Molecular Surveillance for Potential Zoonotic Pathogens in Troglophilus Bats: Detection and Molecular Characterization of Bat Coronaviruses in Southern Italy
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Location and Samples Collection
2.2. Samples Processing
2.3. Virus Screening
2.3.1. Lyssavirus Screening
2.3.2. Coronavirus Screening
2.4. Sequence and Phylogenetic Analyses
3. Results
3.1. Virus Detection
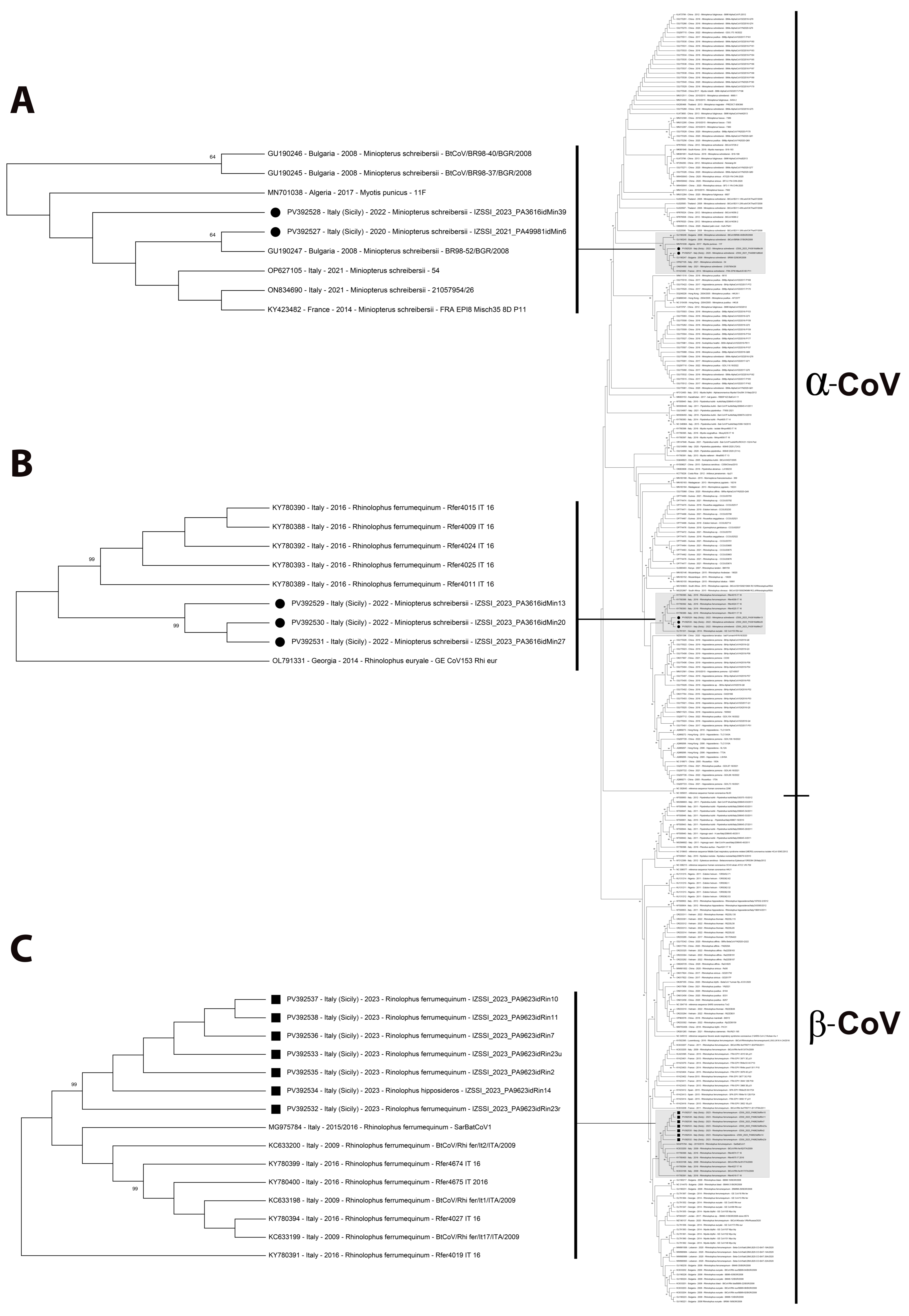
3.2. Coronavirus Sequence and Phylogenetic Analyses
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO); Food and Agriculture Organization of the United Nations (FAO); World Organisation for Animal Health (OIE). Taking a Multisectoral One Health Approach: A Tripartite Guide to Addressing Zoonotic Diseases in Countries; World Organisation for Animal Health: Paris, France, 2019; Available online: https://www.woah.org/en/document/en_tripartitezoonosesguide_webversion/ (accessed on 18 March 2025).
- Jones, K.E.; Patel, N.G.; Levy, M.A.; Storeygard, A.; Balk, D.; Gittleman, J.L.; Daszak, P. Global Trends in Emerging Infectious Diseases. Nature 2008, 451, 990–993. [Google Scholar] [CrossRef] [PubMed]
- World Organisation for Animal Health (WOAH). Policy Brief: The Importance of the One Health Approach in Tackling Emerging and Re-Emerging Zoonotic Epidemics and Pandemics. 2024. Available online: https://doc.woah.org/dyn/portal/index.xhtml?page=alo&aloId=43901 (accessed on 18 March 2025).
