Cloning and Expression of a Truncated Form of the p72 Protein of the African Swine Fever Virus (ASFV) for Application in an Efficient Indirect ELISA System
Abstract
1. Introduction
2. Materials and Methods
2.1. Reference Swine Serum Samples
2.2. In Silico and Bioinformatics Analysis of ASF-p72-Truncated Protein
2.3. Cloning, Subcloning, and Overexpression of ASF-p72-Truncated Protein
2.4. Antigenic Evaluation of ASF-p72-Truncated Protein in a Mouse Model
2.5. Standardization of Indirect ELISA (iELISA) of ASF-p72-Truncated Protein with Pig Serum Samples
2.6. Statistical Analysis (Sensitivity, Specificity, and Index Kappa) of iELISA
2.7. Determination of the iELISA Cut-Off Point
3. Results
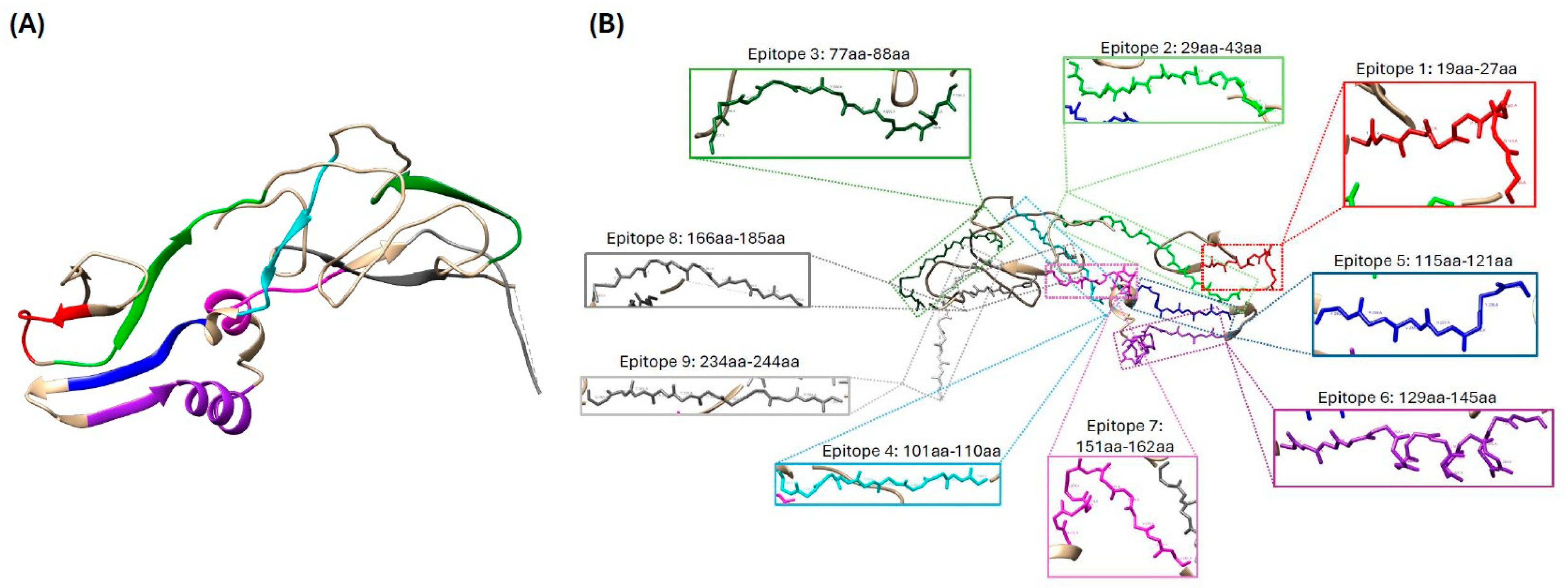
3.1. In Silico Analysis of ASF-p72-Truncated Protein
3.2. Bioinformatics Analysis of ASF-p72-Truncated Protein
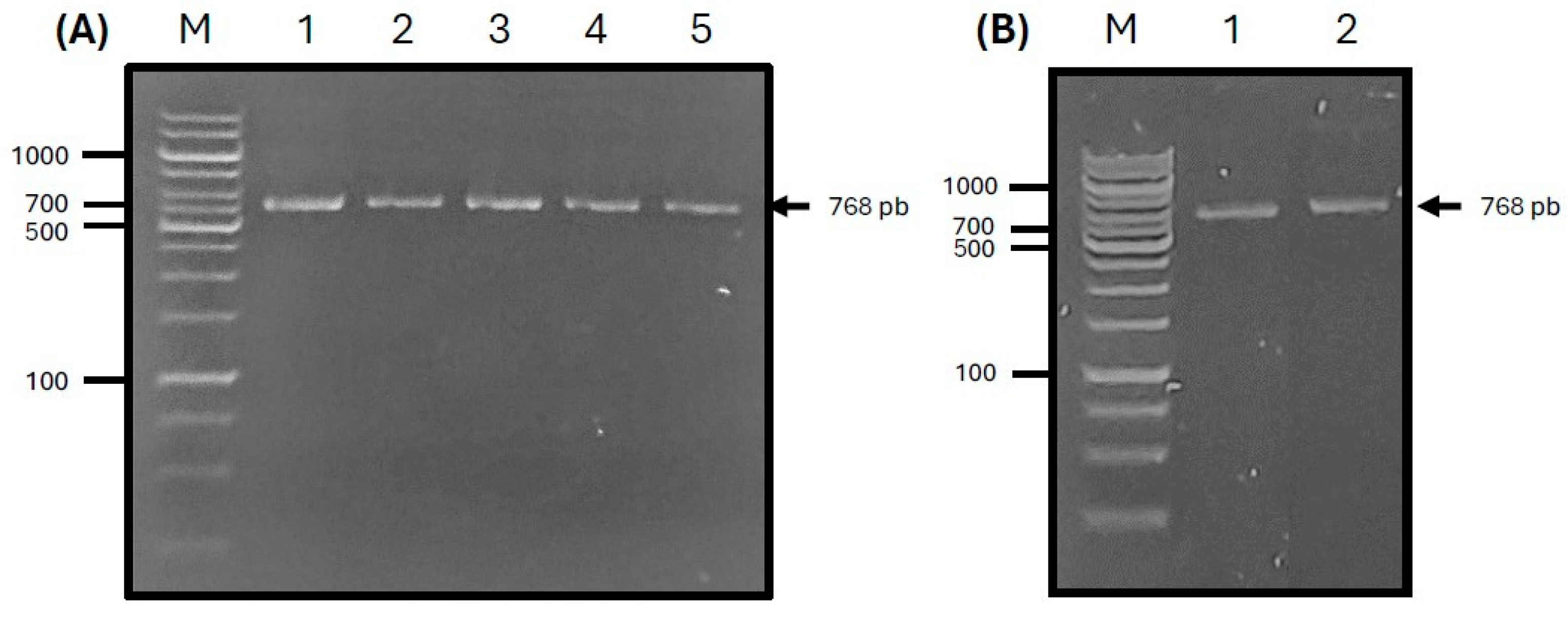
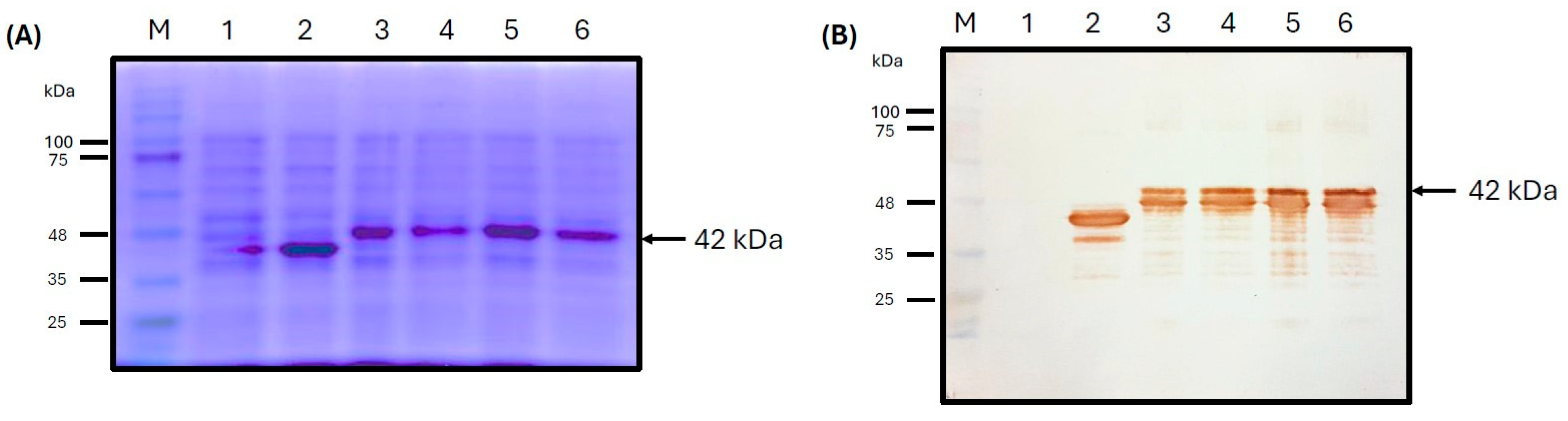
3.3. Cloning and Expression of ASF-p72-Truncated Protein
3.4. Antigenicity of ASF-p72-Truncated Protein by Mice Immunization
3.5. Standardization and Validation of Indirect ELISA (iELISA) of ASF-p72-Truncated Protein
3.6. Determination of Sensitivity, Specificity, and Concordance of iELISA
3.7. Determination of Cut-Off Values
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASF | African swine fever |
| ASFV | African swine fever virus |
| CReSA | Centre de Recerca en Sanitat Animal |
| CV | coefficient of variation |
| DP | double positive |
| ELISA | enzyme-linked immunosorbent assay |
| EURL | European Union Reference Laboratory |
| HRP | horseradish peroxidase |
| IB | immunoblotting |
| iELISA | indirect enzyme-linked immunosorbent assay |
| IIF | indirect immunofluorescence |
