Epidemiological and Clinical Manifestations of Acute Rheumatic Fever in Far North Queensland, Australia
Abstract
1. Introduction
2. Methods
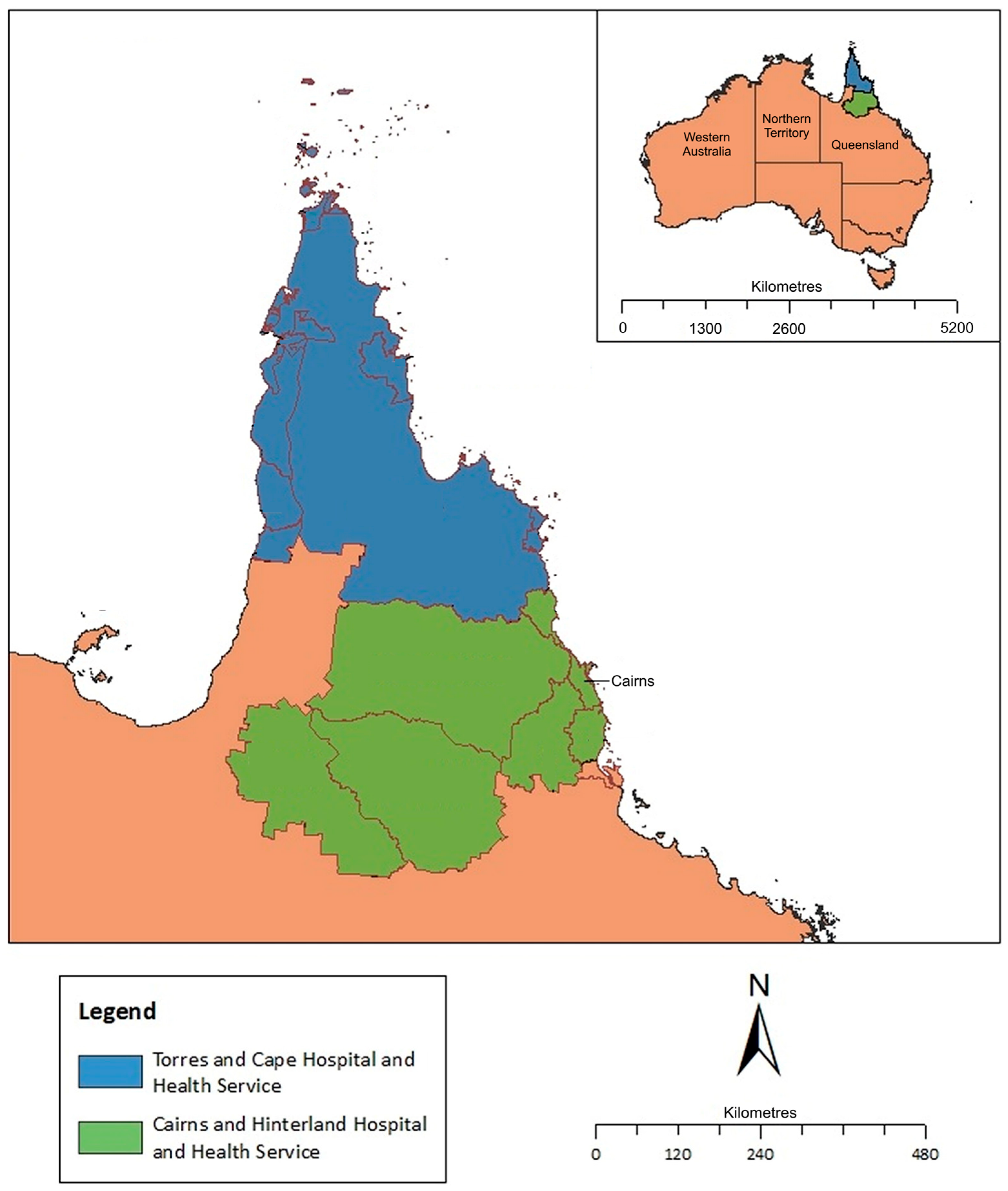
2.1. Setting
2.2. Data Collection
2.3. Statistical Analysis
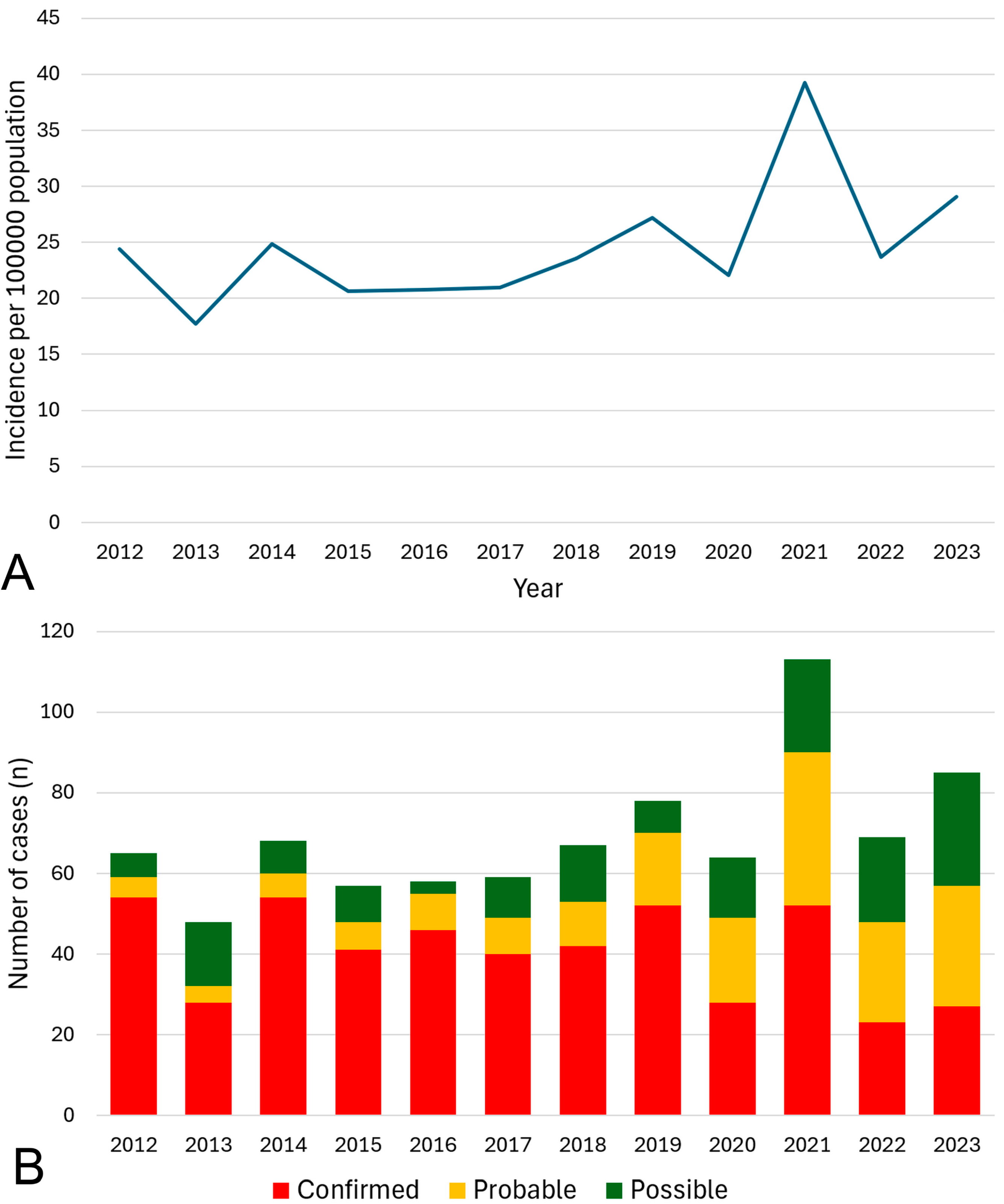
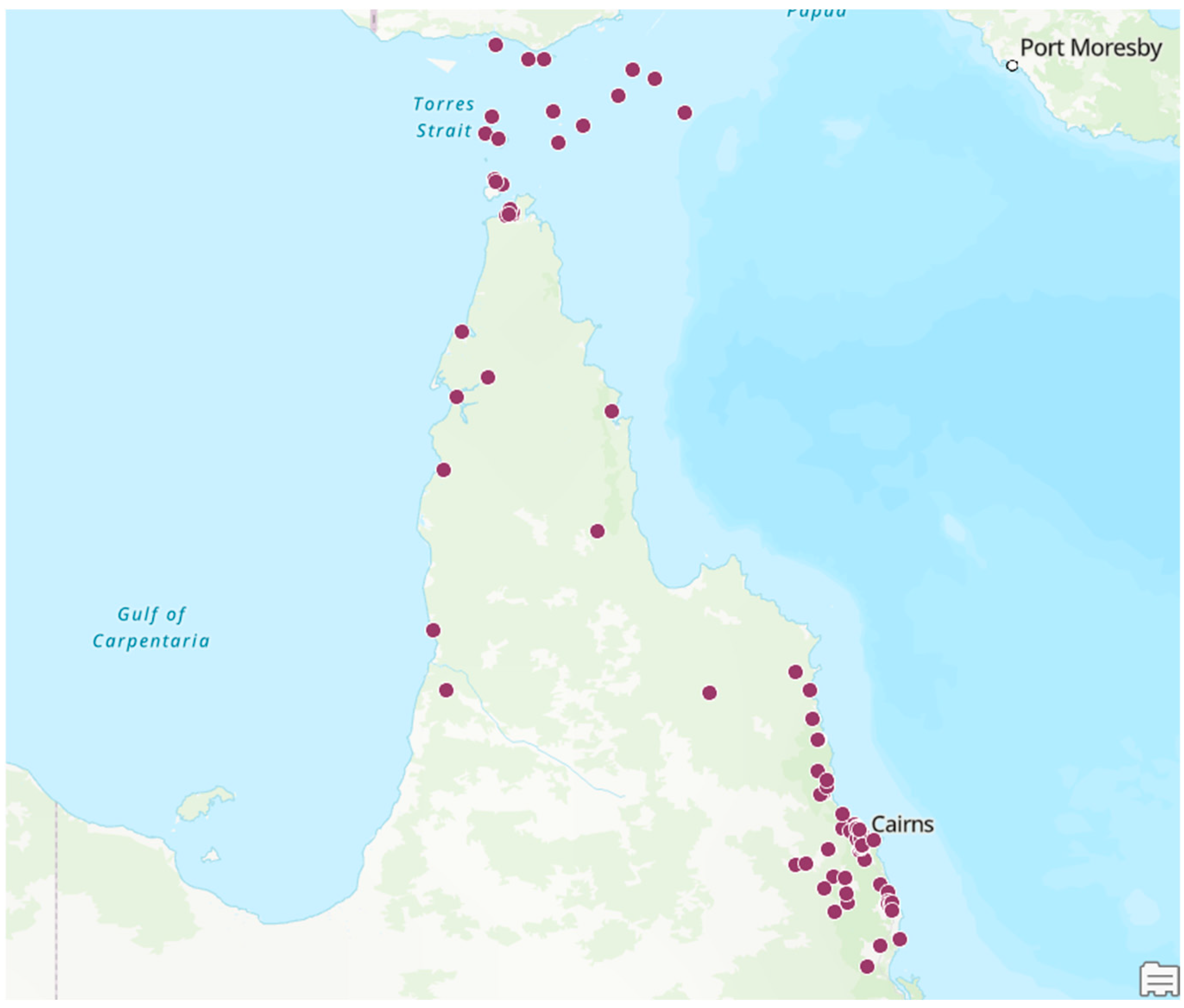
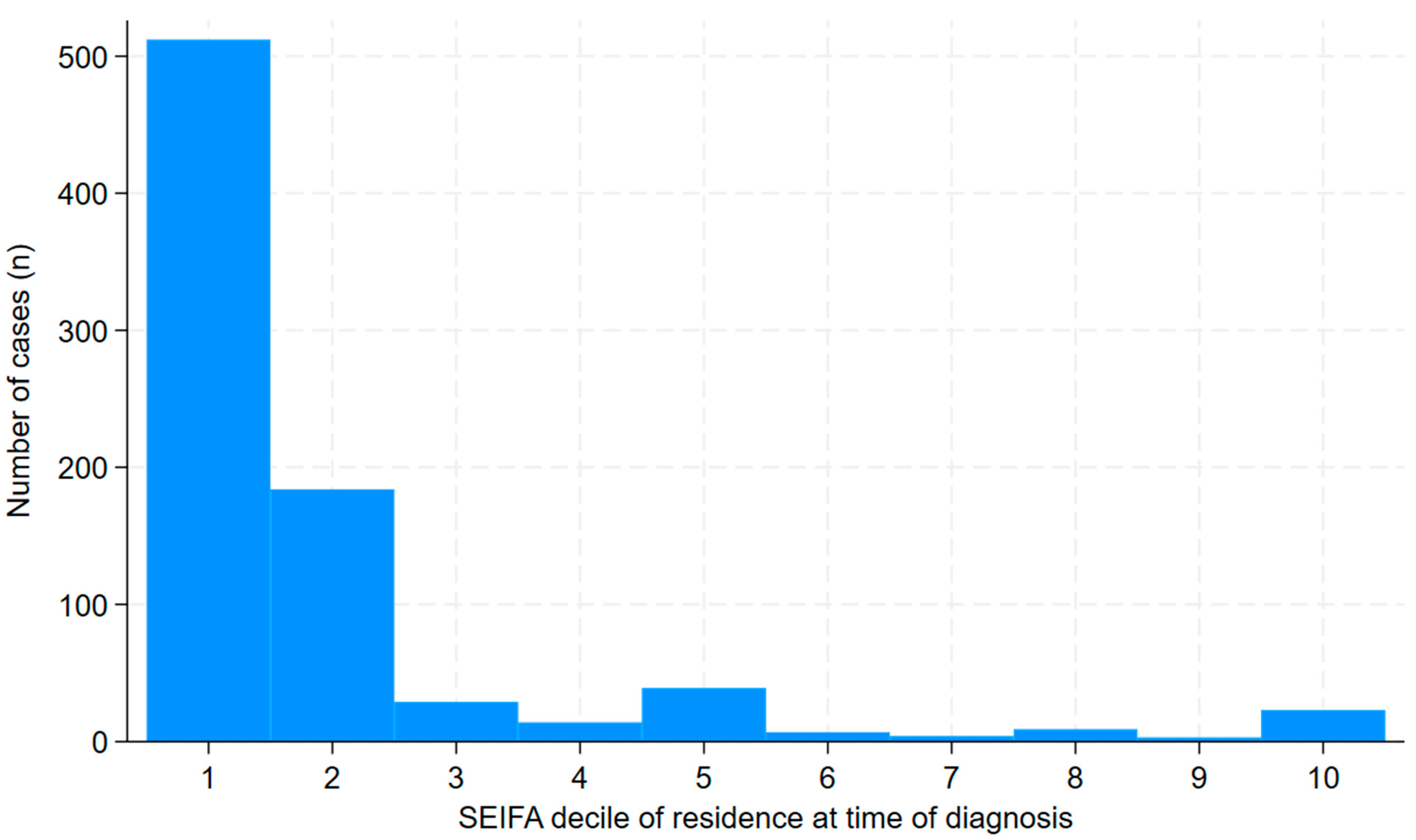
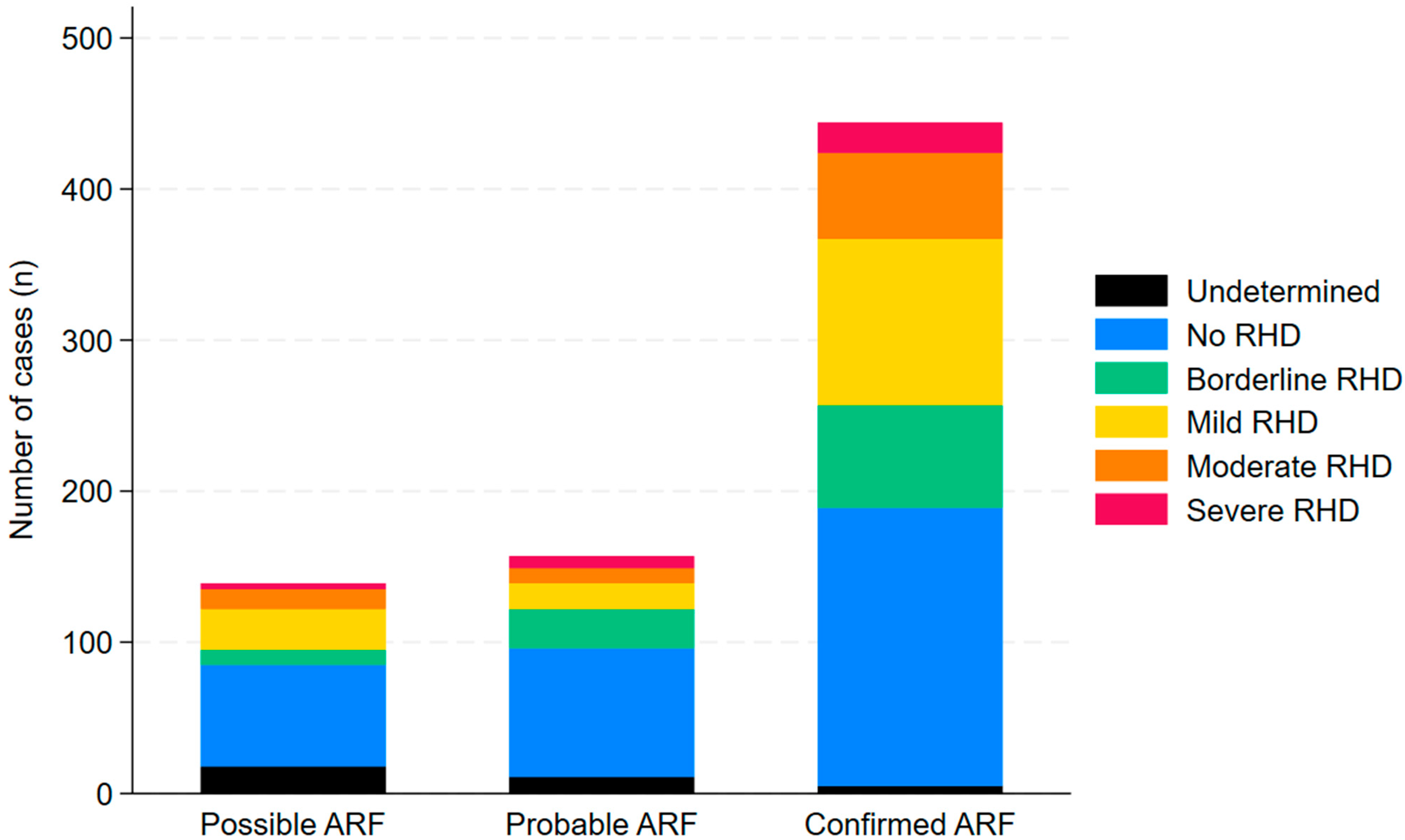
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Auala, T.; Zavale, B.L.G.; Mbakwem, A.