Rotavirus Infections: Pathophysiology, Symptoms, and Vaccination
Abstract
1. Introduction
2. Materials and Methods
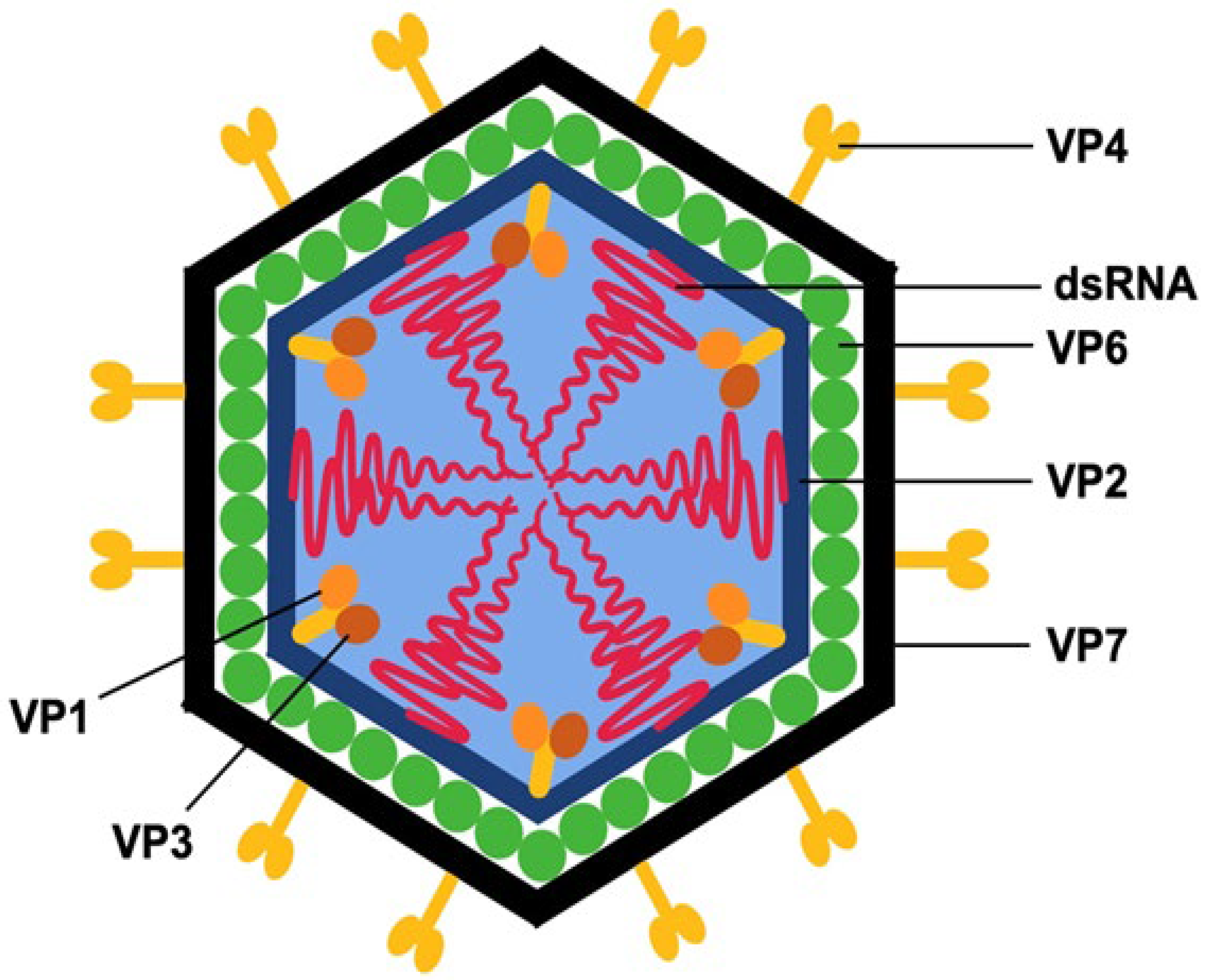
3. RVs Structure
4. Local and Systemic Symptoms Caused by RV
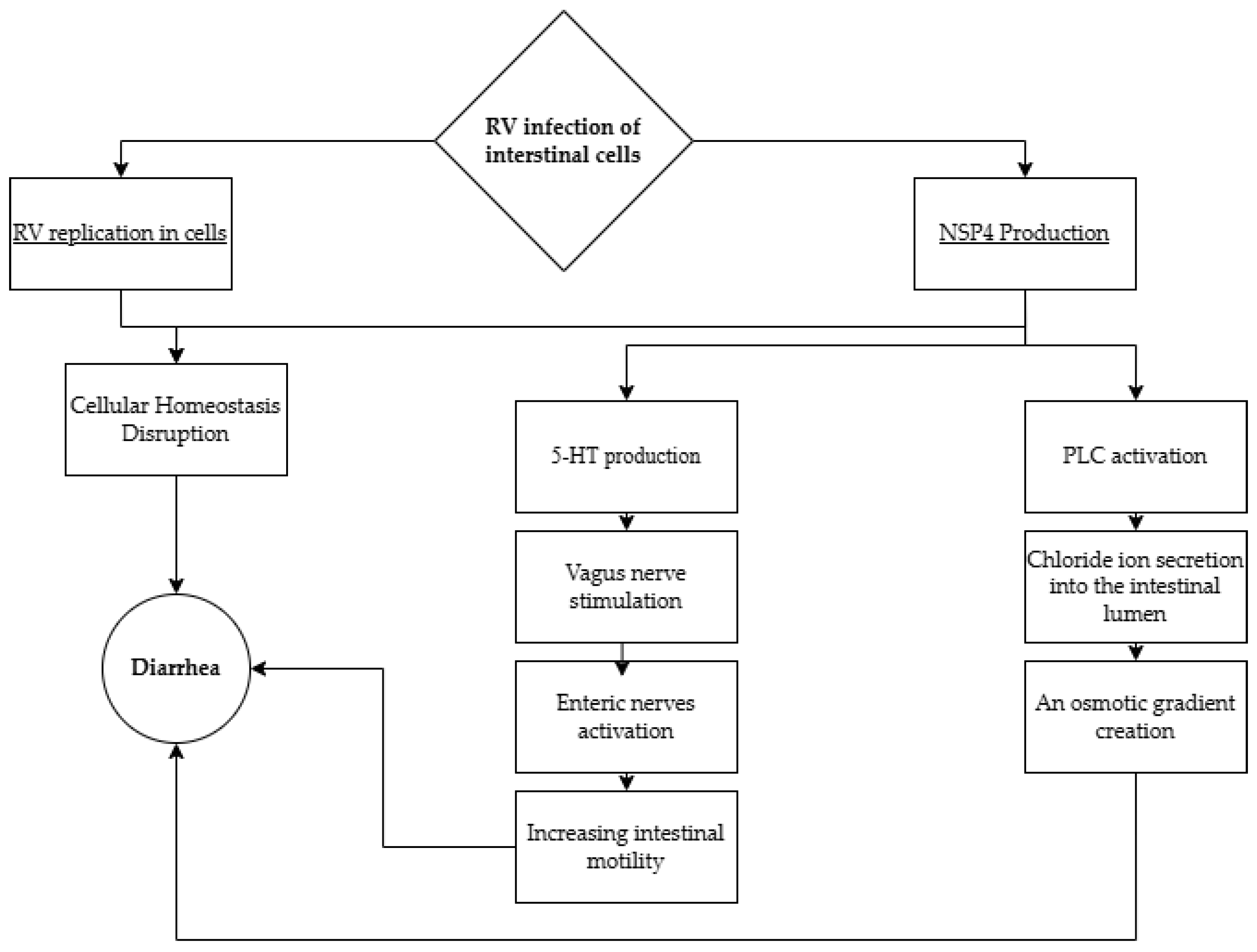
4.1. Diarrhea, Vomiting, and Dehydration
4.2. RV Infection and Central Nervous System (CNS)
4.3. RV, Autoimmunity, and Biliary Atresia
4.4. RV and Respiratory Tract Infections (RTIs)
5. AGE RV+ Progression Across Various Age Groups
5.1. Disease Progression in Children Under 5 Years of Age
5.2. Disease Progression in Children over 5 Years of Age
6. Vaccination and Its Efficacy in Preventing RV Infections
6.1. The Types of Available Vaccines with Dosing Schedules
6.2. Adverse Effects Associated with Vaccine Administration
6.3. Contraindications to Vaccine Administration
6.4. Effectiveness
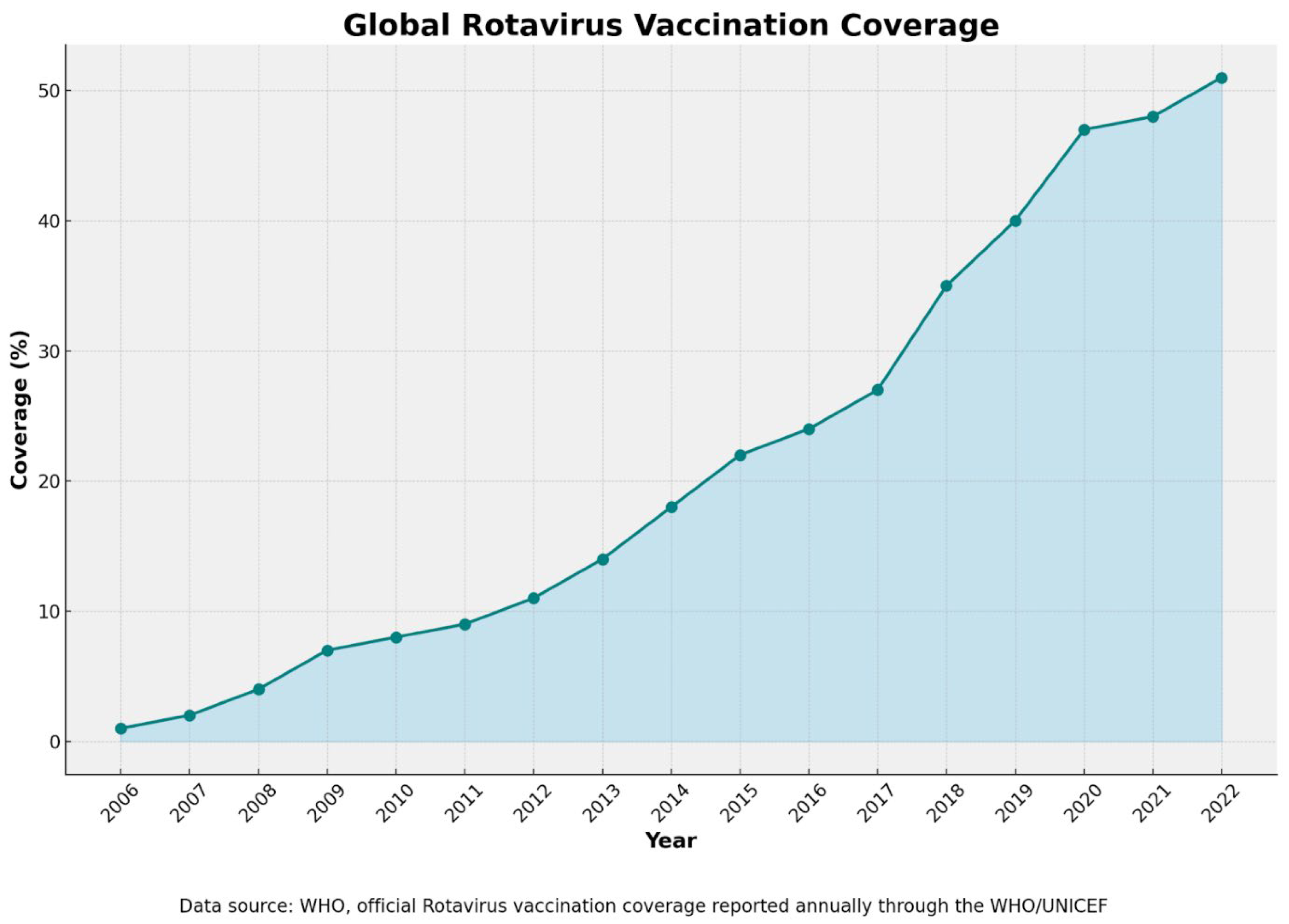
6.5. Vaccination Coverage
6.6. Vaccination Dropout
6.7. RV Vaccines and Dilemmas: Vaccinating Immunocompromised Children, Premature Infants, and High-Medical-Risk Infants
6.8. Perspectives on Next-Generation RV Vaccines
7. Concluding Remarks and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jesudason, T.; Sharomi, O.; Fleetwood, K.; Cheuk, A.L.; Bermudez, M.; Schirrmacher, H.; Hauck, C.; Matthijnssens, J.; Hungerford, D.; Tordrup, D.; et al. Systematic literature review and meta-analysis on the prevalence of rotavirus genotypes in Europe and the Middle East in the post-licensure period. Hum. Vaccines Immunother. 2024, 20, 2389606. [Google Scholar] [CrossRef] [PubMed]
- Hall, E.; Wodi, A.P.; Hamborsky, J.; Morelli, V.; Schillie, S. RV. In Epidemiology and Prevention of Vaccine-Preventable Diseases, 14th ed.; Cortese, M.M., Haber, P., Eds.; Public Health Foundation: Washington, DC, USA, 2021; pp. 311–322. [Google Scholar]
- Burnett, E.; Parashar, U.D.; Tate, J.E. Global impact of rotavirus vaccination on diarrhea hospitalizations and deaths among children < 5 years old: 2006–2019. J. Infect. Dis. 2020, 222, 1731–1739. [Google Scholar]
- Clark, A.; Mahmud, S.; Debellut, F.; Pecenka, C.; Jit, M.; Perin, J.; Tate, J.; Soeters, H.M.; Black, R.E.; Santosham, M.; et al. Estimating the Global Impact of RV Vaccines on Child Mortality. Int. J. Infect. Dis. 2023, 137, 90–97. [Google Scholar] [CrossRef]
- Du, Y.; Chen, C.; Zhang, X.; Yan, D.; Jiang, D.; Liu, X.; Yang, M.; Ding, C.; Lan, L.; Hecht, R.; et al. Global Burden and Trends of RV Infection-Associated Deaths from 1990 to 2019: An Observational Trend Study. Virol. J. 2022, 19, 166. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Kumar, M.; Santiana, M.; Mishra, A.; Zhang, M.; Labayo, H.; Chibly, A.M.; Nakamura, H.; Tanaka, T.; Henderson, W.; et al. Enteric viruses replicate in salivary glands and infect through saliva. Nature 2022, 607, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Nyblade, C.; Zhou, P.; Frazier, M.; Frazier, A.; Hensley, C.; Fantasia-Davis, A.; Shahrudin, S.; Hoffer, M.; Agbemabiese, C.A.; LaRue, L.; et al. Human Rotavirus Replicates in Salivary Glands and Primes Immune Responses in Facial and Intestinal Lymphoid Tissues of Gnotobiotic Pigs. Viruses 2023, 15, 1864. [Google Scholar] [CrossRef]
- American Academy of Pediatrics, A. Practice parameter: The management of acute gastroenteritis in young children. Pediatrics 1996, 97, 424–435. [Google Scholar]