| IMAC | immobilized metal affinity chromatography |
| IPT | indirect immunoperoxidase test |
| IPTG | isopropyl β-D-1-thiogalactopyranoside |
| IRTA | Institute for Research and Technology in Food and Agriculture |
| MAb | monoclonal antibody |
| OIE | Organization for Animal Health |
| ORF | open reading frame |
| SC | subcutaneous |
| SD | standard deviations |
| TMB | 3,3′,5,5′-tetramethylbenzidine |
| κ | kappa |
References
- Sánchez-Cordón, P.J.; Montoya, M.; Reis, A.L.; Dixon, L.K. African swine fever: A re-emerging viral disease threatening the global pig industry. Vet. J. 2018, 233, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, M.C.; Reoyo, A.T.; Fernández-Pinero, J.; Iglesias, I.; Muñoz, M.J.; Arias, M.L. African swine fever: A global view of the current challenge. Porcine Health Manag. 2015, 1, 21. [Google Scholar] [CrossRef]
- Ge, S.; Li, J.; Fan, X.; Liu, F.; Li, L.; Wang, Q.; Ren, W.; Bao, J.; Liu, C.; Wang, H.; et al. Molecular characterization of African swine fever virus, China, 2018. Emerg. Infect. Dis. 2018, 24, 2131–2133. [Google Scholar] [CrossRef] [PubMed]
- Global African Swine Fever Research Alliance. Available online: https://www.ars.usda.gov/GARA/reports.htm (accessed on 21 April 2025).
- Gonzales, W.; Moreno, C.; Duran, U.; Henao, N.; Bencosme, M.; Lora, P.; Reyes, R.; Núñez, R.; De Gracia, A.; Perez, A.M. African swine fever in the Dominican Republic. Transbound. Emerg. Dis. 2021, 68, 3018–3019. [Google Scholar] [CrossRef] [PubMed]
- Dixon, L.K.; Escribano, J.M.; Martins, C.; Rock, D.L.; Salas, M.L.; Wilkinson, P.J. Asfarviridae. In Virus Taxonomy, VIIIth Report of the ICTV; Fauquet, C.M., Mayo, M.A., Maniloff, J., Desselberger, U., Ball, L.A., Eds.; Elsevier/Academic Press: London, UK, 2005; pp. 135–143. [Google Scholar]