- Mackenzie, J.S.; Field, H.E. Emerging Encephalitogenic Viruses: Lyssaviruses and Henipaviruses Transmitted by Frugivorous Bats. Arch. Virol. Suppl. 2004, 18, 97–111. [Google Scholar] [CrossRef]
- Woo, P.C.Y.; Lau, S.K.P.; Huang, Y.; Yuen, K.-Y. Coronavirus Diversity, Phylogeny and Interspecies Jumping. Exp. Biol. Med. (Maywood) 2009, 234, 1117–1127. [Google Scholar] [CrossRef]
- Letko, M.; Seifert, S.N.; Olival, K.J.; Plowright, R.K.; Munster, V.J. Bat-Borne Virus Diversity, Spillover and Emergence. Nat. Rev. Microbiol. 2020, 18, 461–471. [Google Scholar] [CrossRef]
- Jiménez-Rico, M.A.; Vigueras-Galván, A.L.; Hernández-Villegas, E.N.; Martínez-Duque, P.; Roiz, D.; Falcón, L.I.; Vázquez-Domínguez, E.; Gaona, O.; Arnal, A.; Roche, B.; et al. Bat Coronavirus Surveillance across Different Habitats in Yucatán, México. Virology 2025, 603, 110401. [Google Scholar] [CrossRef] [PubMed]
- Burgin, C.J.; Colella, J.P.; Kahn, P.L.; Upham, N.S. How Many Species of Mammals Are There? J. Mammal. 2018, 99, 1–14. [Google Scholar] [CrossRef]
- Kerth, G. Animal Sociality: Bat Colonies Are Founded by Relatives. Curr. Biol. 2008, 18, R740–R742. [Google Scholar] [CrossRef] [PubMed]
- Platto, S.; Zhou, J.; Wang, Y.; Wang, H.; Carafoli, E. Biodiversity Loss and COVID-19 Pandemic: The Role of Bats in the Origin and the Spreading of the Disease. Biochem. Biophys. Res. Commun. 2021, 538, 2–13. [Google Scholar] [CrossRef]
- Wong, S.; Lau, S.; Woo, P.; Yuen, K.-Y. Bats as a Continuing Source of Emerging Infections in Humans. Rev. Med. Virol. 2007, 17, 67–91. [Google Scholar] [CrossRef]
- Kandeil, A.; Abi-Said, M.; Badra, R.; El-Shesheny, R.; Al-Karmalawy, A.A.; Alnajjar, R.; Khalid, Z.; Kamel, M.N.; Abi Habib, W.; Abdallah, J.; et al. Detection of Coronaviruses in Bats in Lebanon during 2020. Pathogens 2023, 12, 876. [Google Scholar] [CrossRef]
- Decaro, N.; Balboni, A.; Bertolotti, L.; Martino, P.A.; Mazzei, M.; Mira, F.; Pagnini, U. SARS-CoV-2 Infection in Dogs and Cats: Facts and Speculations. Front. Vet. Sci. 2021, 8, 619207. [Google Scholar] [CrossRef] [PubMed]
- Abay, Z.; Sadikaliyeva, S.; Nurpeisova, A.; Jekebekov, K.; Shorayeva, K.; Yespembetov, B.; Nurabayev, S.; Kerimbayev, A.; Khairullin, B.; Yoo, H.; et al. Breaking the Barrier: SARS-CoV-2 Infections in Wild and Companion Animals and Their Implications for Public Health. Viruses 2024, 16, 956. [Google Scholar] [CrossRef] [PubMed]
- Marchi, S.; Guarducci, G.; Marotta, M.G.; Peccetti, B.; Viviani, S.; Messina, G.; Montomoli, E.; Martella, V.; Camero, M.; Trombetta, C.M. Improving the ONE HEALTH Approach: A Lesson from SARS-CoV-2 Pandemic. J. Prev. Med. Hyg. 2024, 65, E312–E322. [Google Scholar] [CrossRef]
- Woo, P.C.Y.; de Groot, R.J.; Haagmans, B.; Lau, S.K.P.; Neuman, B.W.; Perlman, S.; Sola, I.; van der Hoek, L.; Wong, A.C.P.; Yeh, S.-H. ICTV Virus Taxonomy Profile: Coronaviridae 2023. J. Gen. Virol. 2023, 104, 001843. [Google Scholar] [CrossRef] [PubMed]
- Balboni, A.; Palladini, A.; Bogliani, G.; Battilani, M. Detection of a Virus Related to Betacoronaviruses in Italian Greater Horseshoe Bats. Epidemiol. Infect. 2011, 139, 216–219. [Google Scholar] [CrossRef]
- Lau, S.K.P.; Woo, P.C.Y.; Li, K.S.M.; Huang, Y.; Tsoi, H.-W.; Wong, B.H.L.; Wong, S.S.Y.; Leung, S.-Y.; Chan, K.-H.; Yuen, K.-Y. Severe Acute Respiratory Syndrome Coronavirus-like Virus in Chinese Horseshoe Bats. Proc. Natl. Acad. Sci. USA 2005, 102, 14040–14045. [Google Scholar] [CrossRef]
- Li, W.; Shi, Z.; Yu, M.; Ren, W.; Smith, C.; Epstein, J.H.; Wang, H.; Crameri, G.; Hu, Z.; Zhang, H.; et al. Bats Are Natural Reservoirs of SARS-like Coronaviruses. Science 2005, 310, 676–679. [Google Scholar] [CrossRef]