- Asensio-Cob, D.; Rodríguez, J.M.; Luque, D. Rotavirus Particle Disassembly and Assembly In Vivo and In Vitro. Viruses 2023, 15, 1750. [Google Scholar] [CrossRef]
- Buttafuoco, A.; Michaelsen, K.; Tobler, K.; Ackermann, M.; Fraefel, C.; Eichwald, C. Conserved Rotavirus NSP5 and VP2 Domains Interact and Affect Viroplasm. J. Virol. 2020, 94, e01965-19. [Google Scholar] [CrossRef]
- McDonald, S.M.; Patton, J.T. Rotavirus VP2 core shell regions critical for viral polymerase activation. J. Virol. 2011, 85, 3095–3105. [Google Scholar] [CrossRef]
- Jenni, S.; Salgado, E.N.; Herrmann, T.; Li, Z.; Grant, T.; Grigorieff, N.; Trapani, S.; Estrozi, L.F.; Harrison, S.C. In situ Structure of Rotavirus VP1 RNA-Dependent RNA Polymerase. J. Mol. Biol. 2019, 431, 3124–3138. [Google Scholar] [CrossRef]
- Murray, P.R.; Pfaller, M.A.; Rosenthal, K.S. Medical Microbiology, 9th ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 510–551. [Google Scholar]
- Dai, J.; Agbemabiese, C.A.; Griffin, A.N.; Patton, J.T. Rotavirus capping enzyme VP3 inhibits interferon expression by inducing MAVS degradation during viral replication. mBio 2023, 14, e0225523. [Google Scholar] [CrossRef] [PubMed]
- Hou, G.; Zeng, Q.; Matthijnssens, J.; Greenberg, H.B.; Ding, S. Rotavirus NSP1 Contributes to Intestinal Viral Replication, Pathogenesis, and Transmission. mBio 2021, 12, e0320821. [Google Scholar] [CrossRef]
- Gratia, M.; Vende, P.; Charpilienne, A.; Baron, H.C.; Laroche, C.; Sarot, E.; Pyronnet, S.; Duarte, M.; Poncet, D. Challenging the Roles of NSP3 and Untranslated Regions in Rotavirus mRNA Translation. PLoS ONE 2016, 11, e0145998. [Google Scholar] [CrossRef] [PubMed]
- Nichols, S.L.; Haller, C.; Borodavka, A.; Esstman, S.M. Rotavirus NSP2: A Master Orchestrator of Early Viral Particle Assembly. Viruses 2024, 16, 814. [Google Scholar] [CrossRef] [PubMed]
- Althof, N.; Trojnar, E.; Johne, R. Rotaviruses in Wild Ungulates from Germany, 2019–2022. Microorganisms 2023, 11, 566. [Google Scholar] [CrossRef]
- Diller, J.R.; Parrington, H.M.; Patton, J.T.; Ogden, K.M. Rotavirus Species B Encodes a Functional Fusion-Associated Small Transmembrane Protein. J. Virol. 2019, 93, e00813-19. [Google Scholar] [CrossRef]
- Mitsutaka, W.; Ide, T.; Sasaki, J.; Komoto, S.; Ishii, J.; Takeshi, S.; Taniguchi, K. Porcine RV Closely Related to Novel Group of Human RVs. Emerg. Infect. Dis. 2011, 17, 1491–1493. [Google Scholar]
- Deol, P.; Kattoor, J.; Sircar, S.; Ghosh, S.; Bányai, K.; Dhama, K.; Malik, Y. Avian Group D RVs: Structure, Epidemiology, Diagnosis, and Perspectives on Future Research Challenges. Pathogens 2017, 6, 53. [Google Scholar] [CrossRef]
- Phan, T.G.; Leutenegger, C.M.; Chan, R.; Delwart, E. RV I in Feces of a Cat with Diarrhea. Virus Genes 2017, 53, 487–490. [Google Scholar] [CrossRef]
- Bányai, K.; Kemenesi, G.; Budinski, I.; Földes, F.; Zana, B.; Marton, S.; Varga-Kugler, R.; Oldal, M.; Kurucz, K.; Jakab, F. Candidate New RV Species in Schreiber’s Bats, Serbia. Infect. Genet. Evol. 2017, 48, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.A.; Springthorpe, V.S.; Sattar, S.A. Survival and Vehicular Spread of Human RVs: Possible Relation to Seasonality of Outbreaks. Clin. Infect. Dis. 1991, 13, 448–461. [Google Scholar] [CrossRef]
- World Health Organization. The Treatment of Diarrhoea: A Manual for Physicians and Other Senior Health Workers. Available online: https://www.who.int/publications/i/item/9241593180 (accessed on 11 August 2024).
