Occupational Transmission of Measles Despite COVID-19 Precautions
Abstract
1. Introduction
Description of Cases
2. Materials and Methods
2.1. Viral RNA Detection and Sequencing
2.2. Data Analysis
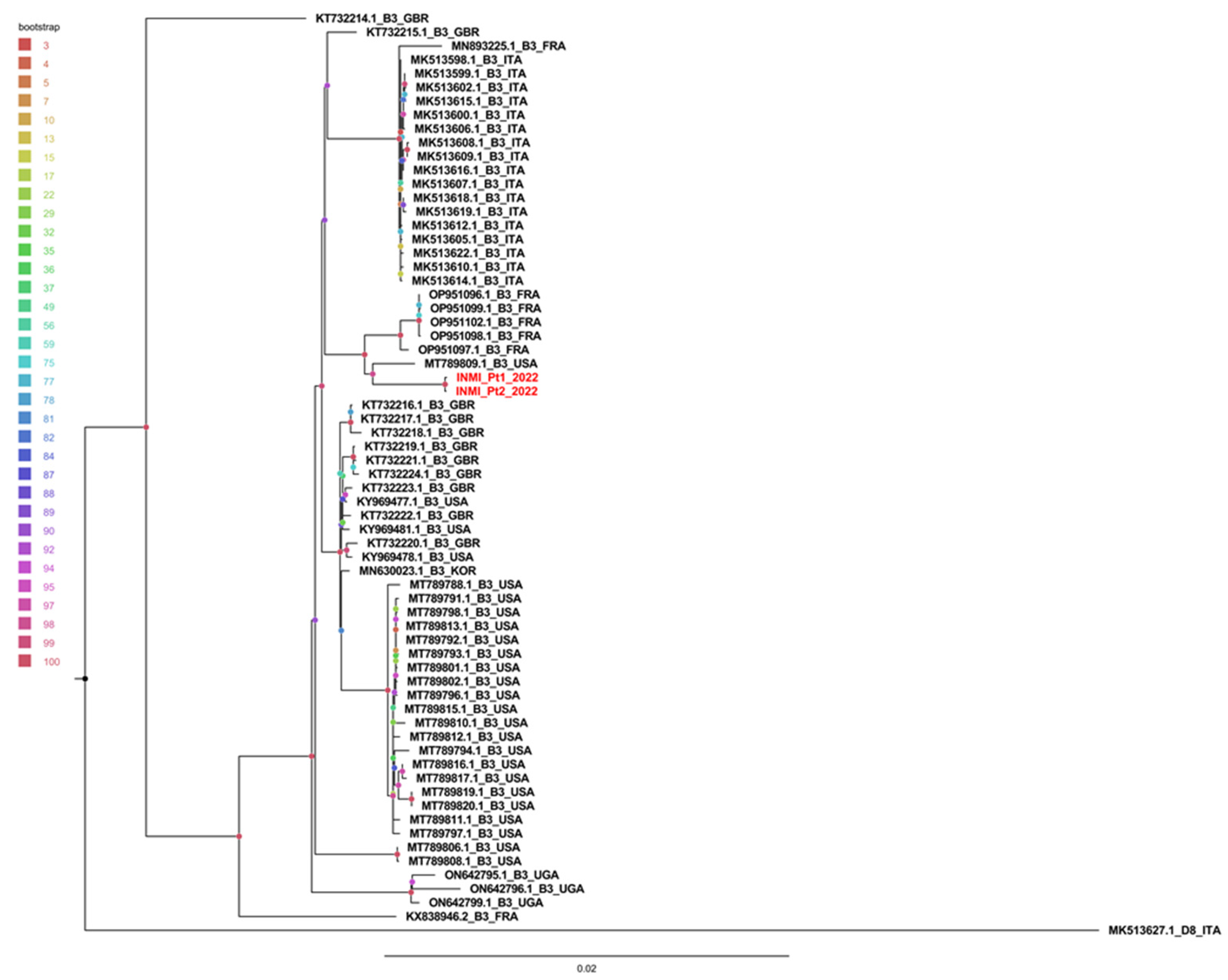
2.3. Phylogenetic Tree and Genetic Distance
3. Results
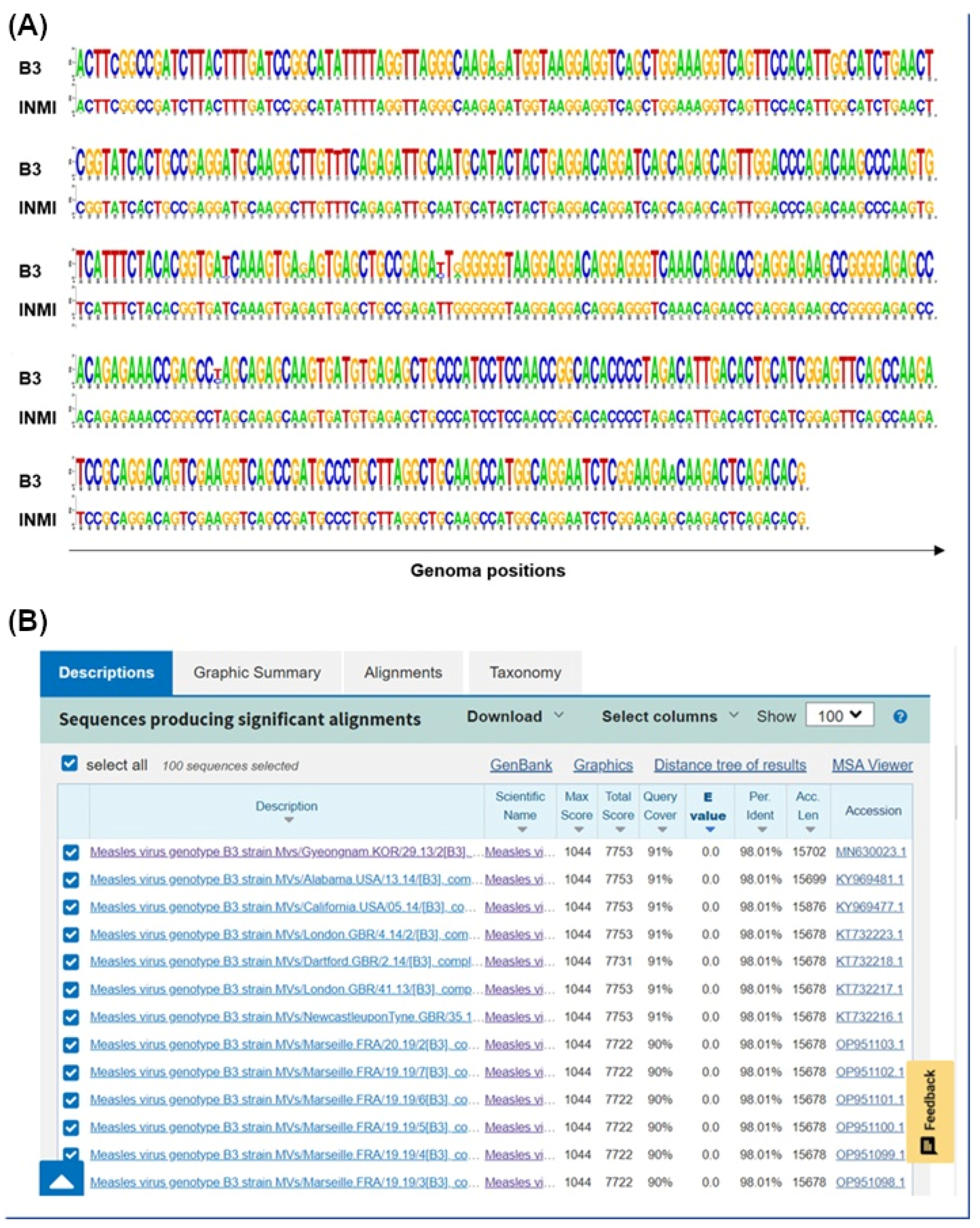
3.1. Molecular Epidemiology
3.2. Phylogenetic and Distance Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Servizio Regionale per l’Epidemiologia Sorveglianza e Controllo Delle Malattie Infettive, SeRESMI. Report dei Casi di Morbillo Notificati Nella Regione Lazio 01/01/2017–31/12/2017. Available online: https://www.inmi.it/wp-content/uploads/2018/12/Report_Morbillo_2017.pdf (accessed on 8 April 2025).