- Drexler, J.F.; Gloza-Rausch, F.; Glende, J.; Corman, V.M.; Muth, D.; Goettsche, M.; Seebens, A.; Niedrig, M.; Pfefferle, S.; Yordanov, S.; et al. Genomic Characterization of Severe Acute Respiratory Syndrome-Related Coronavirus in European Bats and Classification of Coronaviruses Based on Partial RNA-Dependent RNA Polymerase Gene Sequences. J. Virol. 2010, 84, 11336–11349. [Google Scholar] [CrossRef]
- Ar Gouilh, M.; Puechmaille, S.J.; Diancourt, L.; Vandenbogaert, M.; Serra-Cobo, J.; Lopez Roïg, M.; Brown, P.; Moutou, F.; Caro, V.; Vabret, A.; et al. SARS-CoV Related Betacoronavirus and Diverse Alphacoronavirus Members Found in Western Old-World. Virology 2018, 517, 88–97. [Google Scholar] [CrossRef]
- Buonocore, M.; Marino, C.; Grimaldi, M.; Santoro, A.; Firoznezhad, M.; Paciello, O.; Prisco, F.; D’Ursi, A.M. New Putative Animal Reservoirs of SARS-CoV-2 in Italian Fauna: A Bioinformatic Approach. Heliyon 2020, 6, e05430. [Google Scholar] [CrossRef]
- Briggs, K.; Sweeney, R.; Blehert, D.S.; Spackman, E.; Suarez, D.L.; Kapczynski, D.R. SARS-CoV-2 Utilization of ACE2 from Different Bat Species Allows for Virus Entry and Replication in Vitro. Virology 2023, 586, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Hamdy, M.E.; El Deeb, A.H.; Hagag, N.M.; Shahein, M.A.; Alaidi, O.; Hussein, H.A. Interspecies Transmission of SARS CoV-2 with Special Emphasis on Viral Mutations and ACE-2 Receptor Homology Roles. Int. J. Vet. Sci. Med. 2023, 11, 55–86. [Google Scholar] [CrossRef]
- Leopardi, S.; Barneschi, E.; Manna, G.; Zecchin, B.; Priori, P.; Drzewnioková, P.; Festa, F.; Lombardo, A.; Parca, F.; Scaravelli, D.; et al. Spillover of West Caucasian Bat Lyssavirus (WCBV) in a Domestic Cat and Westward Expansion in the Palearctic Region. Viruses 2021, 13, 2064. [Google Scholar] [CrossRef] [PubMed]
- Walker, P.J.; Freitas-Astúa, J.; Bejerman, N.; Blasdell, K.R.; Breyta, R.; Dietzgen, R.G.; Fooks, A.R.; Kondo, H.; Kurath, G.; Kuzmin, I.V.; et al. ICTV Virus Taxonomy Profile: Rhabdoviridae 2022. J. Gen. Virol. 2022, 103, 001689. [Google Scholar] [CrossRef] [PubMed]
- Rupprecht, C.E.; Turmelle, A.; Kuzmin, I.V. A Perspective on Lyssavirus Emergence and Perpetuation. Curr. Opin. Virol. 2011, 1, 662–670. [Google Scholar] [CrossRef]
- Fooks, A.R.; Cliquet, F.; Finke, S.; Freuling, C.; Hemachudha, T.; Mani, R.S.; Müller, T.; Nadin-Davis, S.; Picard-Meyer, E.; Wilde, H.; et al. Rabies. Nat. Rev. Dis. Primers 2017, 3, 17091. [Google Scholar] [CrossRef]
- Eblé, P.; Dekker, A.; van den End, S.; Visser, V.; Engelsma, M.; Harders, F.; van Keulen, L.; van Weezep, E.; Holwerda, M. A Case Report of a Cat Infected with European Bat Lyssavirus Type 1, the Netherlands, October 2024. Euro. Surveill. 2025, 30, 2500154. [Google Scholar] [CrossRef]
- Fooks, A.R.; Brookes, S.M.; Johnson, N.; McElhinney, L.M.; Hutson, A.M. European Bat Lyssaviruses: An Emerging Zoonosis. Epidemiol. Infect. 2003, 131, 1029–1039. [Google Scholar] [CrossRef]
- Agnelli, P.; Martinoli, A.; Patriarca, E.; Scaravelli, D.; Genovesi, P. Linee Guida per Il Monitoraggio Dei Chirotteri: Indicazioni Metodologiche per Lo Studio e La Conservazione Dei Pipistrelli in Italia; In Quaderni di Conservazione della Natura; Ministero dell’Ambiente—Istituto Nazionale per la Fauna Selvatica. 2004. Available online: https://www.isprambiente.gov.it/files/pubblicazioni/quaderni/conservazione-natura/files/6730_19_qcn_monitoraggio_chirotteri.pdf (accessed on 1 November 2020).