- Florez, I.D.; Niño-Serna, L.F.; Beltrán-Arroyave, C.P. Acute Infectious Diarrhea and Gastroenteritis in Children. Curr. Infect. Dis. Rep. 2020, 22, 4. [Google Scholar] [CrossRef] [PubMed]
- Crawford, S.E.; Ramani, S.; Tate, J.E.; Parashar, U.D.; Svensson, L.; Hagbom, M.; Franco, M.A.; Greenberg, H.B.; O’Ryan, M.; Kang, G.; et al. RV infection. Nat. Rev. Dis. Primers 2017, 3, 17083. [Google Scholar]
- Lv, Y.J.; Hu, Q.L.; Huang, R.; Zhang, L.; Wu, L.F.; Fu, S. The Diagnostic and Therapeutic Value of the Detection of Serum Amyloid A and C-Reactive Protein in Infants with RV Diarrhea. Int. J. Gen. Med. 2021, 14, 3611–3617. [Google Scholar] [CrossRef]
- Hyser, J.M.; Collinson-Pautz, M.R.; Utama, B.; Estes, M.K. Rotavirus disrupts calcium homeostasis by NSP4 viroporin activity. mBio 2010, 1, e00265-10. [Google Scholar] [CrossRef] [PubMed]
- Boshuizen, J.A.; Reimerink, J.H.J.; Korteland-van Male, A.M.; van Ham, V.J.J.; Koopmans, M.P.G.; Büller, H.A.; Dekker, J.; Einerhand, A.W.C. Changes in Small Intestinal Homeostasis, Morphology, and Gene Expression during RV Infection of Infant Mice. J. Virol. 2003, 77, 13005–13016. [Google Scholar] [CrossRef]
- Bishop, R.F.; Davidson, G.P.; Holmes, I.H.; Ruck, B.J. Virus particles in epithelial cells of duodenal mucosa from children with viral gastroenteritis. Lancet 1973, 1, 1281–1283. [Google Scholar] [CrossRef]
- O’Ryan, M.G. Clinical Manifestations and Diagnosis of Rotavirus Infection: An Update. Wolters Kluwers. Available online: https://www.uptodate.com/contents/clinical-manifestations-and-diagnosis-of-rotavirus-infection#H3096970958 (accessed on 12 March 2025).
- Tafazoli, F.; Zeng, C.Q.; Estes, M.K.; Magnusson, K.E.; Svensson, L. NSP4 Enterotoxin of RV Induces Paracellular Leakage in Polarized Epithelial Cells. J. Virol. 2001, 75, 1540–1546. [Google Scholar] [CrossRef]
- Boshuizen, J.A.; Rossen, J.W.A.; Sitaram, C.K.; Kimenai, F.F.P.; Simons-Oosterhuis, Y.; Laffeber, C.; Büller, H.A.; Einerhand, A.W.C. RV Enterotoxin NSP4 Binds to the Extracellular Matrix Proteins Laminin-Beta3 and Fibronectin. J. Virol. 2004, 78, 10045–10053. [Google Scholar] [CrossRef]
- Bialowas, S.; Hagbom, M.; Nordgren, J.; Karlsson, T.; Sharma, S.; Magnusson, K.-E.; Svensson, L. RV and Serotonin Cross-Talk in Diarrhoea. PLoS ONE 2016, 11, e0159660. [Google Scholar] [CrossRef] [PubMed]
- Hagbom, M.; Istrate, C.; Engblom, D.; Karlsson, T.; Rodriguez-Diaz, J.; Buesa, J.; Taylor, J.A.; Loitto, V.-M.; Magnusson, K.-E.; Ahlman, H.; et al. RV Stimulates Release of Serotonin (5-HT) from Human Enterochromaffin Cells and Activates Brain Structures Involved in Nausea and Vomiting. PLoS Pathog. 2011, 7, e1002115. [Google Scholar] [CrossRef] [PubMed]
- Hagbom, M.; Hellysaz, A.; Istrate, C.; Nordgren, J.; Sharma, S.; De-Faria, F.M.; Magnusson, K.-E.; Svensson, L. The 5-HT3 Receptor Affects Rotavirus-Induced Motility. J. Virol. 2021, 95, e0075121. [Google Scholar] [CrossRef]
- Marie, H.; Claudia, I.; David, E.; Thommie, K.; Jesus, R.; Javier, B.; John, A.T. Rotavirus stimulates release of serotonin from human enterochromaffin cells and activates brain cells involved in nausea and vomiting. PLoS Pathog. 2011, 7, 10–131. [Google Scholar]
- Zaman, K.; Dang, D.A.; Victor, J.C.; Shin, S.; Yunus, M.; Dallas, M.J.; Podder, G.; Vu, D.T.; Le, T.P.M.; Luby, S.P.; et al. Efficacy of Pentavalent RV Vaccine against Severe RV Gastroenteritis in Infants in Developing Countries in Asia: A Randomised, Double-Blind, Placebo-Controlled Trial. Lancet 2010, 376, 615–623. [Google Scholar] [CrossRef]
- Guarino, A.; Ashkenazi, S.; Gendrel, D.; Lo Vecchio, A.; Shamir, R.; Szajewska, H. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition/European Society for Pediatric Infectious Diseases Evidence-Based Guidelines for the Management of Acute Gastroenteritis in Children in Europe: Update 2014. J. Pediatr. Gastroenterol. Nutr. 2014, 59, 132–152. [Google Scholar] [CrossRef]
- Ruuska, T.; Vesikari, T. RV Disease in Finnish Children: Use of Numerical Scores for Clinical Severity of Diarrhoeal Episodes. Scand. J. Infect. Dis. 1990, 22, 259–267. [Google Scholar] [CrossRef]
- Clark, H.F.; Borian, F.E.; Bell, L.M.; Modesto, K.; Gouvea, V.; Plotkin, S.A. Protective effect of WC3 vaccine against RV diarrhea in infants during a predominantly serotype 1 RV season. J. Infect. Dis. 1988, 158, 570–587. [Google Scholar] [CrossRef]
- Aslan, A.; Kurugol, Z.; Cetin, H.; Karakaşlilar, S.; Koturoğlu, G. Comparison of Vesikari and Clark Scales Regarding the Definition of Severe RV Gastroenteritis in Children. Infect. Dis. 2015, 47, 332–337. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, Y.; Li, Y.; Zhang, S.; Wang, X.; Zong, H.; Huang, W.; Kong, D.; Jiang, Y.; Liu, P.; et al. Development of a Rapid Homogeneous Immunoassay for Detection of RV in Stool Samples. Front. Public Health 2022, 10, 975720. [Google Scholar]
- World Health Organization. Generic Protocol for Monitoring Impact of RV Vaccination on Gastroenteritis Disease Burden and Viral Strains. Available online: http://whqlibdoc.who.int/hq/2008/WHO_IVB_08.16_eng.pdf (accessed on 11 August 2024).
