The Dual Burden of Hepatitis B and C Among Drug Users in Asia: The First Systematic Review and Meta-Analysis
Abstract
1. Background
2. Methods
2.1. Research Design
2.2. Eligibility Criteria
2.3. Information Sources
2.4. Search Strategy
2.5. Selection of Studies
2.6. Data Extraction
2.7. Risk of Bias Assessment
2.8. Statistical Analysis
3. Result
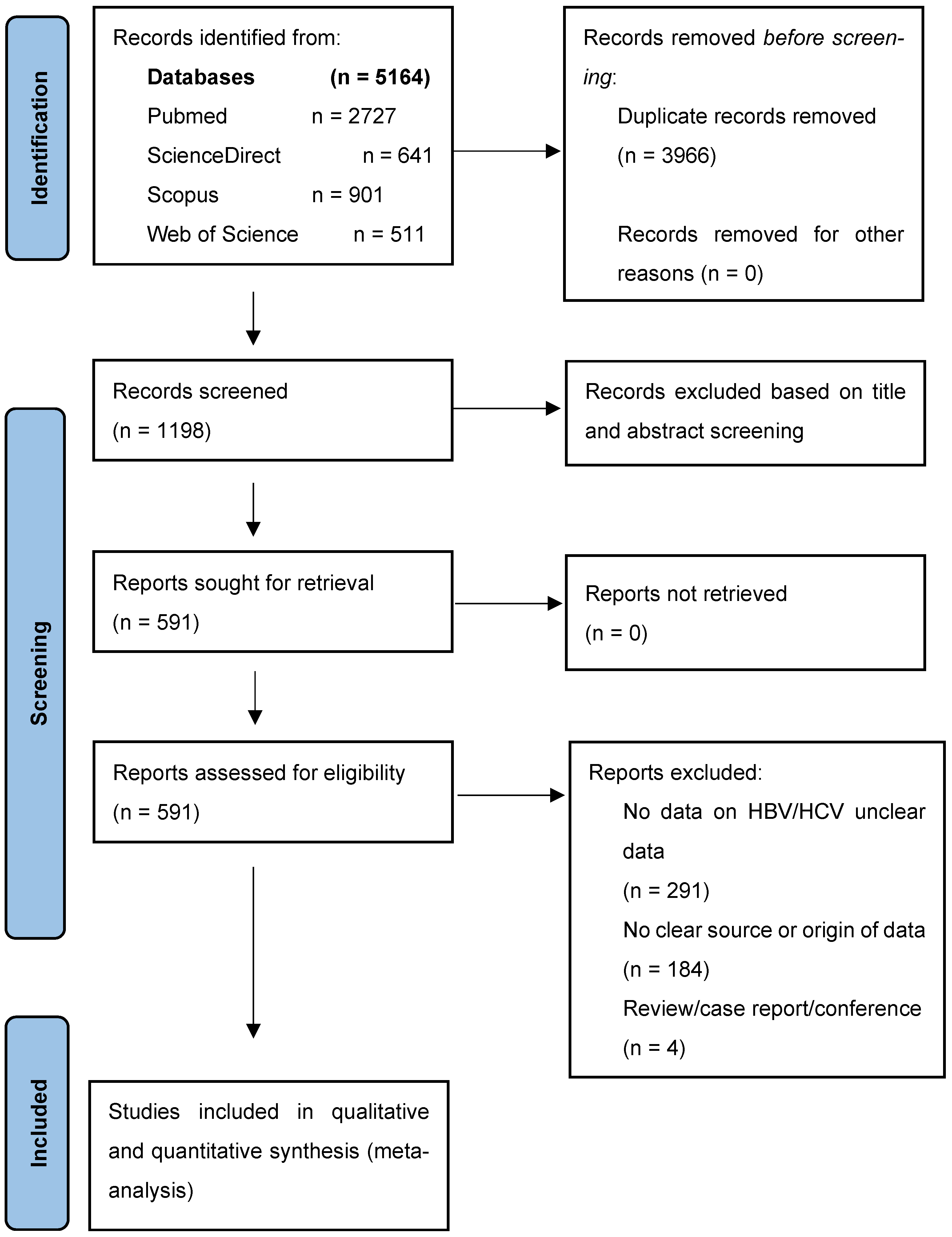
3.1. Study Selection
3.2. Characteristics of the Included Studies
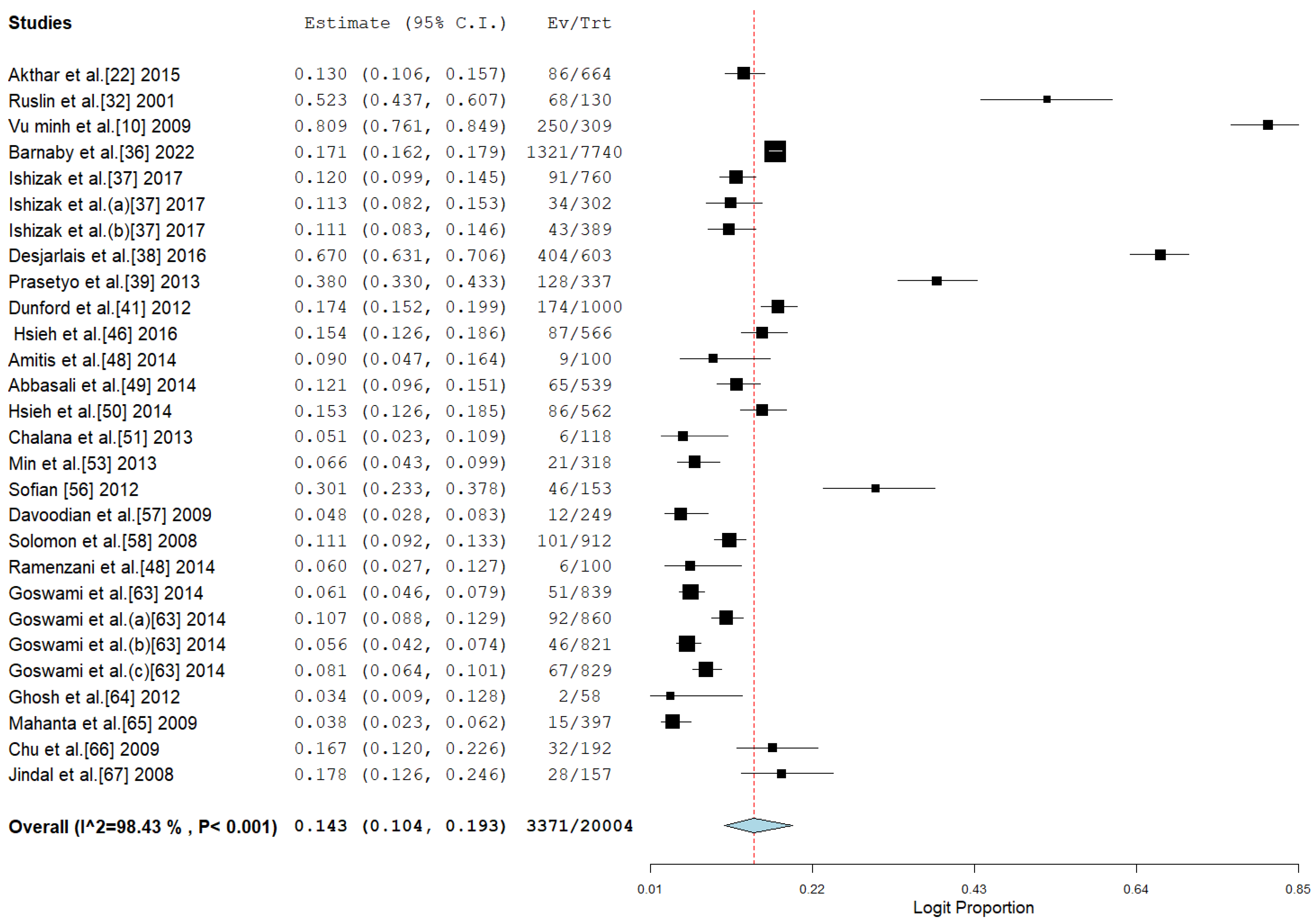
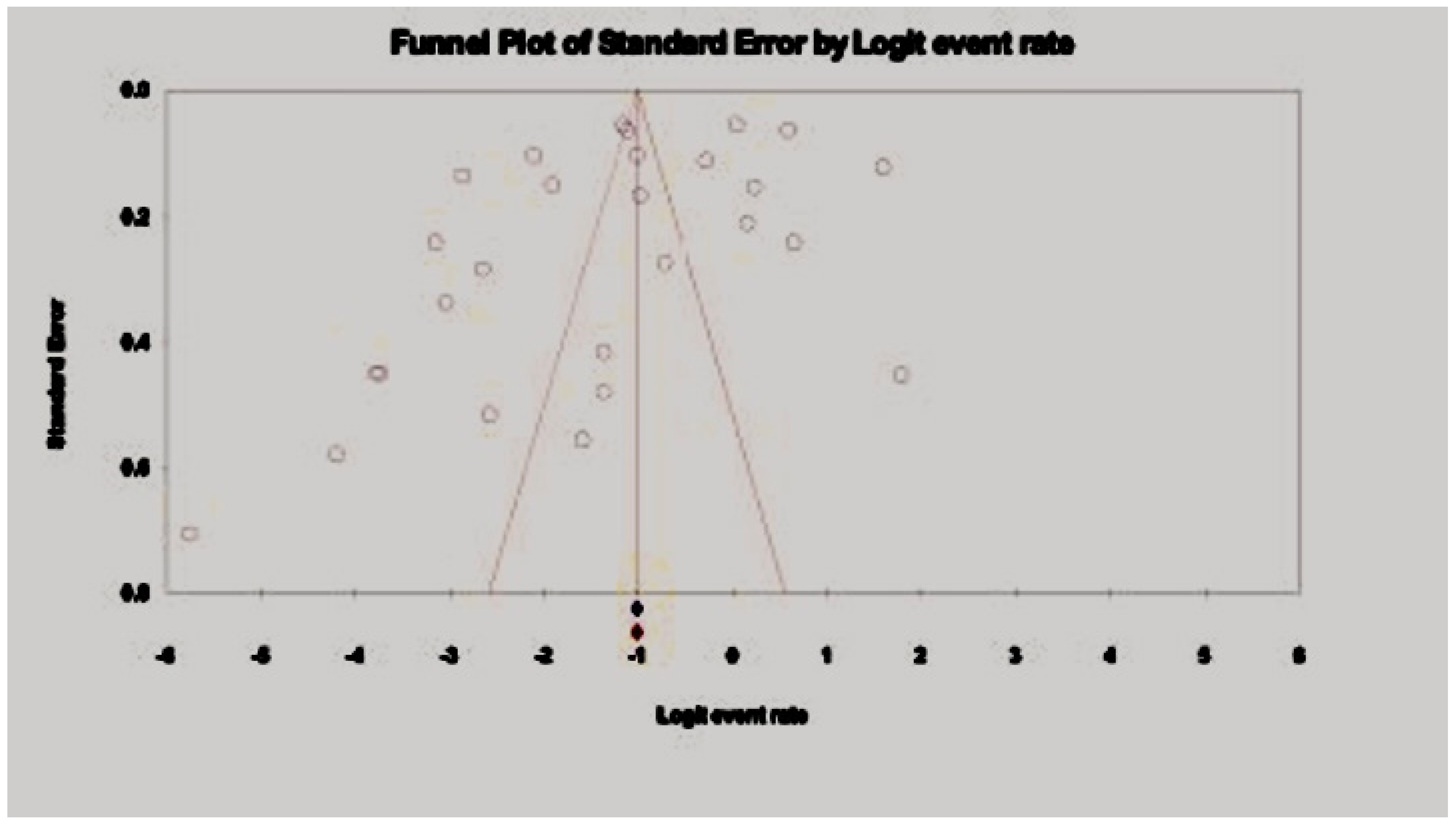
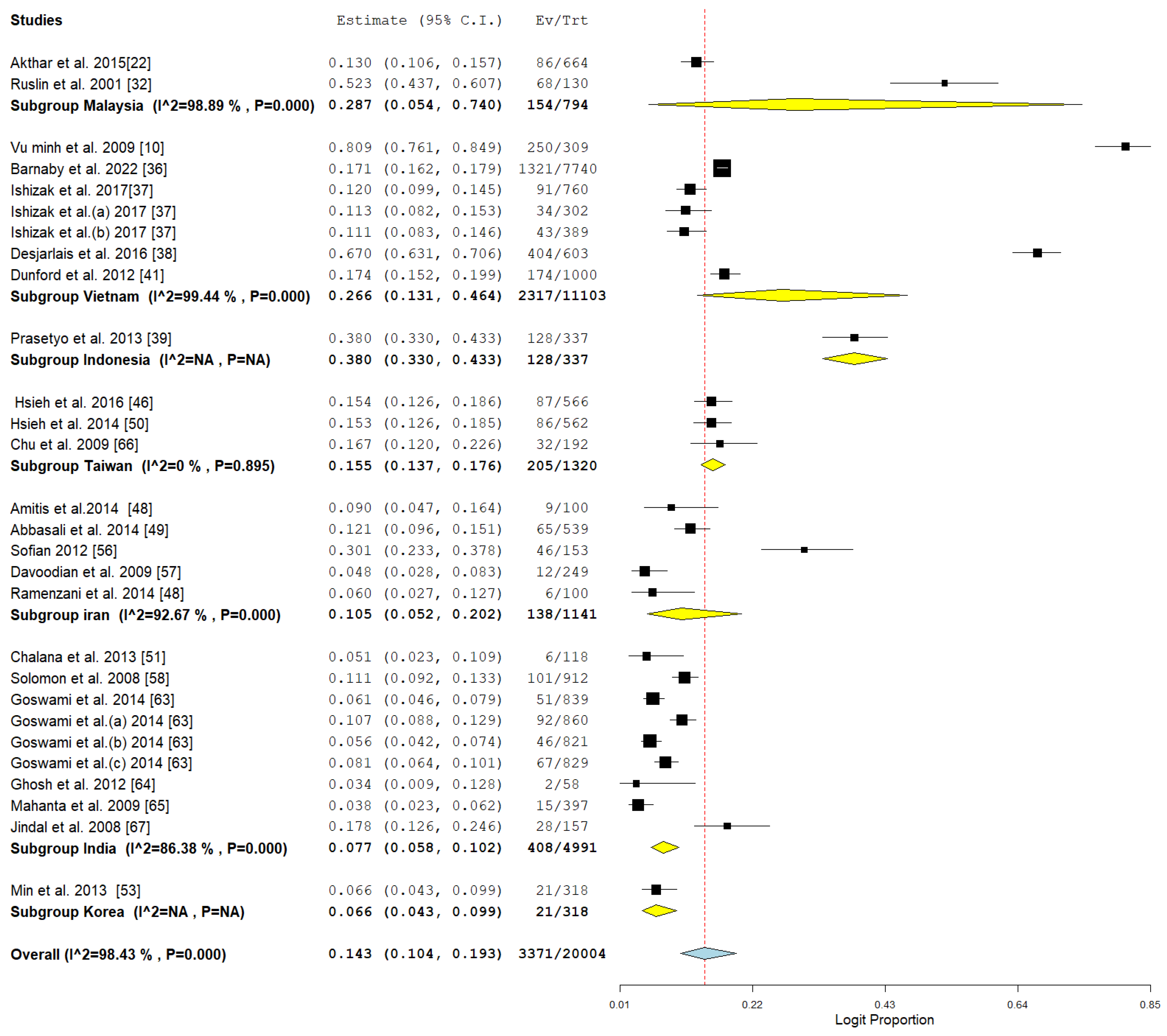
3.3. Pooled Prevalence of HBV Among Drug Users
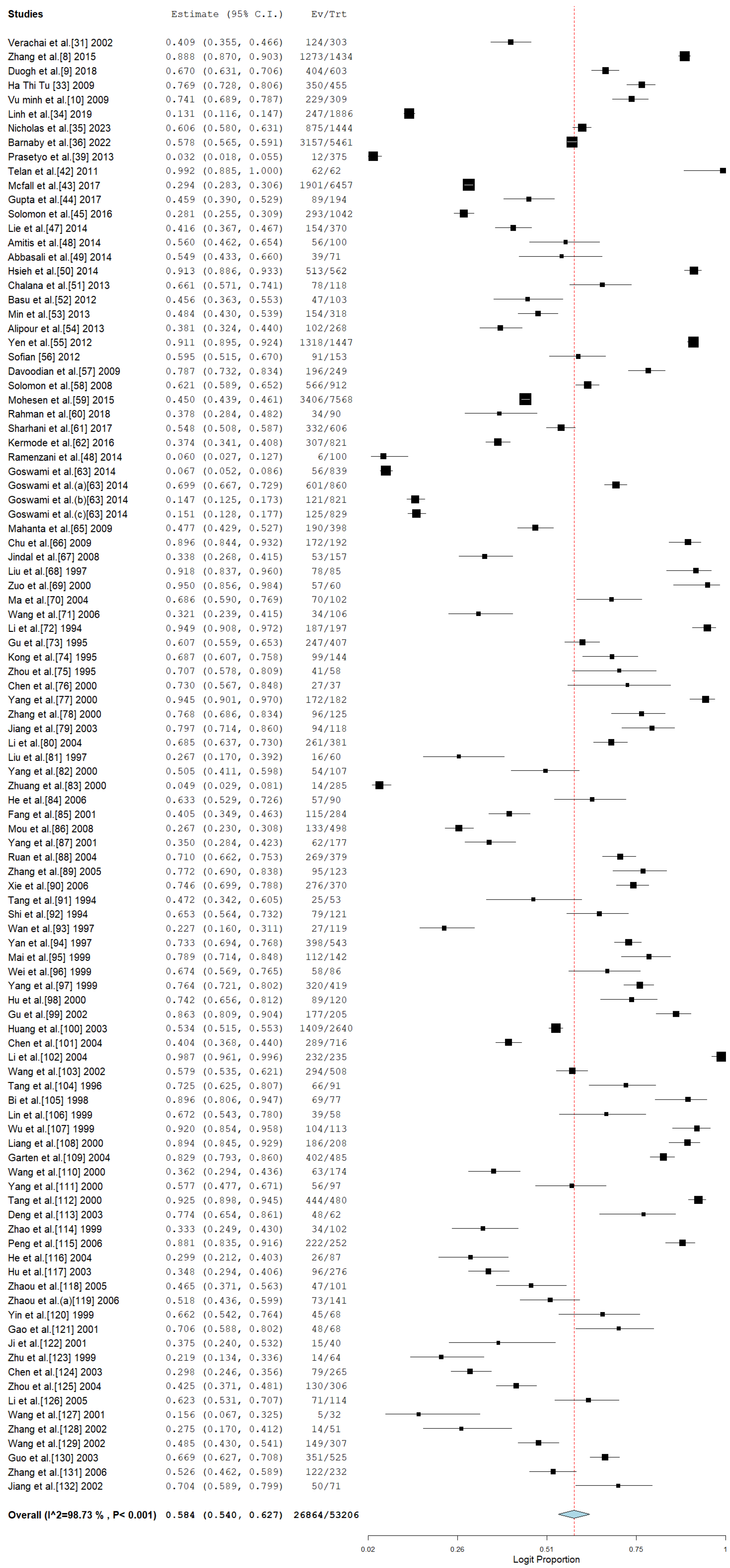
3.4. Pooled Prevalence of HCV Among Drug Users in Asia
3.5. Subgroup Meta-Analysis
3.5.1. HBV Subgroup
3.5.2. HCV Subgroup
4. Discussion
5. Strengths and Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Torbenson, M.; Kannangai, R.; Astemborski, J.; Strathdee, S.A.; Vlahov, D.; Thomas, D.L. High Prevalence of Occult Hepatitis B in Baltimore Injection Drug Users. Hepatology 2004, 39, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Drysdale, K.; Bryant, J.; Holt, M.; Dowsett, G.W.; Holt, M.; Lea, T.; Aggleton, P.; Treloar, C. Destabilising the ‘problem’ of chemsex: Diversity in settings, relations and practices revealed in Australian gay and bisexual men’s crystal methamphetamine use. Int. J. Drug Policy 2020, 78, 102697. [Google Scholar] [CrossRef] [PubMed]
- Curtis, T.J.; Rodger, A.J.; Burns, F.; Nardone, A.; Copas, A.; Wayal, S. Patterns of sexualised recreational drug use and its association with risk behaviours and sexual health outcomes in men who have sex with men in London, UK: A comparison of cross-sectional studies conducted in 2013 and 2016. Sex. Transm. Infect. 2020, 96, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Sun, L.; Mu, T.; Yi, J.; Ma, C.; Xie, H.; Liu, M.; Tang, H. An HBV-encoded miRNA activates innate immunity to restrict HBV replication. J. Mol. Cell Biol. 2020, 12, 263–276. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Hepatitis B Vaccines: WHO Position Paper—Recommendations. Vaccine 2010, 28, 589–590. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, T.; Pan, S.W.; Tang, W.; Guo, W.; Tucker, J.D. HBV and HCV test uptake and correlates among men who have sex with men in China: A nationwide cross-sectional online survey. Sex. Transm. Infect. 2018, 94, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Bello, K.E.; Mat Jusoh, T.N.A.; Irekeola, A.A.; Abu, N.; Amin, N.A.Z.M.; Mustaffa, N.; Shueb, R.H. A Recent Prevalence of Hepatitis B Virus (HBV) Genotypes and Subtypes in Asia: A Systematic Review and Meta-Analysis. Healthcare 2023, 11, 1011. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Celentano, D.D.; Le Minh, N.; Latkin, C.A.; Mehta, S.H.; Frangakis, C.; Ha, T.V.; Mo, T.T.; Sripaipan, T.; Davis, W.W.; et al. Prevalence and correlates of HCV monoinfection and HIV and HCV coinfection among persons who inject drugs in Vietnam. Eur. J. Gastroenterol. Hepatol. 2015, 27, 550–556. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Drive Study Group; Duong, H.T.; Jarlais, D.D.; Khuat, O.H.T.; Arasteh, K.; Feelemyer, J.; Khue, P.M.; Giang, H.T.; Laureillard, D.; Hai, V.V.; et al. Risk behaviors for hiv and hcv infection among people who inject drugs in Hai Phong, Viet Nam, 2014. AIDS Behav. 2018, 22, 2161–2171. [Google Scholar] [CrossRef] [PubMed]
- Quan, V.M.; Go, V.F.; Van Nam, L.; Bergenstrom, A.; Thuoc, N.P.; Zenilman, J.; Latkin, C.; Celentano, D.D. Risks for HIV, HBV, and HCV infections among male injection drug users in northern Vietnam: A case–control study. AIDS Care 2009, 21, 7–16. [Google Scholar] [CrossRef]
- Suppiah, J.; Zain, R.M.; Nawi, S.H.; Bahari, N.; Saat, Z. Drug-resistance associated mutations in polymerase (P) gene of hepatitis B virus isolated from Malaysian HBV carriers. Hepat. Mon. 2014, 14, e13173. [Google Scholar] [CrossRef] [PubMed]
- Trung, N.T.; Hai, L.T.; Giang, D.P.; Hoan, P.Q.; Binh, M.T.; Hoan, N.X.; Toan, N.L.; Meyer, C.G.; Velavan, T.P.; Bang, M.H.; et al. No expression of HBV-human chimeric fusion transcript (HBx-LINE1) among Vietnamese patients with HBV-associated hepatocellular carcinoma. Ann. Hepatol. 2019, 18, 404–405. [Google Scholar] [CrossRef] [PubMed]
- Candotti, D.; Assennato, S.M.; Laperche, S.; Allain, J.P.; Levicnik-Stezinar, S. Multiple HBV transfusion transmissions from undetected occult infections: Revising the minimal infectious dose. Gut 2019, 68, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Keryakos, H.K.H.; Mohammed, A.A.; Higazi, A.M.; Mahmoud, E.A.M.; Saad, Z.M. Serum and ascitic fluid interleukin-17 in spontaneous bacterial peritonitis in Egyptian patients with HCV-related liver cirrhosis. Curr. Res. Transl. Med. 2020, 68, 237–243. [Google Scholar] [CrossRef]
- Johnson, D.F.; Ratnam, I.; Matchett, E.; Earnest-Silveria, L.; Christiansen, D.; Leder, K.; Grayson, M.L.; Torresi, J. The incidence of HBV and HCV infection in Australian travelers to Asia. J. Travel Med. 2013, 20, 203–205. [Google Scholar] [CrossRef][Green Version]
- Irekeola, A.A.; Malek, N.A.; Wada, Y.; Mustaffa, N.; Muhamad, N.I.; Shueb, R.H. Prevalence of HCV genotypes and subtypes in Southeast Asia: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0251673. [Google Scholar] [CrossRef]
- Wei, L.; Rao, H.Y.; Wang, Y.; Yang, M.; Gao, Y.H. Molecular epidemiology of HCV in Asia. Curr. Hepat. Rep. 2013, 12, 133–142. [Google Scholar] [CrossRef]
- Abdala, N.; Krasnoselskikh, T.V.; Durante, A.J.; Timofeeva, M.Y.; Verevochkin, S.V.; Kozlov, A.P. Sexually transmitted infections, sexual risk behaviors and the risk of heterosexual spread of HIV among and beyond IDUs in St. Petersburg, Russia. Eur. Addict. Res. 2008, 14, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Khodjaeva, M.; Ibadullaeva, N.; Khikmatullaeva, A.; Joldasova, E.; Ismoilov, U.; Colombo, M.; Caviglia, G.P.; Rizzetto, M.; Musabaev, E. The medical impact of hepatitis D virus infection in Uzbekistan. Liver Int. 2019, 39, 2077–2081. [Google Scholar] [CrossRef] [PubMed]
