Temporal Variation and Human Host Predominance in Aedes aegypti from Coastal and Western Kenya: Insights from Pooled Blood Meal Metagenomics
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Mosquito Collection Methods
2.3. DNA Extraction
2.4. Next Generation Sequencing [NGS]
2.5. Data Analysis
3. Results
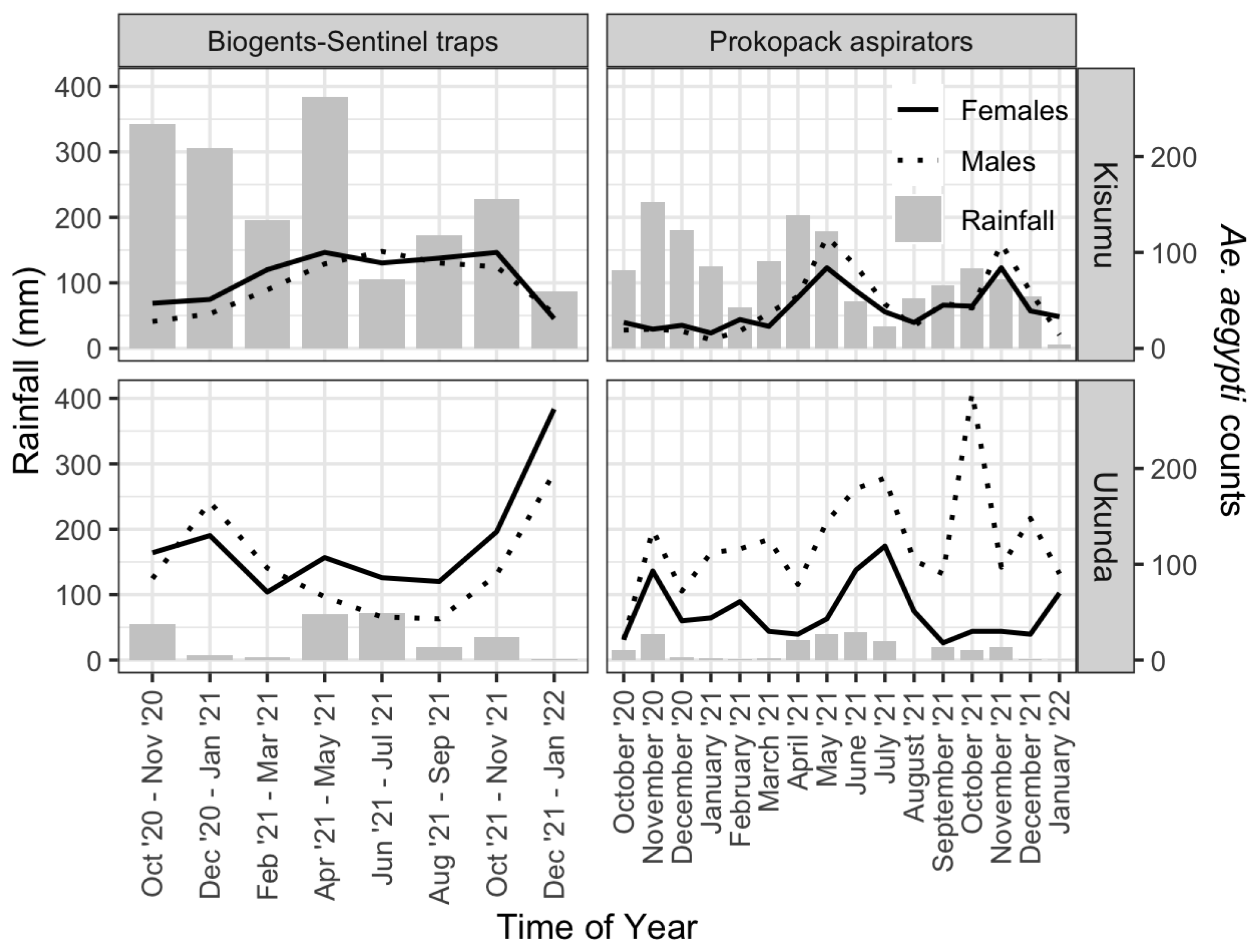
3.1. Mosquito Abundance and Spatial–Temporal Distribution
3.2. Vertebrate Food Sources
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CD-HIT | Cluster Database at High Identity with tolerance |
| CO1 | Cytochrome c oxidase subunit 1 gene |
| Cytb | Cytochrome b |
| DNA | Deoxy-ribonucleic acid |
| ELISA | Enzyme-linked immunosorbent assay |
| HRM | High resolution melting |
| MiSeq | Next generation sequencing platform for genomic analysis |
| NACOSTI | National commission of science, technology and innovation |
| NCBI | National Centre for Biotechnology Information |
| NGS | Next generation sequencing |
| OTU | Operational taxonomic unit |
| PCR | Polymerase chain reaction |
References
- Jansen, C.C.; Beebe, N.W. The dengue vector Aedes aegypti: What comes next. Microbes Infect. 2010, 12, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Ngugi, H.N.; Mutuku, F.M.; Ndenga, B.A.; Musunzaji, P.S.; Mbakaya, J.O.; Aswani, P.; Irungu, L.W.; Mukoko, D.; Vulule, J.; Kitron, U.; et al. Characterization and productivity profiles of Aedes aegypti L., breeding habitats across rural and urban landscapes in western and coastal Kenya. Parasites Vectors 2017, 10, 331. [Google Scholar] [CrossRef]
- Dengue and Severe Dengue. Available online: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue (accessed on 25 September 2024).