Ç.; Mocumbi, A.O. Acute Rheumatic Fever and Rheumatic Heart Disease: Highlighting the Role of Group A Streptococcus in the Global Burden of Cardiovascular Disease. Pathogens 2022, 11, 496. [Google Scholar] [CrossRef]
- Karthikeyan, G.; Guilherme, L. Acute rheumatic fever. Lancet 2018, 392, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Ruan, R.; Liu, X.; Zhang, Y.; Tang, M.; He, B.; Zhang, Q.W.; Shu, T. Global, Regional, and National Advances Toward the Management of Rheumatic Heart Disease Based on the Global Burden of Disease Study 2019. J. Am. Heart Assoc. 2023, 12, e028921. [Google Scholar] [CrossRef] [PubMed]
- Steer, A.C. Historical aspects of rheumatic fever. J. Paediatr. Child health 2015, 51, 21–27. [Google Scholar] [CrossRef]
- Wyber, R.; Noonan, K.; Halkon, C.; Enkel, S.; Cannon, J.; Haynes, E.; Mitchell, A.G.; Bessarab, D.C.; Katzenellenbogen, J.M.; Bond-Smith, D.; et al. Ending rheumatic heart disease in Australia: The evidence for a new approach. Med. J. Aust. 2020, 213, S3–S31. [Google Scholar] [CrossRef] [PubMed]
- Parnaby, M.G.; Carapetis, J.R. Rheumatic fever in indigenous Australian children. J. Paediatr. Child Health 2010, 46, 527–533. [Google Scholar] [CrossRef]
- Aboriginal and Torres Strait islander Health Performance Framework. In Tier 1—Health Status and Outcomes. 1.06 Acute Rheumatic Fever and Rheumatic Heart Disease; The Australian Institute of Health and Welfare: Canberra, Australia, 2025. Available online: https://www.indigenoushpf.gov.au/measures/1-06-rheumatic-fever-rheumatic-heart-disease (accessed on 30 March 2025).