- Sowmyanarayanan, T.V.; Ramani, S.; Sarkar, R.; Arumugam, R.; Warier, J.P.; Moses, P.D.; Simon, A.; Agarwal, I.; Bose, A.; Arora, R.; et al. Severity of RV Gastroenteritis in Indian Children Requiring Hospitalization. Vaccine 2012, 30, 167–172. [Google Scholar] [CrossRef]
- Armah, G.E.; Sow, S.O.; Breiman, R.F.; Dallas, M.J.; Tapia, M.D.; Feikin, D.R.; Binka, F.N.; Steele, A.D.; Laserson, K.F.; Ansah, N.A.; et al. Efficacy of Pentavalent RV Vaccine against Severe RV Gastroenteritis in Infants in Developing Countries in Sub-Saharan Africa: A Randomised, Double-Blind, Placebo-Controlled Trial. Lancet 2010, 376, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Madhi, S.A.; Cunliffe, N.A.; Steele, D.; Witte, D.; Kirsten, M.; Louw, C.; Ngwira, B.; Victor, J.C.; Gillard, P.H.; Cheuvart, B.B.; et al. Effect of Human RV Vaccine on Severe Diarrhea in African Infants. N. Engl. J. Med. 2010, 362, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.C.; Glavis-Bloom, J.; Modi, P.; Nasrin, S.; Rege, S.; Chu, C.; Schmid, C.H.; Alam, N.H. Empirically Derived Dehydration Scoring and Decision Tree Models for Children with Diarrhea: Assessment and Internal Validation in a Prospective Cohort Study in Dhaka, Bangladesh. Glob. Health Sci. Pract. 2015, 3, 405–418. [Google Scholar] [CrossRef]
- Newburg, D.S.; Peterson, J.A.; Ruiz-Palacios, G.M.; Matson, D.O.; Morrow, A.L.; Shults, J.; Guerrero, M.D.L.; Chaturvedi, P.; Newburg, S.O.; Scallan, C.D.; et al. Role of Human-Milk Lactadherin in Protectoin against Symptomatic RV Infection. Lancet 1998, 351, 1160–1164. [Google Scholar] [CrossRef] [PubMed]
- St Jean, D.T.; Chilyabanyama, O.N.; Bosomprah, S.; Asombang, M.; Velu, R.M.; Chibuye, M.; Mureithi, F.; Sukwa, N.; Chirwa, M.; Mokha, P.; et al. Development of a diarrhoea severity scoring scale in a passive health facility-based surveillance system. PLoS ONE 2022, 17, e0272981. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yin, J.; Yang, J.; Tian, L.; Li, D.; Zhang, Q.; Chen, J.; Xu, W.; Zhou, X. Epidemiology and genetic diversity of group A RV in acute diarrhea patients in pre-vaccination era in southwest China. J. Med. Virol. 2017, 89, 71–78. [Google Scholar] [CrossRef]
- Kamei, K.; Ogura, M.; Ishimori, S.; Kaito, H.; Iijima, K.; Ito, S. Acute kidney injury after acute gastroenteritis in an infant with hereditary hypouricemia. Eur. J. Pediatr. 2014, 173, 247–249. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.A.; Offit, P.A. Rotavirus-specific proteins are detected in murine macrophages in both intestinal and extraintestinal lymphoid tissues. Microb. Pathog. 1998, 24, 327–331. [Google Scholar] [CrossRef]
- Hemming, M.; Huhti, L.; Räsänen, S.; Salminen, M.; Vesikari, T. RV Antigenemia in Children Is Associated with More Severe Clinical Manifestations of Acute Gastroenteritis. Pediatr. Infect. Dis. J. 2014, 33, 366–371. [Google Scholar] [CrossRef]
- Sugata, K.; Taniguchi, K.; Yui, A.; Miyake, F.; Suga, S.; Asano, Y.; Ohashi, M.; Suzuki, K.; Nishimura, N.; Ozaki, T.; et al. Analysis of RV Antigenemia and Extraintestinal Manifestations in Children with RV Gastroenteritis. Pediatrics 2008, 122, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Yeom, J.S.; Kim, Y.-S.; Park, J.S.; Seo, J.-H.; Park, E.S.; Lim, J.-Y.; Park, C.-H.; Woo, H.-O.; Youn, H.-S. Role of Ca2+ Homeostasis Disruption in RV-Associated Seizures. J. Child Neurol. 2014, 29, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Weclewicz, K.; Kristensson, K.; Greenberg, H.B.; Svensson, L. The Endoplasmic Reticulum-Associated VP7 of RV Is Targeted to Axons and Dendrites in Polarized Neurons. J. Neurocytol. 1993, 22, 616–626. [Google Scholar] [CrossRef] [PubMed]
- Kashiwagi, Y.; Kawashima, H.; Suzuki, S.; Nishimata, S.; Takekuma, K.; Hoshika, A. Marked Elevation of Excitatory Amino Acids in Cerebrospinal Fluid Obtained from Patients with Rotavirus-Associated Encephalopathy. J. Clin. Lab. Anal. 2015, 29, 328–333. [Google Scholar] [CrossRef]
- Gross, P.M. Circumventricular organ capillaries. In Progress in Brain Research; Ermisch, A., Landgraf, R., Rühle, H.J., Eds.; Elsevier: Amsterdam, The Netherlands, 1992; Volume 91, pp. 219–233. [Google Scholar]
- Hellysaz, A.; Hagbom, M. Understanding the Central Nervous System Symptoms of RV: A Qualitative Review. Viruses 2021, 13, 658. [Google Scholar] [CrossRef]
- Salmi, T.T.; Arstila, P.; Koivikko, A. Central nervous system involvement in patients with RV gastroenteritis. Scand. J. Infect. Dis. 1978, 10, 29–31. [Google Scholar] [CrossRef]
- Castellazzi, L.; Principi, N.; Agostoni, C.; Esposito, S. Benign convulsions in children with mild gastroenteritis. Eur. J. Paediatr. Neurol. 2016, 20, 690–695. [Google Scholar] [CrossRef]
- Kang, B.; Kim, D.H.; Hong, Y.J.; Son, B.K.; Kim, D.W.; Kwon, Y.S. Comparison between febrile and afebrile seizures associated with mild rotavirus gastroenteritis. Seizure 2013, 22, 560–564. [Google Scholar] [CrossRef]
- Takanashi, J.; Miyamoto, T.; Ando, N.; Kubota, T.; Oka, M.; Kato, Z.; Hamano, S.; Hirabayashi, S.; Kikuchi, M.; Barkovich, A.J. Clinical and radiological features of rotavirus cerebellitis. AJNR Am. J. Neuroradiol. 2010, 31, 1591–1595. [Google Scholar] [CrossRef]
- Lee, K.Y.; Oh, K.W.; Weon, Y.C.; Choi, S.H. Neonatal seizures accompanied by diffuse cerebral white matter lesions on diffusion-weighted imaging are associated with rotavirus infection. Eur. J. Paediatr. Neurol. 2014, 18, 624–631. [Google Scholar] [CrossRef]
- Tada, H.; Takanashi, J.; Barkovich, A.J.; Oba, H.; Maeda, M.; Tsukahara, H.; Suzuki, M.; Yamamoto, T.; Shimono, T.; Ichiyama, T.; et al. Clinically mild encephalitis/encephalopathy with a reversible splenial lesion. Neurology 2004, 63, 1854–1858. [Google Scholar] [CrossRef] [PubMed]
- Takanashi, J. Two newly proposed infectious encephalitis/encephalopathy syndromes. Brain Dev. 2009, 31, 521–528. [Google Scholar] [CrossRef]
- Mizuguchi, M.; Ichiyama, T.; Imataka, G.; Okumura, A.; Goto, T.; Sakuma, H.; Takanashi, J.I.; Murayama, K.; Yamagata, T.; Yamanouchi, H.; et al. Guidelines for the diagnosis and treatment of acute encephalopathy in childhood. Brain Dev. 2021, 43, 2–31. [Google Scholar] [CrossRef] [PubMed]
- Harrison, L.C.; Perrett, K.P.; Jachno, K.; Nolan, T.M.; Honeyman, M.C. Does rotavirus turn on type 1 diabetes? PLoS Pathog. 2019, 15, e1007965. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-D.; Feng, N.; Sen, A.; Balan, M.; Tseng, H.-C.; McElrath, C.; Smirnov, S.V.; Peng, J.; Yasukawa, L.L.; Durbin, R.K.; et al. Distinct Roles of Type I and Type III Interferons in Intestinal Immunity to Homologous and Heterologous RV Infections. PLoS Pathog. 2016, 12, e1005600. [Google Scholar]
- Gómez-Rial, J.; Rivero-Calle, I.; Salas, A.; Martinón-Torres, F. Rotavirus and autoimmunity. J. Infect. 2020, 81, 183–189. [Google Scholar] [CrossRef]
- Kohm, A.P.; Fuller, K.G.; Miller, S.D. Mimicking the way to autoimmunity: An evolving theory of sequence and structural homology. Trends Microbiol. 2003, 11, 101–105. [Google Scholar] [CrossRef]
- Honeyman, M.C.; Stone, N.L.; Harrison, L.C. T-cell epitopes in type 1 diabetes autoantigen tyrosine phosphatase IA-2: Potential for mimicry with rotavirus and other environmental agents. Mol. Med. 1998, 4, 231–239. [Google Scholar] [CrossRef]
- Honeyman, M.C.; Stone, N.L.; Falk, B.A.; Nepom, G.; Harrison, L.C. Evidence for molecular mimicry between human T cell epitopes in rotavirus and pancreatic islet autoantigens. J. Immunol. 2010, 184, 2204–2210. [Google Scholar] [CrossRef]
- Zanoni, G.; Navone, R.; Lunardi, C.; Tridente, G.; Bason, C.; Sivori, S.; Beri, R.; Dolcino, M.; Valletta, E.; Corrocher, R.; et al. In celiac disease, a subset of autoantibodies against transglutaminase binds toll-like receptor 4 and induces activation of monocytes. PLoS Med. 2006, 3, e358. [Google Scholar] [CrossRef]
- Dolcino, M.; Zanoni, G.; Bason, C.; Tinazzi, E.; Boccola, E.; Valletta, E.; Contreas, G.; Lunardi, C.; Puccetti, A. A subset of anti-rotavirus antibodies directed against the viral protein VP7 predicts the onset of celiac disease and induces typical features of the disease in the intestinal epithelial cell line T84. Immunol. Res. 2013, 56, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.R.; Brindley, S.M.; Tucker, R.M.; Lambert, C.L.; Mack, C.L. α-enolase autoantibodies cross-reactive to viral proteins in a mouse model of biliary atresia. Gastroenterology 2010, 139, 1753–1761. [Google Scholar] [CrossRef] [PubMed]
