An Introduction to the Influence of Nutritional Factors on the Pathogenesis of Opportunist Fungal Pathogens in Humans
Abstract
1. Introduction
2. Fungal Pathogens
2.1. Classification of Fungal Infections
2.2. Infections Caused by Opportunist Fungal Pathogens in Immunocompetent Hosts
2.3. Opportunist Fungal Pathogens
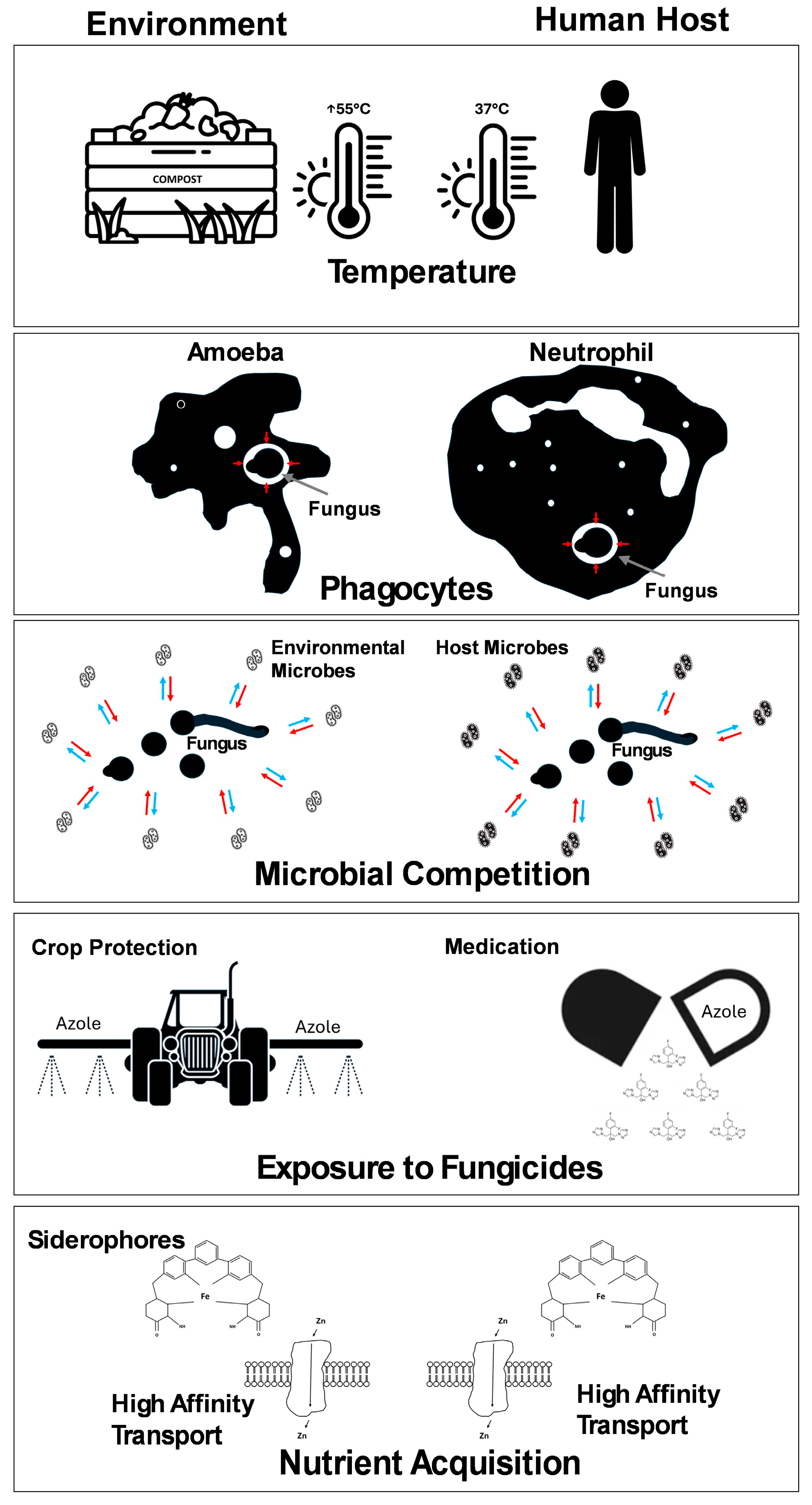
3. Virulence in Opportunist Pathogens
4. Nutritional Immunity
5. Nutrition and Pathogenesis
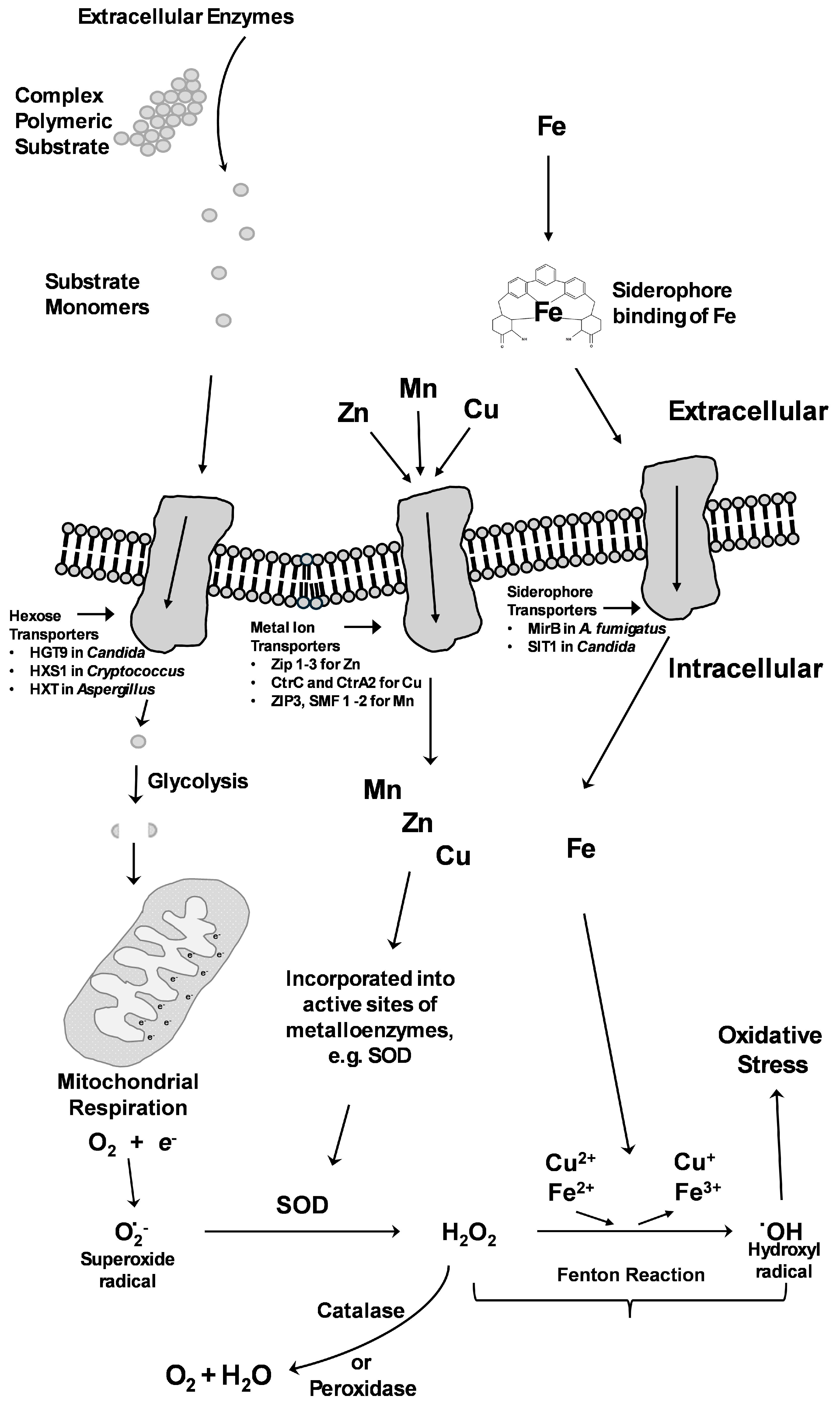
6. Macronutrient Acquisition
7. Micronutrient Acquisition
7.1. Iron Acquisition
7.2. Zinc Acquisition
Zinc in Transcription Factors
7.3. Copper Acquisition
Adaptation to High Copper Concentrations
7.4. Manganese Acquisition
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Denning, D.W. Global incidence and mortality of severe fungal disease. Lancet Infect. Dis. 2024, 24, e428–e438. [Google Scholar] [CrossRef] [PubMed]
- Park, B.J.; Wannemuehler, K.A.; Marston, B.J.; Govender, N.; Pappas, P.G.; Chiller, T.M. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS 2009, 23, 525–530. [Google Scholar] [CrossRef]
- Liu, S.; Garcia-Palacios, P.; Tedersoo, L.; Guirado, E.; van der Heijden, M.G.A.; Wagg, C.; Chen, D.; Wang, Q.; Wang, J.; Singh, B.K.; et al. Phylotype diversity within soil fungal functional groups drives ecosystem stability. Nat. Ecol. Evol. 2022, 6, 900–909. [Google Scholar] [CrossRef]
- Smith, S.E.; Jakobsen, I.; Gronlund, M.; Smith, F.A. Roles of arbuscular mycorrhizas in plant phosphorus nutrition: Interactions between pathways of phosphorus uptake in arbuscular mycorrhizal roots have important implications for understanding and manipulating plant phosphorus acquisition. Plant Physiol. 2011, 156, 1050–1057. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zheng, X.; Tian, M.; Zhang, K. Ammonia and Nematode Ascaroside Are Synergistic in Trap Formation in Arthrobotrys oligospora. Pathogens 2023, 12, 1114. [Google Scholar] [CrossRef]
- Segers, R.; Butt, T.M.; Kerry, B.R.; Peberdy, J.F. The nematophagous fungus Verticillium chlamydosporium produces a chymoelastase-like protease which hydrolyses host nematode proteins in situ. Microbiology 1994, 140 Pt 10, 2715–2723. [Google Scholar] [CrossRef] [PubMed]
- St Leger, R.J.; Wang, J.B. Metarhizium: Jack of all trades, master of many. Open Biol. 2020, 10, 200307. [Google Scholar] [CrossRef]
- Kim, S.W.; Kim, S.; Lee, H.J.; Park, J.W.; Ro, H.S. Isolation of Fungal Pathogens to an Edible Mushroom, Pleurotus eryngii, and Development of Specific ITS Primers. Mycobiology 2013, 41, 252–255. [Google Scholar] [CrossRef]
- Kumamoto, C.A.; Gresnigt, M.S.; Hube, B. The gut, the bad and the harmless: Candida albicans as a commensal and opportunistic pathogen in the intestine. Curr. Opin. Microbiol. 2020, 56, 7–15. [Google Scholar] [CrossRef]
- Chai, Y.; Pardey, P.G.; Hurley, T.M.; Senay, S.D.; Beddow, J.M. A Probabilistic Bio-Economic Assessment of the Global Consequences of Wheat Leaf Rust. Phytopathology 2020, 110, 1886–1896. [Google Scholar] [CrossRef]
- Frick, W.F.; Pollock, J.F.; Hicks, A.C.; Langwig, K.E.; Reynolds, D.S.; Turner, G.G.; Butchkoski, C.M.; Kunz, T.H. An emerging disease causes regional population collapse of a common North American bat species. Science 2010, 329, 679–682. [Google Scholar] [CrossRef] [PubMed]
- Olson, D.H.; Aanensen, D.M.; Ronnenberg, K.L.; Powell, C.I.; Walker, S.F.; Bielby, J.; Garner, T.W.; Weaver, G.; Bd Mapping, G.; Fisher, M.C. Mapping the global emergence of Batrachochytrium dendrobatidis, the amphibian chytrid fungus. PLoS ONE 2013, 8, e56802. [Google Scholar] [CrossRef]
- Havlickova, B.; Czaika, V.A.; Friedrich, M. Epidemiological trends in skin mycoses worldwide. Mycoses 2008, 51 (Suppl. S4), 2–15. [Google Scholar] [CrossRef] [PubMed]
- Deng, R.; Wang, X.; Li, R. Dermatophyte infection: From fungal pathogenicity to host immune responses. Front. Immunol. 2023, 14, 1285887. [Google Scholar] [CrossRef]
- Bonifaz, A.; Vazquez-Gonzalez, D.; Perusquia-Ortiz, A.M. Endemic systemic mycoses: Coccidioidomycosis, histoplasmosis, paracoccidioidomycosis and blastomycosis. J. Dtsch. Dermatol. Ges. 2011, 9, 705–714. [Google Scholar] [CrossRef] [PubMed]
- de Hoog, S.; Tang, C.; Zhou, X.; Jacomel, B.; Lustosa, B.; Song, Y.; Kandemir, H.; Ahmed, S.A.; Zhou, S.; Belmonte-Lopes, R.; et al. Fungal primary and opportunistic pathogens: An ecological perspective. FEMS Microbiol. Rev. 2024, 48, fuae022. [Google Scholar] [CrossRef]
- Schwartz, R.A. Superficial fungal infections. Lancet 2004, 364, 1173–1182. [Google Scholar] [CrossRef]
- La Hoz, R.M.; Baddley, J.W. Subcutaneous Fungal Infections. Curr. Infect. Dis. Rep. 2012, 14, 530–539. [Google Scholar] [CrossRef]
- Thompson, G.R., 3rd; Le, T.; Chindamporn, A.; Kauffman, C.A.; Alastruey-Izquierdo, A.; Ampel, N.M.; Andes, D.R.; Armstrong-James, D.; Ayanlowo, O.; Baddley, J.W.; et al. Global guideline for the diagnosis and management of the endemic mycoses: An initiative of the European Confederation of Medical Mycology in cooperation with the International Society for Human and Animal Mycology. Lancet Infect. Dis. 2021, 21, e364–e374. [Google Scholar] [CrossRef]
- Rautemaa-Richardson, R.; Richardson, M.D. Systemic fungal infections. Medicine 2017, 45, 757–762. [Google Scholar]