- Massaad, M.; da Silveira Bueno, R.; Bentaleb, I.; La Mantia, T. Bats of Sicily: Historical evidence, current knowledge, research biases and trends. Nat. Hist. Sci. 2023, 10, 45–58. [Google Scholar] [CrossRef]
- Caruso, D.; Grasso, R. La fauna delle grotte. In Atti del Convegno su “La fauna degli Iblei”, Ente Fauna Siciliana, Italy, 13–14 May 1995; Ragonese, B., Ed.; Libreria Editrice Urso: Avola, Italy, 1995; pp. 201–281. [Google Scholar]
- Agnelli, P.; Di Salvo, I.; Russo, D.; Sarà, M. Chirotterofauna della Sicilia (Mammalia Chiroptera). In Atlante Della Biodiversità della Sicilia: Vertebrati Terrestri; Sicilia, A., Ed.; Studi e Ricerche, 6; Arpa Sicilia: Palermo, Italy, 2008; pp. 25–41. [Google Scholar]
- Spena, M.T.; Allegra Filosico, M.; Brogna, F.; Dipasquale, C.; Puma, A.; Grasso, R.; Agnelli, P. I chirotteri della Grotta dei Pipistrelli (SR): Un unicum nella Sicilia sud-orientale. In Proceedings of the 74° Congresso Nazionale dell’Unione Zoologica Italiana, Atti della Società dei Naturalisti e Matematici di Modena, Modena, Italy, 30 September–3 October 2013. [Google Scholar]
- Sperlinga, G.; Fichera, G.; Reitano, A. I chirotteri delle grotte dell’Etna. In Le grotte dell’Etna; Cavallaro, F., Reitano, A., Danaus, E., Eds.; Casa Editrice DANAUS: Palermo, Italy, 2013; pp. 235–252. [Google Scholar]
- Ait-Abdesselam, M.; Dalhoumi, R.; Ouabed, A.; Bounaceur, F.; Aulagnier, S. Bat species richness and activity in a Mediterranean area of Northwest Africa: Urban–rural shifts. Anim. Taxon. Ecol. 2025, 71, 36–50. [Google Scholar] [CrossRef]
- Hemnani, M.; da Silva, P.G.; Thompson, G.; Poeta, P.; Rebelo, H.; Mesquita, J.R. First Report of Alphacoronavirus Circulating in Cavernicolous Bats from Portugal. Viruses 2023, 15, 1521. [Google Scholar] [CrossRef] [PubMed]
- Dakroub, H.; Russo, D.; Cistrone, L.; Serra, F.; Fusco, G.; De Carlo, E.; Amoroso, M.G. A First Assessment of SARS-CoV-2 Circulation in Bats of Central-Southern Italy. Pathogens 2022, 11, 742. [Google Scholar] [CrossRef]
- Lecis, R.; Mucedda, M.; Pidinchedda, E.; Pittau, M.; Alberti, A. Molecular Identification of Betacoronavirus in Bats from Sardinia (Italy): First Detection and Phylogeny. Virus Genes 2019, 55, 60–67. [Google Scholar] [CrossRef]
- Amoroso, M.G.; Russo, D.; Lanave, G.; Cistrone, L.; Pratelli, A.; Martella, V.; Galiero, G.; Decaro, N.; Fusco, G. Detection and Phylogenetic Characterization of Astroviruses in Insectivorous Bats from Central-Southern Italy. Zoonoses Public Health 2018, 65, 702–710. [Google Scholar] [CrossRef]
- Leopardi, S.; Desiato, R.; Mazzucato, M.; Orusa, R.; Obber, F.; Averaimo, D.; Berjaoui, S.; Canziani, S.; Capucchio, M.T.; Conti, R.; et al. One Health Surveillance Strategy for Coronaviruses in Italian Wildlife. Epidemiol. Infect. 2023, 151, e96. [Google Scholar] [CrossRef]
- Di Bella, S.; Giacchino, I.; Blanda, V.; Gucciardi, F.; Scibetta, S.; La Russa, F.; Lastra, A.; Purpari, G.; Grasso, R.; Spena, M.T.; et al. Zoonotic Bacteria and Vector-Borne Protozoa in Troglophilus Bat Colonies in Sicily (Southern Italy): A Biomolecular Survey. Animals 2025, 15, 488. [Google Scholar] [CrossRef] [PubMed]
- Gigante, C.M.; Dettinger, L.; Powell, J.W.; Seiders, M.; Condori, R.E.C.; Griesser, R.; Okogi, K.; Carlos, M.; Pesko, K.; Breckenridge, M.; et al. Multi-Site Evaluation of the LN34 Pan-Lyssavirus Real-Time RT-PCR Assay for Post-Mortem Rabies Diagnostics. PLoS ONE 2018, 13, e0197074. [Google Scholar] [CrossRef] [PubMed]
- Wadhwa, A.; Wilkins, K.; Gao, J.; Condori Condori, R.E.; Gigante, C.M.; Zhao, H.; Ma, X.; Ellison, J.A.; Greenberg, L.; Velasco-Villa, A.; et al. A Pan-Lyssavirus Taqman Real-Time RT-PCR Assay for the Detection of Highly Variable Rabies Virus and Other Lyssaviruses. PLoS Negl. Trop. Dis. 2017, 11, e0005258. [Google Scholar] [CrossRef]
