Plasma-Activated Water Against Carbapenem-Resistant Klebsiella pneumoniae and Vancomycin-Resistant Enterococcus faecalis
Abstract
1. Introduction
2. Materials and Methods
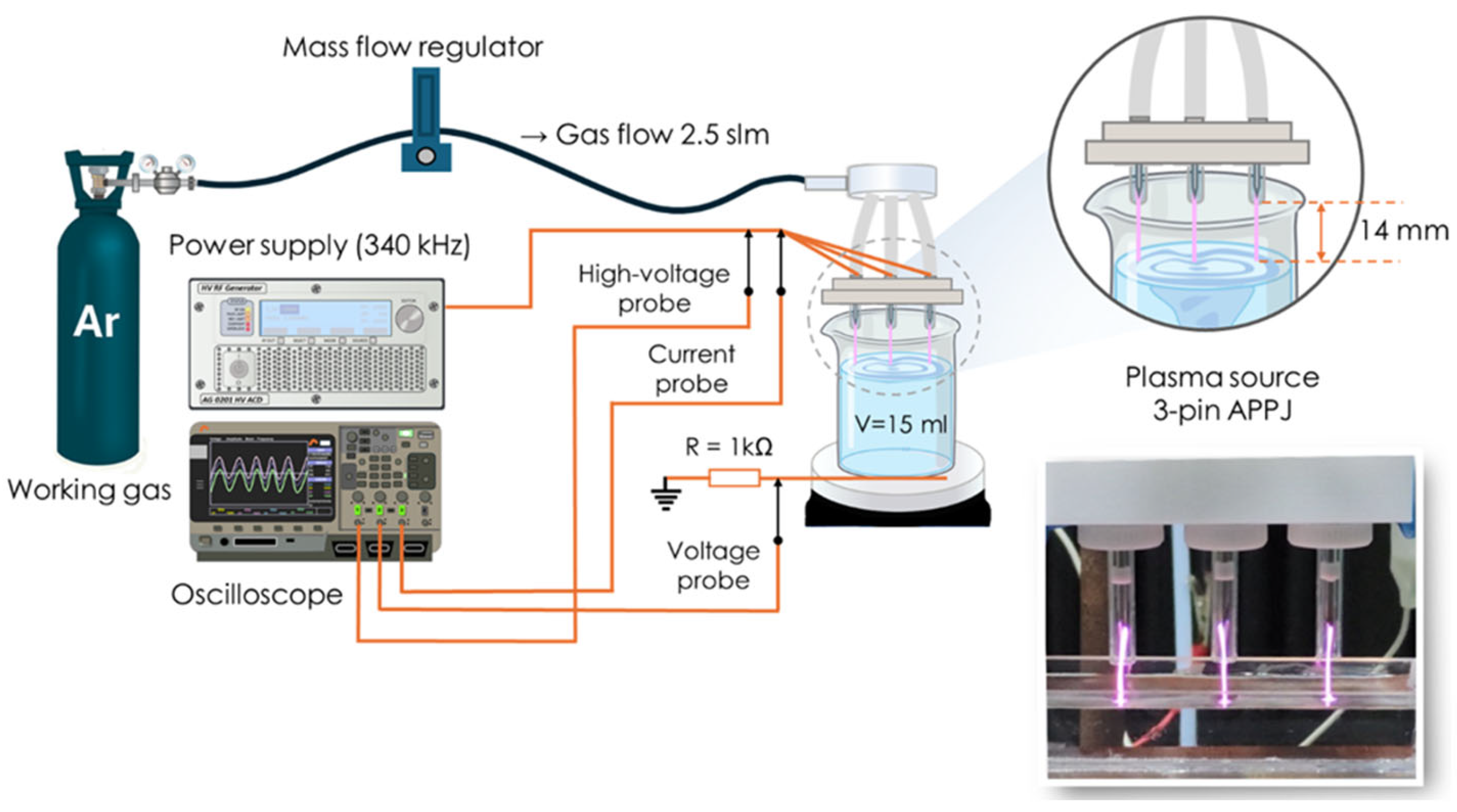
2.1. Experimental Setup and Plasma Treatment of Water Sample
2.2. Characterization of Plasma-Activated Water
2.3. Bacterial Strains and Growth Conditions
2.4. PAW Treatment and Assessment of Bacterial Inactivation
2.5. Determination of Minimum Inhibitory Concentrations (MICs)
2.6. Detection of Intracellular RONS Generated by the PAW Treatment
2.7. Statistical Analyses
3. Results
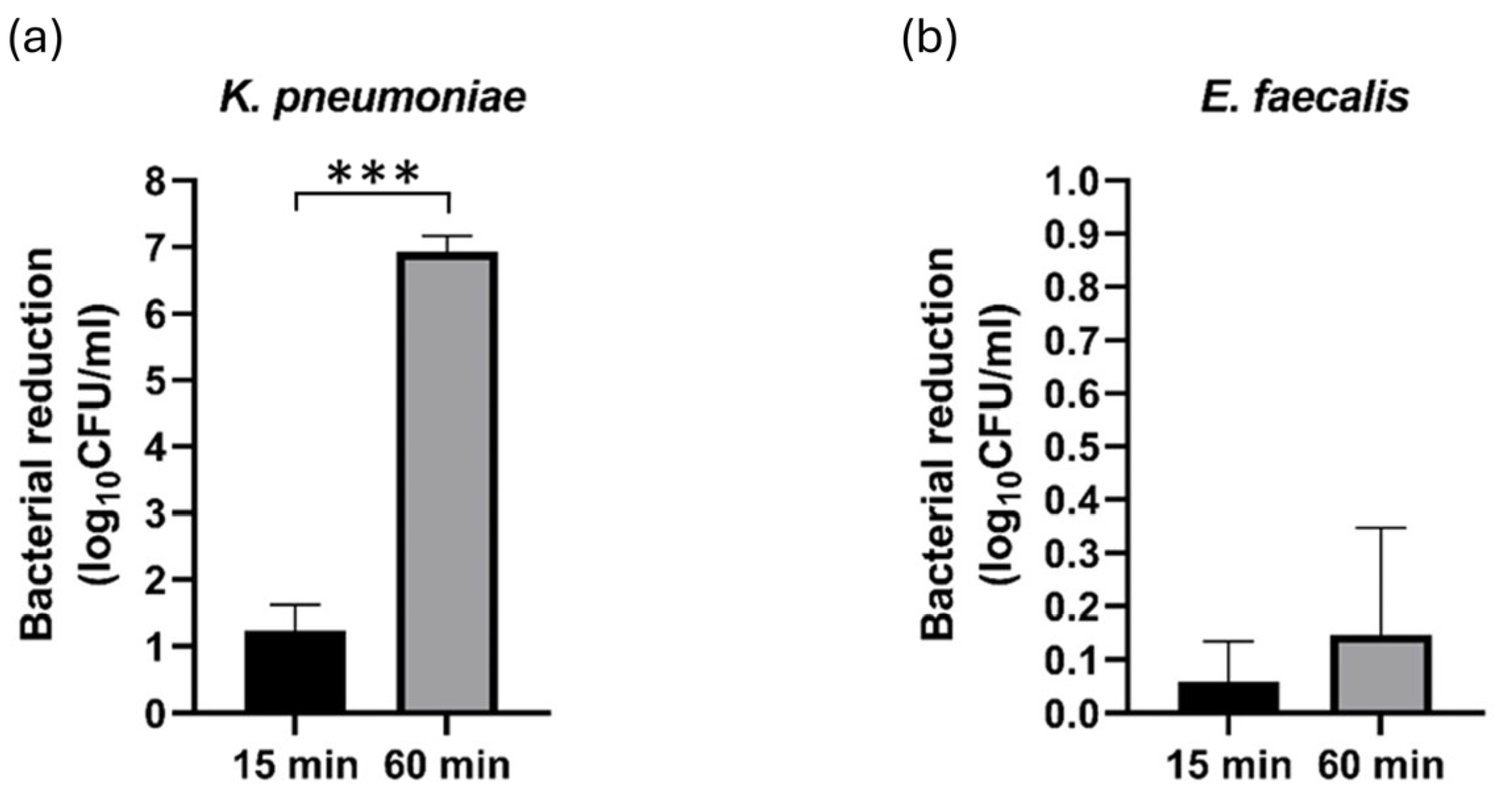
3.1. Inactivation Efficacy of PAW
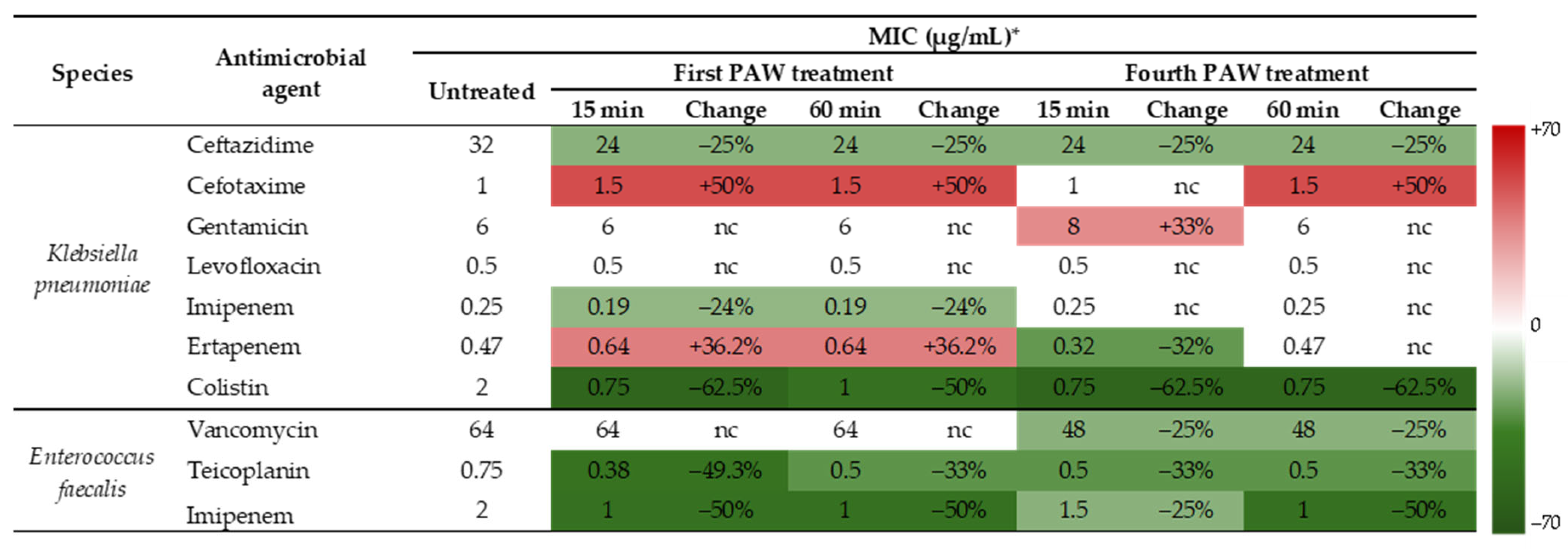
3.2. Effects of PAW on Susceptibility to Antibiotics
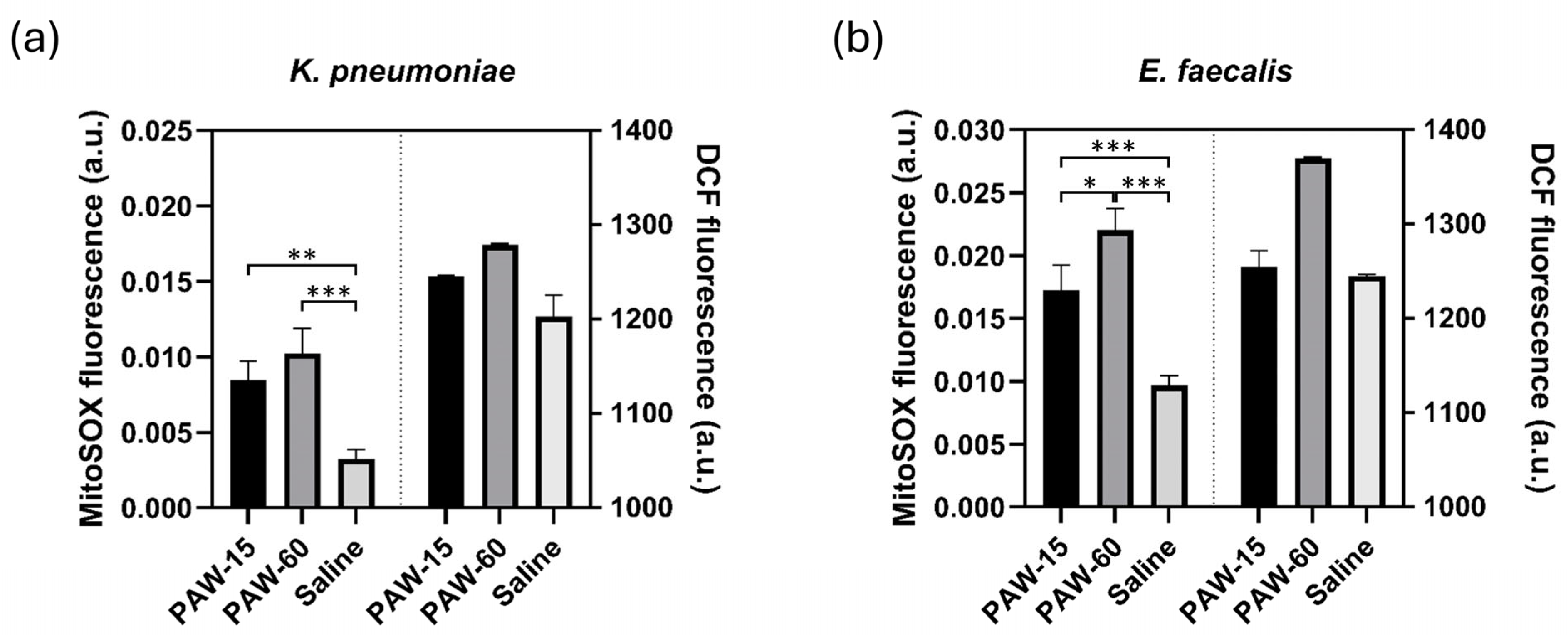
3.3. Assessment of Intracellular RONS Generated by the PAW Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PAW | Plasma-activated water |
| MDR | Multidrug-resistant |
| MICs | Minimum inhibitory concentration |
| AMR | Antimicrobial resistance |
| WHO | World Health Organization |
| CAP | Cold atmospheric plasma |
| RONS | Reactive oxygen and nitrogen species |
| ORP | Oxidation reduction potential |
| PALs | Plasma-activated liquids |
| HAIs | Healthcare-associated infections |
| APPJ | Atmospheric pressure plasma jet |
| slm | Standard liters per minute |
| ATCC | American Type Culture Collection |
| TSA | Tryptic soy agar |
| TSB | Tryptic soy broth |
| LB | Luria Bertani |
| CFU | Colony forming unit |
| MH | Mueller Hinton |
| DCF | Dichlorodihydrofluorescein |
| SPSS | Statistical Package for the Social Sciences |
| EUCAST | European Committee on Antimicrobial Susceptibility Testing |
| MRSA | Methicillin-resistant S. aureus |
References
- World Health Organization. Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report 2022, 1st ed.; World Health Organization: Geneva, Switzerland, 2022; ISBN 978-92-4-006270-2. [Google Scholar]
- Oliveira, M.; Antunes, W.; Mota, S.; Madureira-Carvalho, Á.; Dinis-Oliveira, R.J.; Dias Da Silva, D. An Overview of the Recent Advances in Antimicrobial Resistance. Microorganisms 2024, 12, 1920. [Google Scholar] [CrossRef] [PubMed]
- Publications Office of the European Union. Antimicrobial Resistance Surveillance in Europe 2023: 2021 Data; Publications Office of the European Union: Luxembourg, 2023; ISBN 978-92-9498-612-2. [Google Scholar]
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Naghavi, M.; Vollset, S.E.; Ikuta, K.S.; Swetschinski, L.R.; Gray, A.P.; Wool, E.E.; Robles Aguilar, G.; Mestrovic, T.; Smith, G.; Han, C.; et al. Global Burden of Bacterial Antimicrobial Resistance 1990–2021: A Systematic Analysis with Forecasts to 2050. Lancet 2024, 404, 1199–1226. [Google Scholar] [CrossRef]