- Francis, J.R.; Fairhurst, H.; Hardefeldt, H.; Brown, S.; Ryan, C.; Brown, K.; Smith, G.; Baartz, R.; Horton, A.; Whalley, G.; et al. Hyperendemic rheumatic heart disease in a remote Australian town identified by echocardiographic screening. Med. J. Aust. 2020, 213, 118–123. [Google Scholar] [CrossRef]
- Beaton, A.; Okello, E.; Rwebembera, J.; Grobler, A.; Engelman, D.; Alepere, J.; Canales, L.; Carapetis, J.; DeWyer, A.; Lwabi, P.; et al. Secondary Antibiotic Prophylaxis for Latent Rheumatic Heart Disease. N. Engl. J. Med. 2022, 386, 230–240. [Google Scholar] [CrossRef]
- AIHW. Acute Rheumatic Fever and Rheumatic Heart Disease in Australia, 2022; AIHW: Canberra, Australia, 2024. [Google Scholar]
- Bray, J.J.; Thompson, S.; Seitler, S.; Ali, S.A.; Yiu, J.; Salehi, M.; Ahmad, M.; Pelone, F.; Gashau, H.; Shokraneh, F.; et al. Long-term antibiotic prophylaxis for prevention of rheumatic fever recurrence and progression to rheumatic heart disease. Cochrane Database Syst. Rev. 2024, 9, CD015779. [Google Scholar]
- Gewitz, M.H.; Baltimore, R.S.; Tani, L.Y.; Sable, C.A.; Shulman, S.T.; Carapetis, J.; Remenyi, B.; Taubert, K.A.; Bolger, A.F.; Beerman, L.; et al. Revision of the Jones Criteria for the diagnosis of acute rheumatic fever in the era of Doppler echocardiography: A scientific statement from the American Heart Association. Circulation 2015, 131, 1806–1818. [Google Scholar] [CrossRef]
- Pulle, J.; Ndagire, E.; Atala, J.; Fall, N.; Okello, E.; Oyella, L.M.; Rwebembera, J.; Sable, C.; Parks, T.; Sarnacki, R.; et al. Specificity of the Modified Jones Criteria. Pediatrics 2024, 153, e2023062624. [Google Scholar] [CrossRef]
- Carapetis, J.R.; Currie, B.J. Rheumatic fever in a high incidence population: The importance of monoarthritis and low grade fever. Arch. Dis. Child. 2001, 85, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Ralph, A.P.; Noonan, S.; Wade, V.; Currie, B.J. The 2020 Australian guideline for prevention, diagnosis and management of acute rheumatic fever and rheumatic heart disease. Med. J. Aust. 2021, 214, 220–227. [Google Scholar] [CrossRef]
- Kang, K.; Chau, K.W.T.; Howell, E.; Anderson, M.; Smith, S.; Davis, T.J.; Starmer, G.; Hanson, J. The temporospatial epidemiology of rheumatic heart disease in Far North Queensland, tropical Australia 1997–2017; impact of socioeconomic status on disease burden, severity and access to care. PLoS Neglected Trop. Dis. 2021, 15, e0008990. [Google Scholar] [CrossRef] [PubMed]
- Hempenstall, A.; Howell, E.; Kang, K.; Chau, K.W.T.; Browne, A.; Kris, E.; Wapau, H.; Pilot, P.; Smith, S.; Reeves, B.; et al. Echocardiographic Screening Detects a Significant Burden of Rheumatic Heart Disease in Australian Torres Strait Islander Children and Missed Opportunities for its Prevention. Am. Soc. Trop. Med. Hyg. 2021, 104, 1211–1214. [Google Scholar] [CrossRef]
- Australian Census; Australian Bureau of Statistics: Canberra, Australia, 2021. Available online: https://www.abs.gov.au/census (accessed on 30 March 2025).
- Bird, K.; Bohanna, I.; McDonald, M.; Wapau, H.; Blanco, L.; Cullen, J.; McLucas, J.; Forbes, S.; Vievers, A.; Wason, A.; et al. A good life for people living with disability: The story from Far North Queensland. Disabil. Rehabil. 2024, 46, 1787–1795. [Google Scholar] [CrossRef]
- Socio-Economic Indexes for Areas (SEIFA); Australian Bureau of Statistics: Canberra, Australia, 2021.