- Anderson, E.C.; Hutchings, G.H.; Mukarati, N.; Wilkinson, P.J. African swine fever virus infection of the bushpig (Potamochoerus porcus) and its significance in the epidemiology of the disease. Vet. Microbiol. 1998, 62, 1–15. [Google Scholar] [CrossRef]
- Kleiboeker, S.B.; Scoles, G.A.; Burrage, T.G.; Sur, J. African swine fever virus replication in the midgut epithelium is required for infection of ornithodoros ticks. J. Virol. 1999, 73, 8587–8598. [Google Scholar] [CrossRef]
- Duan, X.; Ru, Y.; Yang, W.; Ren, J.; Hao, R.; Qin, X.; Li, D.; Zheng, H. Research progress on the proteins involved in african swine fever virus infection and replication. Front. Immunol. 2022, 13, 947180. [Google Scholar] [CrossRef]
- Alejo, A.; Matamoros, T.; Guerra, M.; Andres, G. A proteomic atlas of the african swine fever virus particle. J. Virol. 2018, 92, e01293-18. [Google Scholar] [CrossRef]
- Spinard, E.; Dinhobl, M.; Tesler, N.; Birtley, H.; Signore, A.V.; Ambagala, A.; Masembe, C.; Borca, M.V.; Gladue, D.P. A Re-Evaluation of African Swine Fever Genotypes Based on P72 Sequences Reveals the Existence of Only Six Distinct P72 Groups. Viruses 2023, 15, 2246. [Google Scholar] [CrossRef]
- Wang, Y.; Kang, W.; Yang, W.; Zhang, J.; Li, D.; Zheng, H. Structure of African Swine Fever Virus and Associated Molecular Mechanisms Underlying Infection and Immunosuppression: A Review. Front. Immunol. 2021, 12, 715582. [Google Scholar] [CrossRef]
- Liu, Q.; Ma, B.; Qian, N.; Zhang, F.; Tan, X.; Lei, J.; Xiang, Y. Structure of the African swine fever virus major capsid protein p72. Cell Res. 2019, 29, 953–955. [Google Scholar] [CrossRef]