- Gainor, E.M.; Harris, E.; LaBeaud, A.D. Uncovering the Burden of Dengue in Africa: Considerations on Magnitude, Misdiagnosis, and Ancestry. Viruses 2022, 14, 233. [Google Scholar] [CrossRef]
- Mordecai, E.A.; Ryan, S.J.; Caldwell, J.M.; Shah, M.M.; LaBeaud, A.D. Climate change could shift disease burden from malaria to arboviruses in Africa. Lancet Planet. Health 2020, 49, e416–e423. [Google Scholar] [CrossRef]
- Bosire, C.; Mutuku, F.; Ndenga, B.; Nzaro, M.; Mwendwa, K.; LaBeaud, A.D. A narrative review of dengue fever infection and epidemic activity in Kenya 2010 to 2020. PAMJ-One Health 2023, 12, 10. [Google Scholar]
- Weeratunga, P.; Rodrigo, C.; Fernando, S.D.; Rajapakse, S. Control methods for Aedes albopictus and Aedes aegypti. Cochrane Database Syst. Rev. 2017, 8, CD012759. [Google Scholar]
- William, W. The Importance of Vector Abundance and Seasonality. EFSA Support. Publ. 2018, 15, EN-1491. [Google Scholar] [CrossRef]
- Tchouassi, D.P.; Kisero, R.O.; Rotich, G.; Dunlap, C.; Torto, B.; Muturi, E.J. Next generation sequencing improves the resolution of detecting mixed host blood meal sources in field collected arboviral mosquito vectors. Med. Vet. Entomol. 2024, 38, 407–415. [Google Scholar] [CrossRef]
- Kent, R.J.; Norris, D.E. Identification of mammalian blood meals in mosquitoes by a multiplexed polymerase chain reaction targeting cytochrome B. Am. J. Trop. Med. Hyg. 2005, 73, 336–342. [Google Scholar] [CrossRef]
- Sham, P.; Bader, J.S.; Craig, I.; O’Donovan, M.; Owen, M. DNA Pooling: A tool for large-scale association studies. Nat. Rev. Genet. 2002, 3, 862–871. [Google Scholar] [CrossRef]
- Khan, A.; Ndenga, B.; Mutuku, F.; Bosire, C.M.; Okuta, V.; Ronga, C.O.; Mutai, N.K.; Musaki, S.K.; Chebii, P.K.; Maina, P.W.; et al. Majority of pediatric dengue virus infections in Kenya do not meet 2009 WHO criteria for dengue diagnosis. PLOS Glob. Public Health 2022, 2, e0000175. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Bisanzio, D.; Mutuku, F.; Ndenga, B.; Grossi-Soyster, E.N.; Jembe, Z.; Maina, P.W.; Chebii, P.K.; Ronga, C.O.; Okuta, V.; et al. Spatiotemporal overlapping of dengue, chikungunya, and malaria infections in children in Kenya. BMC Infect. Dis. 2023, 23, 183. [Google Scholar] [CrossRef] [PubMed]
- Kiener, M.; Shayegh, N.; Nyathi, S.V.; Ndenga, B.A.; Mutuku, F.M.; LaBeaud, A.D. Low Rate of Asymptomatic Dengue Infection Detected in Coastal Kenya Using Pooled Polymerase Chain Reaction Testing. Am. J. Trop. Med. Hyg. 2024, 1104, 738–740. [Google Scholar] [CrossRef] [PubMed]
- Gudo, E.S.; Ali, S.; António, V.S.; Chelene, I.R.; Chongo, I.; Demanou, M.; Falk, K.; Guiliche, O.C.; Heinrich, N.; Monteiro, V.; et al. Seroepidemiological Studies of Arboviruses in Africa. Adv. Exp. Med. Biol. 2018, 1062, 361–371. [Google Scholar]
- Grossi-Soyster, E.N.; Cook, E.A.J.; de Glanville, W.A.; Thomas, L.F.; Krystosik, A.R.; Lee, J.; Wamae, C.N.; Kariuki, S.; Fèvre, E.M.; LaBeaud, A.D. Serological and spatial analysis of alphavirus and flavivirus prevalence and risk factors in a rural community in western Kenya. PLoS Negl. Trop. Dis. 2017, 11, e0005998. [Google Scholar] [CrossRef]