- Kucirka, L.M.; Farzadegan, H.; Feld, J.J.; Mehta, S.H.; Winters, M.; Glenn, J.S.; Kirk, G.D.; Segev, D.L.; Nelson, K.E.; Marks, M.; et al. Prevalence, correlates, and viral dynamics of hepatitis delta among injection drug users. J. Infect. Dis. 2010, 202, 845–852. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, F.; Guo, F.; Zhao, Q.; Chang, J.; Guo, J.T. Characterization of novel hepadnaviral RNA species accumulated in hepatoma cells treated with viral DNA polymerase inhibitors. Antivir. Res. 2016, 131, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, A.; Khan, A.H.; Sulaiman, S.A.S.; Soo, C.T.; Khan, K. HBV and HIV co-infection: Prevalence and clinical outcomes in tertiary care hospital Malaysia. J. Med. Virol. 2016, 88, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yu, Y.; Li, G.; Shen, C.; Li, J.; Chen, S.; Zhang, X.; Zhu, M.; Zheng, J.; Song, Z.; et al. Natural history of serum HBV-RNA in chronic HBV infection. J. Viral Hepat. 2018, 25, 1038–1047. [Google Scholar] [CrossRef]
- Alzahrani, F.M.; Muzaheed Shaikh, S.S.; Alomar, A.I.; Acharya, S.; Elhadi, N. Prevalence of Hepatitis B Virus (HBV) among blood donors in eastern Saudi Arabia: Results from a five-year retrospective study of HBV seromarkers. Ann. Lab. Med. 2018, 39, 81–85. [Google Scholar] [CrossRef]
- Brouard, C.; Saboni, L.; Gautier, A.; Chevaliez, S.; Rahib, D.; Richard, J.-B.; Barin, F.; Larsen, C.; Sommen, C.; Pillonel, J.; et al. HCV and HBV prevalence based on home blood self-sampling and screening history in the general population in 2016: Contribution to the new French screening strategy. BMC Infect. Dis. 2019, 19, 896. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Mude, A.S.A.; Nageye, Y.A.; Bello, K.E. Prevalence of hepatitis B virus among people in Somalia and among Somalian immigrants in diaspora: A systematic review and meta-analysis. Microbes Infect. Dis. 2024, 5, 532–546. [Google Scholar] [CrossRef]
- Fletcher, J. What is heterogeneity and is it important? BMJ 2007, 334, 94–96. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Hunter, J.; Saratzis, A.; Sutton, A.; Boucher, R.; Sayers, R.; Bown, M. In meta-analyses of proportion studies, funnel plots were found to be an inaccurate method of assessing publication bias. J. Clin. Epidemiol. 2014, 67, 897–903. [Google Scholar] [PubMed]
- Verachai, V.; Phutiprawan, T.; Theamboonlers, A.; Chinchai, T.; Tanprasert, S.; Haagmans, B.L.; E Osterhaus, A.D.M.; Poovorawan, Y. Prevalence and genotypes of hepatitis C virus infection among drug addicts and blood donors in Thailand. Southeast Asian J. Trop. Med. Public Health 2002, 33, 849–851. [Google Scholar] [PubMed]
- Nordin, R.B.; Rahman Bin Isa, A.; Rusli Bin Abdullah, M. Prevalence of sexually transmitted diseases among new female drug abusers in a rehabilitation centre. Malays. J. Med. Sci. 2001, 8, 9–13. [Google Scholar] [PubMed]
- Phan, H.T.T. Hepatitis C and Human Immunodeficiency Virus Infections in Injecting Drug Users in Drug Treatment Centers in Vietnam. Ph.D. Thesis, The University of Texas School of Public Health, Dallas, TX, USA, 2009. [Google Scholar]
- Le, L.V.N.; O’Connor, S.; Tran, T.H.; Maher, L.; Kaldor, J.; Sabin, K.; Tran, H.V.; Tran, Q.D.; Ho, V.A.T.; Nguyen, T.A. High hepatitis C virus infection among female sex workers in Viet Nam: Strong correlation with HIV and injection drug use. West. Pac. Surveill. Response J. 2019, 10, 9. [Google Scholar]
- Nagot, N.; Binh, N.T.; Hong, T.T.; Vinh, V.H.; Quillet, C.; Vallo, R.; Huong, D.T.; Oanh, K.T.H.; Thanh, N.T.T.; Rapoud, D.; et al. A community-based strategy to eliminate hepatitis C among people who inject drugs in Vietnam. Lancet Reg. Health West. Pac. 2023, 37, 100801. [Google Scholar] [CrossRef]
- Flower, B.; Du Hong, D.; Kim, H.V.T.; Minh, K.P.; Geskus, R.B.; Day, J.; Cooke, G.S. Seroprevalence of Hepatitis B, C and D in Vietnam: A systematic review and meta-analysis. Lancet Reg. Health West. Pac. 2022, 24, 100468. [Google Scholar] [CrossRef] [PubMed]
- Ishizaki, A.; Tran, V.T.; Nguyen, C.H.; Tanimoto, T.; Hoang, H.T.T.; Pham, H.V.; Phan, C.T.T.; Bi, X.; Van Pham, T.; Ichimura, H. Discrepancies in prevalence trends for HIV, hepatitis B virus, and hepatitis C virus in Haiphong, Vietnam from 2007 to 2012. PLoS ONE 2017, 12, e0179616. [Google Scholar] [CrossRef]
- Des Jarlais, D.C.; Huong, D.T.; Oanh, K.T.H.; Pham, M.K.; Giang, H.T.; Thanh, N.T.; Arasteh, K.; Feelemyer, J.; Hammett, T.; Peries, M.; et al. Prospects for ending the HIV epidemic among persons who inject drugs in Haiphong, Vietnam. Int. J. Drug Policy 2016, 32, 50–56. [Google Scholar] [CrossRef]
- Agung Prasetyo, A.; Dirgahayu, P.; Sari, Y.; Kageyama, S. Molecular epidemiology of HIV, HBV, HCV, and HTLV-1/2 in drug abuser inmates in central Javan prisons, Indonesia. J. Infect. Dev. Ctries. 2013, 7, 453–467. [Google Scholar]
- Dunford, L.; Carr, M.J.; Dean, J.; Waters, A.; Nguyen, L.T.; Do, H.D.; Thi, T.T.D.; Nguyen, H.T.; Do, T.T.D.; Luu, Q.P.; et al. Hepatitis C virus in Vietnam: High prevalence of infection in dialysis and multi-transfused patients involving diverse and novel virus variants. PLoS ONE 2012, 7, e41266. [Google Scholar] [CrossRef]
- Dunford, L.; Carr, M.J.; Dean, J.; Nguyen, L.T.; Thi, T.H.T.; Nguyen, B.T.; Connell, J.; Coughlan, S.; Nguyen, H.T.; Hall, W.W.; et al. A multicentre molecular analysis of hepatitis B and blood-borne virus coinfections in Viet Nam. PLoS ONE 2012, 7, e39027. [Google Scholar]
- Telan, E.F.O.; Samonte, G.M.J.; Abellanosa-Tac-An, I.P.; Alesna, E.T.; Leaño, P.S.A.; Emphasis, Y.E.E.; Tsuneki, A.; Matsumoto, K.; Kageyama, S. The early phase of an HIV epidemic in a population exposed previously to HCV in the Philippines. J. Med. Virol. 2011, 83, 941–947. [Google Scholar] [CrossRef] [PubMed]
- McFall, A.M.; Solomon, S.S.; Lucas, G.M.; Celentano, D.D.; Srikrishnan, A.K.; Kumar, M.S.; Mehta, S.H. Epidemiology of HIV and hepatitis C infection among women who inject drugs in Northeast India: A respondent-driven sampling study. Addiction 2017, 112, 1480–1487. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Saha, K.; Biswas, A.; Firdaus, R.; Ghosh, M.; Sadhukhan, P.C. Recombination in hepatitis C virus is not uncommon among people who inject drugs in Kolkata, India. Infect. Genet. Evol. 2017, 48, 156–163. [Google Scholar] [CrossRef]
- Solomon, S.S.; Srikrishnan, A.K.; McFall, A.M.; Kumar, M.S.; Saravanan, S.; Balakrishnan, P.; Solomon, S.; Thomas, D.L.; Sulkowski, M.S.; Mehta, S.H. Burden of liver disease among community-based people who inject drugs (PWID) in Chennai, India. PLoS ONE 2016, 11, e0147879. [Google Scholar] [CrossRef]
