Syzygium aromaticum Essential Oil as a Safe Natural Solution to Control Bacteria in Hatching Eggs
Abstract
1. Introduction
2. Materials and Methods
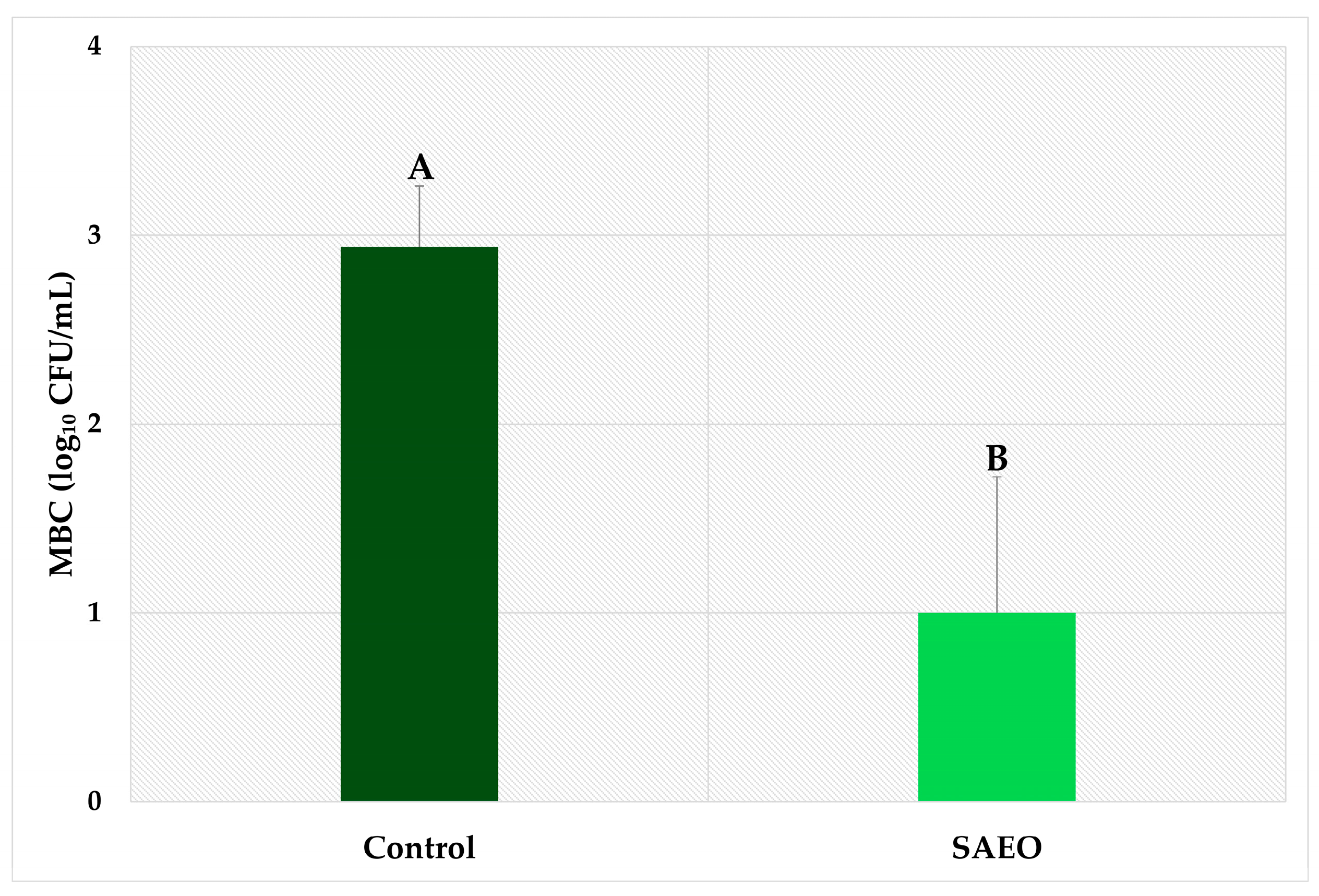
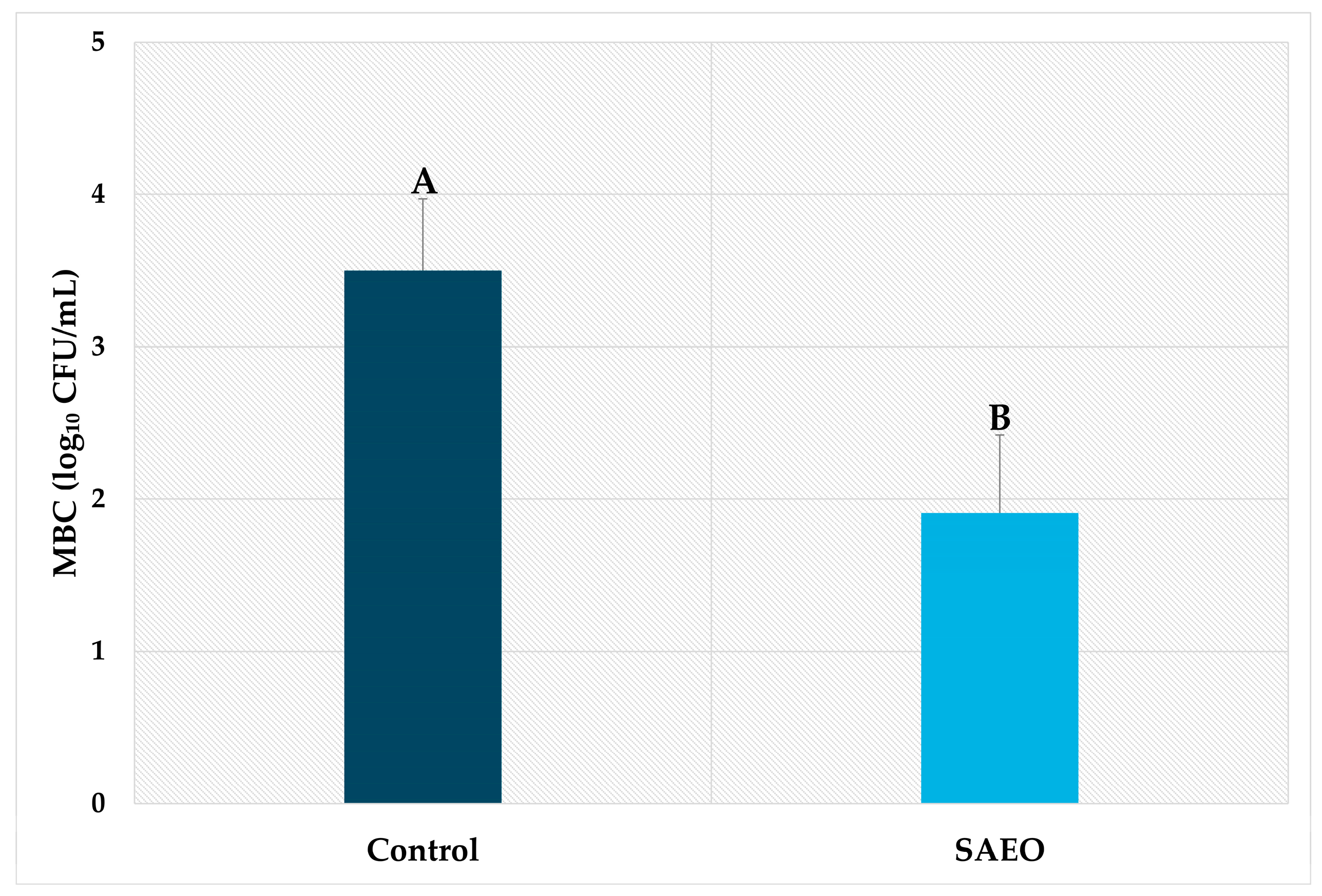
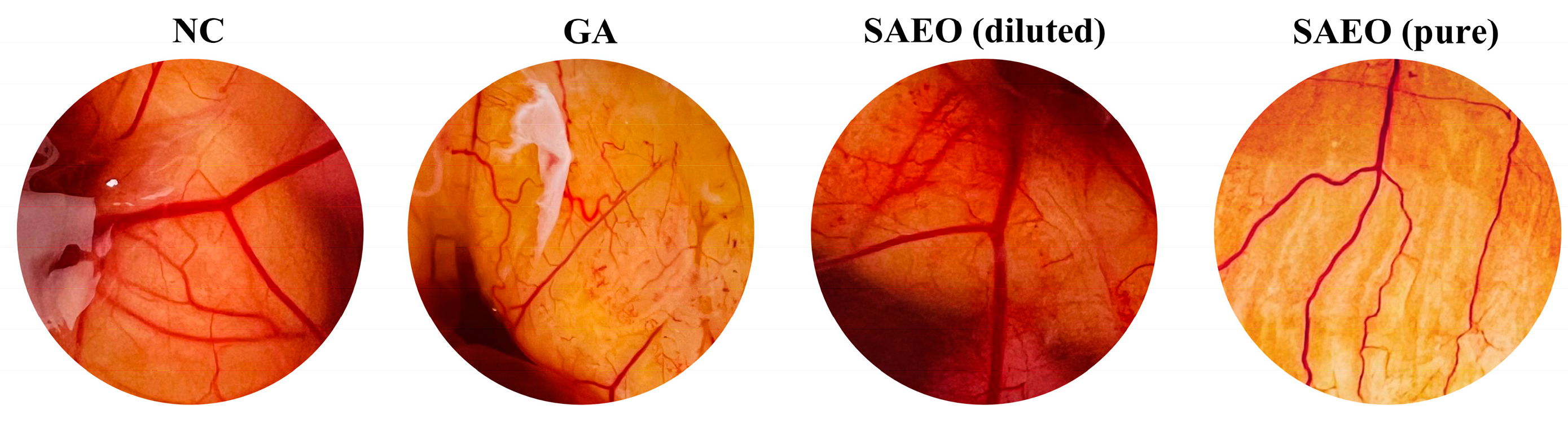
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdelhamid, M.K.; Hess, C.; Bilic, I.; Glösmann, M.; Rehman, H.U.; Liebhart, D.; Hess, M.; Paudel, S. A comprehensive study of colisepticaemia progression in layer chickens applying novel tools elucidates pathogenesis and transmission of Escherichia coli into eggs. Sci. Rep. 2024, 14, 8111. [Google Scholar] [CrossRef]
- Board, R.G.; Tranter, H.S. The microbiology of eggs. In Egg Science and Technology, 4th ed.; Stadelman, W.J., Cotterill, O.J., Eds.; CRC Press: Boca Raton, FL, USA, 1995; pp. 81–104. [Google Scholar]
- Katiyo, W.; de Kock, H.L.; Coorey, R.; Buys, E.M. Sensory implications of chicken meat spoilage in relation to microbial and physicochemical characteristics during refrigerated storage. LWT 2020, 128, 109468. [Google Scholar] [CrossRef]
- Cony, H.C.; Vieira, S.L.; Berres, J.; Gomes, H.A.; Coneglian, J.L.B.; Freitas, D.M.D. Técnicas de pulverização e imersão com distintos desinfetantes sobre ovos incubáveis. Ciênc. Rural 2008, 38, 1407–1412. [Google Scholar] [CrossRef]
- Aygun, A.; Sert, D.; Copur, G. Effects of propolis on eggshell microbial activity, hatchability, and chick performance in japanese quail (Coturnix coturnix japonica) eggs. Poult. Sci. 2012, 91, 1018–1025. [Google Scholar] [CrossRef]
- Koc, S.; Aygun, A. Effects of ozone on eggshell microbial load, hatching traits and chick performance in quail eggs. Innoriginal Int. J. Sci. 2013, 8, 47–52. [Google Scholar]
- Hrnčár, C.; Hanusová, E.; Hanus, A.; Arpášová, H.; Kokoszyński, D.; Bujko, J. The effect of various disinfectants on hatching results in chickens. Sci. Pap. Anim. Sci. Biotechnol. 2021, 54, 193–196. [Google Scholar]