- Oliveira, M.; Oliveira, D.; Lisboa, C.; Boechat, J.L.; Delgado, L. Clinical Manifestations of Human Exposure to Fungi. J. Fungi 2023, 9, 381. [Google Scholar] [CrossRef] [PubMed]
- Stack, C.M.; Morton, C.O. Diagnostics for Fungal Infections in Solid Organ Transplants (SOT). Curr. Fungal Infect. R. 2021, 15, 127–135. [Google Scholar] [CrossRef]
- Hope, W.W.; Walsh, T.J.; Denning, D.W. The invasive and saprophytic syndromes due to Aspergillus spp. Med. Mycol. 2005, 43 (Suppl. S1), S207–S238. [Google Scholar] [CrossRef]
- Shahhosseini, N.; Khabiri, A.; Bagheri, F. The spectrum of diseases caused by Aspergillus fumigatus. Iran. J. Clin. Infect. Dis. 2011, 6, 136–141. [Google Scholar]
- Kidd, S.E.; Abdolrasouli, A.; Hagen, F. Fungal Nomenclature: Managing Change is the Name of the Game. Open Forum Infect. Dis. 2023, 10, ofac559. [Google Scholar] [CrossRef]
- Borman, A.M.; Johnson, E.M. Name Changes for Fungi of Medical Importance, 2018 to 2019. J. Clin. Microbiol. 2021, 59, 10-1128. [Google Scholar] [CrossRef]
- Borman, A.M.; Johnson, E.M. Name Changes for Fungi of Medical Importance, 2020 to 2021. J. Clin. Microbiol. 2023, 61, e0033022. [Google Scholar] [CrossRef]
- Kruithoff, C.; Gamal, A.; McCormick, T.S.; Ghannoum, M.A. Dermatophyte Infections Worldwide: Increase in Incidence and Associated Antifungal Resistance. Life 2023, 14, 1. [Google Scholar] [CrossRef]
- Wang, R.; Huang, C.; Zhang, Y.; Li, R. Invasive dermatophyte infection: A systematic review. Mycoses 2021, 64, 340–348. [Google Scholar] [CrossRef]
- Batard, E.; Renaudin, K.; Morin, O.; Desjars, P.; Germaud, P. Fatal acute granulomatous pulmonary aspergillosis in a healthy subject after inhalation of vegetal dust. Eur. J. Clin. Microbiol. Infect. Dis. 2003, 22, 357–359. [Google Scholar] [CrossRef]
- Zuk, J.A.; King, D.; Zakhour, H.D.; Delaney, J.C. Locally invasive pulmonary aspergillosis occurring in a gardener: An occupational hazard? Thorax 1989, 44, 678–679. [Google Scholar] [CrossRef] [PubMed]
- Galanis, E.; Macdougall, L.; Kidd, S.; Morshed, M.; British Columbia Cryptococcus gattii Working, G. Epidemiology of Cryptococcus gattii, British Columbia, Canada, 1999–2007. Emerg. Infect. Dis. 2010, 16, 251–257. [Google Scholar] [CrossRef]
- Bartlett, K.H.; Cheng, P.Y.; Duncan, C.; Galanis, E.; Hoang, L.; Kidd, S.; Lee, M.K.; Lester, S.; MacDougall, L.; Mak, S.; et al. A decade of experience: Cryptococcus gattii in British Columbia. Mycopathologia 2012, 173, 311–319. [Google Scholar] [CrossRef]
- Perfect, J.R.; Casadevall, A. Cryptococcosis. Infect. Dis. Clin. N. Am. 2002, 16, 837–874. [Google Scholar] [CrossRef]
- Ascioglu, S.; Rex, J.H.; de Pauw, B.; Bennett, J.E.; Bille, J.; Crokaert, F.; Denning, D.W.; Donnelly, J.P.; Edwards, J.E.; Erjavec, Z.; et al. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: An international consensus. Clin. Infect. Dis. 2002, 34, 7–14. [Google Scholar] [CrossRef]
- Hope, W.; Natarajan, P.; Goodwin, L. Invasive fungal infections. Clin. Med. 2013, 13, 507–510. [Google Scholar] [CrossRef] [PubMed]
- Hoenigl, M. Invasive Fungal Disease Complicating Coronavirus Disease 2019: When It Rains, It Spores. Clin. Infect. Dis. 2021, 73, e1645–e1648. [Google Scholar] [CrossRef]
- Yan, H.; Guo, L.; Pang, Y.; Liu, F.; Liu, T.; Gao, M. Clinical characteristics and predictive model of pulmonary tuberculosis patients with pulmonary fungal coinfection. BMC Pulm. Med. 2023, 23, 56. [Google Scholar] [CrossRef]
- Badiee, P.; Hashemizadeh, Z. Opportunistic invasive fungal infections: Diagnosis & clinical management. Indian. J. Med. Res. 2014, 139, 195–204. [Google Scholar]
- Gnat, S.; Lagowski, D.; Nowakiewicz, A.; Dylag, M. A global view on fungal infections in humans and animals: Opportunistic infections and microsporidioses. J. Appl. Microbiol. 2021, 131, 2095–2113. [Google Scholar] [CrossRef]
- Beffa, T.; Staib, F.; Lott Fischer, J.; Lyon, P.F.; Gumowski, P.; Marfenina, O.E.; Dunoyer-Geindre, S.; Georgen, F.; Roch-Susuki, R.; Gallaz, L.; et al. Mycological control and surveillance of biological waste and compost. Med. Mycol. 1998, 36 (Suppl. S1), 137–145. [Google Scholar] [PubMed]
- Novohradska, S.; Ferling, I.; Hillmann, F. Exploring Virulence Determinants of Filamentous Fungal Pathogens through Interactions with Soil Amoebae. Front. Cell Infect. Microbiol. 2017, 7, 497. [Google Scholar] [CrossRef]
- Slesiona, S.; Gressler, M.; Mihlan, M.; Zaehle, C.; Schaller, M.; Barz, D.; Hube, B.; Jacobsen, I.D.; Brock, M. Persistence versus escape: Aspergillus terreus and Aspergillus fumigatus employ different strategies during interactions with macrophages. PLoS ONE 2012, 7, e31223. [Google Scholar] [CrossRef]
- Willger, S.D.; Puttikamonkul, S.; Kim, K.H.; Burritt, J.B.; Grahl, N.; Metzler, L.J.; Barbuch, R.; Bard, M.; Lawrence, C.B.; Cramer, R.A., Jr. A sterol-regulatory element binding protein is required for cell polarity, hypoxia adaptation, azole drug resistance, and virulence in Aspergillus fumigatus. PLoS Pathog. 2008, 4, e1000200. [Google Scholar] [CrossRef]
- Grahl, N.; Puttikamonkul, S.; Macdonald, J.M.; Gamcsik, M.P.; Ngo, L.Y.; Hohl, T.M.; Cramer, R.A. In vivo hypoxia and a fungal alcohol dehydrogenase influence the pathogenesis of invasive pulmonary aspergillosis. PLoS Pathog. 2011, 7, e1002145. [Google Scholar] [CrossRef]
