. Staphylococcus aureus in veterinary medicine. Infect. Genet. Evol. 2014, 21, 602–615. [Google Scholar] [CrossRef] [PubMed]
- Campos, B.; Pickering, A.C.; Rocha, L.S.; Aguilar, A.P.; Fabres-Klein, M.H.; de Oliveira Mendes, T.A.; Fitzgerald, J.R.; de Oliveira Barros Ribon, A. Diversity and pathogenesis of Staphylococcus aureus from bovine mastitis: Current understanding and future perspectives. BMC Vet. Res. 2022, 18, 115. [Google Scholar] [CrossRef]
- Kaczorek-Łukowska, E.; Małaczewska, J.; Sowińska, P.; Szymańska, M.; Wójcik, E.A.; Siwicki, A.K. Staphylococcus aureus from Subclinical Cases of Mastitis in Dairy Cattle in Poland, What Are They Hiding? Antibiotic Resistance and Virulence Profile. Pathogens 2022, 11, 1404. [Google Scholar] [CrossRef] [PubMed]
- Kwon, A.S.; Park, G.C.; Ryu, S.Y.; Lim, D.H.; Lim, D.Y.; Choi, C.H.; Park, Y.; Lim, Y. Higher biofilm formation in multidrug-resistant clinical isolates of Staphylococcus aureus. Int. J. Antimicrob. Agents 2008, 32, 68–72. [Google Scholar] [CrossRef]
- Mirghani, R.; Saba, T.; Khaliq, H.; Mitchell, J.; Do, L.; Chambi, L.; Diaz, K.; Kennedy, T.; Alkassab, K.; Huynh, T.; et al. Biofilms: Formation, drug resistance and alternatives to conventional approaches. AIMS Microbiol. 2022, 8, 239–277. [Google Scholar] [CrossRef]
- Shin, J.M.; Gwak, J.W.; Kamarajan, P.; Fenno, J.C.; Rickard, A.H.; Kapila, Y.L. Biomedical applications of nisin. J. Appl. Microbiol. 2016, 120, 1449–1465. [Google Scholar] [CrossRef]
- Fernandes, A.; Jobby, R. Bacteriocins from lactic acid bacteria and their potential clinical applications. Appl. Biochem. Biotechnol. 2022, 194, 4377–4399. [Google Scholar] [CrossRef]
- Waack, U.; Nicholson, T.L. Subinhibitory Concentrations of Amoxicillin, Lincomycin, and Oxytetracycline Commonly Used to Treat Swine Increase Streptococcus suis Biofilm Formation. Front. Microbiol. 2018, 9, 2707. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Fang, J.; Tian, Y.; Lu, X.Y. Mechanisms of nisin resistance in Gram-positive bacteria. Ann. Microbiol. 2014, 64, 413–420. [Google Scholar] [CrossRef]
- Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gundert-Remy, U.; Kuhnle, G.G.; et al. Safety of nisin (E234) as a food additive in the light of new toxicological data and the proposed extension of use. Efsa J. 2017, 15, e05063. [Google Scholar]
- CLSI document M100-S23; Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Third Informational Supplement. Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2013.
- Ebrahimi, A.; Moatamedi, A.; Lotfalian, S.; Mirshokraei, P. Biofilm formation, hemolysin production and antimicrobial susceptibilities of Streptococcus agalactiae isolated from the mastitis milk of dairy cows in Shahrekord district, Iran. Vet. Res. Forum 2013, 4, 269–272. [Google Scholar] [PubMed]
- Duquenne, M.; Fleurot, I.; Aigle, M.; Darrigo, C.; Borezée-Durant, E.; Derzelle, S.; Bouix, M.; Deperrois-Lafarge, V.; Delacroix-Buchet, A. Tool for quantification of staphylococcal enterotoxin gene expression in cheese. Appl. Env. Environ. Microbiol. 2010, 76, 1367–1374. [Google Scholar] [CrossRef]
- Kot, B.; Sytykiewicz, H.; Sprawka, I. Expression of the Biofilm-Associated Genes in Methicillin-Resistant Staphylococcus aureus in Biofilm and Planktonic Conditions. Int. J. Mol. Sci. 2018, 19, 3487. [Google Scholar] [CrossRef] [PubMed]
- Prinzi, A.; Rohde, R. ASM Articles 2023. Available online: https://asm.org/articles/2023/march/the-role-of-bacterial-biofilms-in-antimicrobial-re (accessed on 13 May 2024).