- Murugaiyan, J.; Kumar, P.A.; Rao, G.S.; Iskandar, K.; Hawser, S.; Hays, J.P.; Mohsen, Y.; Adukkadukkam, S.; Awuah, W.A.; Jose, R.A.M.; et al. Progress in Alternative Strategies to Combat Antimicrobial Resistance: Focus on Antibiotics. Antibiotics 2022, 11, 200. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Bacterial Priority Pathogens List 2024: Bacterial Pathogens of Public Health Importance, to Guide Research, Development, and Strategies to Prevent and Control Antimicrobial Resistance, 1st ed.; World Health Organization: Geneva, Switzerland, 2024; ISBN 978-92-4-009346-1. [Google Scholar]
- World Health Organization. 2023 Antibacterial Agents in Clinical and Preclinical Development: An Overwiev and Analysis; World Health Organization: Geneva, Switzerland, 2024; ISBN 978-92-4-009400-0. [Google Scholar]
- Ermolaeva, S.A.; Varfolomeev, A.F.; Chernukha, M.Y.; Yurov, D.S.; Vasiliev, M.M.; Kaminskaya, A.A.; Moisenovich, M.M.; Romanova, J.M.; Murashev, A.N.; Selezneva, I.I.; et al. Bactericidal Effects of Non-Thermal Argon Plasma In Vitro, in Biofilms and in the Animal Model of Infected Wounds. J. Med. Microbiol. 2011, 60, 75–83. [Google Scholar] [CrossRef]
- Mai-Prochnow, A.; Murphy, A.B.; McLean, K.M.; Kong, M.G.; Ostrikov, K.K. Atmospheric Pressure Plasmas: Infection Control and Bacterial Responses. Int. J. Antimicrob. Agents 2014, 43, 508–517. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, C.; Han, Q. Mechanisms of Bacterial Inhibition and Tolerance around Cold Atmospheric Plasma. Appl. Microbiol. Biotechnol. 2023, 107, 5301–5316. [Google Scholar] [CrossRef]
- Ehlbeck, J.; Schnabel, U.; Polak, M.; Winter, J.; Von Woedtke, T.; Brandenburg, R.; Von Dem Hagen, T.; Weltmann, K.-D. Low Temperature Atmospheric Pressure Plasma Sources for Microbial Decontamination. J. Phys. D Appl. Phys. 2011, 44, 013002. [Google Scholar] [CrossRef]
- Simoncelli, E.; Barbieri, D.; Laurita, R.; Liguori, A.; Stancampiano, A.; Viola, L.; Tonini, R.; Gherardi, M.; Colombo, V. Preliminary Investigation of the Antibacterial Efficacy of a Handheld Plasma Gun Source for Endodontic Procedures. Clin. Plasma Med. 2015, 3, 77–86. [Google Scholar] [CrossRef]
- Laurita, R.; Barbieri, D.; Gherardi, M.; Colombo, V.; Lukes, P. Chemical Analysis of Reactive Species and Antimicrobial Activity of Water Treated by Nanosecond Pulsed DBD Air Plasma. Clin. Plasma Med. 2015, 3, 53–61. [Google Scholar] [CrossRef]
- Capelli, F.; Laghi, G.; Laurita, R.; Puač, N.; Gherardi, M. Recommendations and Guidelines for the Description of Cold Atmospheric Plasma Devices in Studies of Their Application in Food Processing. Innov. Food Sci. Emerg. Technol. 2024, 97, 103818. [Google Scholar] [CrossRef]
- Machala, Z.; Chládeková, L.; Pelach, M. Plasma Agents in Bio-Decontamination by Dc Discharges in Atmospheric Air. J. Phys. D Appl. Phys. 2010, 43, 222001. [Google Scholar] [CrossRef]