- Rwebembera, J.; Marangou, J.; Mwita, J.C.; Mocumbi, A.O.; Mota, C.; Okello, E.; Nascimento, B.; Thorup, L.; Beaton, A.; Kado, J.; et al. 2023 World Heart Federation guidelines for the echocardiographic diagnosis of rheumatic heart disease. Nat. Rev. Cardiol. 2024, 21, 250–263. [Google Scholar] [CrossRef]
- Kumar, M.; Little, J.; Pearce, S.; MacDonald, B.; Greenland, M.; Tarca, A.; Ramsay, J.; Katzenellenbogen, J.; Yim, D. Clinical profile of paediatric acute rheumatic fever and rheumatic heart disease in Western Australia: 1987 to 2020. J. Paediatr. Child Health 2024, 60, 375–383. [Google Scholar] [CrossRef]
- Stacey, I.; Ralph, A.; de Dassel, J.; Nedkoff, L.; Wade, V.; Francia, C.; Wyber, R.; Murray, K.; Hung, J.; Katzenellenbogen, J.; et al. The evidence that rheumatic heart disease control programs in Australia are making an impact. Aust. N. Z. J. Public Health 2023, 47, 100071. [Google Scholar] [CrossRef]
- Quinn, E.; Girgis, S.; Van Buskirk, J.; Matthews, V.; Ward, J.E. Clinic factors associated with better delivery of secondary prophylaxis in acute rheumatic fever management. Aust. J. Gen. Pract. 2019, 48, 859–865. [Google Scholar] [CrossRef]
- Lindholm, D.E.; Whiteman, I.J.; Oliver, J.; Cheung, M.M.H.; Hope, S.A.; Brizard, C.P.; Horton, A.E.; Sheridan, B.; Hardy, M.; Osowicki, J.; et al. Acute rheumatic fever and rheumatic heart disease in children and adolescents in Victoria, Australia. J. Paediatr. Child Health 2023, 59, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Jack, S.; Moreland, N.J.; Meagher, J.; Fittock, M.; Galloway, Y.; Ralph, A.P. Streptococcal Serology in Acute Rheumatic Fever Patients: Findings From 2 High-income, High-burden Settings. Pediatr. Infect. Dis. J. 2019, 38, e1–e6. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J.G.; Carapetis, J.R.; Griffiths, K.; Edwards, K.; Condon, J.R. Acute Rheumatic Fever and Rheumatic Heart Disease: Incidence and Progression in the Northern Territory of Australia, 1997 to 2010. Circulation 2013, 128, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Davidson, L.; Knight, J.; Bowen, A.C. Skin infections in Australian Aboriginal children: A narrative review. Med. J. Aust. 2020, 212, 231–237. [Google Scholar] [CrossRef]
- Ricciardo, B.M.; Kessaris, H.L.; Cherian, S.; Kumarasinghe, S.P.; Amgarth-Duff, I.; Sron, D.; Oladokun, R.; Tatian, A.H.; Bowen, A.C. Healthy skin for children and young people with skin of colour starts with clinician knowledge and recognition: A narrative review. Lancet Child Adolesc. Health 2025, 9, 262–273. [Google Scholar] [CrossRef]
- Engel, M.E.; Stander, R.; Vogel, J.; Adeyemo, A.A.; Mayosi, B.M. Genetic susceptibility to acute rheumatic fever: A systematic review and meta-analysis of twin studies. PLoS ONE 2011, 6, e25326. [Google Scholar] [CrossRef]
- Baker, M.G.; Gurney, J.; Moreland, N.J.; Bennett, J.; Oliver, J.; Williamson, D.A.; Pierse, N.; Wilson, N.; Merriman, T.R.; Percival, T.; et al. Risk factors for acute rheumatic fever: A case-control study. Lancet Reg. Health West. Pac. 2022, 26, 100508. [Google Scholar]
- Oliver, J.; Bennett, J.; Thomas, S.; Zhang, J.; Pierse, N.; Moreland, N.J.; Williamson, D.A.; Jack, S.; Baker, M. Preceding group A streptococcus skin and throat infections are individually associated with acute rheumatic fever: Evidence from New Zealand. BMJ Glob. Health 2021, 6, e007038. [Google Scholar] [CrossRef]
- Parks, T.; Smeesters, P.R.; Steer, A.C. Streptococcal skin infection and rheumatic heart disease. Curr. Opin. Infect. Dis. 2012, 25, 145–153. [Google Scholar] [CrossRef]
- Thomas, H.M.M.; Enkel, S.L.; Mullane, M.; McRae, T.; Barnett, T.C.; Carapetis, J.R.; Christophers, R.; Coffin, J.; Famlonga, R.; Jacky, J.; et al. Trimodal skin health programme for childhood impetigo control in remote Western Australia (SToP): A cluster randomised, stepped-wedge trial. Lancet Child Adolesc. Health 2024, 8, 809–820. [Google Scholar] [CrossRef]