- Oura, C.A.L.; Denyer, M.S.; Takamatsu, H.; Parkhouse, R.M.E. In vivo depletion of CD8+ T lymphocytes abrogates protective immunity to African swine fever virus. J. Gen. Virol. 2005, 86, 2445–2450. [Google Scholar] [CrossRef]
- Montoya, M.; Franzoni, G.; Pérez-Nuñez, D.; Revilla, Y.; Galindo, I.; Alonso, C.; Netherton, C.L.; Blohm, U. 3. Immune responses against African swine fever virus infection. In Understanding and Combatting African Swine Fever; Brill: Leiden, The Netherlands, 2021. [Google Scholar] [CrossRef]
- Gladue, D.P.; Borca, M.V. Recombinant ASF Live Attenuated Virus Strains as Experimental Vaccine Candidates. Viruses 2022, 14, 878. [Google Scholar] [CrossRef]
- Revilla, Y.; Pérez-Núñez, D.; Richt, J.A. African Swine Fever Virus Biology and Vaccine Approaches. Adv. Virus Res. 2018, 100, 41–74. [Google Scholar] [CrossRef] [PubMed]
- Oura, C.A.; Edwards, L.; Batten, C.A. Virological diagnosis of African swine fever--comparative study of available tests. Virus Res. 2013, 173, 150–158. [Google Scholar] [CrossRef] [PubMed]
- VALIDATION GUIDELINE 3.6.2.: Development and Optimisation of Antigen Detection Assays. Available online: https://www.woah.org/fileadmin/Home/eng/Health_standards/aahm/current/GUIDELINE_3.6.2_ANTIGEN_DETECT.pdf (accessed on 21 April 2025).
- Muzykina, L.; Barrado-Gil, L.; Gonzalez-Bulnes, A.; Crespo-Piazuelo, D.; Cerón, J.J.; Alonso, C.; Montoya, M. Overview of Modern Commercial Kits for Laboratory Diagnosis of African Swine Fever and Swine Influenza A Viruses. Viruses 2024, 16, 505. [Google Scholar] [CrossRef] [PubMed]
- African Swine Fever (Infection with African Swine Fever Virus). Available online: https://www.gob.mx/cms/uploads/attachment/file/870460/e-bsc-23_anexo16.pdf (accessed on 21 April 2025).
