Immunogenicity, Pathogenesis, and Host’s Immuno-Responses to Marburg Virus Infection
Abstract
1. Introduction
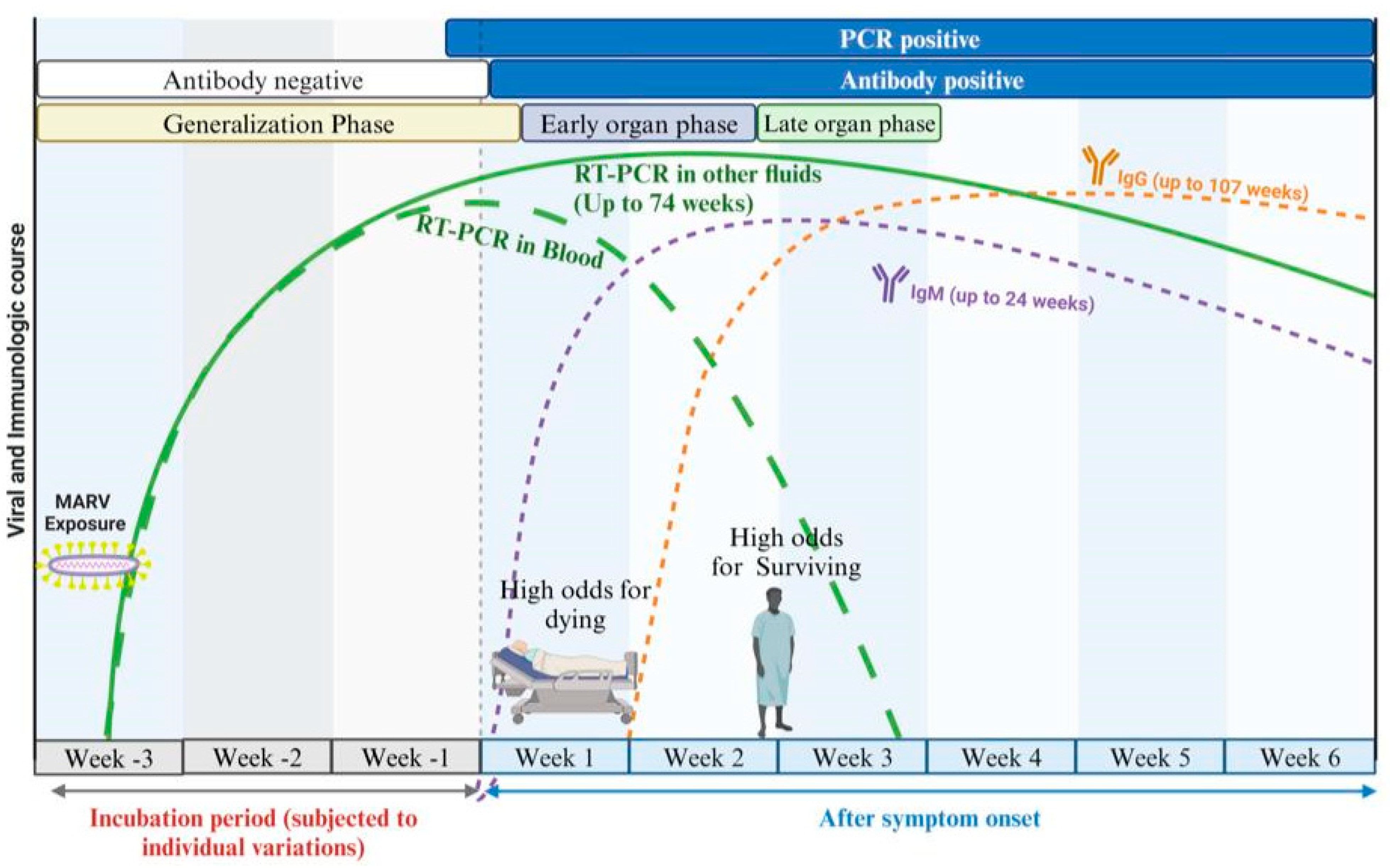
2. Overview of MARV Disease Progression
3. Viral Entry and Budding
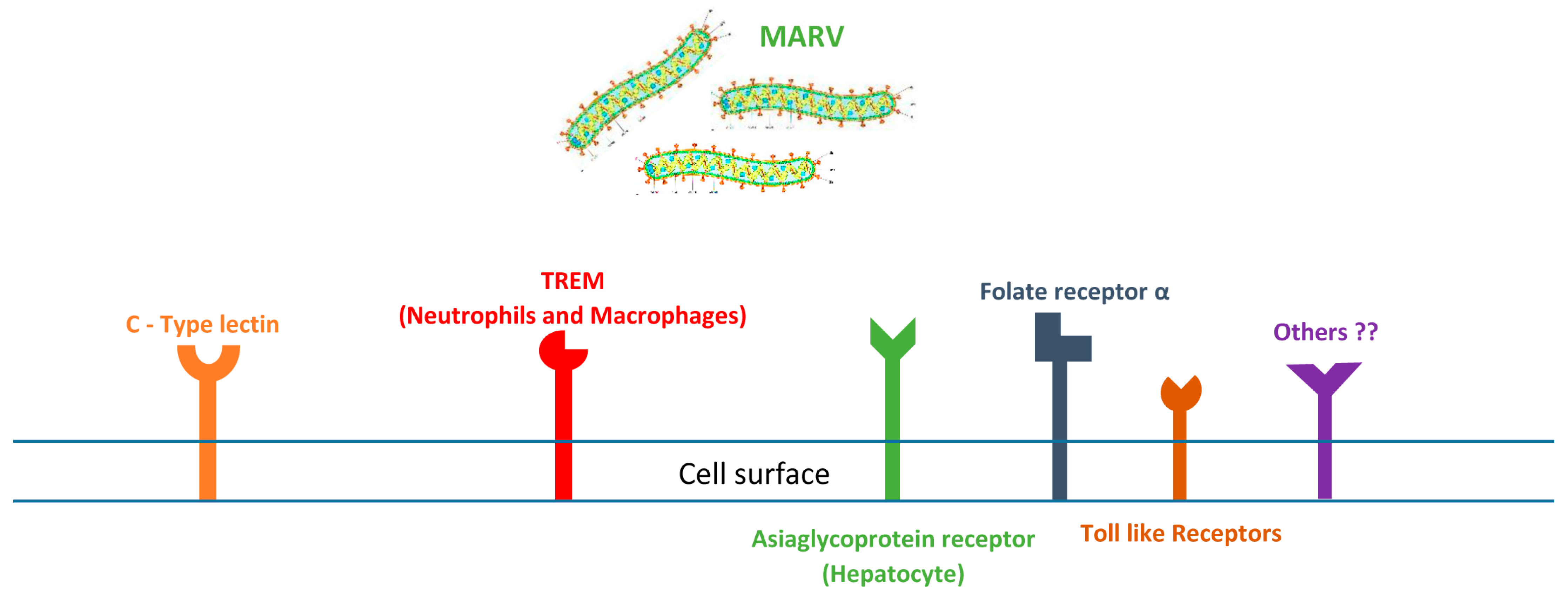
4. How Marburg Virus Targets Cells: Receptor-Mediated Infection
5. Cellular Injury and Viral Targeting
5.1. Liver
5.2. Adrenal Gland
5.3. Pancreas
5.4. Spleen
5.5. Gastrointestinal Tract
5.6. Kidney
5.7. Skin and Mucous Membrane
5.8. Reproductive System
5.9. Bone Marrow
5.10. Cardiovascular and Nervous System
6. Host Immune Response
6.1. Innate Immunity and MARV
6.2. Adaptive Immune Response
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Biedenkopf, N.; Bukreyev, A.; Chandran, K.; Paola, N.D.; Formenty, P.B.H.; Griffiths, A.; Hume, A.J.; Mühlberger, E.; Netesov, S.V.; Palacios, G.; et al. ICTV Virus Taxonomy Profile: Filoviridae 2024. J. Gen. Virol. 2024, 105, 001955. [Google Scholar] [CrossRef] [PubMed]