- Woods, J.A.; Sodhi-Berry, N.; MacDonald, B.R.; Ralph, A.P.; Francia, C.; Stacey, I.; Katzenellenbogen, J.M. Are we missing opportunities to detect acute rheumatic fever and rheumatic heart disease in hospital care? A multijurisdictional cohort study. Aust. Health Rev. 2024, 49, AH23273. [Google Scholar] [CrossRef] [PubMed]
- Ben-Dov, I.; Berry, E. Acute rheumatic fever in adults over the age of 45 years: An analysis of 23 patients together with a review of the literature. Semin. Arthritis Rheum. 1980, 10, 100–110. [Google Scholar] [CrossRef]
- Chan, K.; Cullen, T.; Ralph, A.; Ilton, M.; Marangou, J. Acute Rheumatic Fever Diagnosis in Older Adults. Heart Lung Circ. 2019, 28, S50. [Google Scholar] [CrossRef]
- Basaglia, A.; Kang, K.; Wilcox, R.; Lau, A.; McKenna, K.; Smith, S.; Chau, K.W.T.; Hanson, J. The aetiology and incidence of infective endocarditis in people living with rheumatic heart disease in tropical Australia. Eur. J. Clin. Microbiol. Infect. Dis. 2023, 42, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
- Bossingham, D. Systemic lupus erythematosus in the far north of Queensland. Lupus 2003, 12, 327–331. [Google Scholar] [CrossRef]
- Price, C.F.; Wood, J.P.; Ismail, I.; Smith, S.; Hanson, J. The Incidence, Aetiology and Clinical Course of Serious Infections Complicating Biological and Targeted Synthetic Disease-Modifying Antirheumatic Drug Therapy in Patients with Rheumatoid Arthritis in Tropical Australia. Pathogens 2024, 13, 943. [Google Scholar] [CrossRef]
- Dadwal, P.; Bonner, B.; Fraser, D.; Loveridge, J.; Withey, G.; Puri, A.; Smith, S.; Hanson, J. Bone and joint infections due to melioidosis; diagnostic and management strategies to optimise outcomes. PLoS Neglected Trop. Dis. 2024, 18, e0012317. [Google Scholar] [CrossRef]
- Casey, D.; Turner, P. Australia’s rheumatic fever strategy three years on. Med. J. Aust. 2024, 220, 170–171. [Google Scholar] [CrossRef]
- de Dassel, J.L.; de Klerk, N.; Carapetis, J.R.; Ralph, A.P. How Many Doses Make a Difference? An Analysis of Secondary Prevention of Rheumatic Fever and Rheumatic Heart Disease. J. Am. Heart Assoc. 2018, 7, e010223. [Google Scholar] [CrossRef]
- Coffey, P.M.; Ralph, A.P.; Krause, V.L. The role of social determinants of health in the risk and prevention of group A streptococcal infection, acute rheumatic fever and rheumatic heart disease: A systematic review. PLoS Neglected Trop. Dis. 2018, 12, e0006577. [Google Scholar] [CrossRef]
- Doran, J.; Canty, D.; Dempsey, K.; Cass, A.; Kangaharan, N.; Remenyi, B.; Brunsdon, G.; McDonald, M.; Heal, C.; Wang, Z.; et al. Surgery for rheumatic heart disease in the Northern Territory, Australia, 1997–2016: What have we gained? BMJ Glob. Health 2023, 8, e011763. [Google Scholar] [CrossRef] [PubMed]
- Hanson, J.; Smith, S.; Stewart, J.; Horne, P.; Ramsamy, N. Melioidosis—A disease of socioeconomic disadvantage. PLoS Neglected Trop. Dis. 2021, 15, e0009544. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.D.K.; Smith, S.; Davis, T.J.; Yarwood, T.; Hanson, J. The efficacy and safety of a shortened duration of antimicrobial therapy for group A Streptococcus bacteremia. Int. J. Infect. Dis. 2023, 128, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Guthridge, I.; Smith, S.; Horne, P.; Hanson, J. Increasing prevalence of methicillin-resistant Staphylococcus aureus in remote Australian communities: Implications for patients and clinicians. Pathology 2019, 51, 428–431. [Google Scholar] [CrossRef]
- Rheumatic Fever and Rheumatic Heart Disease: Report by the Director-General; World Health Organisation (WHO) Executive Board: Geneva, Switzerland, 2018.
- Agenson, T.; Katzenellenbogen, J.M.; Seth, R.; Dempsey, K.; Anderson, M.; Wade, V.; Bond-Smith, D. Case Ascertainment on Australian Registers for Acute Rheumatic Fever and Rheumatic Heart Disease. Int. J. Environ. Res. Public Health 2020, 17, 5505. [Google Scholar] [CrossRef]

| High-Risk Individuals a | All Other Individuals |
|---|---|
| Major manifestations | |
|
|
| Minor manifestations | |
|
|
| All n = 830 | Confirmed n = 492 | Probable n = 180 | Possible n = 158 | |
|---|---|---|---|---|
| Age | ||||