- Hortion, J.; Mutuku, F.M.; Eyherabide, A.L.; Vu, D.M.; Boothroyd, D.B.; Grossi-Soyster, E.N.; King, C.H.; Ndenga, B.A.; LaBeaud, A.D. Acute Flavivirus and Alphavirus Infections among Children in Two Different Areas of Kenya, 2015. Am. J. Trop. Med. Hyg. 2019, 100, 170–173. [Google Scholar] [CrossRef]
- Gerken, K.N.; Mutuku, F.M.; Ndenga, B.A.; Agola, G.A.; Migliore, E.; Fabre, E.P.; Malumbo, S.; Shaita, K.N.; Rezende, I.M.; LaBeaud, A.D. Urban risk factors for human Rift Valley fever virus exposure in Kenya. PLoS Glob. Public Health 2022, 2, e0000505. [Google Scholar] [CrossRef]
- LaBeaud, A.D.; Banda, T.; Brichard, J.; Muchiri, E.M.; Mungai, P.L.; Mutuku, F.M.; Borland, E.; Gildengorin, G.; Pfeil, S.; Teng, C.Y.; et al. High rates of o’nyong nyong and Chikungunya virus transmission in coastal Kenya. PLoS Negl. Trop. Dis. 2015, 9, e0003436. [Google Scholar] [CrossRef]
- Waggoner, J.; Heath, C.J.; Ndenga, B.; Mutuku, F.; Sahoo, M.K.; Mohamed-Hadley, A.; Vulule, J.; Mukoko, D.; LaBeaud, A.D.; Pinsky, B.A. Development of a Real-Time Reverse Transcription Polymerase Chain Reaction for O’nyong-nyong Virus and Evaluation with Clinical and Mosquito Specimens from Kenya. Am. J. Trop. Med. Hyg. 2017, 97, 121–124. [Google Scholar] [CrossRef]
- Heath, C.J.; Grossi-Soyster, E.N.; Ndenga, B.A.; Mutuku, F.M.; Sahoo, M.K.; Ngugi, H.N.; Mbakaya, J.O.; Siema, P.; Kitron, U.; Zahiri, N.; et al. Evidence of transovarial transmission of Chikungunya and Dengue viruses in field-caught mosquitoes in Kenya. PLoS Negl. Trop. Dis. 2020, 14, e0008362. [Google Scholar] [CrossRef]
- Musunzaji, P.S.; Ndenga, B.A.; Mzee, S.; Abubakar, L.U.; Kitron, U.D.; Labeaud, A.D.; Mutuku, F.M. Oviposition Preferences of Aedes aegypti in Msambweni, Kwale County, Kenya. J. Am. Mosq. Control Assoc. 2023, 39, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Mwakutwaa, A.S.; Ngugi, H.N.; Ndenga, B.A.; Krystosik, A.; Ngari, M.; Abubakar, L.U.; Yonge, S.; Kitron, U.; LaBeaud, A.D.; Mutuku, F.M. Pupal productivity of larval habitats of Aedes aegypti in Msambweni, Kwale County, Kenya. Parasitol. Res. 2023, 122, 801–814. [Google Scholar] [CrossRef]
- Ndenga, B.A.; Mutuku, F.M.; Ngugi, H.N.; Mbakaya, J.O.; Aswani, P.; Musunzaji, P.S.; Vulule, J.; Mukoko, D.; Kitron, U.; LaBeaud, A.D. Characteristics of Aedes aegypti adult mosquitoes in rural and urban areas of western and coastal Kenya. PLoS ONE 2017, 12, e0189971. [Google Scholar] [CrossRef]
- Karisa, J.; Muriu, S.; Omuoyo, D.; Karia, B.; Ngari, M.; Nyamwaya, D.; Rono, M.; Warimwe, G.; Mwangangi, J.; Mbogo, C.M. Urban Ecology of Arboviral Mosquito Vectors Along the Kenyan Coast. J. Med. Entomol. 2021, 58, 428–438. [Google Scholar] [CrossRef]
- 2019 Kenya Population and Housing Census Volume IV. Available online: https://www.google.com/search?q=2019+kenya+population+and+housing+census+volume+iv (accessed on 9 March 2025).