- Hsieh, M.H.; Hsieh, M.Y.; Huang, C.F.; Yeh, M.L.; Wang, S.C.; Yang, J.F.; Chang, K.; Lin, W.R.; Lin, C.Y.; Chen, T.C.; et al. Anti-HIV seropositivity was related to HBsAg seropositivity among injecting drug users in Taiwan. Kaohsiung J. Med. Sci. 2016, 32, 96–102. [Google Scholar]
- Li, L.; Assanangkornchai, S.; Duo, L.; McNeil, E.; Li, J. Risk behaviors, prevalence of HIV and hepatitis C virus infection and population size of current injection drug users in a China-Myanmar border city: Results from a Respondent-Driven Sampling Survey in 2012. PLoS ONE 2014, 9, e106899. [Google Scholar] [CrossRef]
- Ramezani, A.; Amirmoezi, R.; Volk, J.E.; Aghakhani, A.; Zarinfar, N.; McFarland, W.; Banifazl, M.; Mostafavi, E.; Eslamifar, A.; Sofian, M. HCV, HBV, and HIV seroprevalence, coinfections, and related behaviors among male injection drug users in Arak, Iran. AIDS Care 2014, 26, 1122–1126. [Google Scholar] [CrossRef] [PubMed]
- Javadi, A.; Ataei, B.; Kassaian, N.; Nokhodian, Z.; Yaran, M. Co-infection of human immunodeficiency virus, hepatitis C and hepatitis B virus among injection drug users in Drop in centers. J. Res. Med. Sci. 2014, 19 (Suppl. S1), S17–S21. [Google Scholar] [PubMed]
- Hsieh, M.H.; Tsai, J.J.; Hsieh, M.Y.; Huang, C.F.; Yeh, M.L.; Yang, J.F.; Chang, K.; Lin, W.R.; Lin, C.Y.; Chen, T.C.; et al. Hepatitis C virus infection among injection drug users with and without human immunodeficiency virus co-infection. PLoS ONE 2014, 9, e94791. [Google Scholar] [CrossRef] [PubMed]
- Chalana, H.; Singh, H.; Sachdeva, J.K.; Sharma, S. Seroprevalence of human immunodeficiency virus, hepatitis B surface antigen, and hepatitis C in substance dependents admitted in a tertiary hospital at Amritsar, India. Asian J. Psychiatry 2013, 6, 552–555. [Google Scholar] [CrossRef]
- Basu, D.; Kumar, V.; Sharma, A.K.; Barnwal, P.K.; Mattoo, S.K. Seroprevalence of anti-hepatitis C virus (anti-HCV) antibody and HCV-related risk in injecting drug users in northern India: Comparison with non-injecting drug users. Asian J. Psychiatry 2013, 6, 52–55. [Google Scholar] [CrossRef] [PubMed]
- Min, J.A.; Yoon, Y.; Lee, H.J.; Choi, J.; Kwon, M.; Kim, K.; Lee, C.; Kim, D.; Yun, H. Prevalence and associated clinical characteristics of hepatitis B, C, and HIV infections among injecting drug users in Korea. J. Med. Virol. 2013, 85, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Alipour, A.; Haghdoost, A.A.; Sajadi, L.; Zolala, F. HIV prevalence and related risk behaviours among female partners of male injecting drugs users in Iran: Results of a bio-behavioural survey, 2010. Sex. Transm. Infect. 2013, 89 (Suppl. S3), iii41–iii44. [Google Scholar] [CrossRef] [PubMed]
- Yen, Y.F.; Yen, M.Y.; Su, L.W.; Li, L.H.; Chuang, P.; Jiang, X.R.; Deng, C.Y. Prevalences and associated risk factors of HCV/HIV co-infection and HCV mono-infection among injecting drug users in a methadone maintenance treatment program in Taipei, Taiwan. BMC Public Health 2012, 12, 1066. [Google Scholar] [CrossRef]
- Sofian, M.; Aghakhani, A.; Banifazl, M.; Azadmanesh, K.; Farazi, A.-A.; McFarland, W.; Eslamifar, A.; Ramezani, A. Viral hepatitis and HIV infection among injection drug users in a central Iranian City. J. Addict. Med. 2012, 6, 292–296. [Google Scholar] [CrossRef]
- Davoodian, P.; Dadvand, H.; Mahoori, K.; Amoozandeh, A.; Salavati, A. Prevalence of selected sexually and blood-borne infections in Injecting drug abuser inmates of bandar abbas and roodan correction facilities, Iran, 2002. Braz. J. Infect. Dis. 2009, 13, 356–358. [Google Scholar] [CrossRef]
- Solomon, S.S.; Srikrishnan, A.K.; Mehta, S.H.; Vasudevan, C.K.B.; Murugavel, K.G.; Thamburaj, E.M.; Anand, S.B.; Kumar, M.S.M.; Latkin, C.; Solomon, S.; et al. High prevalence of HIV, HIV/hepatitis C virus coinfection, and risk behaviors among injection drug users in Chennai, India: A cause for concern. J. Acquir. Immune Defic. Syndr. 2008, 49, 327–332. [Google Scholar] [CrossRef]
- Malekinejad, M.; Navadeh, S.; Lotfizadeh, A.; Rahimi-Movaghar, A.; Amin-Esmaeili, M.; Noroozi, A. High hepatitis C virus prevalence among drug users in Iran: Systematic review and meta-analysis of epidemiological evidence (2001–2012). Int. J. Infect. Dis. 2015, 40, 116–130. [Google Scholar] [CrossRef]
- Rahman, M.; Hossain, M.E.; Afrad, M.H.; Hasan, R.; Rahman, M.; Sarker, S.; Azim, T. Hepatitis C virus infections among clients attending an HIV testing and counseling center in Dhaka, Bangladesh. J. Med. Virol. 2018, 90, 383–387. [Google Scholar] [CrossRef]
- Sharhani, A.; Mehrabi, Y.; Noroozi, A.; Nasirian, M.; Higgs, P.; Hajebi, A.; Hamzeh, B.; Khademi, N.; Noroozi, M.; Shakiba, E.; et al. Hepatitis C virus seroprevalence and associated risk factors among male drug injectors in Kermanshah, Iran. Hepat. Mon. 2017, 17, e58739. [Google Scholar] [CrossRef]
- Kermode, M.; Nuken, A.; Medhi, G.K.; Akoijam, B.S.; Sharma, H.U.; Mahanta, J. High burden of hepatitis C & HIV co-infection among people who inject drugs in Manipur, Northeast India. Indian J. Med. Res. 2016, 143, 348–356. [Google Scholar] [PubMed]
- Goswami, P.; Medhi, G.K.; Armstrong, G.; Setia, M.S.; Mathew, S.; Thongamba, G.; Ramakrishnan, L.; George, B.; Singh, R.K.; Paranjape, R.S.; et al. An assessment of an HIV prevention intervention among People Who Inject Drugs in the states of Manipur and Nagaland, India. Int. J. Drug Policy 2014, 25, 853–864. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ghosh, I.; Ghosh, P.; Bharti, A.C.; Mandal, R.; Biswas, J.; Basu, P. Prevalence of human papillomavirus and co-existent sexually transmitted infections among female sex workers, men having sex with men and injectable drug abusers from eastern India. Asian Pac. J. Cancer Prev. 2012, 13, 799–802. [Google Scholar] [CrossRef] [PubMed]
- Mahanta, J.; Borkakoty, B.; Das, H.K.; Chelleng, P.K. The risk of HIV and HCV infections among injection drug users in northeast India. AIDS Care 2009, 21, 1420–1424. [Google Scholar] [CrossRef] [PubMed]
- Chu, F.Y.; Chiang, S.C.; Su, F.H.; Chang, Y.Y.; Cheng, S.H. Prevalence of human immunodeficiency virus and its association with hepatitis B, C, and D virus infections among incarcerated male substance abusers in Taiwan. J. Med. Virol. 2009, 81, 973–978. [Google Scholar] [CrossRef]