- Chun, C.D.; Liu, O.W.; Madhani, H.D. A link between virulence and homeostatic responses to hypoxia during infection by the human fungal pathogen Cryptococcus neoformans. PLoS Pathog. 2007, 3, e22. [Google Scholar] [CrossRef]
- Fleck, C.B.; Schobel, F.; Brock, M. Nutrient acquisition by pathogenic fungi: Nutrient availability, pathway regulation, and differences in substrate utilization. Int. J. Med. Microbiol. 2011, 301, 400–407. [Google Scholar] [CrossRef]
- Warn, P.A.; Sharp, A.; Guinea, J.; Denning, D.W. Effect of hypoxic conditions on in vitro susceptibility testing of amphotericin B, itraconazole and micafungin against Aspergillus and Candida. J. Antimicrob. Chemother. 2004, 53, 743–749. [Google Scholar] [CrossRef]
- Ren, J.; Jin, X.; Zhang, Q.; Zheng, Y.; Lin, D.; Yu, Y. Fungicides induced triazole-resistance in Aspergillus fumigatus associated with mutations of TR46/Y121F/T289A and its appearance in agricultural fields. J. Hazard. Mater. 2017, 326, 54–60. [Google Scholar] [CrossRef]
- Verweij, P.E.; Snelders, E.; Kema, G.H.; Mellado, E.; Melchers, W.J. Azole resistance in Aspergillus fumigatus: A side-effect of environmental fungicide use? Lancet Infect. Dis. 2009, 9, 789–795. [Google Scholar] [CrossRef]
- Stevanovic, J.; Stanimirovic, Z.; Radakovic, M.; Stojic, V. In vitro evaluation of the clastogenicity of fumagillin. Environ. Mol. Mutagen. 2008, 49, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Svahn, K.S.; Goransson, U.; Chryssanthou, E.; Olsen, B.; Sjolin, J.; Stromstedt, A.A. Induction of gliotoxin secretion in Aspergillus fumigatus by bacteria-associated molecules. PLoS ONE 2014, 9, e93685. [Google Scholar] [CrossRef]
- Haas, H.; Eisendle, M.; Turgeon, B.G. Siderophores in fungal physiology and virulence. Annu. Rev. Phytopathol. 2008, 46, 149–187. [Google Scholar] [CrossRef] [PubMed]
- Chow, E.W.L.; Pang, L.M.; Wang, Y. Impact of the host microbiota on fungal infections: New possibilities for intervention? Adv. Drug Deliv. Rev. 2023, 198, 114896. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.D.; Ballou, E.R.; Bates, S.; Bignell, E.M.; Borman, A.M.; Brand, A.C.; Brown, A.J.P.; Coelho, C.; Cook, P.C.; Farrer, R.A.; et al. The pathobiology of human fungal infections. Nat. Rev. Microbiol. 2024, 22, 687–704. [Google Scholar] [CrossRef]
- Hood, M.I.; Skaar, E.P. Nutritional immunity: Transition metals at the pathogen-host interface. Nat. Rev. Microbiol. 2012, 10, 525–537. [Google Scholar] [CrossRef]
- Mandarano, A.H.; McGargill, M.A. The critical role of copper homeostasis during the immune response. J. Immunol. 2023, 210, 148.113. [Google Scholar] [CrossRef]
- Richards, T.A.; Talbot, N.J. Osmotrophy. Curr. Biol. 2018, 28, R1179–R1180. [Google Scholar] [CrossRef]
- Stergiopoulou, T.; Meletiadis, J.; Roilides, E.; Kleiner, D.E.; Schaufele, R.; Roden, M.; Harrington, S.; Dad, L.; Segal, B.; Walsh, T.J. Host-dependent patterns of tissue injury in invasive pulmonary aspergillosis. Am. J. Clin. Pathol. 2007, 127, 349–355. [Google Scholar] [CrossRef]
- Fu, M.S.; Coelho, C.; De Leon-Rodriguez, C.M.; Rossi, D.C.P.; Camacho, E.; Jung, E.H.; Kulkarni, M.; Casadevall, A. Cryptococcus neoformans urease affects the outcome of intracellular pathogenesis by modulating phagolysosomal pH. PLoS Pathog. 2018, 14, e1007144. [Google Scholar] [CrossRef]
- Xiong, Z.; Zhang, N.; Xu, L.; Deng, Z.; Limwachiranon, J.; Guo, Y.; Han, Y.; Yang, W.; Scharf, D.H. Urease of Aspergillus fumigatus Is Required for Survival in Macrophages and Virulence. Microbiol. Spectr. 2023, 11, e0350822. [Google Scholar] [CrossRef]
- Scott, J.A.; Untereiner, W.A. Determination of keratin degradation by fungi using keratin azure. Med. Mycol. 2004, 42, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Kruger, W.; Vielreicher, S.; Kapitan, M.; Jacobsen, I.D.; Niemiec, M.J. Fungal-Bacterial Interactions in Health and Disease. Pathogens 2019, 8, 70. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Regan, J.; Cai, C.; Palmer, G.E.; Williams, D.L.; Kruppa, M.D.; Peters, B.M. Glycogen Metabolism in Candida albicans Impacts Fitness and Virulence during Vulvovaginal and Invasive Candidiasis. mBio 2023, 14, e0004623. [Google Scholar] [CrossRef] [PubMed]
- Ries, L.N.A.; Beattie, S.; Cramer, R.A.; Goldman, G.H. Overview of carbon and nitrogen catabolite metabolism in the virulence of human pathogenic fungi. Mol. Microbiol. 2018, 107, 277–297. [Google Scholar] [CrossRef]
- Wilson, W.A.; Roach, P.J.; Montero, M.; Baroja-Fernandez, E.; Munoz, F.J.; Eydallin, G.; Viale, A.M.; Pozueta-Romero, J. Regulation of glycogen metabolism in yeast and bacteria. FEMS Microbiol. Rev. 2010, 34, 952–985. [Google Scholar] [CrossRef]
- Man, A.; Ciurea, C.N.; Pasaroiu, D.; Savin, A.I.; Toma, F.; Sular, F.; Santacroce, L.; Mare, A. New perspectives on the nutritional factors influencing growth rate of Candida albicans in diabetics. An in vitro study. Mem. Inst. Oswaldo Cruz 2017, 112, 587–592. [Google Scholar] [CrossRef]
- Balan, P.; Gogineni, S.B.; Kumari, N.S.; Shetty, V.; Rangare, A.L.; Castelino, R.L.; Areekat, K.F. Candida Carriage Rate and Growth Characteristics of Saliva in Diabetes Mellitus Patients: A Case-Control Study. J. Dent. Res. Dent. Clin. Dent. Prospect 2015, 9, 274–279. [Google Scholar] [CrossRef]