- Regione Lazio-Direzione Salute e Integrazione Sociosanitaria. Nota Regionale U.0523849.17-10-2017. Available online: https://www.vaccinarsinlazio.org/assets/uploads/files/7/06.circolare-0523849-17-10-2017-pnpv-2017-2019-operatori-sanitari.pdf (accessed on 8 April 2025).
- Legge 31 Luglio 2017, n. 119. Conversione in Legge, con Modificazioni, del Decreto-Legge 7 Giugno 2017, n. 73, Recante Disposizioni Urgenti in Materia di Prevenzione Vaccinale. (17G00132). Available online: http://www.trovanorme.salute.gov.it/norme/dettaglioAtto?id=60201 (accessed on 8 April 2025).
- Piano Nazionale per la Prevenzione Vaccinale (PNPV) 2017–2019. Available online: http://www.salute.gov.it/imgs/C_17_pubblicazioni_2571_allegato.pdf (accessed on 8 April 2025).
- Nota Regionale U.0397892.24-05-2019; Situazione Epidemiologica Morbillo. Regione Lazio—Indicazioni di Sorveglianza, Controllo e Prevenzione. Regione Lazio-Direzione Salute e Integrazione Sociosanitaria: Rome, Italy, 2019.
- Magurano, F.; Baggieri, M.; Mazzilli, F.; Bucci, P.; Marchi, A.; Nicoletti, L.; MoRoNet Group. Measles in Italy: Viral strains and crossing borders. Int. J. Infect. Dis. 2019, 79, 199–201. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Genetic Analysis of Measles Viruses. Available online: https://www.cdc.gov/measles/php/laboratories/genetic-analysis.html?CDC_AAref_Val=https://www.cdc.gov/measles/lab-tools/genetic-analysis.html (accessed on 8 April 2025).
- Bankamp, B.; Byrd-Leotis, L.A.; Lopareva, E.N.; Woo, G.K.; Liu, C.; Jee, Y.; Ahmed, H.; Lim, W.W.; Ramamurty, N.; Mulders, M.N.; et al. Improving molecular tools for global surveillance of measles virus. J. Clin. Virol. 2013, 58, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Penedos, A.R.; Myers, R.; Hadef, B.; Aladin, F.; Brown, K.E. Assessment of the Utility of Whole Genome Sequencing of Measles Virus in the Characterisation of Outbreaks. PLoS ONE 2015, 10, e0143081. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Beaty, S.M.; Lee, B. Constraints on the Genetic and Antigenic Variability of Measles Virus. Viruses 2016, 8, 109. [Google Scholar] [CrossRef] [PubMed]
- Regione Lazio-Direzione Salute e Integrazione Sociosanitaria. Nota Regionale 0133296.14-02-2020: Infezione da Nuovo Coronavirus 2019 nCoV (COVID-19). Indicazioni Operative per la Gestione e la Sorveglianza Nella Regione Lazio. Available online: https://www.snamiroma.org/wp1/wp-content/uploads/2020/02/Prot.0133296_Coronavirus_Indicazioni-operative_14-02-2020.pdf (accessed on 8 April 2025).
- Regione Lazio-Direzione Salute e Integrazione Sociosanitaria. Determinazione 31 Ottobre 2022, n. G14889. Misure per la Prevenzione e Gestione dei Contagi da COVID-19: Utilizzo Delle Mascherine Nelle Strutture Sanitarie, Socio-Sanitarie e Socio-Assistenziali, nel Periodo 1° Novembre 2022–31 Marzo 2023. Available online: https://www.inapp.gov.it/strumenti-normativa/norme-regionali/lazio-determinazione-direttoriale-31-ottobre-2022-n-g14889/ (accessed on 8 April 2025).