- Patrzałek, M.; Kosecka-Strojek, M.; Lisowska-Łysiak, K.; Trela, M.; Kot, M.; Gawlak, M.; Liszka, D.; Sajewicz, M.; Tombarkiewicz, B.; Pawlak, K.; et al. Preliminary evaluation of application of a 3-dimensional network structure of siloxanes dergall preparation on chick embryo development and microbiological status of eggshells. Poult. Sci. 2020, 99, 1581–1590. [Google Scholar] [CrossRef]
- Wlazlo, L.; Drabik, K.; Al-Shammari, K.I.A.; Batkowska, J.; Nowakowicz-Debek, B.; Gryzińska, M. Use of reactive oxygen species (ozone, hydrogen peroxide) for disinfection of hatching eggs. Poult. Sci. 2020, 99, 2478–2484. [Google Scholar] [CrossRef]
- Taşdemir, A.N.; Onbaşılar, E.E.; Yalçın, S.; Boyalı, B.; Aygören, H.; Tülek, E.; Sarıçam, S.; Akan, M. Effects of oregano juice on eggshell microbial load, layer embryo development, hatching results, and growth at the first 2 weeks after hatch. Trop. Anim. Health Prod. 2021, 53, 404. [Google Scholar] [CrossRef]
- Ayuningtyas, G.; Martini, R.; Yulianti, W. The role of dipping duck hatching eggs with cherry leaf extract as natural sanitizers on hatching performance and eggshell bacterial counts. E3S Web Conf. 2022, 348, 00023. [Google Scholar] [CrossRef]
- Fouad, W.; Kassab, A.Y.; El-damrawy, S.Z. Effect of spraying moringa oil on embryonic development, hatchability, physiological parameters, post-hatch chick growth and bacterial contamination of fertile quail eggs. Egypt. J. Nutr. Feeds 2023, 26, 203–221. [Google Scholar] [CrossRef]
- Wang, L.; Dong, Q.; Tang, K.; Han, K.; Bai, H.; Yin, Y.; Wang, X. Effect of phage spray on hatchability and chick quality of eggs contaminated with Salmonella typhimurium. Viruses 2024, 16, 1338. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, G.d.S.; McManus, C.; Salgado, C.B.; dos Santos, V.M. Effects of sanitizers on microbiological control of hatching eggshells and poultry health during embryogenesis and early stages after hatching in the last decade. Animals 2022, 12, 2826. [Google Scholar] [CrossRef]