- Drzewnioková, P.; Festa, F.; Panzarin, V.; Lelli, D.; Moreno, A.; Zecchin, B.; De Benedictis, P.; Leopardi, S. Best Molecular Tools to Investigate Coronavirus Diversity in Mammals: A Comparison. Viruses 2021, 13, 1975. [Google Scholar] [CrossRef]
- Woo, P.C.Y.; Lau, S.K.P.; Chu, C.; Chan, K.; Tsoi, H.; Huang, Y.; Wong, B.H.L.; Poon, R.W.S.; Cai, J.J.; Luk, W.; et al. Characterization and Complete Genome Sequence of a Novel Coronavirus, Coronavirus HKU1, from Patients with Pneumonia. J. Virol. 2005, 79, 884–895. [Google Scholar] [CrossRef]
- Chu, D.K.W.; Poon, L.L.M.; Chan, K.H.; Chen, H.; Guan, Y.; Yuen, K.Y.; Peiris, J.S.M. Coronaviruses in Bent-Winged Bats (Miniopterus spp.). J. Gen. Virol. 2006, 87, 2461–2466. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Zhu, C.; Ai, L.; He, T.; Wang, Y.; Ye, F.; Yang, L.; Ding, C.; Zhu, X.; Lv, R.; et al. Genomic Characterization and Infectivity of a Novel SARS-like Coronavirus in Chinese Bats. Emerg. Microbes Infect. 2018, 7, 154. [Google Scholar] [CrossRef]
- Zhang, Z.; Schwartz, S.; Wagner, L.; Miller, W. A Greedy Algorithm for Aligning DNA Sequences. J. Comput. Biol. 2000, 7, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.X.; Liang, J.Q.; Fung, T.S. Human Coronavirus-229E, -OC43, -NL63, and -HKU1 (Coronaviridae). Encycl. Virol. 2021, 428–440. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, Q.; Wang, H.; Yao, X. Severe Zoonotic Viruses Carried by Different Species of Bats and Their Regional Distribution. Clin. Microbiol. Infect. 2024, 30, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Wickenhagen, A.; van Tol, S.; Munster, V. Molecular Determinants of Cross-Species Transmission in Emerging Viral Infections. Microbiol. Mol. Biol. Rev. 2024, 88, e0000123. [Google Scholar] [CrossRef]
- Leopardi, S.; Dacheux, L.; Serra-Cobo, J.; Ábrahám, Á.; Bajić, B.; Bourhy, H.; Bücs, S.-L.; Budinski, I.; Castellan, M.; Drzewniokova, P.; et al. European Distribution and Intramuscular Pathogenicity of Divergent Lyssaviruses West Caucasian Bat Virus and Lleida Bat Lyssavirus. iScience 2025, 28, 111738. [Google Scholar] [CrossRef]
- Hu, H.; Jung, K.; Wang, Q.; Saif, L.J.; Vlasova, A.N. Development of a One-Step RT-PCR Assay for Detection of Pancoronaviruses (α-, β-, γ-, and δ-Coronaviruses) Using Newly Designed Degenerate Primers for Porcine and Avian `fecal Samples. J. Virol. Methods 2018, 256, 116–122. [Google Scholar] [CrossRef]
- Tang, G.; Liu, Z.; Chen, D. Human Coronaviruses: Origin, Host and Receptor. J. Clin. Virol. 2022, 155, 105246. [Google Scholar] [CrossRef]
- Li, Q.; Hou, Y.; Huang, B.; Le, X.; Wang, B.; Xia, X. Identification and Genetic Characterization of Five Novel Bat Coronaviruses from Yunnan, China. BMC Vet. Res. 2024, 20, 466. [Google Scholar] [CrossRef]
- Seifert, S.N.; Bai, S.; Fawcett, S.; Norton, E.B.; Zwezdaryk, K.J.; Robinson, J.; Gunn, B.; Letko, M. An ACE2-Dependent Sarbecovirus in Russian Bats Is Resistant to SARS-CoV-2 Vaccines. PLoS Pathog. 2022, 18, e1010828. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, W.; Li, Y.; Liu, C.; Dong, T.; Chen, H.; Wu, C.; Su, J.; Li, B.; Zhang, W.; et al. Bat-Infecting Merbecovirus HKU5-CoV Lineage 2 Can Use Human ACE2 as a Cell Entry Receptor. Cell 2025, 188, 1729–1742.e16. [Google Scholar] [CrossRef] [PubMed]
- Leopardi, S.; Holmes, E.C.; Gastaldelli, M.; Tassoni, L.; Priori, P.; Scaravelli, D.; Zamperin, G.; De Benedictis, P. Interplay between Co-Divergence and Cross-Species Transmission in the Evolutionary History of Bat Coronaviruses. Infect. Genet. Evol. 2018, 58, 279–289. [Google Scholar] [CrossRef]