- Machala, Z.; Tarabová, B.; Sersenová, D.; Janda, M.; Hensel, K. Chemical and Antibacterial Effects of Plasma Activated Water: Correlation with Gaseous and Aqueous Reactive Oxygen and Nitrogen Species, Plasma Sources and Air Flow Conditions. J. Phys. D Appl. Phys. 2019, 52, 034002. [Google Scholar] [CrossRef]
- Bruggeman, P.; Leys, C. Non-Thermal Plasmas in and in Contact with Liquids. J. Phys. D Appl. Phys. 2009, 42, 053001. [Google Scholar] [CrossRef]
- Van Gils, C.A.J.; Hofmann, S.; Boekema, B.K.H.L.; Brandenburg, R.; Bruggeman, P.J. Mechanisms of Bacterial Inactivation in the Liquid Phase Induced by a Remote RF Cold Atmospheric Pressure Plasma Jet. J. Phys. D Appl. Phys. 2013, 46, 175203. [Google Scholar] [CrossRef]
- Ma, M.; Zhang, Y.; Lv, Y.; Sun, F. The Key Reactive Species in the Bactericidal Process of Plasma Activated Water. J. Phys. D Appl. Phys. 2020, 53, 185207. [Google Scholar] [CrossRef]
- Rathore, V.; Patel, D.; Butani, S.; Nema, S.K. Investigation of Physicochemical Properties of Plasma Activated Water and Its Bactericidal Efficacy. Plasma Chem. Plasma Process. 2021, 41, 871–902. [Google Scholar] [CrossRef]
- Yang, L.; Niyazi, G.; Qi, Y.; Yao, Z.; Huang, L.; Wang, Z.; Guo, L.; Liu, D. Plasma-Activated Saline Promotes Antibiotic Treatment of Systemic Methicillin-Resistant Staphylococcus aureus Infection. Antibiotics 2021, 10, 1018. [Google Scholar] [CrossRef]
- Vaňková, E.; Julák, J.; Machková, A.; Obrová, K.; Klančnik, A.; Smole Možina, S.; Scholtz, V. Overcoming Antibiotic Resistance: Non-Thermal Plasma and Antibiotics Combination Inhibits Important Pathogens. Pathog. Dis. 2024, 82, ftae007. [Google Scholar] [CrossRef]
- Mai-Prochnow, A.; Zhou, R.; Zhang, T.; Ostrikov, K.; Mugunthan, S.; Rice, S.A.; Cullen, P.J. Interactions of Plasma-Activated Water with Biofilms: Inactivation, Dispersal Effects and Mechanisms of Action. npj Biofilms Microbiomes 2021, 7, 11. [Google Scholar] [CrossRef]
- Puač, N.; Miletić, M.; Mojović, M.; Popović-Bijelić, A.; Vuković, D.; Miličić, B.; Maletić, D.; Lazović, S.; Malović, G.; Petrović, Z.L. Sterilization of Bacteria Suspensions and Identification of Radicals Deposited Duringplasma Treatment. Open Chem. 2015, 13, 000010151520150041. [Google Scholar] [CrossRef]
- Vanraes, P.; Bogaerts, A. Plasma Physics of Liquids—A Focused Review. Appl. Phys. Rev. 2018, 5, 031103. [Google Scholar] [CrossRef]
- Tendero, C.; Tixier, C.; Tristant, P.; Desmaison, J.; Leprince, P. Atmospheric Pressure Plasmas: A Review. Spectrochim. Acta Part B At. Spectrosc. 2006, 61, 2–30. [Google Scholar] [CrossRef]
- Schutze, A.; Jeong, J.Y.; Babayan, S.E.; Park, J.; Selwyn, G.S.; Hicks, R.F. The Atmospheric-Pressure Plasma Jet: A Review and Comparison to Other Plasma Sources. IEEE Trans. Plasma Sci. 1998, 26, 1685–1694. [Google Scholar] [CrossRef]