- Rogers, M.A.M.; Basu, T.; Kim, C. Lower incidence rate of type 1 diabetes after receipt of the RV vaccine in the United States, 2001–2017. Sci. Rep. 2019, 9, 7727. [Google Scholar] [CrossRef] [PubMed]
- Inns, T.; Fleming, K.M.; Iturriza-Gomara, M.; Hungerford, D. Paediatric RV vaccination, coeliac disease and type 1 diabetes in children: A population-based cohort study. BMC Med. 2021, 19, 147. [Google Scholar] [CrossRef]
- Stene, L.C.; Honeyman, M.C.; Hoffenberg, E.J.; Haas, J.E.; Sokol, R.J.; Emery, L.; Taki, I.; Norris, J.M.; Erlich, H.A.; Eisenbarth, G.S.; et al. RV Infection Frequency and Risk of Celiac Disease Autoimmunity in Early Childhood: A Longitudinal Study. Am. J. Gastroenterol. 2006, 101, 2333–2340. [Google Scholar] [CrossRef] [PubMed]
- Ziberna, F.; De Lorenzo, G.; Schiavon, V.; Arnoldi, F.; Quaglia, S.; De Leo, L.; Vatta, S.; Martelossi, S.; Burrone, O.R.; Ventura, A.; et al. Lack of evidence of rotavirus-dependent molecular mimicry as a trigger of coeliac disease. Clin. Exp. Immunol. 2016, 186, 356–363. [Google Scholar] [CrossRef]
- Hertel, P.M.; Crawford, S.E.; Finegold, M.J.; Estes, M.K. Osteopontin upregulation in rotavirus-induced murine biliary atresia requires replicating virus but is not necessary for development of biliary atresia. Virology 2011, 417, 281–292. [Google Scholar] [CrossRef]
- Shivakumar, P.; Campbell, K.M.; Sabla, G.E.; Miethke, A.; Tiao, G.; McNeal, M.M.; Ward, R.L.; Bezerra, J.A. Obstruction of extrahepatic bile ducts by lymphocytes is regulated by IFN-gamma in experimental biliary atresia. J. Clin. Investig. 2004, 114, 322–329. [Google Scholar] [CrossRef]
- Wang, W.; Donnelly, B.; Bondoc, A.; Mohanty, S.K.; McNeal, M.; Ward, R.; Sestak, K.; Zheng, S.; Tiao, G. The rhesus rotavirus gene encoding VP4 is a major determinant in the pathogenesis of biliary atresia in newborn mice. J. Virol. 2011, 85, 9069–9077. [Google Scholar] [CrossRef]
- Allen, S.R.; Jafri, M.; Donnelly, B.; McNeal, M.; Witte, D.; Bezerra, J.; Ward, R.; Tiao, G.M. Effect of rotavirus strain on the murine model of biliary atresia. J. Virol. 2007, 81, 1671–1679. [Google Scholar] [CrossRef]
- GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef] [PubMed]
- Dennehy, P.H. Rotavirus vaccines: An overview. Clin. Microbiol. Rev. 2008, 21, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Zhaori, G.T.; Fu, L.T.; Xu, Y.H.; Guo, Y.R.; Peng, Z.J.; Shan, W.S. Detection of rotavirus antigen in tracheal aspirates of infants and children with pneumonia. Chin. Med. J. 1991, 104, 830–833. [Google Scholar] [PubMed]
- Zheng, B.J.; Chang, R.X.; Ma, G.Z.; Xie, J.M.; Liu, Q.; Liang, X.R.; Ng, M.H. Rotavirus infection of the oropharynx and respiratory tract in young children. J. Med. Virol. 1991, 34, 29–37. [Google Scholar] [CrossRef]
- Brandt, C.D.; Kim, H.W.; Rodriguez, W.J.; Arrobio, J.O.; Jeffries, B.C.; Parrott, R.H. Simultaneous infections with different enteric and respiratory tract viruses. J. Clin. Microbiol. 1986, 23, 177–179. [Google Scholar] [CrossRef]
- Isa, P.; Arias, C.F.; López, S. Role of sialic acids in rotavirus infection. Glycoconj. J. 2006, 23, 27–37. [Google Scholar] [CrossRef]
- Holloway, G.; Coulson, B.S. Innate cellular responses to rotavirus infection. J. Gen. Virol. 2013, 94 Pt 6, 1151–1160. [Google Scholar] [CrossRef]
- Staat, M.A.; Azimi, P.H.; Berke, T.; Roberts, N.; Bernstein, D.I.; Ward, R.L.; Pickering, L.K.; Matson, D.O. Clinical presentations of rotavirus infection among hospitalized children. Pediatr. Infect. Dis. J. 2002, 21, 221–227. [Google Scholar] [CrossRef]
- Bass, E.S.; Pappano, D.A.; Humiston, S.G. RV. Pediatr. Rev. 2007, 28, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Omatola, C.A.; Olaniran, A.O. Rotaviruses: From Pathogenesis to Disease Control-A Critical Review. Viruses 2022, 14, 875. [Google Scholar] [CrossRef]
- Mathew, S.; Al Ansari, K.; Al Thani, A.A.; Zaraket, H.; Yassine, H.M. Epidemiological, Molecular, and Clinical Features of RV Infections among Pediatrics in Qatar. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 1177–1190. [Google Scholar] [CrossRef] [PubMed]
- Sahiledengle, B.; Atlaw, D.; Mwanri, L.; Petrucka, P.; Kumie, A.; Tekalegn, Y.; Desta, F.; Zenbaba, D.; Mesfin, T.; Gomora, D.; et al. Burden of Childhood Diarrhea and Its Associated Factors in Ethiopia: A Review of Observational Studies. Int. J. Public Health 2024, 69, 1606399. [Google Scholar] [CrossRef]
- Arístegui, J.; Ferrer, J.; Salamanca, I.; Garrote, E.; Partidas, A.; San-Martin, M.; San-Jose, B. Multicenter Prospective Study on the Burden of RV Gastroenteritis in Children Less than 3 Years of Age in Spain. BMC Infect. Dis. 2016, 16, 549. [Google Scholar] [CrossRef]
- Omatola, C.A.; Ogunsakin, R.E.; Onoja, A.B.; Okolo, M.O.; Abraham-Oyiguh, J.; Mofolorunsho, K.C.; Akoh, P.Q.; Adejo, O.P.; Idakwo, J.; Okeme, T.O.; et al. Enteropathogenic viruses associated with acute gastroenteritis among African children under 5 years of age: A systematic review and meta-analysis. J. Infect. 2024, 88, 106169. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.-L.; Ma, Y.; Li, Z.; Qi, Y.-Y.; Wang, S.-J.; Fu, L.-J.; Wang, S.-M.; von Seidlein, L.; Wang, X.-Y. Cost-of-Illness of Gastroenteritis Caused by RV in Chinese Children Less than 5 Years. Hum. Vaccines Immunother. 2023, 19, 2276619. [Google Scholar] [CrossRef]
- Liu, J.; Platts-Mills, J.A.; Juma, J.; Kabir, F.; Nkeze, J.; Okoi, C.; Operario, D.J.; Uddin, J.; Ahmed, S.; Alonso, P.L.; et al. Use of Quantitative Molecular Diagnostic Methods to Identify Causes of Diarrhoea in Children: A Reanalysis of the GEMS Case-Control Study. Lancet 2016, 388, 1291–1301. [Google Scholar] [CrossRef]
- Black, R.E.; Perin, J.; Yeung, D.; Rajeev, T.; Miller, J.; Elwood, S.E.; Platts-Mills, J.A. Estimated Global and Regional Causes of Deaths from Diarrhoea in Children Younger than 5 Years during 2000–21: A Systematic Review and Bayesian Multinomial Analysis. Lancet Glob. Health 2024, 12, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Arakaki, L.; Tollefson, D.; Kharono, B.; Drain, P.K. Prevalence of RV among older children and adults with diarrhea: A systematic review and meta-analysis. Vaccine 2021, 39, 4577–4590. [Google Scholar] [CrossRef]
- Kyo, K.; Takano, C.; Kasuga, Y.; Ogawa, E.; Ishige, M.; Pham, N.T.K.; Okitsu, S.; Ushijima, H.; Urakami, T.; Fuchigami, T.; et al. Severe RV Gastroenteritis in Children Older than 5 Years after Vaccine Introduction. J. Infect. Chemother. 2021, 27, 598–603. [Google Scholar] [CrossRef]
- European Medicines Agency. RotaTeq Summary of Product Characteristics, 02/05/2024 Update. Available online: https://www.ema.europa.eu/en/documents/product-information/rotateq-epar-product-information_en.pdf (accessed on 12 March 2025).
- European Medicines Agency. Rotarix® Summary of Product Characteristics, 27/05/2024 Update. Available online: https://www.ema.europa.eu/en/documents/product-information/rotarix-epar-product-information_en.pdf (accessed on 12 March 2025).
- Bhandari, N.; Rongsen-Chandola, T.; Bavdekar, A.; John, J.; Antony, K.; Taneja, S. Efficacy of a monovalent human-bovine (116E) rotavirus vaccine in indian children in the second year of life. Vaccine 2014, 32 (Suppl. S1), A110–A116. [Google Scholar] [CrossRef]
- Isanaka, S.; Guindo, O.; Langendorf, C.; Matar Seck, A.; Plikaytis, B.D.; Sayinzoga-Makombe, N. Efficacy of a low-cost, heat-stable oral rotavirus vaccine in Niger. N. Engl. J. Med. 2017, 376, 1121–1130. [Google Scholar] [CrossRef]
- Dang, D.A.; Nguyen, V.T.; Vu, D.T.; Nguyen, T.H.; Nguyen, D.M.; Yuhuan, W. A dose-escalation safety and immunogenicity study of a new live attenuated human rotavirus vaccine (Rotavin-M1) in Vietnamese children. Vaccine 2012, 30 (Suppl. S1), A114–A121. [Google Scholar] [PubMed]
- Fu, C.; He, Q.; Xu, J.; Xie, H.; Ding, P.; Hu, W. Effectiveness of the Lanzhou lamb rotavirus vaccine against gastroenteritis among children. Vaccine 2012, 31, 154–158. [Google Scholar] [CrossRef]
- RV Vaccines: WHO Position Paper. Available online: https://www.who.int/publications/i/item/WHO-WER9628 (accessed on 11 August 2024).