- De Benedictis, P.; Marciano, S.; Scaravelli, D.; Priori, P.; Zecchin, B.; Capua, I.; Monne, I.; Cattoli, G. Alpha and Lineage C BetaCoV Infections in Italian Bats. Virus Genes 2014, 48, 366–371. [Google Scholar] [CrossRef]
- Lelli, D.; Papetti, A.; Sabelli, C.; Rosti, E.; Moreno, A.; Boniotti, M.B. Detection of Coronaviruses in Bats of Various Species in Italy. Viruses 2013, 5, 2679–2689. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, F.; Edenborough, K.M.; Toffoli, R.; Culasso, P.; Zoppi, S.; Dondo, A.; Robetto, S.; Rosati, S.; Lander, A.; Kurth, A.; et al. Coronavirus and Paramyxovirus in Bats from Northwest Italy. BMC Vet. Res. 2017, 13, 396. [Google Scholar] [CrossRef] [PubMed]
- Leopardi, S.; Priori, P.; Zecchin, B.; Zamperin, G.; Milani, A.; Tonon, F.; Giorgiutti, M.; Beato, M.S.; De Benedictis, P. Interface between Bats and Pigs in Heavy Pig Production. Viruses 2020, 13, 4. [Google Scholar] [CrossRef]
- Leopardi, S.; Priori, P.; Zecchin, B.; Poglayen, G.; Trevisiol, K.; Lelli, D.; Zoppi, S.; Scicluna, M.T.; D’Avino, N.; Schiavon, E.; et al. Active and Passive Surveillance for Bat Lyssaviruses in Italy Revealed Serological Evidence for Their Circulation in Three Bat Species. Epidemiol. Infect. 2018, 147, e63. [Google Scholar] [CrossRef]
- Colombino, E.; Lelli, D.; Canziani, S.; Quaranta, G.; Guidetti, C.; Leopardi, S.; Robetto, S.; De Benedictis, P.; Orusa, R.; Mauthe von Degerfeld, M.; et al. Main Causes of Death of Free-Ranging Bats in Turin Province (North-Western Italy): Gross and Histological Findings and Emergent Virus Surveillance. BMC Vet. Res. 2023, 19, 200. [Google Scholar] [CrossRef]
- Kemenesi, G.; Dallos, B.; Görföl, T.; Boldogh, S.; Estók, P.; Kurucz, K.; Kutas, A.; Földes, F.; Oldal, M.; Németh, V.; et al. Molecular Survey of RNA Viruses in Hungarian Bats: Discovering Novel Astroviruses, Coronaviruses, and Caliciviruses. Vector Borne Zoonotic Dis. 2014, 14, 846–855. [Google Scholar] [CrossRef] [PubMed]
- Cohen, L.E.; Fagre, A.C.; Chen, B.; Carlson, C.J.; Becker, D.J. Coronavirus Sampling and Surveillance in Bats from 1996-2019: A Systematic Review and Meta-Analysis. Nat. Microbiol. 2023, 8, 1176–1186. [Google Scholar] [CrossRef]
- Amengual, B.; López-Roig, M.; Serra-Cobo, J. First record of seasonal over sea migration of Miniopterus schreibersii and Myotis capaccinii between Balearic Islands (Spain). Acta Chiropterologica 2007, 9, 319–322. [Google Scholar] [CrossRef]
- Urushadze, L.; Babuadze, G.; Shi, M.; Escobar, L.E.; Mauldin, M.R.; Natradeze, I.; Machablishvili, A.; Kutateladze, T.; Imnadze, P.; Nakazawa, Y.; et al. A Cross Sectional Sampling Reveals Novel Coronaviruses in Bat Populations of Georgia. Viruses 2021, 14, 72. [Google Scholar] [CrossRef]
- Hemnani, M.; da Silva, P.G.; Thompson, G.; Poeta, P.; Rebelo, H.; Mesquita, J.R. Detection and Prevalence of Coronaviruses in European Bats: A Systematic Review. Ecohealth 2024, 21, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Huang, G.; Huang, M.; Dai, Q.; Hu, Y.; Zhou, J.; Wei, F. Global Patterns of Phylogenetic Diversity and Transmission of Bat Coronavirus. Sci. China Life Sci. 2023, 66, 861–874. [Google Scholar] [CrossRef]
- Begeman, L.; Geschiere, M.J.M.; de Boer, W.F.; van den Brand, J.M.A.; Eblé, P.L.; van der Kerkhof, J.H.T.C.; Keur, I.; Lina, P.H.C.; Reusken, C.B.E.M.; de Rosa, M.; et al. Human-Bat Contacts in the Netherlands, and Potential Risks for Virus Exchange. One Health Outlook 2025, 7, 7. [Google Scholar] [CrossRef]
- Mira, F.; Gucciardi, F.; Vaiana, C.; Anzà, D.; Di Paola, L.; Schirò, G.; Di Bella, S.; Guercio, A.; Purpari, G. Molecular surveillance for potential zoonotic pathogens in bats: Detection and characterization of bat-borne coronaviruses in southern Italy. In Proceedings of the 8° Congresso Nazionale della Società Italiana di Virologia (SIV), Bologna, Italy, 7–9 July 2024. [Google Scholar]