- Laroussi, M.; Akan, T. Arc-Free Atmospheric Pressure Cold Plasma Jets: A Review. Plasma Process. Polym. 2007, 4, 777–788. [Google Scholar] [CrossRef]
- Winter, J.; Brandenburg, R.; Weltmann, K.-D. Atmospheric Pressure Plasma Jets: An Overview of Devices and New Directions. Plasma Sources Sci. Technol. 2015, 24, 064001. [Google Scholar] [CrossRef]
- Gierke, A.-M.; Lingenfelder, C.; Hessling, M. Investigation of the Antimicrobial Impact of Cold Atmospheric Plasma on Wet and Dry Microorganisms. Biol. Life Sci. Forum 2024, 31, 6. [Google Scholar]
- Puač, N.; Škoro, N. Plasma–Liquid Interaction for Agriculture—A Focused Review. Plasma Process. Polym. 2024, 22, e2400208. [Google Scholar] [CrossRef]
- Lu, P.; Boehm, D.; Bourke, P.; Cullen, P.J. Achieving Reactive Species Specificity within Plasma-activated Water through Selective Generation Using Air Spark and Glow Discharges. Plasma Process. Polym. 2017, 14, 1600207. [Google Scholar] [CrossRef]
- Kovačević, V.V.; Sretenović, G.B.; Obradović, B.M.; Kuraica, M.M. Low-Temperature Plasmas in Contact with Liquids—A Review of Recent Progress and Challenges. J. Phys. D Appl. Phys. 2022, 55, 473002. [Google Scholar] [CrossRef]
- Adamovich, I.; Agarwal, S.; Ahedo, E.; Alves, L.L.; Baalrud, S.; Babaeva, N.; Bogaerts, A.; Bourdon, A.; Bruggeman, P.J.; Canal, C.; et al. The 2022 Plasma Roadmap: Low Temperature Plasma Science and Technology. J. Phys. D Appl. Phys. 2022, 55, 373001. [Google Scholar] [CrossRef]
- Bradu, C.; Kutasi, K.; Magureanu, M.; Puač, N.; Živković, S. Reactive Nitrogen Species in Plasma-Activated Water: Generation, Chemistry and Application in Agriculture. J. Phys. D Appl. Phys. 2020, 53, 223001. [Google Scholar] [CrossRef]
- Weltmann, K.D.; Kindel, E.; Von Woedtke, T.; Hähnel, M.; Stieber, M.; Brandenburg, R. Atmospheric-Pressure Plasma Sources: Prospective Tools for Plasma Medicine. Pure Appl. Chem. 2010, 82, 1223–1237. [Google Scholar] [CrossRef]
- Mentheour, R.; Machala, Z. Coupled Antibacterial Effects of Plasma-Activated Water and Pulsed Electric Field. Front. Phys. 2022, 10, 895813. [Google Scholar] [CrossRef]
- Lee, H.R.; Lee, Y.S.; You, Y.S.; Huh, J.Y.; Kim, K.; Hong, Y.C.; Kim, C.-H. Antimicrobial Effects of Microwave Plasma-Activated Water with Skin Protective Effect for Novel Disinfectants in Pandemic Era. Sci. Rep. 2022, 12, 5968. [Google Scholar] [CrossRef]
- Chiappim, W.; Sampaio, A.D.G.; Miranda, F.; Fraga, M.; Petraconi, G.; Da Silva Sobrinho, A.; Kostov, K.; Koga-Ito, C.; Pessoa, R. Antimicrobial Effect of Plasma-Activated Tap Water on Staphylococcus aureus, Escherichia coli, and Candida albicans. Water 2021, 13, 1480. [Google Scholar] [CrossRef]