- Zeweil, H.; Rizk, R.; Bekhet, G.; Ahmed, R. Effect of egg disinfection on hatching performance for Bandarah chicken strain. Egypt. Poult. Sci. J. 2013, 33, 289–307. [Google Scholar]
- Al-Shammari, K.I.A.; Batkowska, J.; Gryzińska, M.; Wlazło, Ł.; Ossowski, M.; Nowakowicz-Dębek, B. The use of selected herbal preparations for the disinfection of japanese quail hatching eggs. Poult. Sci. 2022, 101, 102066. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, I.; Ozsan, M.; Yetisir, R. The use of oregano (Origanum vulgare L.) essential oil as alternative hatching egg disinfectant versus formaldehyde fumigation in quails (Coturnix coturnix japonica). Eggs. Rev. Med. Vet. 2003, 154, 367–370. [Google Scholar]
- Oliveira, G.D.S.; Nascimento, S.T.; dos Santos, V.M.; Silva, M.G. Clove essential oil in the sanitation of fertile eggs. Poult. Sci. 2020, 99, 5509–5516. [Google Scholar] [CrossRef]
- Bekhet, G.M. Impact of hatching egg disinfection on hatching characteristics and chick embryos. Indian J. Anim. Res. 2021, 55, 353–358. [Google Scholar] [CrossRef]
- Susilowati, M.; Wahyuni, S.; Setiadi, A.; Bermawie, N. Yield and morphological characteristics of cloves from Semarang plantation, Indonesia. In IOP Conf. Ser. Earth Environ. Sci. 2024, 1377, 012097. [Google Scholar] [CrossRef]
- Abdullah, B.H.; Hatem, S.F.; Jumaa, W. A Comparative study of the antibacterial activity of clove and rosemary essential oils on multidrug resistant bacteria. Pharm. Biosci. J. 2015, 3, 18–22. [Google Scholar] [CrossRef]
- Oliveira, G.D.S.; McManus, C.; dos Santos, V.M. Maintaining safe antibacterial standards in poultry production using Syzygium aromaticum essential oil. Worlds Poult. Sci. J. 2024, 80, 885–895. [Google Scholar] [CrossRef]