- Jindal, N.; Arora, U.; Singh, K. Prevalence of human immunodeficiency virus (HIV), hepatitis B virus, and hepatitis C virus in three groups of populations at high risk of HIV infection in Amritsar (Punjab), Northern India. Jpn. J. Infect. Dis. 2008, 61, 79–81. [Google Scholar] [CrossRef]
- Liu, W.; Hai, T.; Li, X.Y. Seroepidemiological investigation of HBV and HCV among drug users in Yinin. Chin. Acad. J. Public Health 1997, 16, 363. (In Chinese) [Google Scholar]
- Zuo, H. A cross-sectional survey of HIV and HCV prevalence among drug users in detoxification centre of Urumchi railway station. J. Chin. AIDS/STD 2000, 6, 376. (In Chinese) [Google Scholar]
- Ma, X.M.; Amanguli, A.; Wang, X. Analysis of TTV, HBV and HCV infections in intravenous drug users in Xinjiang. J. Xinjiang Med. Univ. 2004, 27, 125–127. (In Chinese) [Google Scholar]
- Wang, L.; Yang, J.S. Study on infection with hepatitis B and C virus among incarcerated intravenous heroin users. Endem. Dis. Bull. 2004, 19, 68–69. [Google Scholar]
- Li, D.; Zheng, X.; Zhang, G. Prevalence of HIV and HCV among injecting drug users (IDUs) in Yunnan, China. Zhonghua Liu Xing Bing Xue Za Zhi 1994, 15, 74–75. [Google Scholar] [PubMed]
- Gu, X.H.; Gao, L.X.; Liu, F.H.; Pan, C.Z.; Li, C.Y.; Yang, Y.M. HCV infection and genotype of intravenous drug users. Chin. J. Infect. Dis. 1995, 13, 228–229. [Google Scholar]
- Kong, J.X.; Tong, L.; Tang, B.Z.; Pan, C.Z.; Zhang, Y.Z. Survey of HBV, HCV infection in drug users in Kunming. Med. Pharm. Yunnan 1995, 16, 45–46. [Google Scholar]
- Zhou, W.Z.; Wang, X.Y.; Wei, T. Investigation on the rates of HAV, HBV, HCV infection in heroin addicts. Chin. J. Drug Depend. 1995, 4, 25–26. [Google Scholar]
- Chen, G.L.; Wei, T.; Li, G.L. Coinfection with HCV and HIV among 37 injecting drug abusers. Chin. J. Drug Abus. Prev. Treat. 2000, 5, 28–30. [Google Scholar]
- Yang, Y.F.; Wang, J.Y.; Zhuang, H.; Ling, B.H.; Jin, L. Study on HBV, HCV and HGV infection in injecting drug users. Chin. J. Exp. Clin. Virol. 2000, 14, 23. [Google Scholar]
- Zhang, Z.X.; Zhu, L.F.; Cheng, L.X.; Sha, L.J. Epidemiological survey of heroin addicts infected by HCV and HBV. Chin. J. Drug Depend. 2000, 9, 144–146. [Google Scholar]
- Jiang, Z.L.; Guo, Y.H.; Li, Y.P.; Yang, H.; Yao, Z.P. The analysis of HCV and HIV infections in 176 heroin addicts. Chin. Mag. Drug Abuse Prev. Treat. 2003, 9, 23–25. [Google Scholar]
- Li, J.R.; Gong, S.Y.; Sun, H.; Guan, W.X.; Huang, H.J. Relationship between T cell subgroups and HCV infection in intravenous drug addicts. Chin. J. Infect. Dis. 2004, 22, 413–414. [Google Scholar]
- Liu, Y.; Zhuang, Y.; Ou, Y.Z. Survey of HCV prevalence among drug addicts in Guiyang. Chin. J. Public Health 1997, 16, 156. [Google Scholar]
- Yang, J.; Ding, J.J.; Li, Y.Y. Co-infection with HCV and HGV among 107 injection drug users. Chin. J. Prev. Med. 2000, 34, 85. [Google Scholar]
- Zhuang, Y.; Hu, L.J.; Cai, X.H. A cross-sectional survey of HCV prevalence among injection drug users in a detoxification centre in Guiyang. Guizhou Med. J. 2000, 24, 573. [Google Scholar]
- He, J.; Li, X.X.; Long, Z.Y.; Yang, Y.X.; Huang, J.M.; Zhang, S. Analysis of HCV infection in 183 heroin addicts. Chin. J. Drug Abus. Prev. Treat. 2006, 12, 224–225. [Google Scholar]
- Fang, Y.F.; Chen, S.; Wang, Y.M.; Li, C.M. Survey of HCV infection in intravenous drug abusers in Chongqing. Chin. J. Drug Depend. 2002, 10, 220–222. [Google Scholar]
- Mou, Q.; Li, X.Y. Analysis of infection with hepatitis C virus in 767 heroin addicts. Chin. J. Drug Abus. Prev. Treat. 2001, 6, 17. [Google Scholar]
- Yang, T.L.; Xu, Y.C.; Hu, X.H. Seroepidemiological survey of HIV, HBV and HCV infection among drug users in Xichang. J. Prev. Med. Inform. 2001, 17, 170–171. [Google Scholar]
- Ruan, Y.H.; Hong, K.X.; Liu, S.Z.; He, Y.X.; Zhou, F.; Qin, G.M. Community-based survey of HCV and HIV coinfection in injection drug abusers in Sichuan Province of China. World J. Gastroenterol. 2004, 10, 1589–1593. [Google Scholar] [CrossRef]
- Zhang, C.T.; Wei, D.Y.; Li, X.H. Investigation on infection status of HIV and HCV in drug users in Liangshan Area. Chin. Public Health 2005, 21, 1287–1288. [Google Scholar]
- Xie, L.Z.; Chen, X.; Hu, W.; Bian, H.Z.; Liu, L.; Xing, Z.H. Investigation into the relationship between the high risk behavior of drug abusing population and HCV infection. Chin. Trop. Med. 2006, 6, 1140–1142. [Google Scholar]
- Tang, X.X.; Yuan, D.F.; Li, L.H. Report of anti-HCV test result and analysis of 200 heroin addicts. J. Branch Campus First Milit. Med. 1994, 17, 75–78. [Google Scholar]
- Shi, X.C.; Liu, J.B.; Cao, Z.W.; Tan, L.X. Seroepidemiological survey of HCV infection among different populations in Guangdong. Chin. Public Health 1998, 14, 8–9. [Google Scholar]
- Wan, P.; Qiu, S.F.; Li, W.; Zeng, D.X. Infections with hepatitis B and C virus in 325 drug addicts. Chin. J. Exp. Clin. Virol. 1997, 11, 90. [Google Scholar]
- Yan, J.; Zeng, C.H.; Lin, P.; Li, H.; Xi, H.F. Relationship between methods of drug use and infections with HIV, HCV and syphilis among drug abusers in Guangdong. Chin. J. Prev. Control STD AIDS 1997, 3, 254–255. [Google Scholar]
- Mai, H.M.; Zhang, Y.P.; Song, A.H. Infections with HBV and HCV in 219 heroin addicts. Guangdong J. Health Epidemiol. Prev. 1999, 25, 29–30. [Google Scholar]
- Wei, L.P.; Zhou, M.; Zhou, D.R.; Zhong, H.B. Relationship between methods of drug use and infections with HIV, HBV, HDV and HGV among heroin abusers. Chin. Public Health 1999, 15, 413–414. [Google Scholar]
- Yang, Y.; Zhang, G.Q.; Chen, S.D.; Wu, B.W. A study on the risk factors for hepatitis C virus infection among drug users. Chin. Public Health 1999, 15, 495–496. [Google Scholar]
- Hu, C.Z.; Ge, B.L.; Li, G. Prevalence of HBV, HCV and HGV infection in 120 intravenous drug abusers in Jiangmen City. J. Clin. Hepatol. 2000, 5, 21–23. [Google Scholar]
- Gu, Y.C.; Wu, B.Y.; Li, G.J.; Luo, X.M.; Gao, S.Z. Drug-using behavior and infections with HIV, HBV, HCV and syphilis among 317 drug users. South China J. Prev. Med. 2002, 28, 26. [Google Scholar]