- Zhao, Y.-M.; Ojha, S.; Burgess, C.M.; Sun, D.-W.; Tiwari, B.K. Inactivation Efficacy and Mechanisms of Plasma Activated Water on Bacteria in Planktonic State. J. Appl. Microbiol. 2020, 129, 1248–1260. [Google Scholar] [CrossRef]
- Droste, N.C.; Hummert, M.; Leenders, P.; Mellmann, A.; Becker, K.; Kuczius, T. Plasma-Activated Tap Water with Oxidative Potential Has an Inactivating Effect on Microbiological Contaminants in Aqueous Suspensions. Pathogens 2024, 13, 535. [Google Scholar] [CrossRef]
- Rothwell, J.G.; Alam, D.; Carter, D.A.; Soltani, B.; McConchie, R.; Zhou, R.; Cullen, P.J.; Mai-Prochnow, A. The Antimicrobial Efficacy of Plasma-Activated Water against Listeria and E. coli is Modulated by Reactor Design and Water Composition. J. Appl. Microbiol. 2022, 132, 2490–2500. [Google Scholar] [CrossRef]
- Gorrie, C.L.; Mirčeta, M.; Wick, R.R.; Judd, L.M.; Lam, M.M.C.; Gomi, R.; Abbott, I.J.; Thomson, N.R.; Strugnell, R.A.; Pratt, N.F.; et al. Genomic Dissection of Klebsiella pneumoniae Infections in Hospital Patients Reveals Insights into an Opportunistic Pathogen. Nat. Commun. 2022, 13, 3017. [Google Scholar] [CrossRef]
- Brinkwirth, S.; Ayobami, O.; Eckmanns, T.; Markwart, R. Hospital-Acquired Infections Caused by Enterococci: A Systematic Review and Meta-Analysis, WHO European Region, 1 January 2010 to 4 February 2020. Eurosurveillance 2021, 26, 2001628. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Xu, R.; Zhao, Y.; Liu, D.; Liu, Z.; Wang, X.; Chen, H.; Kong, M.G. Gas Plasma Pre-Treatment Increases Antibiotic Sensitivity and Persister Eradication in Methicillin-Resistant Staphylococcus aureus. Front. Microbiol. 2018, 9, 537. [Google Scholar] [CrossRef] [PubMed]
- Hoon Park, J.; Kumar, N.; Hoon Park, D.; Yusupov, M.; Neyts, E.C.; Verlackt, C.C.W.; Bogaerts, A.; Ho Kang, M.; Sup Uhm, H.; Ha Choi, E.; et al. A Comparative Study for the Inactivation of Multidrug Resistance Bacteria Using Dielectric Barrier Discharge and Nano-Second Pulsed Plasma. Sci. Rep. 2015, 5, 13849. [Google Scholar] [CrossRef] [PubMed]
- Maybin, J.-A.; Thompson, T.P.; Flynn, P.B.; Skvortsov, T.; Hickok, N.J.; Freeman, T.A.; Gilmore, B.F. Cold Atmospheric Pressure Plasma-Antibiotic Synergy in Pseudomonas aeruginosa Biofilms is Mediated via Oxidative Stress Response. Biofilm 2023, 5, 100122. [Google Scholar] [CrossRef]
- Kumar, A.; Škoro, N.; Gernjak, W.; Jovanović, O.; Petrović, A.; Živković, S.; Lumbaque, E.C.; Farré, M.J.; Puač, N. Degradation of Diclofenac and 4-Chlorobenzoic Acid in Aqueous Solution by Cold Atmospheric Plasma Source. Sci. Total Environ. 2023, 864, 161194. [Google Scholar] [CrossRef]
- Jovanović, O.; Puač, N.; Škoro, N. A Comparison of Power Measurement Techniques and Electrical Characterization of an Atmospheric Pressure Plasma Jet. Plasma Sci. Technol. 2022, 24, 105404. [Google Scholar] [CrossRef]