- Onisk, D.V.; Borca, M.V.; Kutish, G.; Kramer, E.; Irusta, P.; Rock, D.L. Passively transferred African swine fever virus antibodies protect swine against lethal infection. Virology 1994, 198, 350–354. [Google Scholar] [CrossRef]
- Jameson, B.A.; Wolf, H. The antigenic index: A novel algorithm for predicting antigenic determinants. Comput. Appl. Biosci. CABIOS 1988, 4, 181–186. [Google Scholar] [CrossRef]
- Kolaskar, A.S.; Tongaonkar, P.C. A semi-empirical method for prediction of antigenic determinants on protein antigens. FEBS Lett. 1990, 276, 172–174. [Google Scholar] [CrossRef]
- Kyte, J.; Doolittle, R.F. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 1982, 157, 105–132. [Google Scholar] [CrossRef]
- Emini, E.A.; Hughes, J.V.; Perlow, D.S.; Boger, J. Induction of hepatitis A virus neutralizing antibody by a virus-specific synthetic peptide. J. Virol. 1985, 55, 836–839. [Google Scholar] [CrossRef]
- Gupta, R.; Brunak, S. Prediction of glycosylation across the human proteome and the correlation to protein function. Pac. Symp. Biocomput. 2001, 310–322. [Google Scholar] [CrossRef]
- Lara-Romero, R.; Cerriteño-Sánchez, J.L.; Mendoza-Elvira, S.; García-Cambrón, J.B.; Castañeda-Montes, M.A.; Pérez-Aguilar, J.M.; Cuevas-Romero, J.S. Development of Novel Recombinant Antigens of Nucleoprotein and Matrix Proteins of Porcine orthorubulavirus: Antigenicity and Structural Prediction. Viruses 2022, 14, 1946. [Google Scholar] [CrossRef] [PubMed]
- Castañeda-Montes, M.A.; Cuevas-Romero, J.S.; Cerriteño-Sánchez, J.L.; de María Ávila-De la Vega, L.; García-Cambrón, J.B.; Ramírez-Álvarez, H. Small ruminant lentivirus capsid protein (SRLV-p25) antigenic structural prediction and immunogenicity to recombinant SRLV-rp25-coupled to immunostimulatory complexes based on glycyrrhizinic acid. Biosci. Biotechnol. Biochem. 2023, 87, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Cuevas-Romero, J.S.; Rivera-Benítez, J.F.; Hernández-Baumgarten, E.; Hernández-Jaúregui, P.; Vega, M.; Blomström, A.L.; Berg, M.; Baule, C. Cloning, expression and characterization of potential immunogenic recombinant hemagglutinin-neuraminidase protein of Porcine rubulavirus. Protein Expr. Purif. 2016, 128, 1–7. [Google Scholar] [CrossRef]
- Norma Oficial Mexicana NOM-062-ZOO-1999. Especificaciones Técnicas Para la Producción, Cuidado y Uso de Los Animales de Laboratorio. Available online: https://www.gob.mx/cms/uploads/attachment/file/203498/NOM-062-ZOO-1999_220801.pdf (accessed on 21 April 2025).
- Crowther, J. Enzyme Linked Immunosorbent Assay (ELISA). In Molecular Biomethods Handbook. Springer Protocols Handbooks; Walker, J.M., Rapley, R., Eds.; Humana Press: Totowa, NJ, USA, 2008. [Google Scholar] [CrossRef]
- Calculating Inter- and Intra-Assay Coefficients of Variability. Available online: https://salimetrics.com/calculating-inter-and-intra-assay-coefficients-of-variability/ (accessed on 21 April 2025).