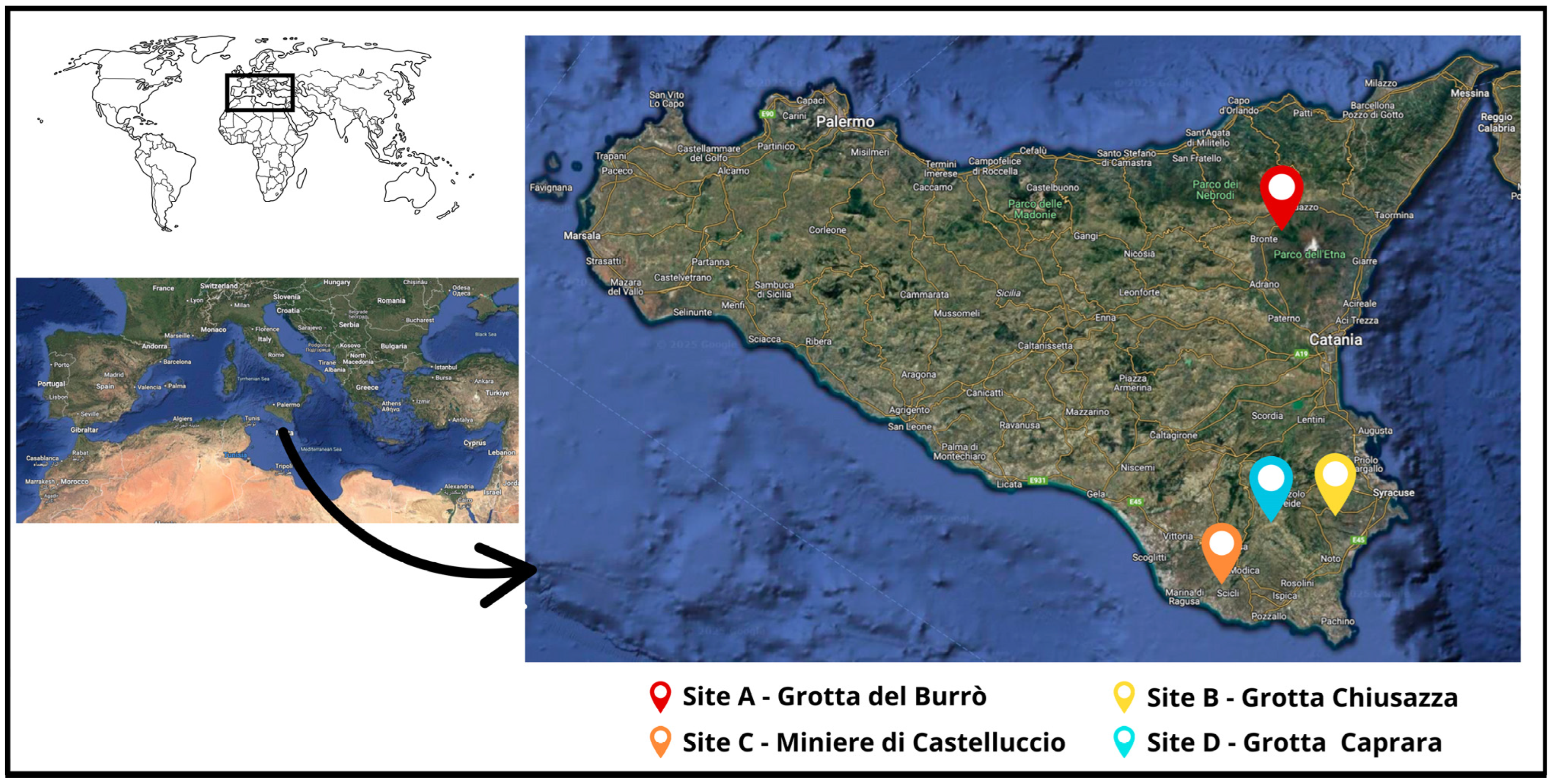
| Site | Site Name | Geographical Location City (Province) | Geographic Coordinates (Longitude—Latitude) | Number of Sampled Bats |
|---|---|---|---|---|
| A | Grotta del Burrò | Randazzo (CT) | 14.934538–37.826880 | 21 |
| B | Grotta Chiusazza | Floridia (SR) | 15.159536–37.026406 | 38 (on 16 March 2022) 41 (on 5 December 2022) |
| C | Miniere di Castelluccio | Modica (RG) | 14.691320–36.837110 | 24 |
| D | Grotta Caprara | Noto (SR) | 14.926690–37.007520 | 26 |
| Total sampled bats: | 150 |
| PCR assay | Target | Primers/Probes | Sequence (5′-3′) | Reference |
|---|---|---|---|---|
| Real-time Lyssavirus | highly conserved non-coding leader region and part of the nucleoprotein (N) coding sequence | LN34 Forward Primer 1 | ACGCTTAACAACCAGATCAAAGAA | [44] |
| LN34 Forward Primer 2 | ACGCTTAACAACAAAATCADAGAAG | |||
| LN34 Reverse Primers | CMGGGTAYTTRTAYTCATAYTGRTC | |||
| LN34 Probe | (FAM)-AA+C+ACCY+C+T+ACA+A+TGGA-(BHQ1) | |||
| LN34lago Probe a | (FAM)-AA+C+ACTA+C+T+ACA+A+TGGA-(BHQ1) | |||
| Real-time host β-actin mRNA | β-actin mRNA | β-actin Forward Primer | CGATGAAGATCAAGATCATTGC | |
| β-actin Reverse Primer | AAGCATTTGCGGTGGAC | |||
| β-actin Probe | (HEX)-TCCACCTTCCAGCAGATGTGGATCA-(BHQ1) | |||
| Nested PCR pan-coronavirus | RNA dependent RNA polymerase (RdRp) | Hu-F | AARTTYTAYGGHHHYTGG | [46] |
| Hu-R | GARCARAATTCATGHGGDCC | |||
| Poon-F | GGTTGGGACTATCCTAAGTGTGA | |||
| Chu06-R1 | CCATCATCAGATAGAATCATCAT |
| Collection Date (Day Month Year) | Site | Detection Frequency | Host Species | Sample | Id |
|---|---|---|---|---|---|
| 15 November 2020 | A | 4.76% | Miniopterus schreibersii | Feces | IZSSI_2021PA49981idMin6 |
| 5 December 2020 | B | 5.06% | Miniopterus schreibersii | Urine/Feces | IZSSI_2023PA3616idMin39 |
| Miniopterus schreibersii | Urine/Feces | IZSSI_2023PA3616idMin13 | |||
| Miniopterus schreibersii | Urine/Feces | IZSSI_2023PA3616idMin20 | |||
| Miniopterus schreibersii | Urine/Feces | IZSSI_2023PA3616idMin27 | |||
| 29 March 2023 | C | 29.16% | Rhinolophus ferrumequinum a | Rectal swab | IZSSI_2023PA9623idRin23r |
| Rhinolophus ferrumequinum a | Urine | IZSSI_2023PA9623idRin23u | |||
| Rhinolophus hipposideros | Urine | IZSSI_2023PA9623idRin14 | |||
| Rhinolophus ferrumequinum | Rectal swab | IZSSI_2023PA9623idRin2 | |||
| Rhinolophus ferrumequinum | Rectal swab | IZSSI_2023PA9623idRin7 | |||
| Rhinolophus ferrumequinum | Rectal swab | IZSSI_2023PA9623idRin10 | |||
| Rhinolophus ferrumequinum | Rectal swab | IZSSI_2023PA9623idRin11 | |||
| Rhinolophus ferrumequinum | Urine | IZSSI_2023PA9623idRin21 |
| Sequence Id | Accession Number | Genera | Site | Host Species |
|---|---|---|---|---|
| IZSSI_2021PA49981idMin6 | PV392527 | Alphacoronavirus | A | Miniopterus schreibersii |
| IZSSI_2023PA3616idMin39 | PV392528 | B | Miniopterus schreibersii | |
| IZSSI_2023PA3616idMin13 | PV392529 | Miniopterus schreibersii | ||
| IZSSI_2023PA3616idMin20 | PV392530 | Miniopterus schreibersii | ||
| IZSSI_2023PA3616idMin27 | PV392531 | Miniopterus schreibersii | ||
| IZSSI_2023PA9623idRin23r | PV392532 | Betacoronavirus | C | Rhinolophus ferrumequinum a |
| IZSSI_2023PA9623idRin23u | PV392533 | Rhinolophus ferrumequinum a | ||
| IZSSI_2023PA9623idRin14 | PV392534 | Rhinolophus hipposideros | ||
| IZSSI_2023PA9623idRin2 | PV392535 | Rhinolophus ferrumequinum | ||
| IZSSI_2023PA9623idRin7 | PV392536 | Rhinolophus ferrumequinum | ||
| IZSSI_2023PA9623idRin10 | PV392537 | Rhinolophus ferrumequinum | ||