- Dornelas Figueira, L.M.; Ricomini Filho, A.P.; da Silva, W.J.; Del Be, L.C.A.A.; Ruiz, K.G.S. Glucose effect on Candida albicans biofilm during tissue invasion. Arch. Oral. Biol. 2020, 117, 104728. [Google Scholar] [CrossRef]
- Ene, I.V.; Brunke, S.; Brown, A.J.; Hube, B. Metabolism in fungal pathogenesis. Cold Spring Harb. Perspect. Med. 2014, 4, a019695. [Google Scholar] [CrossRef]
- Kammer, P.; McNamara, S.; Wolf, T.; Conrad, T.; Allert, S.; Gerwien, F.; Hunniger, K.; Kurzai, O.; Guthke, R.; Hube, B.; et al. Survival Strategies of Pathogenic Candida Species in Human Blood Show Independent and Specific Adaptations. mBio 2020, 11, 10-1128. [Google Scholar] [CrossRef]
- Ortiz-Ramirez, J.A.; Cuellar-Cruz, M.; Villagomez-Castro, J.C.; Lopez-Romero, E. Fungal Glycosidases in Sporothrix Species and Candida albicans. J. Fungi 2023, 9, 919. [Google Scholar] [CrossRef]
- Wang, C.; Typas, M.A.; Butt, T.M. Detection and characterisation of pr1 virulent gene deficiencies in the insect pathogenic fungus Metarhizium anisopliae. FEMS Microbiol. Lett. 2002, 213, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Jany, K.D.; Mayer, B. Proteinase K from Tritirachium album limber. I. Molecular mass and sequence around the active site serine residue. Biol. Chem. Hoppe Seyler 1985, 366, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Tomee, J.F.; Wierenga, A.T.; Hiemstra, P.S.; Kauffman, H.K. Proteases from Aspergillus fumigatus induce release of proinflammatory cytokines and cell detachment in airway epithelial cell lines. J. Infect. Dis. 1997, 176, 300–303. [Google Scholar] [CrossRef]
- Basu, T.; Seyedmousavi, S.; Sugui, J.A.; Balenga, N.; Zhao, M.; Kwon Chung, K.J.; Biardel, S.; Laviolette, M.; Druey, K.M. Aspergillus fumigatus alkaline protease 1 (Alp1/Asp f13) in the airways correlates with asthma severity. J. Allergy Clin. Immunol. 2018, 141, 423–425. [Google Scholar] [CrossRef]
- Bergmann, A.; Hartmann, T.; Cairns, T.; Bignell, E.M.; Krappmann, S. A regulator of Aspergillus fumigatus extracellular proteolytic activity is dispensable for virulence. Infect. Immun. 2009, 77, 4041–4050. [Google Scholar] [CrossRef]
- Beauvais, A.; Monod, M.; Debeaupuis, J.P.; Diaquin, M.; Kobayashi, H.; Latge, J.P. Biochemical and antigenic characterization of a new dipeptidyl-peptidase isolated from Aspergillus fumigatus. J. Biol. Chem. 1997, 272, 6238–6244. [Google Scholar] [CrossRef]
- Portaels, J.; Van Crombrugge, E.; Van Den Broeck, W.; Lagrou, K.; Laval, K.; Nauwynck, H. Aspergillus Fumigatus Spore Proteases Alter the Respiratory Mucosa Architecture and Facilitate Equine Herpesvirus 1 Infection. Viruses 2024, 16, 1208. [Google Scholar] [CrossRef]
- Bozza, S.; Clavaud, C.; Giovannini, G.; Fontaine, T.; Beauvais, A.; Sarfati, J.; D’Angelo, C.; Perruccio, K.; Bonifazi, P.; Zagarella, S.; et al. Immune sensing of Aspergillus fumigatus proteins, glycolipids, and polysaccharides and the impact on Th immunity and vaccination. J. Immunol. 2009, 183, 2407–2414. [Google Scholar] [CrossRef]
- Vernel-Pauillac, F.; Laurent-Winter, C.; Fiette, L.; Janbon, G.; Aimanianda, V.; Dromer, F. Cryptococcus neoformans infections: Aspartyl protease potential to improve outcome in susceptible hosts. mBio 2024, 15, e0273324. [Google Scholar] [CrossRef]
- Naglik, J.R.; Challacombe, S.J.; Hube, B. Candida albicans secreted aspartyl proteinases in virulence and pathogenesis. Microbiol. Mol. Biol. Rev. 2003, 67, 400–428. [Google Scholar] [CrossRef] [PubMed]
- Naglik, J.R.; Rodgers, C.A.; Shirlaw, P.J.; Dobbie, J.L.; Fernandes-Naglik, L.L.; Greenspan, D.; Agabian, N.; Challacombe, S.J. Differential expression of Candida albicans secreted aspartyl proteinase and phospholipase B genes in humans correlates with active oral and vaginal infections. J. Infect. Dis. 2003, 188, 469–479. [Google Scholar] [CrossRef]
- Behnsen, J.; Lessing, F.; Schindler, S.; Wartenberg, D.; Jacobsen, I.D.; Thoen, M.; Zipfel, P.F.; Brakhage, A.A. Secreted Aspergillus fumigatus protease Alp1 degrades human complement proteins C3, C4, and C5. Infect. Immun. 2010, 78, 3585–3594. [Google Scholar] [CrossRef] [PubMed]
- Redes, J.L.; Basu, T.; Ram-Mohan, S.; Ghosh, C.C.; Chan, E.C.; Sek, A.C.; Zhao, M.; Krishnan, R.; Rosenberg, H.F.; Druey, K.M. Aspergillus fumigatus-Secreted Alkaline Protease 1 Mediates Airways Hyperresponsiveness in Severe Asthma. Immunohorizons 2019, 3, 368–377. [Google Scholar] [CrossRef]
- Muller, L.M.; Harrison, M.J. Phytohormones, miRNAs, and peptide signals integrate plant phosphorus status with arbuscular mycorrhizal symbiosis. Curr. Opin. Plant Biol. 2019, 50, 132–139. [Google Scholar] [CrossRef]
- Secco, D.; Wang, C.; Shou, H.; Whelan, J. Phosphate homeostasis in the yeast Saccharomyces cerevisiae, the key role of the SPX domain-containing proteins. FEBS Lett. 2012, 586, 289–295. [Google Scholar] [CrossRef]
- Toh-e, A.; Ohkusu, M.; Li, H.M.; Shimizu, K.; Takahashi-Nakaguchi, A.; Gonoi, T.; Kawamoto, S.; Kanesaki, Y.; Yoshikawa, H.; Nishizawa, M. Identification of genes involved in the phosphate metabolism in Cryptococcus neoformans. Fungal Genet. Biol. 2015, 80, 19–30. [Google Scholar] [CrossRef]