- Tighe, P.J.; Ryder, R.R.; Todd, I.; Fairclough, L.C. ELISA in the multiplex era: Potentials and pitfalls. Proteomics. Clin. Appl. 2015, 9, 406–422. [Google Scholar] [CrossRef]
- Abraira, V. El Índice Kappa. Medicina de Familia. Semergen 2001, 27, 247–249. [Google Scholar] [CrossRef]
- Dierksheide, C.W. Medical decisions: Interpreting clinical test; in clinical practice, the predictive value helps reduce uncertainties when tests are used in diagnosis. ASM News 1987, 53, 677–680. [Google Scholar]
- Power, M.; Fell, G.; Wright, M. Principles for high-quality, high-value testing. Evid.-Based Med. 2013, 18, 5–10. [Google Scholar] [CrossRef]
- López-Ratón, M.; Rodríguez-Álvarez, M.X.; Cadarso-Suárez, C.; Gude-Sampedro, F. Optimal Cutpoints: An R Package for Selecting Optimal Cutpoints in Diagnostic Tests. J. Stat. Softw. 2014, 61, 1–36. [Google Scholar] [CrossRef]
- Spinard, E.; O’Donnell, V.; Vuono, E.; Rai, A.; Davis, C.; Ramirez-Medina, E.; Espinoza, N.; Valladares, A.; Borca, M.V.; Gladue, D.P. Full genome sequence for the African swine fever virus outbreak in the Dominican Republic in 1980. Sci. Rep. 2023, 13, 1024. [Google Scholar] [CrossRef]
- Gallardo, C.; Fernández-Pinero, J.; Pelayo, V.; Gazaev, I.; Markowska-Daniel, I.; Pridotkas, G.; Nieto, R.; Fernández-Pacheco, P.; Bokhan, S.; Nevolko, O.; et al. Genetic variation among African swine fever genotype II viruses, eastern and central Europe. Emerg. Infect. Dis. 2014, 20, 1544–1547. [Google Scholar] [CrossRef]
- Qu, H.; Ge, S.; Zhang, Y.; Wu, X.; Wang, Z. A systematic review of genotypes and serogroups of African swine fever virus. Virus Genes 2022, 58, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Miao, C.; Yang, S.; Shao, J.; Zhou, G.; Ma, Y.; Wen, S.; Hou, Z.; Peng, D.; Guo, H.; Liu, W.; et al. Identification of p72 epitopes of African swine fever virus and preliminary application. Front. Microbiol. 2023, 14, 1126794. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zhao, D.; Wang, J.; Zhang, Y.; Wang, M.; Gao, Y.; Li, F.; Wang, J.; Bu, Z.; Rao, Z.; et al. Architecture of African swine fever virus and implications for viral assembly. Science 2019, 366, 640–644. [Google Scholar] [CrossRef] [PubMed]
- OIE Terrestrial Manual. Chapter, 2019, 3(8):1. Available online: https://www.woah.org/fileadmin/Home/fr/Health_standards/tahm/3.08.01_ASF.pdf (accessed on 21 April 2025).
- Gallardo, C.; Soler, A.; Nieto, R.; Carrascosa, A.L.; De Mia, G.M.; Bishop, R.P.; Martins, C.; Fasina, F.O.; Couacy-Hymman, E.; Heath, L.; et al. Comparative evaluation of novel African swine fever virus (ASF) antibody detection techniques derived from specific ASF viral genotypes with the OIE internationally prescribed serological tests. Vet. Microbiol. 2013, 162, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jiao, J.; Liu, N.; Ren, S.; Zeng, H.; Peng, J.; Zhang, Y.; Guo, L.; Liu, F.; Lv, T.; et al. Novel p22 and p30 dual-proteins combination based indirect ELISA for detecting antibodies against African swine fever virus. Front. Vet. Sci. 2023, 10, 1093440. [Google Scholar] [CrossRef]
- Zhou, L.; Song, J.; Wang, M.; Sun, Z.; Sun, J.; Tian, P.; Zhuang, G.; Zhang, A.; Wu, Y.; Zhang, G. Establishment of a Dual-Antigen Indirect ELISA Based on p30 and pB602L to Detect Antibodies against African Swine Fever Virus. Viruses 2023, 15, 1845. [Google Scholar] [CrossRef]
- Gallardo, C.; Nieto, R.; Soler, A.; Pelayo, V.; Fernández-Pinero, J.; Markowska-Daniel, I.; Pridotkas, G.; Nurmoja, I.; Granta, R.; Simón, A.; et al. Assessment of African Swine Fever Diagnostic Techniques as a Response to the Epidemic Outbreaks in Eastern European Union Countries: How To Improve Surveillance and Control Programs. J. Clin. Microbiol. 2015, 53, 2555–2565. [Google Scholar] [CrossRef]
- Onyilagha, C.; Quizon, K.; Zhmendak, D.; El Kanoa, I.; Truong, T.; Ambagala, T.; Clavijo, A.; Le, V.P.; Babiuk, S.; Ambagala, A. Development and Validation of an Indirect and Blocking ELISA for the Serological Diagnosis of African Swine Fever. Pathogens 2024, 13, 981. [Google Scholar] [CrossRef]
- Weeks, S.D.; Drinker, M.; Loll, P.J. Ligation independent cloning vectors for expression of SUMO fusions. Protein Expr. Purif. 2007, 53, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Stertman, L.; Palm, A.-K.E.; Zarnegar, B.; Carow, B.; Lunderius Andersson, C.; Magnusson, S.E.; Carnrot, C.; Shinde, V.; Smith, G.; Glenn, G.; et al. The Matrix-MTM adjuvant: A critical component of vaccines for the 21st century. Hum. Vaccines Immunother. 2023, 19, 2189885. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Liu, Q.; Li, L.; Zhang, R.; Chang, Y.; Zhao, J.; Liu, S.; Zhao, X.; Chen, X.; Sun, Y.; et al. Nanobodies against African swine fever virus p72 and CD2v proteins as reagents for developing two cELISAs to detect viral antibodies. Virol. Sin. 2024, 39, 478–489. [Google Scholar] [CrossRef] [PubMed]
- WOAH. African Swine Fever: WOAH Warns Veterinary Authorities and Pig Industry of Risk from Use of Sub-Standard Vaccines. Available online: https://www.woah.org/en/african-swine-fever-woah-warns-veterinary-authorities-and-pig-industry-of-risk-from-use-of-sub-standard-vaccines/ (accessed on 21 April 2025).