- Muvunyi, C.M.; Mohamed, N.S.; Siddig, E.E.; Ahmed, A. Genomic Evolution and Phylodynamics of the Species Orthomarburgvirus Marburgense (Marburg and Ravn Viruses) to Understand Viral Adaptation and Marburg Virus Disease’s Transmission Dynamics. Pathogens 2024, 13, 1107. [Google Scholar] [CrossRef] [PubMed]
- Muvunyi, C.M.; Ngabonziza, J.C.S.; Bigirimana, N.; Ndembi, N.; Siddig, E.E.; Kaseya, J.; Ahmed, A. Evidence-Based Guidance for One Health Preparedness, Prevention, and Response Strategies to Marburg Virus Disease Outbreaks. Diseases 2024, 12, 309. [Google Scholar] [CrossRef]
- Muvunyi, C.M.; Bigirimana, N.; Tuyishime, A.; Mukagatare, I.; Ngabonziza, J.C.; Ahmed, A. Initiatives and Strategies to Strengthen the National, Regional, and International Global Health Security: A Case Study of Rwanda Biomedical Centre. 2024. Available online: https://ssrn.com/abstract=4957490 (accessed on 3 October 2024). [CrossRef]
- Gashegu, M.; Ahmed, A.; Clarisse, M.; Remera, E.; Tuyishime, A.; Rwagasore, E.; Muhizi, D.; Kanesa, N.; Ndayisenga, F.; Thadee, T.; et al. One Health Prioritization for Zoonotic Diseases of Public Health Importance in Rwanda. 2024. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4957490 (accessed on 13 November 2024).
- Pigott, D.M.; Golding, N.; Mylne, A.; Huang, Z.; Weiss, D.J.; Brady, O.J.; Kraemer, M.U.G.; Hay, S.I. Mapping the Zoonotic Niche of Marburg Virus Disease in Africa. Trans. R. Soc. Trop. Med. Hyg. 2015, 109, 366–378. [Google Scholar] [CrossRef]
- WHO. List of Blueprint Priority Diseases. Available online: https://www.who.int/activities/prioritizing-diseases-for-research-and-development-in-emergency-contexts/ (accessed on 19 May 2019).
- The Global Alliance for Vaccines and Immunizations (GAVI). The Next Pandemic: Marburg? Available online: https://www.gavi.org/vaccineswork/next-pandemic/marburg (accessed on 10 October 2024).
- The United Nations Environment Programme (UNEP). Preventing the Next Pandemic—Zoonotic Diseases and How to Break the Chain of Transmission. Available online: http://www.unenvironment.org/resources/report/preventing-future-zoonotic-disease-outbreaks-protecting-environment-animals-and (accessed on 7 July 2020).