| IZSSI_2023PA9623idRin11 | PV392538 | Rhinolophus ferrumequinum |
| Country (Area) and Year of Collection | Identified CoV Genera | Positive Bat Species (Family) | Reference | Bat Positivity Rate |
|---|---|---|---|---|
| Portugal a 2022 | α-CoV a | Miniopterus schreibersii * (Miniopteridae); Myotis myotis * (Vespertilionidae) | [38] | 8.9% |
| Italy 2020–2022 | α-CoV and β-CoV | Hypsugo savii, Pipistrellus kuhlii, Myotis crypticus, Plecotus auritus, Pipistrellus pipistrellus (Vespertilionidae) | [42] | 3.1% |
| Italy a (Central) 2021 | α-CoV a | Miniopterus schreibersii * | Unpublished (acc.nr. ON834690, OP627105) | Not available |
| Lebanon a 2020 | α-CoV a and β-CoV | Miniopterus schreibersii * | [12] | 18.3% |
| Italy (Central–Southern) 2021 | None | None | [39] | 0% |
| Italy (Northern–Eastern) 2018 | α-CoV | Pipistrellus khulii (Vespertilionidae) | [65] | undetermined |
| Italy a (Sardinia) 2015–2016 | β-CoV a | Rhinolophus ferrumequinum a (Rhinolophidae); Plecotus auritus, Tadarida teniotis (Vespertilionidae) | [40] | 11% |
| Italy (Central–Southern) year not available | Not determined | Data not available | [41] | 6.8% |
| Italy (Northwestern) a 2013–2016 | α-CoV a and β-CoV a | Pipistrellus kuhlii, Pipistrellus pipistrellus; Myotis myotis, Myotis nattereri, Myotis daubentonii, Myotis oxygnathus; Plecotus auritus (Vespertilionidae); Rhinolophus ferrumequinum a | [64] | 12% |
| France a, Spain, Morocco, Tunisia 2008–2016 | α-CoV a and β-CoV | Miniopterus schreibersii * | [21] | 13.6% |
| Italy (Northern and Sicily, Southern) 2009–2012 | α-CoV and β-CoV | Myotis myotis, Myotis blythii; Eptesicus serotinus (Vespertilionidae) | [62] | 2.7% |
| Hungary a 2012–2013 | α-CoV a and β-CoV | Rhinolophus ferrumequinum *; Rhinolophus hipposideros * (Rhinolophidae) | [68] | 1.8% |
| Italy (Northern) 2010–2012 | α-CoV and β-CoV | Rhinolophus hipposideros; Nyctalus noctula; Hypsugo savii; P. kuhlii (Vespertilionidae) | [63] | 8.2% |
| Italy (Northern and central) a 2009 | β-CoV a | Rhinolophus ferrumequinum a | [17] | 3.8% |
| Bulgaria a 2008 | α-CoV a and β-CoV a | Miniopterus schreibersii * | [20] | 40.4% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mira, F.; Gucciardi, F.; Schiró, G.; Grasso, R.; Spena, M.T.; Kemenesi, G.; Vaiana, C.; Anzá, D.; Di Paola, L.; Di Bella, S.; et al. Molecular Surveillance for Potential Zoonotic Pathogens in Troglophilus Bats: Detection and Molecular Characterization of Bat Coronaviruses in Southern Italy. Pathogens 2025, 14, 457. https://doi.org/10.3390/pathogens14050457
Mira F, Gucciardi F, Schiró G, Grasso R, Spena MT, Kemenesi G, Vaiana C, Anzá D, Di Paola L, Di Bella S, et al. Molecular Surveillance for Potential Zoonotic Pathogens in Troglophilus Bats: Detection and Molecular Characterization of Bat Coronaviruses in Southern Italy. Pathogens. 2025; 14(5):457. https://doi.org/10.3390/pathogens14050457
Chicago/Turabian StyleMira, Francesco, Francesca Gucciardi, Giorgia Schiró, Rosario Grasso, Maria Teresa Spena, Gábor Kemenesi, Claudia Vaiana, Davide Anzá, Laura Di Paola, Santina Di Bella, and et al. 2025. "Molecular Surveillance for Potential Zoonotic Pathogens in Troglophilus Bats: Detection and Molecular Characterization of Bat Coronaviruses in Southern Italy" Pathogens 14, no. 5: 457. https://doi.org/10.3390/pathogens14050457
APA StyleMira, F., Gucciardi, F., Schiró, G., Grasso, R., Spena, M. T., Kemenesi, G., Vaiana, C., Anzá, D., Di Paola, L., Di Bella, S., Guercio, A., & Purpari, G. (2025). Molecular Surveillance for Potential Zoonotic Pathogens in Troglophilus Bats: Detection and Molecular Characterization of Bat Coronaviruses in Southern Italy. Pathogens, 14(5), 457. https://doi.org/10.3390/pathogens14050457