| Strong-Positive ASF Serum (C++) IPT Value 1:1280 | Weak-Positive ASF Serum (C+) IPT Value 1:180 | Negative ASF Serum (C−) C− |
|---|---|---|
| 1.143 ± 0.178 (1 SD) | 0.506 ± 0.089 (1 SD) | 0.147 ± 0.030 (1 SD) |
| 1.143 ± 0.357 (2 SD) | 0.506 ± 0.179 (2 SD) | 0.147 ± 0.060 (2 SD) |
| Range C++ 0.786 to 1.50 OD | Range C+ 0.326 to 0.596 OD | Range C− 0.08 to 0.208 OD |
| CV 17% | CV 3% |
| Laboratory “A” | Laboratory “B” | ||||||
|---|---|---|---|---|---|---|---|
| ID | Description | ELISA*-Ingezim PPA Compac Corta | ELISA*-Ingezim PPA Compac Larga | ELISA* Indirect % Bloqueo Compet | ELISA* Indirect Bloqueo Compet | OD of ASF-p72t Protein iELISA | ASF-p72t Protein iELISA |
| 2019.1 | Pig serum experimentally infected with PPA strain NH/68. Bleeding 30 dpi. | Neg/DUD | Pos | 65.97 | pos | 0.883 | Pos |
| 2019.2 | Pig serum (C2) infected and experimentally re-infected with ASFV (NHV/68 strain). Bleeding 63 dpi/28 dpi. | Pos | Pos | 94.04 | Pos | 0.73 | Pos |
| 2019.8 | Uninfected pork serum. Undiluted | Neg | Neg | 0.27 | Neg | 0.12 | Neg |
| 2019.10 | Uninfected pork serum. Undiluted | Neg | Neg | −2.07 | Neg | 0.179 | Neg |
| 2020.1 | Sow Filtered Serum 3161/NEG) LCV Animal Facility | Neg | Neg | 18.6 | Neg | 0.122 | Neg |
| 2020.3 | Sow Filtered Serum 3161/NEG) LCV Animal Facility | Neg | Neg | 9.9 | Neg | 0.168 | Neg |
| 2020.4 | 27 February 2020 and lyophilized on 6 January 2020. Proceeds from infection (and re-infection) experimentally with VPPA strain NHV/68. Indentation 63 dpi -28 dpi. | Pos | Pos | 102.8 | Pos | 0.558 | Pos |
| 2020.5 | 7 April 2019 and lyophilized on 7 September 2019. Proceeds of an infection (and re-infection) experimentally with the VPPA strain NHV/68. Bleeding 63 dpi -28 dpi. | Pos | Pos | 97.9 | Pos | 0.597 | Pos |
| 2020.6 | 27 February 2020 and lyophilized on 1 June 2020. Proceeds of an infection (and re-infection) experimentally with VPPA strain NHV/68. Bleeding 63 dpi -28 dpi. | DUD | Pos | 77.7 | Pos | 0.506 | Pos |
| 2020.8 | 27 February 2020 and lyophilized on 1 June 2020. Proceeds from infection (and re-infection) experimentally with VPPA strain NHV/68. Bleeding 63 dpi -28 dpi. | NEG/DUD | Pos | 63.2 | Pos | 0.337 | Pos |
| 2020.9 | 7 April 2019 and lyophilized on 7 September 2019. It is from an experimentally infected with VPPA strain NHV/68. Bleeding 30 dpi. | Pos | Pos | 96.9 | Pos | 0.614 | Pos |
| 2021.1 | 7 May 2021 and lyophilized on 19 July 2021. It is from an infection (and re-infection) experimentally with VPPA strain NHV/68. Bleeding 63 dpi -28 dpi. | Pos | Pos | 89.9 | Pos | 0.862 | Pos |
| 2021.2 | 27 February 2020 and lyophilized on 2 June 2020. It is from an infection (and re-infection) experimentally with VPPA strain NHV/68. Bleeding 63 dpi -28 dpi. | Neg/DUD | Pos | 75.2 | Pos | 0.97 | Pos |