- Hassan, A.S.I.; Morsy, E.A.; El Moustafa, K.M.; Ibrahim, F.A.; Elmenawey, M.A. Effects of clove essential oil on eggshell bacterial load, antibacterial sensitivity, and hatchability. Egypt. Pharm. J. 2023, 22, 650–658. [Google Scholar] [CrossRef]
- El-Soufi, A.; Al Khatib, A.; Khazaal, S.; El Darra, N.; Raafat, K. Evaluation of essential oils as natural antibacterial agents for eggshell sanitization and quality preservation. Processes 2025, 13, 224. [Google Scholar] [CrossRef]
- Oliveira, G.S.; Nascimento, S.T.; dos Santos, V.M.; Dallago, B.S.L. Spraying hatching eggs with clove essential oil does not compromise the quality of embryos and one-day-old chicks or broiler performance. Animals 2021, 11, 2045. [Google Scholar] [CrossRef]
- Bauer, A.W.; Kirby, W.M.M.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef]
- Derouiche, M.T.T.; Abdennour, S. HET-CAM test. Application to shampoos in developing countries. Toxicol. Vitr. 2017, 45, 393–396. [Google Scholar] [CrossRef]
- Oliveira, G.D.; McManus, C.; Santos, P.H.; de Sousa, D.E.; Jivago, J.L.; de Castro, M.B.; Dos Santos, V.M. Hatching egg sanitizers based on essential oils: Microbiological parameters, hatchability, and poultry health. Antibiotics 2024, 13, 1066. [Google Scholar] [CrossRef]
- Bai, J.; Li, J.; Chen, Z.; Bai, X.; Yang, Z.; Wang, Z.; Yang, Y. Antibacterial activity and mechanism of clove essential oil against foodborne pathogens. LWT 2023, 173, 114249. [Google Scholar] [CrossRef]
- Franco, C.M.; Araújo, I.C.; Lopes, T.S.; Gonçalves, T.F.; Costa, B.T.; Sousa, L.S.; Araújo, I.M.; Souza, M.R.; Lara, L.J. Sanitization at different times after floor egg collection for hatchability, microbiological quality, and performance of broilers. Poult. Sci. 2023, 102, 103156. [Google Scholar] [CrossRef]