- Ikeh, M.A.; Kastora, S.L.; Day, A.M.; Herrero-de-Dios, C.M.; Tarrant, E.; Waldron, K.J.; Banks, A.P.; Bain, J.M.; Lydall, D.; Veal, E.A.; et al. Pho4 mediates phosphate acquisition in Candida albicans and is vital for stress resistance and metal homeostasis. Mol. Biol. Cell 2016, 27, 2784–2801. [Google Scholar] [CrossRef]
- de Gouvea, P.F.; Soriani, F.M.; Malavazi, I.; Savoldi, M.; Goldman, M.H.; Loss, O.; Bignell, E.; da Silva Ferreira, M.E.; Goldman, G.H. Functional characterization of the Aspergillus fumigatusPHO80 homologue. Fungal Genet. Biol. 2008, 45, 1135–1146. [Google Scholar] [CrossRef]
- Thewes, S.; Kretschmar, M.; Park, H.; Schaller, M.; Filler, S.G.; Hube, B. In vivo and ex vivo comparative transcriptional profiling of invasive and non-invasive Candida albicans isolates identifies genes associated with tissue invasion. Mol. Microbiol. 2007, 63, 1606–1628. [Google Scholar] [CrossRef] [PubMed]
- Urrialde, V.; Prieto, D.; Pla, J.; Alonso-Monge, R. The Candida albicans Pho4 Transcription Factor Mediates Susceptibility to Stress and Influences Fitness in a Mouse Commensalism Model. Front. Microbiol. 2016, 7, 1062. [Google Scholar] [CrossRef]
- Lev, S.; Kaufman-Francis, K.; Desmarini, D.; Juillard, P.G.; Li, C.; Stifter, S.A.; Feng, C.G.; Sorrell, T.C.; Grau, G.E.; Bahn, Y.S.; et al. Pho4 Is Essential for Dissemination of Cryptococcus neoformans to the Host Brain by Promoting Phosphate Uptake and Growth at Alkaline pH. mSphere 2017, 2, 10-1128. [Google Scholar] [CrossRef]
- Morton, C.O.; Varga, J.J.; Hornbach, A.; Mezger, M.; Sennefelder, H.; Kneitz, S.; Kurzai, O.; Krappmann, S.; Einsele, H.; Nierman, W.C.; et al. The temporal dynamics of differential gene expression in Aspergillus fumigatus interacting with human immature dendritic cells in vitro. PLoS ONE 2011, 6, e16016. [Google Scholar] [CrossRef]
- Fan, W.; Kraus, P.R.; Boily, M.J.; Heitman, J. Cryptococcus neoformans gene expression during murine macrophage infection. Eukaryot. Cell 2005, 4, 1420–1433. [Google Scholar] [CrossRef]
- Weiss, G.; Carver, P.L. Role of divalent metals in infectious disease susceptibility and outcome. Clin. Microbiol. Infect. 2018, 24, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Philpott, C.C.; Leidgens, S.; Frey, A.G. Metabolic remodeling in iron-deficient fungi. Biochim. Biophys. Acta 2012, 1823, 1509–1520. [Google Scholar] [CrossRef]
- Ramos-Alonso, L.; Romero, A.M.; Martinez-Pastor, M.T.; Puig, S. Iron Regulatory Mechanisms in Saccharomyces cerevisiae. Front. Microbiol. 2020, 11, 582830. [Google Scholar] [CrossRef]
- Duval, C.; Macabiou, C.; Garcia, C.; Lesuisse, E.; Camadro, J.M.; Auchere, F. The adaptive response to iron involves changes in energetic strategies in the pathogen Candida albicans. Microbiologyopen 2020, 9, e970. [Google Scholar] [CrossRef]
- Wells, M.L.; Washington, O.L.; Hicks, S.N.; Nobile, C.J.; Hartooni, N.; Wilson, G.M.; Zucconi, B.E.; Huang, W.; Li, L.; Fargo, D.C.; et al. Post-transcriptional regulation of transcript abundance by a conserved member of the tristetraprolin family in Candida albicans. Mol. Microbiol. 2015, 95, 1036–1053. [Google Scholar] [CrossRef]
- Schrettl, M.; Bignell, E.; Kragl, C.; Joechl, C.; Rogers, T.; Arst, H.N., Jr.; Haynes, K.; Haas, H. Siderophore biosynthesis but not reductive iron assimilation is essential for Aspergillus fumigatus virulence. J. Exp. Med. 2004, 200, 1213–1219. [Google Scholar] [CrossRef] [PubMed]
- Heymann, P.; Gerads, M.; Schaller, M.; Dromer, F.; Winkelmann, G.; Ernst, J.F. The siderophore iron transporter of Candida albicans (Sit1p/Arn1p) mediates uptake of ferrichrome-type siderophores and is required for epithelial invasion. Infect. Immun. 2002, 70, 5246–5255. [Google Scholar] [CrossRef]
- Duggan, S.; Leonhardt, I.; Hunniger, K.; Kurzai, O. Host response to Candida albicans bloodstream infection and sepsis. Virulence 2015, 6, 316–326. [Google Scholar] [CrossRef]
- Pendrak, M.L.; Chao, M.P.; Yan, S.S.; Roberts, D.D. Heme oxygenase in Candida albicans is regulated by hemoglobin and is necessary for metabolism of exogenous heme and hemoglobin to alpha-biliverdin. J. Biol. Chem. 2004, 279, 3426–3433. [Google Scholar] [CrossRef]
- Weissman, Z.; Kornitzer, D. A family of Candida cell surface haem-binding proteins involved in haemin and haemoglobin-iron utilization. Mol. Microbiol. 2004, 53, 1209–1220. [Google Scholar] [CrossRef]
- Weissman, Z.; Shemer, R.; Conibear, E.; Kornitzer, D. An endocytic mechanism for haemoglobin-iron acquisition in Candida albicans. Mol. Microbiol. 2008, 69, 201–217. [Google Scholar] [CrossRef] [PubMed]
- Bairwa, G.; Caza, M.; Horianopoulos, L.; Hu, G.; Kronstad, J. Role of clathrin-mediated endocytosis in the use of heme and hemoglobin by the fungal pathogen Cryptococcus neoformans. Cell Microbiol. 2019, 21, e12961. [Google Scholar] [CrossRef]
- Xue, P.; Sanchez-Leon, E.; Damoo, D.; Hu, G.; Jung, W.H.; Kronstad, J.W. Heme sensing and trafficking in fungi. Fungal Biol. Rev. 2023, 43, 100286. [Google Scholar] [CrossRef]
- Almeida, R.S.; Brunke, S.; Albrecht, A.; Thewes, S.; Laue, M.; Edwards, J.E.; Filler, S.G.; Hube, B. the hyphal-associated adhesin and invasin Als3 of Candida albicans mediates iron acquisition from host ferritin. PLoS Pathog. 2008, 4, e1000217. [Google Scholar] [CrossRef]