| 2021.3 | 5 July 2021 and lyophilized on 19 July 2021. It is from an infection (and re-infection) experimentally with the VPPA strain NHV/68. Bleeding 63 dpi -28 dpi. | Pos | Pos | 93.4 | Pos | 0.543 | Pos |
| 2021.5 | 27 February 2020 and lyophilized on 2 June 2020. Proceeds from infection (and re-infection) experimentally with VPPA strain NHV/68. Bleeding 63 dpi -28 dpi. | Pos | Pos | 97.3 | Pos | 0.562 | Pos |
| 2021.7 | 27 June 2020 and lyophilized on 5 June 2020. Proceeds from infection (and re-infection) experimentally with VPPA strain NHV/68. Bleeding 30 dpi. | Pos | Pos | 99.2 | Pos | 0.476 | Pos |
| 2022.1 | Uninfected pork serum. Undiluted. | Neg | Neg | 0 | Neg | 0.147 | Neg |
| 2022.5 | 7 April 2022 and lyophilized on 11 May 2022. It is derived from an experimental infection (and re-infection) with the VPPA strain NHV/68. | Pos | Pos | 99 | Pos | 0.938 | Pos |
| IPT-Positive Serum | IPT-Negative Serum | Total | |
|---|---|---|---|
| Positive serums | a (22) | b (1) | r = a + b (23) |
| Negative serums | c (3) | d (43) | s = c + d (46) |
| Total | t = a + b (25) | u = b + d (44) | N = a + b + c + d (69) |
| Sensitivity | |||
| Specificity | |||
| Kappa index | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuevas-Romero, J.S.; Zavala-Ocampo, P.L.; Pina-Pedrero, S.; Ganges, L.; Muñoz-Aguilera, A.; García-Cambrón, J.B.; Rodriguez, F.; Ambagala, A.; Cerriteño-Sánchez, J.L. Cloning and Expression of a Truncated Form of the p72 Protein of the African Swine Fever Virus (ASFV) for Application in an Efficient Indirect ELISA System. Pathogens 2025, 14, 542. https://doi.org/10.3390/pathogens14060542
Cuevas-Romero JS, Zavala-Ocampo PL, Pina-Pedrero S, Ganges L, Muñoz-Aguilera A, García-Cambrón JB, Rodriguez F, Ambagala A, Cerriteño-Sánchez JL. Cloning and Expression of a Truncated Form of the p72 Protein of the African Swine Fever Virus (ASFV) for Application in an Efficient Indirect ELISA System. Pathogens. 2025; 14(6):542. https://doi.org/10.3390/pathogens14060542
Chicago/Turabian StyleCuevas-Romero, Julieta Sandra, Perla Lucero Zavala-Ocampo, Sonia Pina-Pedrero, Llilianne Ganges, Adriana Muñoz-Aguilera, José Bryan García-Cambrón, Fernando Rodriguez, Aruna Ambagala, and José Luis Cerriteño-Sánchez. 2025. "Cloning and Expression of a Truncated Form of the p72 Protein of the African Swine Fever Virus (ASFV) for Application in an Efficient Indirect ELISA System" Pathogens 14, no. 6: 542. https://doi.org/10.3390/pathogens14060542
APA StyleCuevas-Romero, J. S., Zavala-Ocampo, P. L., Pina-Pedrero, S., Ganges, L., Muñoz-Aguilera, A., García-Cambrón, J. B., Rodriguez, F., Ambagala, A., & Cerriteño-Sánchez, J. L. (2025). Cloning and Expression of a Truncated Form of the p72 Protein of the African Swine Fever Virus (ASFV) for Application in an Efficient Indirect ELISA System. Pathogens, 14(6), 542. https://doi.org/10.3390/pathogens14060542