- Huang, H.Y.; Zhang, P.; Li, L. Analysis of co-infection of HIV with hepatitis virus among intravenous drug users. Chin. Public Health 2003, 19, 191–192. [Google Scholar]
- Chen, Y.; Lian, W.; Chen, J.L.; Cao, X.J. An analysis of HCV infection and ALT alteration in intravenous drug addicts in Zhanjiang City. Prac. Prev. Med. 2004, 11, 483–484. [Google Scholar]
- Li, W.J.; Fan, Z.F.; Lin, P.; Wang, Y.; Yan, J.; Mai, R.J. A survey of HIV and HCV infections and related knowledge and behavior among drug abusers in Yangjiang City. South China J. Prev. Med. 2004, 30, 17–19. [Google Scholar]
- Wang, L.R.; Chen, J.L.; Chen, Y.; Li, S.H. Prevalence of HBV and HCV infections and ALT elevation in drug users. Chin. J. Misdiagnost. 2004, 4, 675–677. [Google Scholar]
- Tang, W.H.; Liang, X.X.; Lin, C.L.; Gu, Z.L. Analysis of abnormal infection in 210 heroin dependents. Milit. Med. J. South China 1996, 10, 363–364. [Google Scholar]
- Bi, F.H. Serological survey of HCV of 77 drug addicts. Guangxi J. Prev. Med. 1998, 4, 91. [Google Scholar]
- Lin, H. The analysis of HCV infection in 111 heroin addicts. Chin. J. Drug Abus. Prev. Treat. 1999, 4, 26–28. [Google Scholar]
- Wu, C.Y.; Huang, Q.C.; Hou, D.Y. A study on infection of HCV among drug users, persons with sex-related crimes and errors and general population. Mod. Prev. Med. 1999, 26, 82–83. [Google Scholar]
- Liang, Y.J.; Chen, Y.H.; Wei, Q.Y.; Lu, Y. A study on infection of hepatitis virus among intravenous drug users in Liuzhou City. J. Guangxi Med. Univ. 2000, 17, 342–343. [Google Scholar]
- Garten, R.J.; Lai, S.; Zhang, J.; Liu, W.; Chen, J.; Vlahov, D. Rapid transmission of hepatitis C virus among young injecting heroin users in Southern China. Int. J. Epidemiol. 2004, 33, 182–188. [Google Scholar]
- Wang, M.; Du, J.W.; Huang, H.Z.; Chen, Y.B. Analysis on investigation between methods of drug use and infection of HIV, HCV, HBV and syphilis of drug addicts of Hainan Province. Chin. Public Health 2000, 16, 854–855. [Google Scholar]
- Yang, Q.X. Causal analysis of intravenous drug use of 97 heroin dependents. Hainan Med. J. 2000, 11, 76. [Google Scholar]
- Tang, W.F.; Tang, L.; Chen, Z.D.; He, H.; Yang, J.H. Seroepidemiological survey of HIV and HCV among 597 drug users. Hubei J. Prev. Med. 2000, 11, 19. [Google Scholar]
- Deng, L.P.; Gui, X.E.; Wang, X.; Luo, J.L. A survey of HIV, HBV, HCV, HGV and TTV infections among drug abusers in Hubei Province. Hubei J. Prev. Med. 2003, 14, 1–2. [Google Scholar]
- Zhao, M.; Wu, D.R.; Yang, D.S.; Hao, W.; He, Y.; Li, P. HBV and HCV infections in heroin addicts. Chin. Ment. Health J. 1999, 13, 98–100. [Google Scholar]
- Peng, D.S.; Fang, K.M.; Wang, H.H.; Wu, R.M.; Zhang, D.Q.; Li, S.X. Epidemiological survey of four blood transmitting infections among injection drug takers in Yueyang City. Prac. Prev. Med. 2006, 13, 336–337. [Google Scholar]
- He, B.; Huang, P.; Jiang, X.Y.; Yang, C.Y. HCV infection in 9 heroin addicts. Chin. J. Drug Abus. Prev. Treat. 2004, 10, 222–223. [Google Scholar]
- Hu, W.H.; Zhao, M.; Lu, G.H.; Xu, L.Y. A study on hepatitis B and C virus infection and liver function abnormalities in heroin addicts. Chin. Mag. Drug Abus. Prev. Treat. 2003, 9, 7–9. [Google Scholar]
- Zhao, M.; Wang, Q.Y.; Lu, G.H.; Xu, P.; Xu, H.; McCoy, C.B. Risk behaviors and HIV/AIDS prevention education among IDUs in drug treatment in Shanghai. J. Urban Health 2005, 82, v84–v91. [Google Scholar] [CrossRef]
- Zhao, M.; Du, J.; Lu, G.H.; Wang, Q.Y.; Xu, H.; Zhu, M.; McCoy, C.B. HIV sexual risk behaviors among injection drug users in Shanghai. Drug Alcohol Depend. 2006, 82 (Suppl. S1), S43–S47. [Google Scholar] [CrossRef]
- Yin, J. Infection with HBV and HCV among intravenous drug addicts. Acta Nanjing Med. Univ. 1999, 19, 166. [Google Scholar]
- Gao, Q.M. Infections with HBV and HCV in 120 heroin addicts. Jiangsu Prev. Med. 2001, 12, 16–17. [Google Scholar]
- Ji, X.S.; Lu, W.M.; He, J.H.; Ding, M.J. Survey of HIV, HBV and HCV infection among STD patients and drug users. J. Prev. Med. Inform. 2001, 17, 59. [Google Scholar]
- Zhu, B.; Wu, N.P.; Wu, L.J.; Fang, J. The serological study on HIV, HBV and HCV in drug addicts of Zhejiang, China. Chin. Public Health 1999, 15, 415–416. [Google Scholar]
- Chen, Z.J.; Zhang, L.H.; Xin, L.J.; Jiang, T.Y. The study of HCV and HIV infections among heroin addicts. Chin. J. Lab. Med. 2003, 26, 270–272. [Google Scholar]
- Zhou, Z.M.; Lin, D.; Pan, F.F.; Tan, Y.X.; Wen, H.J.; Liao, X.W. Infection of diseases transmitted through blood of venous drug addicts. Dis. Surveill. 2004, 19, 412–414. [Google Scholar]
- Li, Z.H.; Tang, Y.X.; Wang, Y.; Wei, X.H. The analysis of HCV infection in 327 heroin addicts. Chin. J. Drug Abus. Prev. Treat. 2005, 11, 153–154. [Google Scholar]
- Wang, N.C.; Qiao, X.C.; Zhang, L.F.; Liu, Y.P.; Wu, L.P. Seroepidemiology of HCV infection among different populations in Shanxi. Chin. Public Health 2001, 17, 703. [Google Scholar]
- Zhang, X.L.; Xie, Z.L.; Mei, L. Analysis on HBV and HCV infection among drug users in Taiyuan. Dis. Surveill. 2002, 17, 211. [Google Scholar]
- Wang, Y.F. Analysis of HAV, HBV and HCV infection in 386 heroin addicts. Chin. J. Drug Depend. 2002, 11, 62–63. [Google Scholar]
- Guo, R.Q.; Chang, Y.L.; Kong, Y.; Xu, B.S. The analysis of HCV infection among 1000 heroin addicts. Chin. J. Drug Depend. 2003, 12, 132–134. [Google Scholar]
- Zhang, M.Y.; Wu, Z.Y.; Ming, Z.Q.; Gu, M.; Wu, J.L.; Mi, G.D. Study of hepatitis C virus infection rate and its risk factors among injecting drug users in Beijing, China. Chin. J. Dis. Control Prev. 2006, 10, 139–141. [Google Scholar]
- Jiang, Y.J.; Shang, H.; Wang, Y.N.; Zhao, M.; Cao, J.J.; Lu, C.M. Investigation of HIV, syphilis and hepatitis virus among high risk populations in Shenyang. J. Chin. AIDS/STD 2002, 8, 42–44. [Google Scholar]
- Yuen, M.F.; Wong, D.K.H.; Lee, C.K.; Tanaka, Y.; Allain, J.P.; Fung, J.; Leung, J.; Lin, C.K.; Sugiyama, M.; Sugauchi, F.; et al. Transmissibility of hepatitis B virus (HBV) infection through blood transfusion from blood donors with occult HBV infection. Clin. Infect. Dis. 2011, 52, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Calvaruso, V.; Ferraro, D.; Licata, A.; Bavetta, M.G.; Petta, S.; Bronte, F.; Colomba, G.; Craxì, A.; Di Marco, V. HBV reactivation in patients with HCV/HBV cirrhosis on treatment with direct-acting antivirals. J. Viral Hepat. 2018, 25, 72–79. [Google Scholar] [CrossRef]