- Eroglu, M.; Erisir, Z.; Simsek, U.G.; Mutlu, S.I.; Baykalir, Y.; Gungoren, A.; Adiyaman, G.J. Effects of washing dirty eggs of geese with boric acid and vinegar on hatchability and microbial loads. Anim. Plant Sci. 2025, 35. [Google Scholar] [CrossRef]
- Gole, V.C.; Chousalkar, K.K.; Roberts, J.R.; Sexton, M.; May, D.; Tan, J.; Kiermeier, A. Effect of egg washing and correlation between eggshell characteristics and egg penetration by various Salmonella typhimurium strains. PLoS ONE 2014, 9, e90987. [Google Scholar] [CrossRef] [PubMed]
- Racea, R.C.; Macasoi, I.G.; Dinu, S.; Pinzaru, I.; Marcovici, I.; Dehelean, C.; Rusu, L.C.; Chioran, D.; Rivis, M.; Buzatu, R. Eugenol: In vitro and in ovo assessment to explore cytotoxic effects on osteosarcoma and oropharyngeal cancer cells. Plants 2023, 12, 3549. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.P.D. Uso de Extratos Vegetais Para Higienização de Alface. Master’s Dissertation, Federal Institute Goiano, Morrinhos, GO, Brazil, 7 November 2018. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, G.d.S.; McManus, C.; dos Santos, V.M. Syzygium aromaticum Essential Oil as a Safe Natural Solution to Control Bacteria in Hatching Eggs. Pathogens 2025, 14, 422. https://doi.org/10.3390/pathogens14050422
Oliveira GdS, McManus C, dos Santos VM. Syzygium aromaticum Essential Oil as a Safe Natural Solution to Control Bacteria in Hatching Eggs. Pathogens. 2025; 14(5):422. https://doi.org/10.3390/pathogens14050422
Chicago/Turabian StyleOliveira, Gabriel da Silva, Concepta McManus, and Vinícius Machado dos Santos. 2025. "Syzygium aromaticum Essential Oil as a Safe Natural Solution to Control Bacteria in Hatching Eggs" Pathogens 14, no. 5: 422. https://doi.org/10.3390/pathogens14050422
APA StyleOliveira, G. d. S., McManus, C., & dos Santos, V. M. (2025). Syzygium aromaticum Essential Oil as a Safe Natural Solution to Control Bacteria in Hatching Eggs. Pathogens, 14(5), 422. https://doi.org/10.3390/pathogens14050422