- Latorre, A.A.; Van Kessel, J.S.; Karns, J.S.; Zurakowski, M.J.; Pradhan, A.K.; Boor, K.J.; Jayarao, B.M.; Houser, B.A.; Daugherty, C.S.; Schukken, Y.H. Biofilm in milking equipment on a dairy farm as a potential source of bulk tank milk contamination with Listeria monocytogenes. J. Dairy. Sci. 2010, 93, 2792–2802. [Google Scholar] [CrossRef]
- Michael, C.K.; Lianou, D.T.; Tsilipounidaki, K.; Gougoulis, D.A.; Giannoulis, T.; Vasileiou, N.G.C.; Mavrogianni, V.S.; Petinaki, E.; Fthenakis, G.C. Recovery of Staphylococci from Teatcups in Milking Parlours in Goat Herds in Greece: Prevalence, Identification, Biofilm Formation, Patterns of Antibiotic Susceptibility, Predictors for Isolation. Antibiotics 2023, 12, 1428. [Google Scholar] [CrossRef]
- Liu, J.; Huang, T.; Xu, Z.; Mao, Y.; Soteyome, T.; Liu, G.; Qu, C.; Yuan, L.; Ma, Q.; Zhou, F.; et al. Sub-MIC streptomycin and tetracycline enhanced Staphylococcus aureus Guangzhou-SAU749 biofilm formation, an in-depth study on transcriptomics. Biofilm 2023, 6, 100156. [Google Scholar] [CrossRef] [PubMed]
- Andersson, D.I.; Hughes, D. Microbiological effects of sublethal levels of antibiotics. Nat. Rev. Microbiol. 2014, 12, 465–478. [Google Scholar] [CrossRef]
- Azzam, A.; Shawky, R.M.; El-Mahdy, T.S. Sub-inhibitory concentrations of ceftriaxone induce morphological alterations and PIA-independent biofilm formation in Staphylococcus aureus. Braz. J. Microbiol. 2024, 55, 297–308. [Google Scholar] [CrossRef]
- Sudagidan, M.; Yemenicioğlu, A. Effects of nisin and lysozyme on growth inhibition and biofilm formation capacity of Staphylococcus aureus strains isolated from raw milk and cheese samples. J. Food Prot. 2012, 75, 1627–1633. [Google Scholar] [CrossRef]
- Pérez-Ibarreche, M.; Field, D.; Ross, R.P.; Hill, C. A Bioengineered Nisin Derivative To Control Streptococcus uberis Biofilms. Appl. Environ. Microbiol. 2021, 87, e0039121. [Google Scholar] [CrossRef]
- Ghapanvari, P.; Taheri, M.; Jalilian, F.A.; Dehbashi, S.; Dezfuli, A.A.Z.; Arabestani, M.R. The effect of nisin on the biofilm production, antimicrobial susceptibility and biofilm formation of Staphylococcus aureus and Pseudomonas aeruginosa. Eur. J. Med. Res. 2022, 27, 173. [Google Scholar] [CrossRef]
- Jesus, C.; Soares, R.; Cunha, E.; Grilo, M.; Tavares, L.; Oliveira, M. Influence of Nisin-Biogel at Subinhibitory Concentrations on Virulence Expression in Staphylococcus aureus Isolates from Diabetic Foot Infections. Antibiotics 2021, 10, 1501. [Google Scholar] [CrossRef] [PubMed]
- Angelopoulou, A.; Field, D.; Pérez-Ibarreche, M.; Warda, A.K.; Hill, C.; Ross, R.P. Vancomycin and nisin A are effective against biofilms of multi-drug resistant Staphylococcus aureus isolates from human milk. PLoS ONE 2020, 15, e0233284. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Jain, A. Glucose & sodium chloride induced biofilm production & ica operon in clinical isolates of staphylococci. Indian. J. Med. Res. 2013, 138, 262–266. [Google Scholar]
- Resch, A.; Rosenstein, R.; Nerz, C.; Götz, F. Differential gene expression profiling of Staphylococcus aureus cultivated under biofilm and planktonic conditions. Appl. Env. Microbiol. 2005, 71, 2663–2676. [Google Scholar] [CrossRef] [PubMed]
- O′Gara, J.P. ica and beyond: Biofilm mechanisms and regulation in Staphylococcus epidermidis and Staphylococcus aureus. FEMS Microbiol. Lett. 2007, 270, 179–188. [Google Scholar] [CrossRef]
- Conlon, K.M.; Humphreys, H.; O′Gara, J.P. icaR encodes a transcriptional repressor involved in environmental regulation of ica operon expression and biofilm formation in Staphylococcus epidermidis. J. Bacteriol. 2002, 184, 4400–4408. [Google Scholar] [CrossRef]
- Jeng, W.Y.; Ko, T.P.; Liu, C.I.; Guo, R.T.; Liu, C.L.; Shr, H.L.; Wang, A.H. Crystal structure of IcaR, a repressor of the TetR family implicated in biofilm formation in Staphylococcus epidermidis. Nucleic Acids Res. 2008, 36, 1567–1577. [Google Scholar] [CrossRef]
- Kaczorek-Łukowska, E.; Foksiński, P.; Małaczewska, J.; Wójcik, R.; Szyryńska, N. Is nisine future salvation or a potential threat? Results of a study of the effect of low concentrations of nisin on ability on biofilm formation by Staphylococcus aureus isolated from dairy cattle. In Proceedings of the XVII Congress of the Polish Society of Veterinary Sciences: “Sanitas Animalium pro Salute Homini”, Olsztyn, Poland, 19–21 September 2024. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaczorek-Łukowska, E.; Foksiński, P.; Małaczewska, J.; Wójcik, R.; Szyryńska, N. The Effects of Low Concentrations of Nisin on Biofilm Formation by Staphylococcus aureus Isolated from Dairy Cattle. Pathogens 2025, 14, 566. https://doi.org/10.3390/pathogens14060566
Kaczorek-Łukowska E, Foksiński P, Małaczewska J, Wójcik R, Szyryńska N. The Effects of Low Concentrations of Nisin on Biofilm Formation by Staphylococcus aureus Isolated from Dairy Cattle. Pathogens. 2025; 14(6):566. https://doi.org/10.3390/pathogens14060566
Chicago/Turabian StyleKaczorek-Łukowska, Edyta, Paweł Foksiński, Joanna Małaczewska, Roman Wójcik, and Natalia Szyryńska. 2025. "The Effects of Low Concentrations of Nisin on Biofilm Formation by Staphylococcus aureus Isolated from Dairy Cattle" Pathogens 14, no. 6: 566. https://doi.org/10.3390/pathogens14060566
APA StyleKaczorek-Łukowska, E., Foksiński, P., Małaczewska, J., Wójcik, R., & Szyryńska, N. (2025). The Effects of Low Concentrations of Nisin on Biofilm Formation by Staphylococcus aureus Isolated from Dairy Cattle. Pathogens, 14(6), 566. https://doi.org/10.3390/pathogens14060566