- Bhattarai, M.; Baniya, J.B.; Aryal, N.; Shrestha, B.; Rauniyar, R.; Adhikari, A.; Koirala, P.; Oli, P.K.; Pandit, R.D.; Stein, D.A.; et al. Epidemiological profile and risk factors for acquiring HBV and/or HCV in HIV-infected population groups in Nepal. BioMed Res. Int. 2018, 2018, 9241679. [Google Scholar] [CrossRef] [PubMed]
- Cotler, S.J.; Cotler, S.; Xie, H.; Luc, B.J.; Layden, T.J.; Wong, S.S. Characterizing hepatitis B stigma in Chinese immigrants. J. Viral Hepat. 2012, 19, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Tang, T.; Patterson, M.; Ho, M.; Heathcote, J.; Shah, H. The impact of hepatitis B knowledge and stigma on screening in Canadian Chinese persons. Can. J. Gastroenterol. 2012, 26, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Huong, N.T.C.; Trung, N.Q.; Luong, B.A.; Tram, D.B.; Vu, H.A.; Bui, H.H.; Le, H.P.T. Mutations in the HBV PreS/S gene related to hepatocellular carcinoma in Vietnamese chronic HBV-infected patients. PLoS ONE 2022, 17, e0266134. [Google Scholar] [CrossRef]
- Ghosh, S.; Banerjee, P.; Deny, P.; Mondal, R.K.; Nandi, M.; RoyChoudhury, A.; Das, K.; Banerjee, S.; Santra, A.; Zoulim, F.; et al. New HBV subgenotype D9, a novel D/C recombinant, identified in patients with chronic HBeAg-negative infection in Eastern India. J. Viral Hepat. 2013, 20, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Ismail, H.; Ahmad, H.; Sanef, A.; Shahabudin, W.; Reffin, N.; Chan, D.; Dawam, D.; Hanan, F.; Nordin, M.; Sahar, L.; et al. The rising threat of illicit amphetamine-type stimulant use among methadone maintenance treatment patients in East Coast Malaysia: A retrospective observational study. Am. J. Drug Alcohol. Abuse 2023, 49, 97–108. [Google Scholar] [CrossRef]
- Rumi, M. Prevalence of infectious diseases and drug abuse among Bangladeshi workers. Southeast Asian J. Trop. Med. Public Health 2000, 31, 571–574. [Google Scholar]
- Yeekian, C.; Geratikornsupak, N.; Chumpongthong, P.; Tongsiri, S.; Dhitavat, J.; Phonrat, B.; Pitisuttithum, P. Medical and economic burden of chronic hepatitis B patients at Queen Savang Vadhana Memorial Hospital. J. Med. Assoc. Thai 2014, 97, 447–455. Available online: https://www.scopus.com/inward/record.uri?eid=2-s2.0-84902285661&partnerID=40&md5=5805f369885db943cc3d763350070eea (accessed on 29 January 2025). [PubMed]
- Sangmala, P.; Chaikledkaew, U.; Tanwandee, T.; Pongchareonsuk, P. Economic evaluation and budget impact analysis of the surveillance program for hepatocellular carcinoma in Thai chronic hepatitis B patients. Asian Pac. J. Cancer Prev. 2014, 15, 8993–9004. [Google Scholar] [CrossRef] [PubMed]
- McDonald, S.A.; Azzeri, A.; Shabaruddin, F.H.; Dahlui, M.; Tan, S.S.; Kamarulzaman, A.; Mohamed, R. Projections of the healthcare costs and disease burden due to hepatitis C infection under different treatment policies in Malaysia, 2018–2040. Appl. Health Econ. Health Policy 2018, 16, 847–857. [Google Scholar] [CrossRef]
- Tessema, B.; Yismaw, G.; Kassu, A.; Amsalu, A.; Mulu, A.; Emmrich, F.; Sack, U. Seroprevalence of HIV, HBV, HCV and syphilis infections among blood donors at Gondar University Teaching Hospital, Northwest Ethiopia: Declining trends over a period of five years. BMC Infect. Dis. 2010, 10, 111. [Google Scholar] [CrossRef]
- Aluora, P.O.; Muturi, M.W.; Gachara, G. Seroprevalence and genotypic characterization of HBV among low risk voluntary blood donors in Nairobi, Kenya. Virol. J. 2020, 17, 176. [Google Scholar] [CrossRef] [PubMed]
- Pondé, R.A.A. Molecular mechanisms underlying HBsAg negativity in occult HBV infection. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 1709–1731. [Google Scholar] [CrossRef] [PubMed]
- Caviglia, G.P.; Abate, M.L.; Tandoi, F.; Ciancio, A.; Amoroso, A.; Salizzoni, M.; Saracco, G.M.; Rizzetto, M.; Romagnoli, R.; Smedile, A. Quantitation of HBV cccDNA in anti-HBc-positive liver donors by droplet digital PCR: A new tool to detect occult infection. J. Hepatol. 2018, 69, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Lang-Meli, J.; Neumann-Haefelin, C.; Thimme, R. Immunotherapy and therapeutic vaccines for chronic HBV infection. Curr. Opin. Virol. 2021, 51, 149–157. [Google Scholar] [CrossRef]
- Qin, Y.; Liao, P. Hepatitis B virus vaccine breakthrough infection: Surveillance of S gene mutants of HBV. Acta Virol. 2018, 62, 115–121. [Google Scholar] [CrossRef]
- Lai, M.W.; Lin, T.Y.; Tsao, K.C.; Huang, C.; Hsiao, M.; Liang, K.; Yeh, C. Increased seroprevalence of HBV DNA with mutations in the s gene among individuals greater than 18 years old after complete vaccination. Gastroenterology 2012, 143, 400–407. [Google Scholar] [CrossRef]
- Mastrodomenico, M.; Muselli, M.; Provvidenti, L.; Scatigna, M.; Bianchi, S.; Fabiani, L. Long-term immune protection against HBV: Associated factors and determinants. Hum. Vaccines Immunother. 2021, 17, 2268–2272. [Google Scholar] [CrossRef] [PubMed]







| Author’s Name | Year of Publication | Country | Total HBV Sampled | HBV Positive | Total HCV Sampled | HCV Positive | Study Types | Method of Detection |
|---|---|---|---|---|---|---|---|---|
| Verachai et al. [31] | 2002 | Thailand | NR | NR | 303 | 124 | Cross sectional | Elisa/PCR |
| Akthar et al. [22] | 2015 | Malaysia | 664 | 86 | NR | NR | Retrospective | ELISA |
| Zhang et al. [8] | 2015 | Vietnam | NR | NR | 1434 | 1273 | Cross sectional | ELISA |
| Ruslin et al. [32] | 2001 | Malaysia | 130 | 68 | NR | NR | Retrospective | ELISA |
| Duogh et al. [9] | 2018 | Vietnam | NR | NR | 603 | 404 | Retrospective | ELISA |
| Ha Thi Tu [33] | 2009 | Vietnam | NR | NR | 455 | 350 | Retrospective | ELISA |
| Vu minh et al. [10] | 2009 | Vietnam | 309 | 250 | 309 | 229 | Retrospective | ELISA |
| Linh et al. [34] | 2019 | Vietnam | NR | NR | 1886 | 242 | Cross sectional | ELISA |
| Nicholas et al. [35] | 2023 | Vietnam | NR | NR | 1444 | 875 | Cross sectional | PCR |
| Barnaby et al. [36] | 2022 | Vietnam | 7740 | 1321 | 5461 | 3157 | Retrospective | ELISA |
| Ishizak et al. [37] | 2017 | Vietnam | 760 | 81 | NR | NR | Retrospective | ELISA |
| Ishizak et al. (a) [37] | 2017 | Vietnam | 302 | 34 | NR | NR | Retrospective | ELISA |