- Peña-García, V.H.; Mutuku, F.M.; Ndenga, B.A.; Mbakaya, J.O.; Ndire, S.O.; Agola, G.A.; Mutuku, P.S.; Malumbo, S.L.; Ng’ang’a, C.M.; Andrews, J.R.; et al. The Importance of Including Non-Household Environments in Dengue Vector Control Activities. Viruses 2023, 15, 1550. [Google Scholar] [CrossRef]
- Onyango, S.A.; Kitron, U.; Mungai, P.; Muchiri, E.M.; Kokwaro, E.; King, C.H.; Mutuku, F.M. Monitoring Malaria Vector Control Interventions: Effectiveness of Five Different Adult Mosquito Sampling Methods. J. Med. Entomol. 2013, 50, 1140–1151. [Google Scholar] [CrossRef]
- Huang, Y. The Subgenus Stegomyia of Aedes in the Afrotropical Region with Keys to the Species Diptera: Culicidae. Zootaxa 2004, 700, 1–120. [Google Scholar] [CrossRef]
- Buxton, P.A. Mosquitoes of the Ethiopian Region. Nature 1941, 148, 125–126. [Google Scholar] [CrossRef]
- Riaz, M.; Lorés-Motta, L.; Richardson, A.J.; Lu, Y.; Montgomery, G.; Omar, A.; Koenekoop, R.K.; Chen, J.; Muether, P.; Altay, L.; et al. GWAS study using DNA pooling strategy identifies association of variant rs4910623 in OR52B4 gene with anti-VEGF treatment response in age-related macular degeneration. Sci. Rep. 2016, 6, 37924. [Google Scholar] [CrossRef]
- Hadj-Henni, L.; De Meulemeester, T.; Depaquit, J.; Noël, P.; Germain, A.; Helder, R.; Augot, D. Comparison of Vertebrate Cytochrome b and Prepronociceptin for Blood Meal Analyses in Culicoides. Front. Vet. Sci. 2015, 2, 15. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, 884–890. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Godzik, A. Cd-hit: A fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics 2006, 22, 1658–1659. [Google Scholar] [CrossRef]
- Agha, S.B.; Tchouassi, D.P.; Turell, M.J.; Bastos, A.D.S.; Sang, R. Entomological assessment of dengue virus transmission risk in three urban areas of Kenya. PLoS Negl. Trop. Dis. 2019, 13, e0007686. [Google Scholar] [CrossRef]
- Barrera, R.; Bingham, A.M.; Hassan, H.K.; Amador, M.; Mackay, A.J.; Unnasch, T.R. Vertebrate hosts of Aedes aegypti and Aedes mediovittatus Diptera: Culicidae, in rural Puerto Rico. J. Med. Entomol. 2012, 49, 917–921. [Google Scholar] [CrossRef]
- Ponlawat, A.; Harrington, L.C. Blood feeding patterns of Aedes aegypti and Aedes albopictus in Thailand. J. Med. Entomol. 2005, 42, 844–849. [Google Scholar] [CrossRef]
- Shah, M.M.; Ndenga, B.A.; Mutuku, F.M.; Vu, D.M.; Grossi-Soyster, E.N.; Okuta, V.; Ronga, C.O.; Chebii, P.K.; Maina, P.; Jembe, Z.; et al. High Dengue Burden and Circulation of 4 Virus Serotypes among Children with Undifferentiated Fever, Kenya, 2014–2017. Emerg. Infect. Dis. 2020, 26, 2638–2650. [Google Scholar] [CrossRef]
- Caldwell, J.M.; LaBeaud, A.D.; Lambin, E.F.; Stewart-Ibarra, A.M.; Ndenga, B.A.; Mutuku, F.M.; Krystosik, A.R.; Ayala, E.B.; Anyamba, A.; Borbor-Cordova, M.J.; et al. Climate predicts geographic and temporal variation in mosquito-borne disease dynamics on two continents. Nat. Commun. 2021, 12, 1233. [Google Scholar] [CrossRef]