- Wang, G.; Zhang, K.; Zhang, R.; Kong, X.; Guo, C. Impact of Vaccination with Different Types of RV Vaccines on the Incidence of Intussusception: A Randomized Controlled Meta-Analysis. Front. Pediatr. 2023, 11, 1239423. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, O.; Zhang, W.; Amponsem-Boateng, C.; Bonney Oppong, T.; Zhao, Q.; Li, D. Evidence of RV Vaccine Impact in Sub-Saharan Africa: Systematic Review and Meta-Analysis. PLoS ONE 2020, 15, e0232113. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.M.; López-Collada, V.R.; Bulhões, M.M.; De Oliveira, L.H.; Márquez, A.B.; Flannery, B.; Esparza-Aguilar, M.; Montenegro Renoiner, E.I.; Luna-Cruz, M.E.; Sato, H.K.; et al. Intussusception Risk and Health Benefits of RV Vaccination in Mexico and Brazil. N. Engl. J. Med. 2011, 364, 2283–2292. [Google Scholar] [CrossRef]
- Latifi, T.; Kachooei, A.; Jalilvand, S.; Zafarian, S.; Roohvand, F.; Shoja, Z. Correlates of Immune Protection against Human RVs: Natural Infection and Vaccination. Arch. Virol. 2024, 169, 72. [Google Scholar] [CrossRef]
- Jonesteller, C.L.; Burnett, E.; Yen, C.; Tate, J.E.; Parashar, U.D. Effectiveness of RV Vaccination: A Systematic Review of the First Decade of Global Postlicensure Data, 2006–2016. Clin. Infect. Diss. 2017, 65, 840–850. [Google Scholar] [CrossRef]
- Clark, A.; Van Zandvoort, K.; Flasche, S.; Sanderson, C.; Bines, J.; Tate, J.; Parashar, U.; Jit, M. Efficacy of Live Oral RV Vaccines by Duration of Follow-up: A Meta-Regression of Randomised Controlled Trials. Lancet Infect. Dis. 2019, 19, 717–727. [Google Scholar] [CrossRef]
- Oliveira, L.H.D.; Camacho, L.A.B.; Coutinho, E.S.F.; Ruiz-Matus, C.; Leite, J.P.G. RV Vaccine Effectiveness in Latin American and Caribbean Countries: A Systematic Review and Meta-Analysis. Vaccine 2015, 33, A248–A254. [Google Scholar] [CrossRef]
- Kulkarni, P.S.; Desai, S.; Tewari, T.; Kawade, A.; Goyal, N.; Garg, B.S.; Kumar, D.; Kanungo, S.; Kamat, V.; Kang, G.; et al. A Randomized Phase III Clinical Trial to Assess the Efficacy of a Bovine-Human Reassortant Pentavalent RV Vaccine in Indian Infants. Vaccine 2017, 35, 6228–6237. [Google Scholar] [CrossRef]
- Lin, A.; Bik, E.M.; Costello, E.K.; Dethlefsen, L.; Haque, R.; Relman, D.A.; Singh, U. Distinct distal gut microbiome diversity and composition in healthy children from Bangladesh and the United States. PLoS ONE 2013, 8, e53838. [Google Scholar] [CrossRef] [PubMed]
- Adlerberth, I.; Carlsson, B.; Man, P.D.; Jalil, F.; Khan, S.R.; Larsson, P.; Mellander, L.; Svanborg, C.; Wold, A.E.; Hanson, L.Å. Intestinal colonization with Enterobacteriaceae in Pakistani and Swedish hospital-delivered infants. Acta Paediatr. Scand. 1991, 80, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Nowrouzian, F.; Hesselmar, B.; Saalman, R.; StrannegÅrd, I.L.; Åberg, N.; Wold, A.E.; Adlerberth, I. Escherichia coli in infants’ intestinal microflora: Colonization rate, strain turnover, and virulence gene carriage. Pediatr. Res. 2003, 54, 8–14. [Google Scholar] [CrossRef]
- Kaufman, D.R.; De Calisto, J.; Simmons, N.L.; Cruz, A.N.; Villablanca, E.J.; Mora, J.R.; Barouch, D.H. Vitamin A Deficiency Impairs Vaccine-Elicited Gastrointestinal Immunity. J. Immunol. 2011, 187, 1877–1883. [Google Scholar] [CrossRef]
- Fraker, P.J.; King, L.E. Reprogramming of the immune system during zinc deficiency. Annu. Rev. Nutr. 2004, 24, 277–298. [Google Scholar] [CrossRef] [PubMed]
- Janko, M.M.; Joffe, J.; Michael, D.; Earl, L.; Rosettie, K.L.; Sparks, G.W.; Albertson, S.B.; Compton, K.; Pedroza Velandia, P.; Stafford, L.; et al. Cost-Effectiveness of RV Vaccination in Children under Five Years of Age in 195 Countries: A Meta-Regression Analysis. Vaccine 2022, 40, 3903–3917. [Google Scholar] [CrossRef]
- Troeger, C.; Khalil, I.A.; Rao, P.C.; Cao, S.; Blacker, B.F.; Ahmed, T.; Armah, G.; Bines, J.E.; Brewer, T.G.; Colombara, D.V.; et al. RV Vaccination and the Global Burden of RV Diarrhea Among Children Younger than 5 Years. JAMA Pediatr. 2018, 172, 958. [Google Scholar] [CrossRef]
- Workneh, B.S.; Mekonen, E.G.; Zegeye, A.F.; Gonete, A.T.; Alemu, T.G.; Tamir, T.T.; Tekeba, B.; Wassie, M.; Kassie, A.T.; Ali, M.S. RV Vaccine Dose-Two Dropout and Its Associated Factors among Children Who Received RV Vaccine Dose-One in Sub-Saharan African Countries: A Multilevel Analysis of the Recent Demographic and Health Survey. Hum. Vaccines Immunother. 2024, 20, 2335730. [Google Scholar] [CrossRef]
- Chanie, M.G.; Ewunetie, G.E.; Molla, A.; Muche, A. Determinants of Vaccination Dropout among Children 12–23 Months Age in North Gondar Zone, Northwest Ethiopia, 2019. PLoS ONE 2021, 16, e0246018. [Google Scholar] [CrossRef]
- Wandera, E.A.; Mohammad, S.; Ouko, J.O.; Yatitch, J.; Taniguchi, K.; Ichinose, Y. Variation in RV Vaccine Coverage by Sub-Counties in Kenya. Trop. Med. Health 2017, 45, 9. [Google Scholar] [CrossRef] [PubMed]
- Chiu, M.; Bao, C.; Sadarangani, M. Dilemmas with RV Vaccine: The Neonate and Immunocompromised. Pediatr. Infect. Dis. J. 2019, 38, S43–S46. [Google Scholar] [CrossRef]
- Bakare, N.; Menschik, D.; Tiernan, R.; Hua, W.; Martin, D. Severe Combined Immunodeficiency (SCID) and RV Vaccination: Reports to the Vaccine Adverse Events Reporting System (VAERS). Vaccine 2010, 28, 6609–6612. [Google Scholar] [CrossRef]
- Patel, N.C.; Hertel, P.M.; Estes, M.K.; de la Morena, M.; Petru, A.M.; Noroski, L.M.; Revell, P.A.; Hanson, I.C.; Paul, M.E.; Rosenblatt, H.M.; et al. Vaccine-Acquired RV in Infants with Severe Combined Immunodeficiency. N. Engl. J. Med. 2010, 362, 314–319. [Google Scholar] [CrossRef]
- Levin, M.J.; Lindsey, J.C.; Kaplan, S.S.; Schimana, W.; Lawrence, J.; McNeal, M.M.; Bwakura-Dangarembizi, M.; Ogwu, A.; Mpabalwani, E.M.; Sato, P.; et al. Safety and Immunogenicity of a Live Attenuated Pentavalent RV Vaccine in HIV-Exposed Infants with or without HIV Infection in Africa. AIDS 2017, 31, 49–59. [Google Scholar] [CrossRef]
- Laserson, K.F.; Nyakundi, D.; Feikin, D.R.; Nyambane, G.; Cook, E.; Oyieko, J.; Ojwando, J.; Rivers, S.B.; Ciarlet, M.; Neuzil, K.M.; et al. Safety of the Pentavalent RV Vaccine (PRV), RotaTeq®, in Kenya, Including among HIV-Infected and HIV-Exposed Infants. Vaccine 2012, 30, A61–A70. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, D.B.; Ananthakrishnan, A.N.; Martin, C.; Cohen, R.D.; Kane, S.V.; Mahadevan, U. Use of Biologic Therapy by Pregnant Women with Inflammatory Bowel Disease Does Not Affect Infant Response to Vaccines. Clin. Gastroenterol. Hepatol. 2018, 16, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Esteve-Solé, A.; Deyà-Martínez, À.; Teixidó, I.; Ricart, E.; Gompertz, M.; Torradeflot, M.; de Moner, N.; Gonzalez, E.A.; Plaza-Martin, A.M.; Yagüe, J.; et al. Immunological Changes in Blood of Newborns Exposed to Anti-TNF-α during Pregnancy. Front. Immunol. 2017, 8, 1123. [Google Scholar] [CrossRef]
- Moens, A.; Van Hoeve, K.; Humblet, E.; Rahier, J.-F.; Bossuyt, P.; Dewit, S.; Franchimont, D.; Macken, E.; Nijs, J.; Posen, A.; et al. Outcome of Pregnancies in Female Patients with Inflammatory Bowel Diseases Treated with Vedolizumab. J. Crohn’s Colitis 2019, 13, 12–18. [Google Scholar] [CrossRef]
- Dinelli, M.I.S.; Dos Santos, A.M.N.; Weckx, L.Y.; de Moraes-Pinto, M.I. Safe administration of RV vaccine in a cohort of infants exposed to immunosuppressive drugs during gestation. Transpl. Infect. Dis. 2018, 20, e12951. [Google Scholar] [CrossRef]
- Immunization Against Infectious Disease. Available online: https://www.gov.uk/government/collections/immunisation-against-infectious-disease-the-green-book (accessed on 30 July 2024).