| <5 years | 20 (2%) | 15 (3%) | 1 (1%) | 4 (3%) |
| 5–9 years | 157 (19%) | 116 (24%) | 25 (14%) | 16 (10%) |
| 10–14 years | 234 (28%) | 163 (33%) | 47 (26%) | 24 (15%) |
| 15–19 years | 143 (17%) | 85 (17%) | 26 (14%) | 32 (20%) |
| 20–24 years | 102 (12%) | 44 (9%) | 24 (13%) | 34 (22%) |
| ≥25 years | 174 (21%) | 69 (14%) | 57 (32%) | 48 (30%) |
| Population group | ||||
| Aboriginal | 429 (52%) | 248 (50%) | 96 (53%) | 85 (54%) |
| Torres Strait Islander | 237 (29%) | 145 (29%) | 52 (29%) | 40 (25%) |
| Aboriginal and Torres Strait Islander | 119 (14%) | 64 (13%) | 25 (14%) | 30 (19%) |
| Māori | 6 (1%) | 5 (1%) | 1 (1%) | 0 |
| Pacific Islander | 12 (1%) | 11 (2%) | 0 | 1 (1%) |
| Other High Risk | 13 (1%) | 10 (2%) | 3 (2%) | 0 |
| Other Low Risk | 14 (1%) | 9 (2%) | 3 (2%) | 2 (1%) |
| Sex | ||||
| Female | 475 (57%) | 264 (54%) | 111 (62%) | 100 (63%) |
| Male | 355 (43%) | 228 (46%) | 69 (38%) | 58 (37%) |
| Geography | ||||
| CHHHS | 371 (45%) | 217 (44%) | 88 (49%) | 66 (42%) |
| TCHHS | 459 (55%) | 275 (56%) | 92 (51%) | 92 (58%) |
| All n = 830 | Confirmed n = 492 | Probable n = 180 | Possible n = 158 | Odds ratio a (95% CI) | p | |
|---|---|---|---|---|---|---|
| Major Criteria | ||||||
| Polyarthralgia | 402 (48%) | 231 (47%) | 95 (53%) | 76 (48%) | 0.86 (0.66–1.14) | 0.30 |
| Monoarthritis | 99 (12%) | 59 (12%) | 17 (9%) | 23 (15%) | 1.02 (0.66–1.56) | 0.95 |
| Polyarthritis | 181 (22%) | 130 (26%) | 37 (21%) | 14 (9%) | 2.02 (1.41–2.89) | <0.001 |
| Carditis | 104 (13%) | 89 (18%) | 13 (7%) | 2 (1%) | 4.76 (2.70–8.38) | <0.001 |
| Sydenham’s Chorea | 53 (6%) | 53 (11%) | 0 | 0 | - | - |
| Erythema Marginatum | 13 (2%) | 8 (2%) | 4 (2%) | 1 (1%) | 1.10 (0.36–3.39) | 0.87 |
| Subcutaneous Nodules | 10 (1%) | 6 (1%) | 3 (2%) | 1 (1%) | 1.03 (0.29–3.68) | 0.96 |
| Minor Criteria | ||||||
| Monoarthralgia | 67 (8%) | 32 (7%) | 14 (8%) | 21 (13%) | 0.60 (0.36–0.99) | 0.047 |
| Fever | 409 (49%) | 295 (60%) | 69 (38%) | 45 (28%) | 2.94 (2.20–3.93) | <0.001 |
| Prolonged PR interval | 196 (24%) | 161 (33%) | 23 (13%) | 12 (8%) | 4.21 (2.83–6.27) | <0.001 |
| CRP > 30 mg/L | 510 (61%) | 358 (73%) | 104 (58%) | 48 (30%) | 3.27 (2.44–4.38) | <0.001 |
| ESR > 30 mm/hr | 450 (54%) | 296 (60%) | 102 (57%) | 52 (33%) | 1.80 (1.36–2.39) | <0.001 |
| Evidence of GAS infection | ||||||
| Median (IQR) ASOT (U/mL) | 649 (385–1053) | 768 (496–1190) | 578 (347–1000) | 395 (223–597) | 1.10 (1.07–1.14) b | <0.001 |
| Median (IQR) Anti-DNase B (U/mL) | 610 (389–957) | 650 (429–1015) | 586 (389–954) | 391 (257–735) | 1.06 (1.03–1.10) b | <0.001 |
| Positive skin swab | 43 (5%) | 26 (5%) | 9 (5%) | 8 (5%) | 1.05 (0.56–1.97) | 0.87 |
| Positive throat swab | 70 (8%) | 43 (9%) | 15 (8%) | 12 (8%) | 1.10 (0.67–1.82) | 0.70 |
| Positive blood culture | 1 (0.1%) | 1 (0.2%) | 0 | 0 | - | - |
| Stated history | 166 (20%) | 96 (20%) | 43 (24%) | 27 (17%) | 0.93 (0.66–1.31) | 0.67 |
| Unknown | 550 (66%) | 326 (66%) | 113 (63%) | 111 (70%) | 1.00 (0.75–1.34) | 1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crous, M.; Hempenstall, A.; Lui-Gamia, N.; Taunton, C.; Hanson, J. Epidemiological and Clinical Manifestations of Acute Rheumatic Fever in Far North Queensland, Australia. Pathogens 2025, 14, 442. https://doi.org/10.3390/pathogens14050442
Crous M, Hempenstall A, Lui-Gamia N, Taunton C, Hanson J. Epidemiological and Clinical Manifestations of Acute Rheumatic Fever in Far North Queensland, Australia. Pathogens. 2025; 14(5):442. https://doi.org/10.3390/pathogens14050442
Chicago/Turabian StyleCrous, Mia, Allison Hempenstall, Nancy Lui-Gamia, Caroline Taunton, and Josh Hanson. 2025. "Epidemiological and Clinical Manifestations of Acute Rheumatic Fever in Far North Queensland, Australia" Pathogens 14, no. 5: 442. https://doi.org/10.3390/pathogens14050442
APA StyleCrous, M., Hempenstall, A., Lui-Gamia, N., Taunton, C., & Hanson, J. (2025). Epidemiological and Clinical Manifestations of Acute Rheumatic Fever in Far North Queensland, Australia. Pathogens, 14(5), 442. https://doi.org/10.3390/pathogens14050442