- Vu, D.M.; Banda, T.; Teng, C.Y.; Heimbaugh, C.; Muchiri, E.M.; Mungai, P.L.; Mutuku, F.M.; Brichard, J.; Gildengorin, G.; Borland, E.M.; et al. Dengue and West Nile Virus Transmission in Children and Adults in Coastal Kenya. Am. J. Trop. Med. Hyg. 2017, 96, 141–143. [Google Scholar] [CrossRef]
- Nyathi, S.; Rezende, I.M.; Walter, K.S.; Thongsripong, P.; Mutuku, F.; Ndenga, B.; Mbakaya, J.O.; Aswani, P.; Musunzaji, P.S.; Chebii, P.K.; et al. Molecular epidemiology and evolutionary characteristics of dengue virus 2 in East Africa. Nat. Commun. 2024, 15, 7832. [Google Scholar] [CrossRef]
- Tariq, A.; Khan, A.; Mutuku, F.; Ndenga, B.; Bisanzio, D.; Grossi-Soyster, E.N.; Jembe, Z.; Maina, P.; Chebii, P.; Ronga, C.; et al. Understanding the factors contributing to dengue virus and chikungunya virus seropositivity and seroconversion among children in Kenya. PLoS Negl. Trop. Dis. 2024, 18, e0012616. [Google Scholar] [CrossRef]
- Agha, S.B.; Tchouassi, D.P.; Bastos, A.D.S.; Sang, R. Dengue and yellow fever virus vectors: Seasonal abundance, diversity and resting preferences in three Kenyan cities. Parasites Vectors 2017, 10, 628. [Google Scholar] [CrossRef] [PubMed]
- Ngugi, H.N.; Nyathi, S.; Krystosik, A.; Ndenga, B.; Mbakaya, J.O.; Aswani, P.; Musunzaji, P.S.; Irungu, L.W.; Bisanzio, D.; Kitron, U.; et al. Risk factors for Aedes aegypti household pupal persistence in longitudinal entomological household surveys in urban and rural Kenya. Parasites Vectors 2020, 13, 499. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.L.; Lippi, C.A.; Stewart-Ibarra, A.M.; Ayala, E.B.; Mordecai, E.A.; Sippy, R.; Heras, F.H.; Blackburn, J.K.; Ryan, S.J. Household and climate factors influence Aedes aegypti presence in the arid city of Huaquillas, Ecuador. PLoS Negl. Trop. Dis. 2021, 15, e0009931. [Google Scholar] [CrossRef] [PubMed]
- Trewin, B.J.; Parry, H.R.; Pagendam, D.E.; Devine, G.J.; Zalucki, M.P.; Darbro, J.M.; Jansen, C.C.; Schellhorn, N.A. Simulating an invasion: Unsealed water storage rainwater tanks, and urban block design facilitate the spread of the dengue fever mosquito, Aedes aegypti, in Brisbane, Australia. Biol. Invasions. 2021, 23, 3891–3906. [Google Scholar] [CrossRef]
- Martinez-Ibarra, J.A.; Rodriguez, M.H.; Arredondo-Jimenez, J.I.; Yuval, B. Influence of plant abundance on nectar feeding by Aedes aegypti Diptera: Culicidae, in southern Mexico. J. Med. Entomol. 1997, 34, 589–593. [Google Scholar] [CrossRef]
- Maciel-de-Freitas, R.; Eiras, A.E.; Lourenço-de-Oliveira, R. Field evaluation of effectiveness of the BG-Sentinel, a new trap for capturing adult Aedes aegypti Diptera: Culicidae. Mem. Inst. Oswaldo Cruz. 2006, 101, 321–325. [Google Scholar] [CrossRef]
- Cilek, J.E.; Jiang, Y.X.; Dejesus, C.E. Field Comparison of Carbon Dioxide Source with Biogents Sentinel-2 and Pro Traps for Adult Aedes Mosquito Surveillance. J. Am. Mosq. Control Assoc. 2024, 40, 75–77. [Google Scholar] [CrossRef]