- Shifflett, K.; Marzi, A. Marburg Virus Pathogenesis—Differences and Similarities in Humans and Animal Models. Virol. J. 2019, 16, 165. [Google Scholar] [CrossRef]
- Mehedi, M.; Groseth, A.; Feldmann, H.; Ebihara, H. Clinical Aspects of Marburg Hemorrhagic Fever. Future Virol. 2011, 6, 1091–1106. [Google Scholar] [CrossRef]
- Stille, W.; Böhle, E. Clinical Course and Prognosis of Marburg Virus (“Green-Monkey”) Disease. In Marburg Virus Disease; Martini, G.A., Siegert, R., Eds.; Springer: Berlin/Heidelberg, Germany, 1971; pp. 10–18. ISBN 978-3-662-01593-3. [Google Scholar]
- Hunter, N.; Rathish, B. Marburg Virus Disease. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Kortepeter, M.G.; Dierberg, K.; Shenoy, E.S.; Cieslak, T.J. Marburg Virus Disease: A Summary for Clinicians. Int. J. Infect. Dis. 2020, 99, 233–242. [Google Scholar] [CrossRef]
- Martini, G.A. Marburg Virus Disease. Postgrad. Med. J. 1973, 49, 542–546. [Google Scholar] [CrossRef] [PubMed]
- Stonier, S.W.; Herbert, A.S.; Kuehne, A.I.; Sobarzo, A.; Habibulin, P.; Dahan, C.V.A.; James, R.M.; Egesa, M.; Cose, S.; Lutwama, J.J. Marburg Virus Survivor Immune Responses Are Th1 Skewed with Limited Neutralizing Antibody Responses. J. Exp. Med. 2017, 214, 2563–2572. [Google Scholar] [CrossRef]
- Gordon, T.B.; Hayward, J.A.; Marsh, G.A.; Baker, M.L.; Tachedjian, G. Host and Viral Proteins Modulating Ebola and Marburg Virus Egress. Viruses 2019, 11, 25. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Hope, T.J.; Young, J.A.T. Differential Requirements for Clathrin Endocytic Pathway Components in Cellular Entry by Ebola and Marburg Glycoprotein Pseudovirions. Virology 2011, 419, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Abir, M.H.; Rahman, T.; Das, A.; Etu, S.N.; Nafiz, I.H.; Rakib, A.; Mitra, S.; Emran, T.B.; Dhama, K.; Islam, A.; et al. Pathogenicity and Virulence of Marburg Virus. Virulence 2022, 13, 609–633. [Google Scholar] [CrossRef]
- Schmidt, K.M.; Mühlberger, E. Marburg Virus Reverse Genetics Systems. Viruses 2016, 8, 178. [Google Scholar] [CrossRef]
- Kolesnikova, L.; Ryabchikova, E.; Shestopalov, A.; Becker, S. Basolateral Budding of Marburg Virus: VP40 Retargets Viral Glycoprotein GP to the Basolateral Surface. J. Infect. Dis. 2007, 196, S232–S236. [Google Scholar] [CrossRef] [PubMed]
- Debroy, B.; Chowdhury, S.; Pal, K. Designing a Novel and Combinatorial Multi-Antigenic Epitope-Based Vaccine “MarVax” against Marburg Virus—A Reverse Vaccinology and Immunoinformatics Approach. J. Genet. Eng. Biotechnol. 2023, 21, 143. [Google Scholar] [CrossRef] [PubMed]
- Mohamadzadeh, M.; Chen, L.; Schmaljohn, A.L. How Ebola and Marburg Viruses Battle the Immune System. Nat. Rev. Immunol. 2007, 7, 556–567. [Google Scholar] [CrossRef]
- Kumar, H.; Kawai, T.; Akira, S. Pathogen Recognition by the Innate Immune System. Int. Rev. Immunol. 2011, 30, 16–34. [Google Scholar] [CrossRef]
- Kolli, D.; Velayutham, T.S.; Casola, A. Host-Viral Interactions: Role of Pattern Recognition Receptors (PRRs) in Human Pneumovirus Infections. Pathogens 2013, 2, 232–263. [Google Scholar] [CrossRef]