| Ishizak et al. (b) [37] | 2017 | Vietnam | 389 | 43 | NR | NR | Retrospective | ELISA |
| Desjarlais et al. [38] | 2016 | Vietnam | 603 | 404 | NR | NR | Retrospective | ELISA |
| Prasetyo et al. [39] | 2013 | Indonesia | 375 | 128 | 375 | 12 | Retrospective | ELISA |
| Dunford [40] | 2012 | Vietnam | NR | NR | 1000 | 556 | Retrospective | ELISA |
| Dunford et al. [41] | 2012 | Vietnam | 1000 | 174 | NR | NR | Retrospective | ELISA |
| Telan et al. [42] | 2011 | Philippines | NR | NR | 62 | 62 | Retrospective | ELISA |
| Mcfall et al. [43] | 2017 | India | NR | NR | 6457 | 1901 | Cross sectional | ELISA |
| Gupta et al. [44] | 2017 | India | NR | NR | 194 | 89 | Cross sectional | PCR |
| Solomon et al. [45] | 2016 | India | NR | NR | 1042 | 293 | Cross sectional | PCR |
| Hsieh et al. [46] | 2016 | Taiwan | 566 | 87 | NR | NR | Retrospective | Elisa/PCR |
| Lie et al. [47] | 2014 | China | NR | NR | 370 | 154 | Retrospective | ELISA |
| Amitis et al. [48] | 2014 | Iran | 100 | 9 | 100 | 56 | Cross sectional | ELISA |
| Abbasali et al. [49] | 2014 | Iran | 539 | 65 | 39 | 71 | Cross sectional | Elisa/PCR |
| Hsieh et al. [50] | 2014 | Taiwan | 562 | 86 | 562 | 513 | Cross sectional | Elisa/PCR |
| Chalana et al. [51] | 2013 | India | 118 | 6 | 118 | 78 | Cross sectional | ELISA |
| Basu et al. [52] | 2012 | India | NR | NR | 103 | 47 | Cross sectional | ELISA |
| Min et al. [53] | 2013 | Korea | 318 | 21 | 318 | 154 | Retrospective | ELISA |
| Alipour et al. [54] | 2013 | Iran | NR | NR | 268 | 102 | Cross sectional | ELISA |
| Yen et al. [55] | 2012 | Taiwan | NR | NR | 1447 | 1318 | Retrospective | ELISA |
| Sofian [56] | 2012 | Iran | 153 | 46 | 153 | 91 | Retrospective | ELISA |
| Davoodian et al. [57] | 2009 | Iran | 249 | 12 | 249 | 196 | Cross sectional | ELISA |
| Solomon et al. [58] | 2008 | India | 912 | 101 | 912 | 566 | Cross sectional | ELISA |
| Mohesen et al. [59] | 2015 | Iran | NR | NR | 7568 | 3406 | Cross sectional | ELISA |
| Rahman et al. [60] | 2018 | Bangladesh | NR | NR | 90 | 34 | Retrospective | ELISA |
| Sharhani et al. [61] | 2017 | Iran | NR | NR | 606 | 332 | Cross sectional | ELISA |
| Kermode et al. [62] | 2016 | India | NR | NR | 821 | 307 | Cross sectional | ELISA |
| Ramenzani et al. [48] | 2014 | iran | 100 | 6 | 100 | 6 | Cross sectional | ELISA |
| Goswami et al. [63] | 2014 | India | 839 | 51 | 839 | 56 | Retrospective | ELISA |
| Goswami et al. (a) [63] | 2014 | India | 860 | 92 | 860 | 601 | Retrospective | ELISA |
| Goswami et al. (b) [63] | 2014 | India | 821 | 46 | 821 | 121 | Retrospective | ELISA |
| Goswami et al. (c) [63] | 2014 | India | 829 | 67 | 829 | 125 | Retrospective | ELISA |
| Ghosh et al. [64] | 2012 | India | 58 | 2 | NR | NR | Retrospective | ELISA |
| Mahanta et al. [65] | 2009 | India | 397 | 15 | 398 | 190 | Retrospective | ELISA |
| Chu et al. [66] | 2009 | Taiwan | 192 | 32 | 192 | 172 | Retrospective | ELISA |
| Jindal et al. [67] | 2008 | India | 157 | 28 | 157 | 53 | Retrospective | ELISA |
| Liu et al. [68] | 1997 | China | NR | NR | 85 | 78 | Retrospective | ELISA |
| Zuo et al. [69] | 2000 | China | NR | NR | 60 | 57 | Retrospective | ELISA |
| Ma et al. [70] | 2004 | China | NR | NR | 102 | 70 | Retrospective | ELISA |
| Wang et al. [71] | 2006 | China | NR | NR | 106 | 34 | Retrospective | ELISA |
| Li et al. [72] | 1994 | China | NR | NR | 197 | 187 | Retrospective | ELISA |
| Gu et al. [73] | 1995 | China | NR | NR | 407 | 247 | Retrospective | ELISA |
| Kong et al. [74] | 1995 | China | NR | NR | 144 | 99 | Retrospective | ELISA |
| Zhou et al. [75] | 1995 | China | NR | NR | 58 | 41 | Retrospective | ELISA |
| Chen et al. [76] | 2000 | China | NR | NR | 37 | 27 | Retrospective | ELISA |
| Yang et al. [77] | 2000 | China | NR | NR | 182 | 172 | Retrospective | ELISA |
| Zhang et al. [78] | 2000 | China | NR | NR | 125 | 96 | Retrospective | ELISA |
| Jiang et al. [79] | 2003 | China | NR | NR | 118 | 94 | Retrospective | ELISA |
| Li et al. [80] | 2004 | China | NR | NR | 381 | 261 | Retrospective | ELISA |
| Liu et al. [81] | 1997 | China | NR | NR | 60 | 16 | Retrospective | ELISA |
| Yang et al. [82] | 2000 | China | NR | NR | 107 | 54 | Retrospective | ELISA |
| Zhuang et al. [83] | 2000 | China | NR | NR | 285 | 14 | Retrospective | ELISA |
| He et al. [84] | 2006 | China | NR | NR | 90 | 57 | Retrospective | ELISA |
| Fang et al. [85] | 2001 | China | NR | NR | 284 | 115 | Retrospective | ELISA |
| Mou et al. [86] | 2008 | China | NR | NR | 498 | 133 | Retrospective | ELISA |
| Yang et al. [87] | 2001 | China | NR | NR | 177 | 62 | Retrospective | ELISA |
| Ruan et al. [88] | 2004 | China | NR | NR | 379 | 269 | Retrospective | ELISA |
| Zhang et al. [89] | 2005 | China | NR | NR | 123 | 95 | Retrospective | ELISA |
| Xie et al. [90] | 2006 | China | NR | NR | 370 | 276 | Retrospective | ELISA |
| Tang et al. [91] | 1994 | China | NR | NR | 53 | 25 | Retrospective | ELISA |
| Shi et al. [92] | 1994 | China | NR | NR | 121 | 79 | Retrospective | ELISA |
| Wan et al. [93] | 1997 | China | NR | NR | 119 | 27 | Retrospective | ELISA |
| Yan et al. [94] | 1997 | China | NR | NR | 543 | 398 | Retrospective | ELISA |
| Mai et al. [95] | 1999 | China | NR | NR | 142 | 112 | Retrospective | ELISA |
| Wei et al. [96] | 1999 | China | NR | NR | 86 | 58 | Retrospective | ELISA |
| Yang et al. [97] | 1999 | China | NR | NR | 419 | 320 | Retrospective | ELISA |
| Hu et al. [98] | 2000 | China | NR | NR | 120 | 89 | Retrospective | ELISA |
| Gu et al. [99] | 2002 | China | NR | NR | 205 | 177 | Retrospective | ELISA |
| Huang et al. [100] | 2003 | China | NR | NR | 2640 | 1409 | Retrospective | ELISA |
| Chen et al. [101] | 2004 | China | NR | NR | 716 | 289 | Retrospective | ELISA |
| Li et al. [102] | 2004 | China | NR | NR | 235 | 232 | Retrospective | ELISA |
| Wang et al. [103] | 2002 | China | NR | NR | 508 | 294 | Retrospective | ELISA |
| Tang et al. [104] | 1996 | China | NR | NR | 91 | 66 | Retrospective | ELISA |
| Bi et al. [105] | 1998 | China | NR | NR | 77 | 69 | Retrospective | ELISA |
| Lin et al. [106] | 1999 | China | NR | NR | 58 | 39 | Retrospective | ELISA |
| Wu et al. [107] | 1999 | China | NR | NR | 113 | 104 | Retrospective | ELISA |