- Reegan, A.D.; Gandhi, M.R.; Balachandar, M.; Farajollahi, A.; Kesavaraju, B.; Ignacimuthu, S. Comparative efficacy of Biogents Sentinel and CDC traps for Aedes and Culex mosquito surveillance in India. J. Basic. Appl. Zool. 2024, 85, 46. [Google Scholar] [CrossRef]
- Scott, T.W.; Morrison, A.C.; Lorenz, L.H.; Clark, G.G.; Strickman, D.; Kittayapong, P.; Zhou, H.; Edman, J.D. Longitudinal Studies of Aedes aegypti Diptera: Culicidae, in Thailand and Puerto Rico: Population Dynamics. J. Med. Entomol. 2000, 37, 89–101. [Google Scholar] [CrossRef]
- Olson, M.F.; Ndeffo-Mbah, M.L.; Juarez, J.G.; Garcia-Luna, S.; Martin, E.; Borucki, M.K.; Frank, M.; Estrada-Franco, J.G.; Rodríguez-Pérez, M.A.; Fernández-Santos, N.A.; et al. High Rate of Non-Human Feeding by Aedes aegypti Reduces Zika Virus Transmission in South Texas. Viruses 2020, 12, 453. [Google Scholar] [CrossRef]
- Kirwa, L.J.; Abkallo, H.M.; Nyamota, R.; Kiprono, E.; Muloi, D.; Akoko, J.; Lord, J.S.; Bett, B. Arboviruses in Kenya: A Systematic Review and Meta-analysis of Prevalence. medRxiv 2024. preprint. [Google Scholar] [CrossRef]
- Musa, A.A.; Muturi, M.W.; Musyoki, A.M.; Ouso, D.O.; Oundo, J.W.; Makhulu, E.E.; Wambua, L.; Villinger, J.; Jeneby, M.M. Arboviruses and Blood Meal Sources in Zoophilic Mosquitoes at Human-Wildlife Interfaces in Kenya. Vector Borne Zoonotic Dis. 2020, 20, 444–453. [Google Scholar] [CrossRef]
- Vu, D.M.; Mutai, N.; Heath, C.J.; Vulule, J.M.; Mutuku, F.M.; Ndenga, B.A.; LaBeaud, A.D. Unrecognized Dengue Virus Infections in Children, Western Kenya, 2014–2015. Emerg. Infect. Dis. 2017, 23, 1915–1917. [Google Scholar] [CrossRef]
- Karisa, J.; Ominde, K.; Tuwei, M.; Bartilol, B.; Ondieki, Z.; Musani, H.; Wanjiku, C.; Mwikali, K.; Babu, L.; Rono, M.; et al. Utility of MALDI-TOF MS for determination of species identity and blood meal sources of primary malaria vectors on the Kenyan coast. Wellcome Open Res. 2024, 8, 151. [Google Scholar] [CrossRef]
- Omondi, D.; Masiga, D.K.; Ajamma, Y.U.; Fielding, B.C.; Njoroge, L.; Villinger, J. Unraveling Host-Vector-Arbovirus Interactions by Two-Gene High Resolution Melting Mosquito Bloodmeal Analysis in a Kenyan Wildlife-Livestock Interface. PLoS ONE 2015, 10, e0134375. [Google Scholar] [CrossRef]
- Lutomiah, J.; Omondi, D.; Masiga, D.; Mutai, C.; Mireji, P.O.; Ongus, J.; Linthicum, K.J.; Sang, R. Blood meal analysis and virus detection in blood-fed mosquitoes collected during the 2006-2007 Rift Valley fever outbreak in Kenya. Vector Borne Zoonotic Dis. 2014, 14, 656–664. [Google Scholar] [CrossRef]
- Katusi, G.C.; Hermy, M.R.G.; Makayula, S.M.; Ignell, R.; Govella, N.J.; Hill, S.R.; Mnyone, L.L. Seasonal variation in abundance and blood meal sources of primary and secondary malaria vectors within Kilombero Valley, Southern Tanzania. Parasites Vectors 2022, 15, 479. [Google Scholar] [CrossRef]
- Gonçalves, A.A.L.M.; Dias, A.H.C.; Monteiro, D.D.S.; Varela, I.B.F.; da Veiga Leal, S. Blood meal survey reveals insights into mosquito-borne diseases on the island of Santiago, Cape Verde. Front. Trop. Dis. 2023, 4, 1070172. [Google Scholar] [CrossRef]