- Jung, W.H.; Kronstad, J.W. Iron and fungal pathogenesis: A case study with Cryptococcus neoformans. Cell Microbiol. 2008, 10, 277–284. [Google Scholar] [CrossRef]
- Song, M.; Thak, E.J.; Kang, H.A.; Kronstad, J.W.; Jung, W.H. Cryptococcus neoformans can utilize ferritin as an iron source. Med. Mycol. 2022, 60, myac056. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.K.; David, M.S.; Yang, S.; Garg, R.; Zhao, H.; Cormack, B.P.; Culotta, V.C. Converging Roles of the Metal Transporter SMF11 and the Ferric Reductase FRE1 in Iron Homeostasis of Candida albicans. Mol. Microbiol. 2024, 122, 879–895. [Google Scholar] [CrossRef] [PubMed]
- Schrettl, M.; Beckmann, N.; Varga, J.; Heinekamp, T.; Jacobsen, I.D.; Jochl, C.; Moussa, T.A.; Wang, S.; Gsaller, F.; Blatzer, M.; et al. HapX-mediated adaption to iron starvation is crucial for virulence of Aspergillus fumigatus. PLoS Pathog. 2010, 6, e1001124. [Google Scholar] [CrossRef]
- Jung, W.H.; Saikia, S.; Hu, G.; Wang, J.; Fung, C.K.; D’Souza, C.; White, R.; Kronstad, J.W. HapX positively and negatively regulates the transcriptional response to iron deprivation in Cryptococcus neoformans. PLoS Pathog. 2010, 6, e1001209. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, J.C.; Ojeda, L.; Balk, J.; Muhlenhoff, U.; Lill, R.; Winge, D.R. Activation of the iron regulon by the yeast Aft1/Aft2 transcription factors depends on mitochondrial but not cytosolic iron-sulfur protein biogenesis. J. Biol. Chem. 2005, 280, 10135–10140. [Google Scholar] [CrossRef]
- Windus, D.W.; Stokes, T.J.; Julian, B.A.; Fenves, A.Z. Fatal Rhizopus infections in hemodialysis patients receiving deferoxamine. Ann. Intern. Med. 1987, 107, 678–680. [Google Scholar] [CrossRef]
- Boelaert, J.R.; de Locht, M.; Van Cutsem, J.; Kerrels, V.; Cantinieaux, B.; Verdonck, A.; Van Landuyt, H.W.; Schneider, Y.J. Mucormycosis during deferoxamine therapy is a siderophore-mediated infection. In vitro and in vivo animal studies. J. Clin. Investig. 1993, 91, 1979–1986. [Google Scholar] [CrossRef]
- Ibrahim, A.S.; Gebermariam, T.; Fu, Y.; Lin, L.; Husseiny, M.I.; French, S.W.; Schwartz, J.; Skory, C.D.; Edwards, J.E., Jr.; Spellberg, B.J. The iron chelator deferasirox protects mice from mucormycosis through iron starvation. J. Clin. Investig. 2007, 117, 2649–2657. [Google Scholar] [CrossRef]
- Wilson, D.; Deepe, G.S., Jr. The intersection of host and fungus through the zinc lens. Curr. Opin. Microbiol. 2019, 52, 35–40. [Google Scholar] [CrossRef]
- Li, Y.H.; Liu, T.B. Zinc Finger Proteins in the Human Fungal Pathogen Cryptococcus neoformans. Int. J. Mol. Sci. 2020, 21, 1361. [Google Scholar] [CrossRef]
- Subramanian Vignesh, K.; Landero Figueroa, J.A.; Porollo, A.; Caruso, J.A.; Deepe, G.S., Jr. Granulocyte macrophage-colony stimulating factor induced Zn sequestration enhances macrophage superoxide and limits intracellular pathogen survival. Immunity 2013, 39, 697–710. [Google Scholar] [CrossRef] [PubMed]
- Joao, M.E.D.; Tavanti, A.G.; Vargas, A.N.; Kmetzsch, L.; Staats, C.C. The influence of amoeba metal homeostasis on antifungal activity against Cryptococcus gattii. Genet. Mol. Biol. 2024, 47, e20230320. [Google Scholar] [CrossRef] [PubMed]
- Pires, A.; Carvalho, A.R.; Vaso, C.O.; Mendes-Giannini, M.J.S.; Singulani, J.L.; Fusco-Almeida, A.M. Influence of Zinc on Histoplasma capsulatum Planktonic and Biofilm Cells. J. Fungi 2024, 10, 361. [Google Scholar] [CrossRef]
- Schneider, R.O.; Diehl, C.; Dos Santos, F.M.; Piffer, A.C.; Garcia, A.W.A.; Kulmann, M.I.R.; Schrank, A.; Kmetzsch, L.; Vainstein, M.H.; Staats, C.C. Effects of zinc transporters on Cryptococcus gattii virulence. Sci. Rep. 2015, 5, 10104. [Google Scholar] [CrossRef]
- Schneider Rde, O.; Fogaca Nde, S.; Kmetzsch, L.; Schrank, A.; Vainstein, M.H.; Staats, C.C. Zap1 regulates zinc homeostasis and modulates virulence in Cryptococcus gattii. PLoS ONE 2012, 7, e43773. [Google Scholar] [CrossRef]
- Garcia, A.W.A.; Kinskovski, U.P.; Diehl, C.; Reuwsaat, J.C.V.; Motta de Souza, H.; Pinto, H.B.; Trentin, D.D.S.; de Oliveira, H.C.; Rodrigues, M.L.; Becker, E.M.; et al. Participation of Zip3, a ZIP domain-containing protein, in stress response and virulence in Cryptococcus gattii. Fungal Genet. Biol. 2020, 144, 103438. [Google Scholar] [CrossRef]
- Diehl, C.; Garcia, A.W.A.; Kinskovski, U.P.; Sbaraini, N.; Schneider, R.O.; Ferrareze, P.A.G.; Gerber, A.L.; de Vasconcelos, A.T.R.; Kmetzsch, L.; Vainstein, M.H.; et al. Zrg1, a cryptococcal protein associated with regulation of growth in nutrient deprivation conditions. Genomics 2021, 113, 805–814. [Google Scholar] [CrossRef]
- Vicentefranqueira, R.; Amich, J.; Marin, L.; Sanchez, C.I.; Leal, F.; Calera, J.A. The Transcription Factor ZafA Regulates the Homeostatic and Adaptive Response to Zinc Starvation in Aspergillus fumigatus. Genes 2018, 9, 318. [Google Scholar] [CrossRef]
- Amich, J.; Vicentefranqueira, R.; Leal, F.; Calera, J.A. Aspergillus fumigatus survival in alkaline and extreme zinc-limiting environments relies on the induction of a zinc homeostasis system encoded by the zrfC and aspf2 genes. Eukaryot. Cell 2010, 9, 424–437. [Google Scholar] [CrossRef]
- Vicentefranqueira, R.; Moreno, M.A.; Leal, F.; Calera, J.A. The zrfA and zrfB genes of Aspergillus fumigatus encode the zinc transporter proteins of a zinc uptake system induced in an acid, zinc-depleted environment. Eukaryot. Cell 2005, 4, 837–848. [Google Scholar] [CrossRef]