- Kutasi, K.; Bencs, L.; Tóth, Z.; Milošević, S. The Role of Metals in the Deposition of Long-lived Reactive Oxygen and Nitrogen Species into the Plasma-activated Liquids. Plasma Process. Polym. 2023, 20, 2200143. [Google Scholar] [CrossRef]
- Eisenberg, G. Colorimetric Determination of Hydrogen Peroxide. Ind. Eng. Chem. Anal. Ed. 1943, 15, 327–328. [Google Scholar] [CrossRef]
- Bruggeman, P.J.; Kushner, M.J.; Locke, B.R.; Gardeniers, J.G.E.; Graham, W.G.; Graves, D.B.; Hofman-Caris, R.C.H.M.; Maric, D.; Reid, J.P.; Ceriani, E.; et al. Plasma–Liquid Interactions: A Review and Roadmap. Plasma Sources Sci. Technol. 2016, 25, 053002. [Google Scholar] [CrossRef]
- Sampaio, A.D.G.; Chiappim, W.; Milhan, N.V.M.; Botan Neto, B.; Pessoa, R.; Koga-Ito, C.Y. Effect of the pH on the Antibacterial Potential and Cytotoxicity of Different Plasma-Activated Liquids. Int. J. Mol. Sci. 2022, 23, 13893. [Google Scholar] [CrossRef]
- Von Woedtke, T.; Bekeschus, S.; Weltmann, K.; Wende, K. Plasma-Treated Liquids for Medicine: A Narrative Review on State and Perspectives. Plasma Process. Polym. 2025, 22, 2400255. [Google Scholar] [CrossRef]
- Foster, J.; Sommers, B.S.; Gucker, S.N.; Blankson, I.M.; Adamovsky, G. Perspectives on the Interaction of Plasmas With Liquid Water for Water Purification. IEEE Trans. Plasma Sci. 2012, 40, 1311–1323. [Google Scholar] [CrossRef]
- Bilea, F.; Garcia-Vaquero, M.; Magureanu, M.; Mihaila, I.; Mildažienė, V.; Mozetič, M.; Pawłat, J.; Primc, G.; Puač, N.; Robert, E.; et al. Non-Thermal Plasma as Environmentally-Friendly Technology for Agriculture: A Review and Roadmap. Crit. Rev. Plant Sci. 2024, 43, 428–486. [Google Scholar] [CrossRef]
- Kim, S.; Kim, C.-H. Applications of Plasma-Activated Liquid in the Medical Field. Biomedicines 2021, 9, 1700. [Google Scholar] [CrossRef]
- Milhan, N.V.M.; Chiappim, W.; Sampaio, A.D.G.; Vegian, M.R.D.C.; Pessoa, R.S.; Koga-Ito, C.Y. Applications of Plasma-Activated Water in Dentistry: A Review. Int. J. Mol. Sci. 2022, 23, 4131. [Google Scholar] [CrossRef]
- Lin, X.; Li, C.; Zhang, S.; Yang, X.; Jiang, M. The Global and Regional Prevalence of Hospital-Acquired Carbapenem-Resistant Klebsiella pneumoniae Infection: A Systematic Review and Meta-analysis. Open Forum Infect. Dis. 2024, 11, ofad649. [Google Scholar] [CrossRef]
- García-Solache, M.; Rice, L.B. The Enterococcus: A Model of Adaptability to Its Environment. Clin. Microbiol. Rev. 2019, 32, e00058-18. [Google Scholar] [CrossRef]
- Flynn, P.B.; Higginbotham, S.; Alshraiedeh, N.H.; Gorman, S.P.; Graham, W.G.; Gilmore, B.F. Bactericidal Efficacy of Atmospheric Pressure Non-Thermal Plasma (APNTP) against the ESKAPE Pathogens. Int. J. Antimicrob. Agents 2015, 46, 101–107. [Google Scholar] [CrossRef]
- Mai-Prochnow, A.; Clauson, M.; Hong, J.; Murphy, A.B. Gram Positive and Gram Negative Bacteria Differ in Their Sensitivity to Cold Plasma. Sci. Rep. 2016, 6, 38610. [Google Scholar] [CrossRef]