- Omondi, S.O. Poultry Value Chain in Two Medium-Sized Cities in Kenya; Insights from Cluster Theory. Front. Vet. Sci. 2022, 9, 601299. [Google Scholar] [CrossRef]
- Omondi, S.O. Economic analysis of small-scale poultry production in Kenyan medium-sized cities of Kisumu and Thika. In Proceedings of the International Association of Agricultural Economists (IAAE), Vancouver, BC, Canada, 28 July–2 August 2018. [Google Scholar]
- Nutritional Diversity of Meat and Eggs. Available online: https://www.google.com/search?client=firefox-b-d&q=Nutritional+diversity+of+meat+and+eggs (accessed on 9 March 2025).
- de Jong, Y.; Butynski, T. The Primates of East Africa: Country Lists and Conservation Priorities. Afr. Primates 2012, 7, 135–155. [Google Scholar]
- Kingdon, J. The Kingdon Field Guide to African Mammals; Princeton University Press: Princeton, NJ, USA, 2015; Available online: https://www.google.com/search?client=firefox-b-d&q=The+Kingdon+field+guide+to+African+mammals (accessed on 9 March 2025).
- Cuff, J.P.; Windsor, F.M.; Tercel, M.P.T.G.; Kitson, J.J.N.; Evans, D.M. Overcoming the pitfalls of merging dietary metabarcoding into ecological networks. Methods Ecol. Evol. 2022, 13, 545–559. [Google Scholar] [CrossRef]
- Lynggaard, C.; Nielsen, M.; Santos-Bay, L.; Gastauer, M.; Oliveira, G.; Bohmann, K. Vertebrate diversity revealed by metabarcoding of bulk arthropod samples from tropical forests. Environ. DNA 2019, 1, 329–341. [Google Scholar] [CrossRef]
- Parker-Allie, F. Strengthening Biodiversity Informatics and Data Mobilisation Efforts Nationally and Regionally through SANBI-GBIF and the African Coordinating Mechanism. Biodivers. Inf. Sci. Stand. 2019, 3, e46908. [Google Scholar] [CrossRef]
- Miyake, T.; Aihara, N.; Maeda, K.; Shinzato, C.; Koyanagi, R.; Kobayashi, H.; Yamahira, K. Bloodmeal host identification with inferences to feeding habits of a fish-fed mosquito, Aedes baisasi. Sci. Rep. 2019, 9, 4002. [Google Scholar] [CrossRef]
- Reeves, L.E.; Burkett-Cadena, N.D. Mosquito Blood Meal Analysis. Cold Spring Harb. Protoc. 2024, 2024, pdb.top107706. [Google Scholar] [CrossRef]
- Fikrig, K.; Harrington, L.C. Understanding and interpreting mosquito blood feeding studies: The case of Aedes albopictus. Trends Parasitol. 2021, 37, 959–975. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, X.; Li, C.; Liu, G.; Wang, S.; Chen, M.; Wei, X.; Wen, H.; Tao, Z.; Xu, Y. Metagenomic sequencing reveals viral diversity of mosquitoes from Shandong Province, China. Microbiol. Spectr. 2024, 12, e03932-23. [Google Scholar] [CrossRef]
- Kent, R.J. Molecular methods for arthropod bloodmeal identification and applications to ecological and vector-borne disease studies. Mol. Ecol. Resour. 2009, 9, 4–18. [Google Scholar] [CrossRef]
- Muturi, E.J.; Dunlap, C.; Tchouassi, D.P.; Swanson, J. Next generation sequencing approach for simultaneous identification of mosquitoes and their blood-meal hosts. J. Vector Ecol. 2021, 46, 116–121. [Google Scholar] [CrossRef]