- Garstka, K.; Hecel, A.; Kozlowski, H.; Rowinska-Zyrek, M. Specific Zn(II)-binding site in the C-terminus of Aspf2, a zincophore from Aspergillus fumigatus. Metallomics 2022, 14, mfac042. [Google Scholar] [CrossRef]
- Segurado, M.; Lopez-Aragon, R.; Calera, J.A.; Fernandez-Abalos, J.M.; Leal, F. Zinc-regulated biosynthesis of immunodominant antigens from Aspergillus spp. Infect. Immun. 1999, 67, 2377–2382. [Google Scholar] [CrossRef] [PubMed]
- Amich, J.; Vicentefranqueira, R.; Mellado, E.; Ruiz-Carmuega, A.; Leal, F.; Calera, J.A. The ZrfC alkaline zinc transporter is required for Aspergillus fumigatus virulence and its growth in the presence of the Zn/Mn-chelating protein calprotectin. Cell Microbiol. 2014, 16, 548–564. [Google Scholar] [CrossRef]
- Citiulo, F.; Jacobsen, I.D.; Miramon, P.; Schild, L.; Brunke, S.; Zipfel, P.; Brock, M.; Hube, B.; Wilson, D. Candida albicans scavenges host zinc via Pra1 during endothelial invasion. PLoS Pathog. 2012, 8, e1002777. [Google Scholar] [CrossRef] [PubMed]
- Sprague, J.L.; Schille, T.B.; Allert, S.; Trumper, V.; Lier, A.; Grossmann, P.; Priest, E.L.; Tsavou, A.; Panagiotou, G.; Naglik, J.R.; et al. Candida albicans translocation through the intestinal epithelial barrier is promoted by fungal zinc acquisition and limited by NFkappaB-mediated barrier protection. PLoS Pathog. 2024, 20, e1012031. [Google Scholar] [CrossRef]
- Shelest, E. Transcription factors in fungi. FEMS Microbiol. Lett. 2008, 286, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.H., Jr.; Giusani, A.D.; Chen, X.; Kumamoto, C.A. Filamentous growth of Candida albicans in response to physical environmental cues and its regulation by the unique CZF1 gene. Mol. Microbiol. 1999, 34, 651–662. [Google Scholar] [CrossRef]
- Sharon, H.; Hagag, S.; Osherov, N. Transcription factor PrtT controls expression of multiple secreted proteases in the human pathogenic mold Aspergillus fumigatus. Infect. Immun. 2009, 77, 4051–4060. [Google Scholar] [CrossRef]
- Staats, C.C.; Kmetzsch, L.; Schrank, A.; Vainstein, M.H. Fungal zinc metabolism and its connections to virulence. Front. Cell Infect. Microbiol. 2013, 3, 65. [Google Scholar] [CrossRef]
- Bertuzzi, M.; Schrettl, M.; Alcazar-Fuoli, L.; Cairns, T.C.; Munoz, A.; Walker, L.A.; Herbst, S.; Safari, M.; Cheverton, A.M.; Chen, D.; et al. The pH-responsive PacC transcription factor of Aspergillus fumigatus governs epithelial entry and tissue invasion during pulmonary aspergillosis. PLoS Pathog. 2014, 10, e1004413. [Google Scholar] [CrossRef]
- Tilburn, J.; Sarkar, S.; Widdick, D.A.; Espeso, E.A.; Orejas, M.; Mungroo, J.; Penalva, M.A.; Arst, H.N., Jr. The Aspergillus PacC zinc finger transcription factor mediates regulation of both acid- and alkaline-expressed genes by ambient pH. EMBO J. 1995, 14, 779–790. [Google Scholar] [CrossRef]
- Chang, Y.C.; Penoyer, L.A.; Kwon-Chung, K.J. The second STE12 homologue of Cryptococcus neoformans is MATa-specific and plays an important role in virulence. Proc. Natl. Acad. Sci. USA 2001, 98, 3258–3263. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.C.; Wright, L.C.; Tscharke, R.L.; Sorrell, T.C.; Wilson, C.F.; Kwon-Chung, K.J. Regulatory roles for the homeodomain and C2H2 zinc finger regions of Cryptococcus neoformans Ste12alphap. Mol. Microbiol. 2004, 53, 1385–1396. [Google Scholar] [CrossRef]
- Jung, W.H.; Sham, A.; White, R.; Kronstad, J.W. Iron regulation of the major virulence factors in the AIDS-associated pathogen Cryptococcus neoformans. PLoS Biol. 2006, 4, e410. [Google Scholar] [CrossRef]
- Garcia-Santamarina, S.; Thiele, D.J. Copper at the Fungal Pathogen-Host Axis. J. Biol. Chem. 2015, 290, 18945–18953. [Google Scholar] [CrossRef]
- Cai, Z.; Du, W.; Zeng, Q.; Long, N.; Dai, C.; Lu, L. Cu-sensing transcription factor Mac1 coordinates with the Ctr transporter family to regulate Cu acquisition and virulence in Aspergillus fumigatus. Fungal Genet. Biol. 2017, 107, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.S.; Kang, S.; Seo, H.; Yun, C.W. A copper transcription factor, AfMac1, regulates both iron and copper homeostasis in the opportunistic fungal pathogen Aspergillus fumigatus. Biochem. J. 2018, 475, 2831–2845. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Seo, H.; Moon, H.S.; Kwon, J.H.; Park, Y.S.; Yun, C.W. The Role of Zinc in Copper Homeostasis of Aspergillus fumigatus. Int. J. Mol. Sci. 2020, 21, 7665. [Google Scholar] [CrossRef]
- Khemiri, I.; Tebbji, F.; Sellam, A. Transcriptome Analysis Uncovers a Link Between Copper Metabolism, and Both Fungal Fitness and Antifungal Sensitivity in the Opportunistic Yeast Candida albicans. Front. Microbiol. 2020, 11, 935. [Google Scholar] [CrossRef]
- Culbertson, E.M.; Bruno, V.M.; Cormack, B.P.; Culotta, V.C. Expanded role of the Cu-sensing transcription factor Mac1p in Candida albicans. Mol. Microbiol. 2020, 114, 1006–1018. [Google Scholar] [CrossRef]
- Ray, S.C.; Rappleye, C.A. Mac1-Dependent Copper Sensing Promotes Histoplasma Adaptation to the Phagosome during Adaptive Immunity. mBio 2022, 13, e0377321. [Google Scholar] [CrossRef]