- Huang, M.; Zhuang, H.; Zhao, J.; Wang, J.; Yan, W.; Zhang, J. Differences in Cellular Damage Induced by Dielectric Barrier Discharge Plasma between Salmonella typhimurium and Staphylococcus aureus. Bioelectrochemistry 2020, 132, 107445. [Google Scholar] [CrossRef]
- Kamgang-Youbi, G.; Herry, J.-M.; Meylheuc, T.; Brisset, J.-L.; Bellon-Fontaine, M.-N.; Doubla, A.; Naïtali, M. Microbial Inactivation Using Plasma-Activated Water Obtained by Gliding Electric Discharges. Lett. Appl. Microbiol. 2009, 48, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Patange, A.; Sun, D.; Tiwari, B. Plasma-activated Water: Physicochemical Properties, Microbial Inactivation Mechanisms, Factors Influencing Antimicrobial Effectiveness, and Applications in the Food Industry. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3951–3979. [Google Scholar] [CrossRef] [PubMed]
- Nicol, M.J.; Brubaker, T.R.; Honish, B.J.; Simmons, A.N.; Kazemi, A.; Geissel, M.A.; Whalen, C.T.; Siedlecki, C.A.; Bilén, S.G.; Knecht, S.D.; et al. Antibacterial Effects of Low-Temperature Plasma Generated by Atmospheric-Pressure Plasma Jet Are Mediated by Reactive Oxygen Species. Sci. Rep. 2020, 10, 3066. [Google Scholar] [CrossRef] [PubMed]
- Tipa, R.S.; Boekema, B.; Middelkoop, E.; Kroesen, G.M. Cold Plasma for Bacterial Inactivation. In Proceedings of the 20th International Symposium on Plasma Chemistry, Philadelphia, PA, USA, 24–29 July 2011. [Google Scholar]
- Rohde, M. The Gram-Positive Bacterial Cell Wall. Microbiol. Spectr. 2019, 7, GPP3-0044-2018. [Google Scholar] [CrossRef]
- Napp, M.; Von Podewils, S.; Klare, I.; Haase, H.; Kasch, R.; Gümbel, D.; Ekkernkamp, A.; Jünger, M.; Daeschlein, G. Does Antibiotic Resistance Impair Plasma Susceptibility of Multi-Drug Resistant Clinical Isolates of Enterococci In Vitro? Gut Pathog. 2016, 8, 41. [Google Scholar] [CrossRef][Green Version]
- Vuković, D.; Toljić, B.; Miletić, M.; Milojević, N.; Jovanović, O.; Škoro, N.; Malović, G.; Đaković, M.; Puač, N. Factors Influencing Bactericidal Effect of Plasma-Activated Water Against Klebsiella pneumoniae. In Proceedings of the 21st International Congress on Plasma Physics (ICPP 2024), Ghent, Belgium, 8–13 September 2024. [Google Scholar]
- Lührmann, A.; Matthes, R.; Kramer, A. Impact of Cold Atmospheric Pressure Argon Plasma on Antibiotic Sensitivity of Methicillin-Resistant Staphylococcus aureus Strains In Vitro. GMS Hyg. Infect. Control 2016, 11, 1–9. [Google Scholar] [CrossRef]
- Doi, Y. Treatment Options for Carbapenem-Resistant Gram-Negative Bacterial Infections. Clin. Infect. Dis. 2019, 69, S565–S575. [Google Scholar] [CrossRef]
- Winterbourn, C.C. Biological Chemistry of Superoxide Radicals. ChemTexts 2020, 6, 7. [Google Scholar] [CrossRef]