- Mweya, C.N.; Kimera, S.I.; Stanley, G.; Misinzo, G.; Mboera, L.E.G. Climate Change Influences Potential Distribution of Infected Aedes aegypti Co-Occurrence with Dengue Epidemics Risk Areas in Tanzania. PLoS ONE 2016, 11, e0162649. [Google Scholar] [CrossRef]
- Mwalugelo, Y.A.; Mponzi, W.P.; Muyaga, L.L.; Mahenge, H.H.; Katusi, G.C.; Muhonja, F.; Omondi, D.; Ochieng, A.O.; Kaindoa, E.W.; Amimo, F.A. Livestock keeping, mosquitoes and community viewpoints: A mixed methods assessment of relationships between livestock management, malaria vector biting risk and community perspectives in rural Tanzania. Malar. J. 2024, 23, 213. [Google Scholar] [CrossRef]
| Organism | Common Name | Species | Kisumu | Ukunda | ||
|---|---|---|---|---|---|---|
| RA (%) | ID (%) | RA (%) | ID (%) | |||
| Mammals | Human | Homo sapiens | 95.88 | 100.00 | 86.55 | 100.00 |
| Goat | Capra hircus | 0.00 | - | 0.06 | 99.72 | |
| Cow | Bos taurus | 0.00 | - | 0.09 | 99.44 | |
| Red deer | Cervus elaphus | 0.00 | - | 12.28 | 95.24 | |
| Gorilla | Gorilla gorilla | 0.05 | 91.64 | 0.00 | - | |
| Chimpanzee | Pan troglodytes | 0.11 | 97.21 | 0.01 | 97.21 | |
| Sheep | Ovis species | 0.00 | - | 0.72 | 100.00 | |
| Wild yak | Bos mutus | 0.00 | - | 0.03 | 100.00 | |
| Aves | Chicken | Gallus gallus | 0.23 | 100.00 | 0.13 | 100.00 |
| Turkey | Meleagris gallopavo | 3.65 | 97.17 | 0.02 | 97.17 | |
| Quail | Cortunix japonica | 0.00 | - | 0.04 | 97.00 | |
| Crow | Corvus moneduloides | 0.00 | - | 0.02 | 93.00 | |
| Pheasant | Phasianus colchicus | 0.08 | 100.00 | 0.02 | 100.00 | |
| Reptiles | Lizard | Acanthodactylus Cf. cantoris | 0.00 | - | 0.02 | 98.02 |
| Fish | Blenny fish | Parablennius sanguinolentus | 0.00 | - | 0.01 | 98.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mwendwa, K.; Mutuku, F.; Wambua, S.; Nzaro, M.; Ndenga, B.A.; Agoi, K.; LaBeaud, A.D.; Bosire, C. Temporal Variation and Human Host Predominance in Aedes aegypti from Coastal and Western Kenya: Insights from Pooled Blood Meal Metagenomics. Pathogens 2025, 14, 505. https://doi.org/10.3390/pathogens14050505
Mwendwa K, Mutuku F, Wambua S, Nzaro M, Ndenga BA, Agoi K, LaBeaud AD, Bosire C. Temporal Variation and Human Host Predominance in Aedes aegypti from Coastal and Western Kenya: Insights from Pooled Blood Meal Metagenomics. Pathogens. 2025; 14(5):505. https://doi.org/10.3390/pathogens14050505
Chicago/Turabian StyleMwendwa, Kavinya, Francis Mutuku, Sammy Wambua, Makenzi Nzaro, Bryson A. Ndenga, Kennedy Agoi, Angelle D. LaBeaud, and Carren Bosire. 2025. "Temporal Variation and Human Host Predominance in Aedes aegypti from Coastal and Western Kenya: Insights from Pooled Blood Meal Metagenomics" Pathogens 14, no. 5: 505. https://doi.org/10.3390/pathogens14050505
APA StyleMwendwa, K., Mutuku, F., Wambua, S., Nzaro, M., Ndenga, B. A., Agoi, K., LaBeaud, A. D., & Bosire, C. (2025). Temporal Variation and Human Host Predominance in Aedes aegypti from Coastal and Western Kenya: Insights from Pooled Blood Meal Metagenomics. Pathogens, 14(5), 505. https://doi.org/10.3390/pathogens14050505