- Kimberlin, D.W.; Barnett, E.D.; Lynfield, R.; Sawyer, M.H. Red Book: 2018–2021 Report of the Committee on Infectious Diseases, 31st ed.; American Academy of Pediatrics: Itasca, IL, USA, 2018; pp. 1–111. [Google Scholar]
- Sharma, R.; Hudak, M.L.; Premachandra, B.R.; Stevens, G.; Monteiro, C.B.; Bradshaw, J.A.; Kaunitz, A.M.; Hollister, R.A. Clinical manifestations of RV infection in the neonatal intensive care unit. Pediatr. Infect. Dis. J. 2002, 21, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Van Dongen, J.A.P.; Rouers, E.D.M.; Schuurman, R.; Band, C.; Watkins, S.M.; Van Houten, M.A.; Bont, L.J.; Norbruis, O.F.; Hemels, M.A.C.; Van Well, G.T.J.; et al. RV Vaccine Safety and Effectiveness in Infants with High-Risk Medical Conditions. Pediatrics 2021, 148, e2021051901. [Google Scholar] [CrossRef]
- Bines, J.E.; Danchin, M.; Jackson, P.; Handley, A.; Watts, E.; Lee, K.J.; West, A.; Cowley, D.; Chen, M.-Y.; Barnes, G.L.; et al. Safety and immunogenicity of RV3-BB human neonatal rotavirus vaccine administered at birth or in infancy: A randomised, double-blind, placebo-controlled trial. Lancet Infect. Dis. 2015, 15, 1389–1397. [Google Scholar] [CrossRef]
- Dhingra, M.; Kundu, R.; Gupta, M.; Kanungo, S.; Ganguly, N.; Singh, M.; Bhattacharya, M.; Ghosh, R.; Kumar, R.; Sur, D.; et al. Evaluation of safety and immunogenicity of a live attenuated tetravalent (G1–G4) Bovine-Human Reassortant Rotavirus vaccine (BRV-TV) in healthy Indian adults and infants. Vaccine 2014, 32 (Suppl. S1), A117–A123. [Google Scholar] [CrossRef]
- Saluja, T.; Palkar, S.; Misra, P.; Gupta, M.; Venugopal, P.; Sood, A.K.; Dhati, R.M.; Shetty, A.; Dhaded, S.M.; Agarkhedkar, S.; et al. Live attenuated tetravalent (G1–G4) bovine-human reassortant rotavirus vaccine (BRV-TV): Randomized, controlled phase III study in Indian infants. Vaccine 2017, 35, 3575–3581. [Google Scholar] [CrossRef]
- Kapikian, A.Z.; Simonsen, L.; Vesikari, T.; Hoshino, Y.; Morens, D.M.; Chanock, R.M.; La Montagne, J.R.; Murphy, B.R. A hexavalent human rotavirus-bovine rotavirus (UK) reassortant vaccine designed for use in developing countries and delivered in a schedule with the potential to eliminate the risk of intussusception. J. Infect. Dis. 2005, 192 (Suppl. S1), S22–S29. [Google Scholar] [CrossRef] [PubMed]
- Luna, E.J.; Frazatti-Gallina, N.M.; Timenetsky, M.C.; Cardoso, M.R.; Veras, M.A.; Miraglia, J.L.; Escobar, A.M.; Grisi, S.J.; Raw, I.; Precioso, A.R. A phase I clinical trial of a new 5-valent rotavirus vaccine. Vaccine 2013, 31, 1100–1105. [Google Scholar] [CrossRef]
- Jiang, B.; Wang, Y.; Glass, R.I. Does a monovalent inactivated human rotavirus vaccine induce heterotypic immunity? evidence from animal studies. Hum. Vaccin. Immunother. 2013, 9, 1634–1637. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Azevedo, M.; Saif, L.J.; Gentsch, J.R.; Glass, R.I.; Jiang, B. Inactivated rotavirus vaccine induces protective immunity in gnotobiotic piglets. Vaccine 2010, 28, 5432–5436. [Google Scholar] [CrossRef]
- Resch, T.K.; Wang, Y.; Moon, S.-S.; Joyce, J.; Li, S.; Prausnitz, M. Inactivated rotavirus vaccine by parenteral administration induces mucosal immunity in mice. Sci. Rep. 2018, 8, 561. [Google Scholar] [CrossRef]
- Wang, Y.; Vlasova, A.; Velasquez, D.E.; Saif, L.J.; Kandasamy, S.; Kochba, E. Skin vaccination against rotavirus using microneedles: Proof of concept in gnotobiotic piglets. PLoS ONE 2016, 11, e0166038. [Google Scholar] [CrossRef] [PubMed]
- PATH PATH Announces Early Closure of Pivotal Phase 3 Study of an Injectable Rotavirus Vaccine Candidate. 2022. Available online: https://www.path.org/our-impact/media-center/path-announces-early-closure-of-pivotal-phase-3-study-of-an-injectable-rotavirus-vaccine-candidate/ (accessed on 12 March 2025).
- Ward, R.L.; McNeal, M.M. VP6: A candidate rotavirus vaccine. J. Infect. Dis. 2010, 202, S101–S107. [Google Scholar] [CrossRef]
- Esteban, L.E.; Temprana, C.F.; Arguelles, M.H.; Glikmann, G.; Castello, A.A. Antigenicity and immunogenicity of rotavirus VP6 protein expressed on the surface of Lactococcus lactis. BioMed Res. Int. 2013, 2013, 298598. [Google Scholar] [CrossRef] [PubMed]
- Temprana, C.F.; Arguelles, M.H.; Gutierrez, N.M.; Barril, P.A.; Esteban, L.E.; Silvestre, D. Rotavirus VP6 protein mucosally delivered by cell wall-derived particles from Lactococcus lactis induces protection against infection in a murine model. PLoS ONE 2018, 13, e0203700. [Google Scholar] [CrossRef]
- Changotra, H.; Vij, A. Rotavirus virus-like particles (RV-VLPs) vaccines: An update. Rev. Med. Virol. 2017, 27, e1954. [Google Scholar] [CrossRef]
- Conner, M.E.; Zarley, C.D.; Hu, B.; Parsons, S.; Drabinski, D.; Greiner, S. Virus-like particles as a rotavirus subunit vaccine. J. Infect. Dis. 1996, 174 (Suppl. S1), S88–S92. [Google Scholar] [CrossRef] [PubMed]
- Lappalainen, S.; Pastor, A.R.; Malm, M.; Lopez-Guerrero, V.; Esquivel-Guadarrama, F.; Palomares, L.A. Protection against live rotavirus challenge in mice induced by parenteral and mucosal delivery of VP6 subunit rotavirus vaccine. Arch. Virol. 2015, 160, 2075–2078. [Google Scholar] [CrossRef]
- Lappalainen, S.; Pastor, A.R.; Tamminen, K.; Lopez-Guerrero, V.; Esquivel-Guadarrama, F.; Palomares, L.A. Immune responses elicited against rotavirus middle layer protein VP6 inhibit viral replication in vitro and in vivo. Hum. Vaccines Immunother. 2014, 10, 2039–2047. [Google Scholar] [CrossRef]
- Tamminen, K.; Lappalainen, S.; Huhti, L.; Vesikari, T.; Blazevic, V. Trivalent combination vaccine induces broad heterologous immune responses to norovirus and rotavirus in mice. PLoS ONE 2013, 8, e70409. [Google Scholar] [CrossRef]
- Dróżdż, M.; Krzyżek, P.; Dudek, B.; Makuch, S.; Janczura, A.; Paluch, E. Current State of Knowledge about Role of Pets in Zoonotic Transmission of SARS-CoV-2. Viruses 2021, 13, 1149. [Google Scholar] [CrossRef]
| Name of Protein | Role of the Protein |
|---|---|
| Structural proteins (VP) | |
| VP4, VP7 | Mediate viral attachment to host cell surface receptors and facilitate membrane penetration during entry. VP4 contributes to viral antigenic diversity, with 58 recognized P genotypes [12]. |
| VP1 | Functions as the RNA-dependent RNA polymerase, catalyzing the synthesis of viral RNA [12]. |
| VP2 | Serves as the inner capsid scaffold and essential cofactor for VP1, enabling initiation of double-stranded RNA genome replication [11]. |
| VP3 | Guanylyl-methyltransferase, capping enzyme [10]. RV proteins NSP1 and VP3 act to suppress interferon (IFN) expression by inducing degradation of transcription factors and other host elements essential for effective innate immune responses [11]. |
| VP6 | Major structural protein [13]. Involved in RV diversity—42 G-types and species classification (RVA–RVJ). |
| Non-structural proteins (NSP) | |
| NSP1 | Interferon antagonist [14]. |
| NSP2 | Involved in RV particle assembly [14,15]. |
| NSP3 | Stimulates translation of both capped and uncapped viral mRNA [16]. |
| NSP4 | Involved in RV particle assembly, enterotoxin [14] |
| NSP5 | Involved in RV particle assembly [14,17]. |
| NSP6 | Expressed from an alternative reading frame of the NSP5 gene in some Group A RV strains [15]. |
| Symptoms | Vesikari Scale | Clark Scale | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 1 | 2 | 3 | |
| Number of stools/days | 1–3 | 4–5 | ≥6 | 2–4 | 5–7 | ≥8 |
| Duration of diarrhea (days) | 1–4 | 5 | ≥6 | 1–4 | 5–7 | ≥8 |
| Number of emesis events/day | 1 | 2–4 | ≥5 | 1–3 | 4–6 | ≥7 |
| Duration of emesis (days) | 1 | 2 | ≥3 | 2 | 3–5 | ≥6 |
| Rectal temperature (°C) | 37.1–38.4 | 38.5–38.9 | ≥39 | 38.1–38.2 | 38.3–38.7 | ≥38.8 |
| Temperature duration (days) | – | – | – | 1–2 | 3–4 | ≥5 |
| Dehydration | – | 1–5% | ≥6% | – | – | – |
| Behavioral symptoms | – | – | – | Irritable/less playful | Lethargic/listless | Seizures |
| Duration of behavioral symptoms (days) | – | – | – | 1–2 | 3–4 | ≥5 |
| Treatment | Rehydration | Hospitalization | – | – | – | – |
| The Vesikari Scale | The Clark Scale | ||
|---|---|---|---|
| Number of Points Obtained in Scale | Severity | Number of Points Obtained in Scale | Severity |
| <11 | Non-severe | 0–8 | Mild |
| - | - | 9–16 | Moderate |