- Smith, A.D.; Logeman, B.L.; Thiele, D.J. Copper Acquisition and Utilization in Fungi. Annu. Rev. Microbiol. 2017, 71, 597–623. [Google Scholar] [CrossRef] [PubMed]
- White, C.; Lee, J.; Kambe, T.; Fritsche, K.; Petris, M.J. A role for the ATP7A copper-transporting ATPase in macrophage bactericidal activity. J. Biol. Chem. 2009, 284, 33949–33956. [Google Scholar] [CrossRef]
- Flemming, A. Copper boosts pro-inflammatory state of macrophages. Nat. Rev. Immunol. 2023, 23, 344. [Google Scholar] [CrossRef] [PubMed]
- Casey, A.L.; Adams, D.; Karpanen, T.J.; Lambert, P.A.; Cookson, B.D.; Nightingale, P.; Miruszenko, L.; Shillam, R.; Christian, P.; Elliott, T.S. Role of copper in reducing hospital environment contamination. J. Hosp. Infect. 2010, 74, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Handelman, M.; Meir, Z.; Shadkchan, Y.; Abo Kandil, A.; Amano, O.; Mariscal, M.; Lopez-Berges, M.S.; Osherov, N. Evolution of the pathogenic mold Aspergillus fumigatus on high copper levels identifies novel resistance genes. mSphere 2024, 9, e0025324. [Google Scholar] [CrossRef]
- Sun, T.S.; Ju, X.; Gao, H.L.; Wang, T.; Thiele, D.J.; Li, J.Y.; Wang, Z.Y.; Ding, C. Reciprocal functions of Cryptococcus neoformans copper homeostasis machinery during pulmonary infection and meningoencephalitis. Nat. Commun. 2014, 5, 5550. [Google Scholar] [CrossRef]
- Ding, C.; Festa, R.A.; Chen, Y.L.; Espart, A.; Palacios, O.; Espin, J.; Capdevila, M.; Atrian, S.; Heitman, J.; Thiele, D.J. Cryptococcus neoformans copper detoxification machinery is critical for fungal virulence. Cell Host Microbe 2013, 13, 265–276. [Google Scholar] [CrossRef]
- Frealle, E.; Noel, C.; Viscogliosi, E.; Camus, D.; Dei-Cas, E.; Delhaes, L. Manganese superoxide dismutase in pathogenic fungi: An issue with pathophysiological and phylogenetic involvements. FEMS Immunol. Med. Microbiol. 2005, 45, 411–422. [Google Scholar] [CrossRef]
- Giles, S.S.; Batinic-Haberle, I.; Perfect, J.R.; Cox, G.M. Cryptococcus neoformans mitochondrial superoxide dismutase: An essential link between antioxidant function and high-temperature growth. Eukaryot. Cell 2005, 4, 46–54. [Google Scholar] [CrossRef]
- Bates, S.; MacCallum, D.M.; Bertram, G.; Munro, C.A.; Hughes, H.B.; Buurman, E.T.; Brown, A.J.; Odds, F.C.; Gow, N.A. Candida albicans Pmr1p, a secretory pathway P-type Ca2+/Mn2+-ATPase, is required for glycosylation and virulence. J. Biol. Chem. 2005, 280, 23408–23415. [Google Scholar] [CrossRef] [PubMed]
- Wildeman, A.S.; Patel, N.K.; Cormack, B.P.; Culotta, V.C. The role of manganese in morphogenesis and pathogenesis of the opportunistic fungal pathogen Candida albicans. PLoS Pathog. 2023, 19, e1011478. [Google Scholar] [CrossRef] [PubMed]
- Henry, M.; Khemiri, I.; Tebbji, F.; Abu-Helu, R.; Vincent, A.T.; Sellam, A. Manganese homeostasis modulates fungal virulence and stress tolerance in Candida albicans. mSphere 2024, 9, e0080423. [Google Scholar] [CrossRef] [PubMed]
- Clark, H.L.; Jhingran, A.; Sun, Y.; Vareechon, C.; de Jesus Carrion, S.; Skaar, E.P.; Chazin, W.J.; Calera, J.A.; Hohl, T.M.; Pearlman, E. Zinc and Manganese Chelation by Neutrophil S100A8/A9 (Calprotectin) Limits Extracellular Aspergillus fumigatus Hyphal Growth and Corneal Infection. J. Immunol. 2016, 196, 336–344. [Google Scholar] [CrossRef]
- Schrettl, M.; Kim, H.S.; Eisendle, M.; Kragl, C.; Nierman, W.C.; Heinekamp, T.; Werner, E.R.; Jacobsen, I.; Illmer, P.; Yi, H.; et al. SreA-mediated iron regulation in Aspergillus fumigatus. Mol. Microbiol. 2008, 70, 27–43. [Google Scholar] [CrossRef]
- Zhai, P.; Du, W.; Long, N.; Lu, L. A GATA-type transcription factor SreA affects manganese susceptibility by regulating the expression of iron uptake-related genes. Fungal Genet. Biol. 2022, 163, 103731. [Google Scholar] [CrossRef]
- Chen, S.C.; Sorrell, T.C. Antifungal agents. Med. J. Aust. 2007, 187, 404–409. [Google Scholar] [CrossRef]
- Zhai, P.; Chai, Y.; Lu, L. Fungal Zinc Homeostasis and Its Potential as an Antifungal Target: A Focus on the Human Pathogen Aspergillus fumigatus. Microorganisms 2022, 10, 2469. [Google Scholar] [CrossRef]
- Hunsaker, E.W.; Franz, K.J. Copper potentiates azole antifungal activity in a way that does not involve complex formation. Dalton Trans. 2019, 48, 9654–9662. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jong, T.; Stack, C.M.; Moffitt, M.C.; Morton, C.O. An Introduction to the Influence of Nutritional Factors on the Pathogenesis of Opportunist Fungal Pathogens in Humans. Pathogens 2025, 14, 335. https://doi.org/10.3390/pathogens14040335
Jong T, Stack CM, Moffitt MC, Morton CO. An Introduction to the Influence of Nutritional Factors on the Pathogenesis of Opportunist Fungal Pathogens in Humans. Pathogens. 2025; 14(4):335. https://doi.org/10.3390/pathogens14040335
Chicago/Turabian StyleJong, Timothy, Colin M. Stack, Michelle C. Moffitt, and Charles Oliver Morton. 2025. "An Introduction to the Influence of Nutritional Factors on the Pathogenesis of Opportunist Fungal Pathogens in Humans" Pathogens 14, no. 4: 335. https://doi.org/10.3390/pathogens14040335
APA StyleJong, T., Stack, C. M., Moffitt, M. C., & Morton, C. O. (2025). An Introduction to the Influence of Nutritional Factors on the Pathogenesis of Opportunist Fungal Pathogens in Humans. Pathogens, 14(4), 335. https://doi.org/10.3390/pathogens14040335