- Ueki, H.; Furusawa, Y.; Iwatsuki-Horimoto, K.; Imai, M.; Kabata, H.; Nishimura, H.; Kawaoka, Y. Effectiveness of Face Masks in Preventing Airborne Transmission of SARS-CoV-2. mSphere 2020, 5, e00637-20. [Google Scholar] [CrossRef] [PubMed]
- Lievano, F.A.; Papania, M.J.; Helfand, R.F.; Harpaz, R.; Walls, L.; Katz, R.S.; Williams, I.; Villamarzo, Y.S.; Rota, P.A.; Bellini, W.J. Lack of evidence of measles virus shedding in people with inapparent measles virus infections. J. Infect. Dis. 2004, 189 (Suppl. S1), S165–S170. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global Technical Consultation Report on Proposed Terminology for Pathogens that Transmit Through the Air; World Health Organization: Geneva, Switzerland, 2024; Available online: https://www.who.int/publications/m/item/global-technical-consultation-report-on-proposed-terminology-for-pathogens-that-transmit-through-the-air (accessed on 8 April 2025).
- Mikszewski, A.; Stabile, L.; Buonanno, G.; Morawska, L. The airborne contagiousness of respiratory viruses: A comparative analysis and implications for mitigation. Geosci. Front. 2022, 13, 101285. [Google Scholar] [CrossRef] [PubMed]
- Morawska, L.; Tang, J.W.; Bahnfleth, W.; Bluyssen, P.M.; Boerstra, A.; Buonanno, G.; Cao, J.; Dancer, S.; Floto, A.; Franchimon, F.; et al. How can airborne transmission of COVID-19 indoors be minimised? Environ. Int. 2020, 142, 105832. [Google Scholar] [CrossRef] [PubMed]
- Schull, M.J.; Redelmeier, D.A. Infection control for the disinterested. CMAJ 2003, 169, 122–123. [Google Scholar] [PubMed] [PubMed Central]

| Panel A—Average intragroup genetic distance | |||||
| Geographic location | Mean genomic distance | Standard deviation | |||
| Europe | 0.0069 | 33.49 × 10−5 | |||
| Italy | 0.0003 | 6.56 × 10−5 | |||
| World | 0.0042 | 26.68 × 10−5 | |||
| INMI | 0.0001 | 9.0681 × 10−5 | |||
| Panel B—Average intergroup genetic distance | |||||
| Geographic location | Europe | Italy | World | ||
| Europe | |||||
| Italy | 0.0075 ± 0.0005 | ||||
| World | 0.0070 ± 0.0003 | 0.0078 ± 0.0006 | |||
| INMI | 0.0088 ± 0.0006 | 0.0097 ± 0.0007 | 0.0095 ± 0.0007 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Carli, G.; Giombini, E.; Colosi, A.; Fusco, M.C.; Lalle, E.; Berno, G.; Rueca, M.; Fabeni, L.; Bordi, L.; Maggi, F.; et al. Occupational Transmission of Measles Despite COVID-19 Precautions. Pathogens 2025, 14, 519. https://doi.org/10.3390/pathogens14060519
De Carli G, Giombini E, Colosi A, Fusco MC, Lalle E, Berno G, Rueca M, Fabeni L, Bordi L, Maggi F, et al. Occupational Transmission of Measles Despite COVID-19 Precautions. Pathogens. 2025; 14(6):519. https://doi.org/10.3390/pathogens14060519
Chicago/Turabian StyleDe Carli, Gabriella, Emanuela Giombini, Alberto Colosi, Maria Concetta Fusco, Eleonora Lalle, Giulia Berno, Martina Rueca, Lavinia Fabeni, Licia Bordi, Fabrizio Maggi, and et al. 2025. "Occupational Transmission of Measles Despite COVID-19 Precautions" Pathogens 14, no. 6: 519. https://doi.org/10.3390/pathogens14060519
APA StyleDe Carli, G., Giombini, E., Colosi, A., Fusco, M. C., Lalle, E., Berno, G., Rueca, M., Fabeni, L., Bordi, L., Maggi, F., D’Amato, M., Vantaggio, V., Scognamiglio, P., & Vairo, F. (2025). Occupational Transmission of Measles Despite COVID-19 Precautions. Pathogens, 14(6), 519. https://doi.org/10.3390/pathogens14060519