- Seixas, A.F.; Quendera, A.P.; Sousa, J.P.; Silva, A.F.Q.; Arraiano, C.M.; Andrade, J.M. Bacterial Response to Oxidative Stress and RNA Oxidation. Front. Genet. 2022, 12, 821535. [Google Scholar] [CrossRef]
- Aranda-Rivera, A.K.; Cruz-Gregorio, A.; Arancibia-Hernández, Y.L.; Hernández-Cruz, E.Y.; Pedraza-Chaverri, J. RONS and Oxidative Stress: An Overview of Basic Concepts. Oxygen 2022, 2, 437–478. [Google Scholar] [CrossRef]
- Ezraty, B.; Gennaris, A.; Barras, F.; Collet, J.-F. Oxidative Stress, Protein Damage and Repair in Bacteria. Nat. Rev. Microbiol. 2017, 15, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P.; Bayir, H.; Belousov, V.; Chang, C.J.; Davies, K.J.A.; Davies, M.J.; Dick, T.P.; Finkel, T.; Forman, H.J.; Janssen-Heininger, Y.; et al. Guidelines for Measuring Reactive Oxygen Species and Oxidative Damage in Cells and In Vivo. Nat. Metab. 2022, 4, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Xia, B.; Vyas, H.K.N.; Zhou, R.; Zhang, T.; Hong, J.; Rothwell, J.G.; Rice, S.A.; Carter, D.; Ostrikov, K.K.; Cullen, P.J.; et al. The Importance of Superoxide Anion for Escherichia Coli Biofilm Removal Using Plasma-Activated Water. J. Environ. Chem. Eng. 2023, 11, 109977. [Google Scholar] [CrossRef]
- Vyas, H.K.N.; Hoque, M.M.; Xia, B.; Alam, D.; Cullen, P.J.; Rice, S.A.; Mai-Prochnow, A. Transcriptional Signatures Associated with the Survival of Escherichia coli Biofilm During Treatment with Plasma-Activated Water. Biofilm 2025, 9, 100266. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vuković, D.; Miletić, M.; Toljić, B.; Milojević, N.; Jovanović, O.; Kuzmanović Pfićer, J.; Škoro, N.; Puač, N. Plasma-Activated Water Against Carbapenem-Resistant Klebsiella pneumoniae and Vancomycin-Resistant Enterococcus faecalis. Pathogens 2025, 14, 410. https://doi.org/10.3390/pathogens14050410
Vuković D, Miletić M, Toljić B, Milojević N, Jovanović O, Kuzmanović Pfićer J, Škoro N, Puač N. Plasma-Activated Water Against Carbapenem-Resistant Klebsiella pneumoniae and Vancomycin-Resistant Enterococcus faecalis. Pathogens. 2025; 14(5):410. https://doi.org/10.3390/pathogens14050410
Chicago/Turabian StyleVuković, Dragana, Maja Miletić, Boško Toljić, Nikola Milojević, Olivera Jovanović, Jovana Kuzmanović Pfićer, Nikola Škoro, and Nevena Puač. 2025. "Plasma-Activated Water Against Carbapenem-Resistant Klebsiella pneumoniae and Vancomycin-Resistant Enterococcus faecalis" Pathogens 14, no. 5: 410. https://doi.org/10.3390/pathogens14050410
APA StyleVuković, D., Miletić, M., Toljić, B., Milojević, N., Jovanović, O., Kuzmanović Pfićer, J., Škoro, N., & Puač, N. (2025). Plasma-Activated Water Against Carbapenem-Resistant Klebsiella pneumoniae and Vancomycin-Resistant Enterococcus faecalis. Pathogens, 14(5), 410. https://doi.org/10.3390/pathogens14050410