- Connor, J.H.; Yen, J.; Caballero, I.S.; Garamszegi, S.; Malhotra, S.; Lin, K.; Hensley, L.; Goff, A.J. Transcriptional Profiling of the Immune Response to Marburg Virus Infection. J. Virol. 2015, 89, 9865. [Google Scholar] [CrossRef]
- Marzi, A.; Gramberg, T.; Simmons, G.; Möller, P.; Rennekamp, A.J.; Krumbiegel, M.; Geier, M.; Eisemann, J.; Turza, N.; Saunier, B.; et al. DC-SIGN and DC-SIGNR Interact with the Glycoprotein of Marburg Virus and the S Protein of Severe Acute Respiratory Syndrome Coronavirus. J. Virol. 2004, 78, 12090–12095. [Google Scholar] [CrossRef]
- Prescott, J.; Guito, J.C.; Spengler, J.R.; Arnold, C.E.; Schuh, A.J.; Amman, B.R.; Sealy, T.K.; Guerrero, L.W.; Palacios, G.F.; Sanchez-Lockhart, M.; et al. Rousette Bat Dendritic Cells Overcome Marburg Virus-Mediated Antiviral Responses by Upregulation of Interferon-Related Genes While Downregulating Proinflammatory Disease Mediators. mSphere 2019, 4, e00728-19. [Google Scholar] [CrossRef] [PubMed]
- Yordanova, I.A.; Lander, A.; Wahlbrink, A.; Towner, J.S.; Albariño, C.G.; Ang, L.T.; Prescott, J.B. Human Macrophages Infected with Egyptian Rousette Bat-Isolated Marburg Virus Display Inter-Individual Susceptibility and Antiviral Responsiveness. npj Viruses 2024, 2, 19. [Google Scholar] [CrossRef]
- Olejnik, J.; Ryabchikova, E.; Corley, R.B.; Mühlberger, E. Intracellular Events and Cell Fate in Filovirus Infection. Viruses 2011, 3, 1501–1531. [Google Scholar] [CrossRef] [PubMed]
- Takada, A.; Fujioka, K.; Tsuiji, M.; Morikawa, A.; Higashi, N.; Ebihara, H.; Kobasa, D.; Feldmann, H.; Irimura, T.; Kawaoka, Y. Human Macrophage C-Type Lectin Specific for Galactose and N-Acetylgalactosamine Promotes Filovirus Entry. J. Virol. 2004, 78, 2943–2947. [Google Scholar] [CrossRef]
- Simmons, G.; Reeves, J.D.; Grogan, C.C.; Vandenberghe, L.H.; Baribaud, F.; Whitbeck, J.C.; Burke, E.; Buchmeier, M.J.; Soilleux, E.J.; Riley, J.L.; et al. DC-SIGN and DC-SIGNR Bind Ebola Glycoproteins and Enhance Infection of Macrophages and Endothelial Cells. Virology 2003, 305, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Becker, S.; Spiess, M.; Klenk, H.-D. The Asialoglycoprotein Receptor Is a Potential Liver-Specific Receptor for Marburg Virus. J. Gen. Virol. 1995, 76, 393–399. [Google Scholar] [CrossRef]
- Matsuno, K.; Kishida, N.; Usami, K.; Igarashi, M.; Yoshida, R.; Nakayama, E.; Shimojima, M.; Feldmann, H.; Irimura, T.; Kawaoka, Y.; et al. Different Potential of C-Type Lectin-Mediated Entry between Marburg Virus Strains. J. Virol. 2010, 84, 5140–5147. [Google Scholar] [CrossRef]
- Mohamadzadeh, M.; Coberley, S.S.; Olinger, G.G.; Kalina, W.V.; Ruthel, G.; Fuller, C.L.; Swenson, D.L.; Pratt, W.D.; Kuhns, D.B.; Schmaljohn, A.L. Activation of Triggering Receptor Expressed on Myeloid Cells-1 on Human Neutrophils by Marburg and Ebola Viruses. J. Virol. 2006, 80, 7235–7244. [Google Scholar] [CrossRef]
- Roe, K.; Gibot, S.; Verma, S. Triggering Receptor Expressed on Myeloid Cells-1 (TREM-1): A New Player in Antiviral Immunity? Front. Microbiol. 2014, 5, 627. [Google Scholar] [CrossRef]