| Liang et al. [108] | 2000 | China | NR | NR | 208 | 186 | Retrospective | ELISA |
| Garten et al. [109] | 2004 | China | NR | NR | 485 | 402 | Retrospective | ELISA |
| Wang et al. [110] | 2000 | China | NR | NR | 174 | 63 | Retrospective | ELISA |
| Yang et al. [111] | 2000 | China | NR | NR | 97 | 56 | Retrospective | ELISA |
| Tang et al. [112] | 2000 | China | NR | NR | 480 | 444 | Retrospective | ELISA |
| Deng et al. [113] | 2003 | China | NR | NR | 62 | 48 | Retrospective | ELISA |
| Zhao et al. [114] | 1999 | China | NR | NR | 102 | 34 | Retrospective | ELISA |
| Peng et al. [115] | 2006 | China | NR | NR | 252 | 222 | Retrospective | ELISA |
| He et al. [116] | 2004 | China | NR | NR | 87 | 26 | Retrospective | ELISA |
| Hu et al. [117] | 2003 | China | NR | NR | 276 | 96 | Retrospective | ELISA |
| Zhaou et al. [118] | 2005 | China | NR | NR | 101 | 47 | Retrospective | ELISA |
| Zhaou et al. (a) [119] | 2006 | China | NR | NR | 141 | 73 | Retrospective | ELISA |
| Yin et al. [120] | 1999 | China | NR | NR | 68 | 45 | Retrospective | ELISA |
| Gao et al. [121] | 2001 | China | NR | NR | 68 | 48 | Retrospective | ELISA |
| Ji et al. [122] | 2001 | China | NR | NR | 40 | 15 | Retrospective | ELISA |
| Zhu et al. [123] | 1999 | China | NR | NR | 64 | 14 | Retrospective | ELISA |
| Chen et al. [124] | 2003 | China | NR | NR | 265 | 79 | Retrospective | ELISA |
| Zhou et al. [125] | 2004 | China | NR | NR | 306 | 130 | Retrospective | ELISA |
| Li et al. [126] | 2005 | China | NR | NR | 114 | 71 | Retrospective | ELISA |
| Wang et al. [127] | 2001 | China | NR | NR | 32 | 5 | Retrospective | ELISA |
| Zhang et al. [128] | 2002 | China | NR | NR | 51 | 14 | Retrospective | ELISA |
| Wang et al. [129] | 2002 | China | NR | NR | 307 | 149 | Retrospective | ELISA |
| Guo et al. [130] | 2003 | China | NR | NR | 525 | 351 | Retrospective | ELISA |
| Zhang et al. [131] | 2006 | China | NR | NR | 232 | 122 | Retrospective | ELISA |
| Jiang et al. [132] | 2002 | China | NR | NR | 71 | 50 | Retrospective | ELISA |
| Parameter | No of Studies | Prevalence (%) | Confidence Interval (%) | Q | I2 | Heterogeneity DF | p |
|---|---|---|---|---|---|---|---|
| Country | |||||||
| Malaysia | 2 | 28.7 | 5.4–74.0 | 90.295 | 98.89 | 1 | <0.001 |
| Vietnam | 7 | 26.6 | 13.1–46.4 | 1070.995 | 99.44 | 6 | <0.001 |
| Indonesia | 1 | 26.6 | 33.0–43.3 | – | – | – | – |
| Taiwan | 3 | 15.5 | 13.7–17.6 | 0.222 | 0 | 2 | 0.895 |
| Iran | 5 | 10.5 | 5.2–20.2 | 54.561 | 92.67 | 4 | <0.001 |
| Korea | 1 | 6.6 | 4.3–9.9 | – | – | – | – |
| India | 9 | 7.7 | 5.8–10.2 | 58.719 | 86.38 | 8 | <0.001 |
| Study design | |||||||
| Retrospective | 21 | 16.7 | 11.5–23.6 | 1613.737 | 98.76 | 20 | <0.001 |
| Cross-sectional | 7 | 9.5 | 7.1–12.5 | 26.312 | 77.2 | 6 | <0.001 |
| Method of detection | |||||||
| ELISA | 25 | 14.3 | 14.3–100.0 | 1695.51 | 98.58 | 24 | <0.001 |
| ELISA/PCR | 3 | 14.3 | 12.3–16.6 | 3.188 | 37.26 | 2 | 0.203 |
| Year of publication | |||||||
| 2011–2015 | 15 | 10.9 | 7.8–15.1 | 329.84 | 95.76 | 14 | <0.001 |
| 2001–2005 | 1 | 52.3 | 43.7–60.7 | – | – | – | – |
| 2006–2010 | 6 | 16 | 4.3–44.5 | 504.76 | 99.01 | 5 | <0.001 |
| 2021–2024 | 1 | 17.1 | 16.2–17.9 | – | – | – | – |
| 2016–2020 | 5 | 19.4 | 6.2–46.9 | 582.795 | 99.31 | 4 | <0.001 |
| Parameter | No of Studies | Prevalence (%) | Confidence Interval (%) | Q | I2 | Heterogeneity DF | p |
|---|---|---|---|---|---|---|---|
| Country | |||||||
| Vietnam | 7 | 63.5 | 44.3–79.2 | 1606.126 | 99.63 | 6 | <0.001 |
| Thailand | 1 | 40.9 | 35.5–46.6 | - | - | - | - |
| Indonesia | 1 | 3.2 | 1.8–5.5 | - | - | - | - |
| Philippines | 1 | 99.2 | 88.5–100.0 | - | - | - | - |
| India | 13 | 35.8 | 26.1–46.9 | 1321.086 | 99.09 | 12 | <0.001 |
| Iran | 8 | 49.3 | 39.8–58.8 | 172.606 | 95.94 | 7 | <0.001 |
| China | 66 | 62.9 | 58.0–67.7 | 2065.8 | 96.85 | 65 | <0.001 |
| Iran | 8 | 49.3 | 39.8–58.8 | 172.606 | 95.94 | 7 | <0.001 |
| Taiwan | 3 | 91 | 89.7–92.1 | 0.536 | 0 | 2 | 0.765 |
| Korea | 1 | 48.4 | 43.0–53.9 | - | - | - | - |
| Bangladesh | 1 | 37.8 | 28.4–48.2 | - | - | - | - |
| Study design | |||||||
| Retrospective | 83 | 60.4 | 55.5–65.1 | 4343.258 | 98.11 | 82 | <0.001 |
| Cross-sectional | 19 | 50 | 40.6–59.4 | 2751.01 | 99.35 | 18 | <0.001 |
| Method of detection | |||||||
| ELISA/PCR | 3 | 67.4 | 24.8–92.9 | 208.093 | 99.04 | 2 | <0.001 |
| ELISA | 96 | 58.6 | 54.0–63.0 | 7462.608 | 98.73 | 95 | <0.001 |
| PRC | 3 | 44.4 | 23.1–68.0 | 245.267 | 99.18 | 2 | <0.001 |
| Year of publication | |||||||
| 2001–2010 | 41 | 57.1 | 49.7–64.5 | 4230.883 | 99.05 | 40 | <0.001 |
| 2011–2020 | 28 | 47.6 | 35.3–59.9 | 19,174.396 | 99.86 | 27 | <0.001 |
| >2020 | 2 | 59 | 56.3–61.7 | 3.695 | 72.94 | 1 | 0.055 |
| <2001 | 31 | 65.6 | 54.1–77.2 | 4239.471 | 99.29 | 30 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rabaan, A.A.; Bello, K.E.; Radwan, Z.; Hassouneh, A.K.; Alrasheed, H.A.; Alotaibi, J.; Basrana, B.; Zaidan, A.A.; Garout, M.A.; Zaidan, T.I.; et al. The Dual Burden of Hepatitis B and C Among Drug Users in Asia: The First Systematic Review and Meta-Analysis. Pathogens 2025, 14, 360. https://doi.org/10.3390/pathogens14040360
Rabaan AA, Bello KE, Radwan Z, Hassouneh AK, Alrasheed HA, Alotaibi J, Basrana B, Zaidan AA, Garout MA, Zaidan TI, et al. The Dual Burden of Hepatitis B and C Among Drug Users in Asia: The First Systematic Review and Meta-Analysis. Pathogens. 2025; 14(4):360. https://doi.org/10.3390/pathogens14040360
Chicago/Turabian StyleRabaan, Ali A., Kizito E. Bello, Zaheda Radwan, Amal K. Hassouneh, Hayam A. Alrasheed, Jawaher Alotaibi, Bashayer Basrana, Ali A. Zaidan, Mohammed A. Garout, Tasneem I. Zaidan, and et al. 2025. "The Dual Burden of Hepatitis B and C Among Drug Users in Asia: The First Systematic Review and Meta-Analysis" Pathogens 14, no. 4: 360. https://doi.org/10.3390/pathogens14040360
APA StyleRabaan, A. A., Bello, K. E., Radwan, Z., Hassouneh, A. K., Alrasheed, H. A., Alotaibi, J., Basrana, B., Zaidan, A. A., Garout, M. A., Zaidan, T. I., Al Amri, K. A., Alshaikh, S. A., Al Alawi, K. H., A. Alalqam, R., Tombuloglu, H., & Bouafia, N. A. (2025). The Dual Burden of Hepatitis B and C Among Drug Users in Asia: The First Systematic Review and Meta-Analysis. Pathogens, 14(4), 360. https://doi.org/10.3390/pathogens14040360