| ≥11 | Severe | >16 | Severe |
| Clinical Dehydration Scale (CDS) | DHAKA Scale | |||||
|---|---|---|---|---|---|---|
| Characteristics/Points | 0 | 1 | 2 | 0 | 2 | 4 |
| General appearance | Normal | Thirsty, restless, or lethargic but irritable when touched | Cold, drowsy, limp, sweaty, or comatose | Normal | Restless, irritable | Lethargic, unconscious |
| Eyes | Normal | Slightly sunken | Extremely sunken | - | - | - |
| Mucous membrane (tondue) | Moist | Sticky | - | - | - | |
| Tears | Normal | Decreased tears | Absent tears | Normal | Decreased | Absent |
| Skin pinch | - | - | - | Normal | Slow | Very slow |
| Respirations | - | - | - | Normal | Deep | - |
| Clinical Parameter | Assessment Criteria | Points |
|---|---|---|
| Vomiting frequency | 2–3 episodes/day | 1 |
| 4–5 episodes/day | 2 | |
| ≥6 episodes/day | 3 | |
| Behavioral status | Restless/irritable | 2 |
| Lethargic | 3 | |
| Skin pinch test | Normal (instant recoil) | 0 |
| Slow recoil (>2 s) | 2 | |
| Very slow | 3 | |
| Tears | Present | 0 |
| Absent | 2 | |
| Respirations | Normal | 0 |
| Deep | 2 |
| Types of CNS Complications | Clinical Features |
|---|---|
| Benign convulsions with mild gastroenteritis | After the onset of gastroenteritis, febrile or afebrile seizures. Seizures may precede the onset of diarrhea by 12 to 24 h or coincide with its initiation. Clinically, seizures predominantly manifest as generalized tonic-clonic episodes [63,64]. |
| Acute cerebellitis | After 1–3 days, the onset of symptoms of gastroenteritis decreased consciousness and subsequent mutism (may last for up to 20 days), slow speech, dysarthria, hypotonia, ataxia, tremors, nystagmus, and dysmetria [65]. |
| Neonatal RV-associated leukoencephalopathy | At around the 5th day after birth, repetitive or clustered focal or multifocal clonic seizures [66]. |
| Acute Encephalopathies/Encephalitis | |
| Mild encephalopathy with a reversible splenial lesion (MERS) | Prodromal symptoms: fever, cough, vomiting, and diarrhea. Decreased consciousness, seizures, and delirious behavior [67,68,69]. |
| Acute encephalopathy with biphasic seizures and late reduced diffusion (AESD) | During days 1–2, a prolonged febrile seizure occurs, which is followed by a cluster of complex partial seizures with impaired consciousness between days 3 and 7. In addition, 20–30% of patients experience an interval during which consciousness remains normal and no neurological symptoms are evident [69]. |
| Acute necrotizing encephalopathy (ANE) | Rapid progression of altered consciousness and seizures. Vomiting and diarrhea are common [69]. |
| Vaccination Name | Basic Concept | National License |
|---|---|---|
| RotaTeq [106] | Live-attenuated, human-bovine reassortant vaccine: 5 reassortant strains in one vaccine containing human G1, G2, G3, G4 (VP7), and P (VP4) inserted into the bovine G6P. | WHO-prequalified vaccine |
| Rotarix [107] | Human, live-attenuated G1P RV vaccine. | WHO-prequalified vaccine |
| Rotavac [108] | Live-attenuated, naturally reassorted human-bovine single strain G9P vaccine, containing one bovine RV gene P and 10 human RV genes, including G9. | WHO-prequalified vaccine |
| ROTASIIL [109] | Live-attenuated, human-bovine reassortant vaccine: 5 reassortant strains in one vaccine containing human G1, G2, G3, G4, and G9 (VP7) inserted into the bovine G6P UK strain. | WHO-prequalified vaccine |
| Rotavin-M1 [110] | Human, live-attenuated G1P RV vaccine. | National license granted by Vietnam in 2012. |
| LLR (Lanzhou lamb RV vaccine) [111] | Lamb, live-attenuated G10P RV vaccine. | National license granted by China in 2000. |
| Vaccination Name | Vaccination Dosing Schedules |
|---|---|
| RotaTeq | Beginning at 6–12 weeks of age, 3 oral doses given 4–10 weeks apart; the series should be completed by the age of 32 weeks. |
| Rotarix | Beginning at 6 weeks of age; 2 oral doses given 4 weeks apart; the series should be given before 16 weeks of age but must be completed by the age of 24 weeks. |
| Rotavac | Beginning at 6 weeks of age; 3 oral doses given 4 weeks apart; the series should be completed before the age of 8 months. |
| ROTASIIL | Beginning at 6 weeks of age, 3 oral doses given 4 weeks apart; the series should be completed during the first year of life. |
| Rotavin-M1 | 2 oral doses administered 2 months apart; the first dose must be administered at 6–12 weeks of age. |
| LLR (Lanzhou lamb RV vaccine) | 3 oral doses: 1 dose per year for 3 consecutive years in children aged 2–36 months. |
| Vaccination Name | Basic Concept | Developer | Development Phase |
|---|---|---|---|
| RV3-BB | Human neonatal G3P[6] RV vaccine. | Murdoch Children’s Research Institute/Biofarma | A Phase II Dose-Ranging Study of Oral RV3-BB RV Vaccine ended [144]. |
| Tetravalent UK-BRV | Live-attenuated, human-bovine reassortant vaccine: 4 reassortant strains, including human G1–4, inserted into the bovine G6P[5] UK strain. | Shantha Biotechnics | A phase I/II development—development abandoned [145,146]. |
| Hexavalent UK-BRV | Live-attenuated, human-bovine reassortant vaccine: 6 reassortant strains, including human G1–4, G8, and G9, inserted into the bovine G6P[5] UK strain. | Developer: Wuhan Institute of Biological Products, China. Development. | A Phase III clinical trial for efficacy and safety in China ended. No further clinical trials have been conducted for this candidate vaccine in Brazil, where the inclusion of Rotarix in the national immunization program has significantly reduced the RV disease burden [147]. |
| Pentavalent UK-BRV | Live-attenuated, human-bovine reassortant vaccine: 5 reassortant strains, including human G1–4 and G9, inserted into the bovine G6P[5] UK strain. | Developer: Instituto Butantan, Brazil. Development. | Phase I clinical trial for the efficacy and safety in Brazil ended [148]. |
| G1P[8], inactivated | Inactivated human RV vaccine. | Centers for Disease Control and Prevention (CDC), USA. | Preclinical development [149,150,151,152]. |
| P2-VP8-P[8] and P2-VP8-P[4/6/8] | Subunit RV vaccines based on recombinant proteins. | PATH RV Vaccine Program, USA. | A phase III safety, immunogenicity, and efficacy study in infants 6 to 8 weeks of age in Ghana, Malawi, and Zambia—development abandoned. Findings indicated insufficient evidence that the trivalent P2-VP8 offers improved protection against severe RV diarrhea compared to currently licensed oral vaccines [153]. |
| MBP::VP6 and pCWA:VP6 | Subunit RV vaccines based on recombinant proteins. | Cincinnati Children’s Hospital, USA; Laboratoria de Immunologia y Virologia (LIV), Argentina. | Preclinical development [153,154,155,156]. |
| VP2/6/7 and VP2/4/6/7VLPs | Subunit RV vaccines based on virus-like particles. | Baylor College of Medicine, USA. | Preclinical development [157,158]. |
| VP6 GI.3/GII.4 RV-NoV VLPs | Subunit RV vaccines based on virus-like particles. | University of Tampere School of Medicine, Finland. | Preclinical development [159,160,161]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pawłuszkiewicz, K.; Ryglowski, P.J.; Idzik, N.; Błaszczyszyn, K.; Kucharczyk, E.; Gaweł-Dąbrowska, D.; Siczek, M.; Widelski, J.; Paluch, E. Rotavirus Infections: Pathophysiology, Symptoms, and Vaccination. Pathogens 2025, 14, 480. https://doi.org/10.3390/pathogens14050480
Pawłuszkiewicz K, Ryglowski PJ, Idzik N, Błaszczyszyn K, Kucharczyk E, Gaweł-Dąbrowska D, Siczek M, Widelski J, Paluch E. Rotavirus Infections: Pathophysiology, Symptoms, and Vaccination. Pathogens. 2025; 14(5):480. https://doi.org/10.3390/pathogens14050480
Chicago/Turabian StylePawłuszkiewicz, Karolina, Piotr Józef Ryglowski, Natalia Idzik, Katarzyna Błaszczyszyn, Emilia Kucharczyk, Dagmara Gaweł-Dąbrowska, Marta Siczek, Jarosław Widelski, and Emil Paluch. 2025. "Rotavirus Infections: Pathophysiology, Symptoms, and Vaccination" Pathogens 14, no. 5: 480. https://doi.org/10.3390/pathogens14050480
APA StylePawłuszkiewicz, K., Ryglowski, P. J., Idzik, N., Błaszczyszyn, K., Kucharczyk, E., Gaweł-Dąbrowska, D., Siczek, M., Widelski, J., & Paluch, E. (2025). Rotavirus Infections: Pathophysiology, Symptoms, and Vaccination. Pathogens, 14(5), 480. https://doi.org/10.3390/pathogens14050480