- Chan, S.Y.; Empig, C.J.; Welte, F.J.; Speck, R.F.; Schmaljohn, A.; Kreisberg, J.F.; Goldsmith, M.A. Folate Receptor-α Is a Cofactor for Cellular Entry by Marburg and Ebola Viruses. Cell 2001, 106, 117–126. [Google Scholar] [CrossRef]
- Olejnik, J.; Hume, A.J.; Leung, D.W.; Amarasinghe, G.K.; Basler, C.F.; Mühlberger, E. Filovirus Strategies to Escape Antiviral Responses. In Marburg- and Ebolaviruses: From Ecosystems to Molecules; Mühlberger, E., Hensley, L.L., Towner, J.S., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 293–322. ISBN 978-3-319-68948-7. [Google Scholar]
- Basler, C.F. Innate Immune Evasion by Filoviruses. Virology 2015, 479–480, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Soltan, M.A.; Abdulsahib, W.K.; Amer, M.; Refaat, A.M.; Bagalagel, A.A.; Diri, R.M.; Albogami, S.; Fayad, E.; Eid, R.A.; Sharaf, S.M.A.; et al. Mining of Marburg Virus Proteome for Designing an Epitope-Based Vaccine. Front. Immunol. 2022, 13, 907481. [Google Scholar] [CrossRef] [PubMed]
- Glaze, E.R.; Roy, M.J.; Dalrymple, L.W.; Lanning, L.L. A Comparison of the Pathogenesis of Marburg Virus Disease in Humans and Nonhuman Primates and Evaluation of the Suitability of These Animal Models for Predicting Clinical Efficacy under the “Animal Rule”. Comp. Med. 2015, 65, 241–259. [Google Scholar]
- Geisbert, T.W.; Jaax, N.K. Marburg Hemorrhagic Fever: Report of a Case Studied by Immunohistochemistry and Electron Microscopy. Ultrastruct. Pathol. 1998, 22, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Bechtelsheimer, H.; Korb, G.; Gedigk, P. The Morphology and Pathogenesis of “Marburg Virus” Hepatitis. Hum. Pathol. 1972, 3, 255–264. [Google Scholar] [CrossRef]
- Borchert, M.; Muyembe-Tamfum, J.J.; Colebunders, R.; Libande, M.; Sabue, M.; Van der Stuyft, P. Short Communication: A Cluster of Marburg Virus Disease Involving an Infant. Trop. Med. Int. Health 2002, 7, 902–906. [Google Scholar] [CrossRef]
- Kortepeter, M.G.; Bausch, D.G.; Bray, M. Basic Clinical and Laboratory Features of Filoviral Hemorrhagic Fever. J. Infect. Dis. 2011, 204, S810–S816. [Google Scholar] [CrossRef]
- Spengler, U.; Fischer, H.-P.; Caselmann, W.H. Chapter 34—Liver Disease Associated with Viral Infections. Zakim Boyer’s Hepatol. 2011, 629–643. [Google Scholar]
- Zumbrun, E.E.; Garvey, C.B.; Wells, J.B.; Lynn, G.C.; Van Tongeren, S.; Steffens, J.T.; Wetzel, K.S.; Gomba, L.M.; O’Brien, K.A.; Rossi, F.D.; et al. Characterization of the Cynomolgus Macaque Model of Marburg Virus Disease and Assessment of Timing for Therapeutic Treatment Testing. Viruses 2023, 15, 2335. [Google Scholar] [CrossRef]
- Mahanty, S.; Bray, M. Pathogenesis of Filoviral Haemorrhagic Fevers. Lancet Infect. Dis. 2004, 4, 487–498. [Google Scholar] [CrossRef]
- Paolo, W.F.; Nosanchuk, J.D. Adrenal Infections. Int. J. Infect. Dis. 2006, 10, 343–353. [Google Scholar] [CrossRef]
- BMJ Best Practice Marburg Virus Infection—Symptoms, Diagnosis and Treatment|BMJ Best Practice. Available online: https://bestpractice.bmj.com/topics/en-gb/1615 (accessed on 16 November 2024).
- Geisbert, T.W.; Hensley, L.E.; Gibb, T.R.; Steele, K.E.; Jaax, N.K.; Jahrling, P.B. Apoptosis Induced in Vitro and in Vivo during Infection by Ebola and Marburg Viruses. Lab. Invest. 2000, 80, 171–186. [Google Scholar] [CrossRef] [PubMed]
- Martines, R.B.; Ng, D.L.; Greer, P.W.; Rollin, P.E.; Zaki, S.R. Tissue and Cellular Tropism, Pathology and Pathogenesis of Ebola and Marburg Viruses. J. Pathol. 2015, 235, 153–174. [Google Scholar] [CrossRef] [PubMed]
- ZAKI, S.R.; GOLDSMITH, C.S. Pathologic Features of Filovirus Infections in Humans. Curr. Top. Microbiol. Immunol. 1999, 235, 97–116. [Google Scholar] [PubMed]
- Rougeron, V.; Feldmann, H.; Grard, G.; Becker, S.; Leroy, E.M. Ebola and Marburg Haemorrhagic Fever. J. Clin. Virol. 2015, 64, 111–119. [Google Scholar] [CrossRef]
- Siegert, R. Marburg Virus. In Canine Distemper Virus: Marburg Virus; Appel, M.J.G., Gillespie, J.H., Siegert, R., Eds.; Springer: Vienna, Austria, 1972; pp. 97–153. ISBN 978-3-7091-8302-1. [Google Scholar]
- Nkoghe, D.; Leroy, E.M.; Toung-Mve, M.; Gonzalez, J.P. Cutaneous Manifestations of Filovirus Infections. Int. J. Dermatol. 2012, 51, 1037–1043. [Google Scholar] [CrossRef]
- Manohar, M.P.M.; Lee, V.J.; Odunukwe, E.U.C.; Singh, P.K.; Mpofu, B.S.; Christine Oxley, M.D. Advancements in Marburg (MARV) Virus Vaccine Research With Its Recent Reemergence in Equatorial Guinea and Tanzania: A Scoping Review. Cureus 2023, 15, e42014. [Google Scholar] [CrossRef]
- Asad, A.; Aamir, A.; Qureshi, N.E.; Bhimani, S.; Jatoi, N.N.; Batra, S.; Ochani, R.K.; Abbasi, M.K.; Tariq, M.A.; Diwan, M.N. Past and Current Advances in Marburg Virus Disease: A Review. Infez. Med. 2020, 28, 332–345. [Google Scholar]
- Groß, J.V.; Slanger, T.E.; Cullen, P.; Erren, M.; Erren, T.C. Stopping Possible Sexual Transmission of Filoviruses. Clin. Infect. Dis. 2015, 60, 1871–1872. [Google Scholar] [CrossRef][Green Version]
- Coffin, K.M.; Liu, J.; Warren, T.K.; Blancett, C.D.; Kuehl, K.A.; Nichols, D.K.; Bearss, J.J.; Schellhase, C.W.; Retterer, C.J.; Weidner, J.M.; et al. Persistent Marburg Virus Infection in the Testes of Nonhuman Primate Survivors. Cell Host Microbe 2018, 24, 405–416.e3. [Google Scholar] [CrossRef]
- Zapata, J.C.; Cox, D.; Salvato, M.S. The Role of Platelets in the Pathogenesis of Viral Hemorrhagic Fevers. PLOS Neglected Trop. Dis. 2014, 8, e2858. [Google Scholar] [CrossRef]
- Schnittler, H.J.; Mahner, F.; Drenckhahn, D.; Klenk, H.D.; Feldmann, H. Replication of Marburg Virus in Human Endothelial Cells. A Possible Mechanism for the Development of Viral Hemorrhagic Disease. J. Clin. Investig. 1993, 91, 1301–1309. [Google Scholar] [CrossRef]
- Zarate-Sanchez, E.; George, S.C.; Moya, M.L.; Robertson, C. Vascular Dysfunction in Hemorrhagic Viral Fevers: Opportunities for Organotypic Modeling. Biofabrication 2024, 16, 032008. [Google Scholar] [CrossRef]
- Manfrini, N.; Notarbartolo, S.; Grifantini, R.; Pesce, E. SARS-CoV-2: A Glance at the Innate Immune Response Elicited by Infection and Vaccination. Antibodies 2024, 13, 13. [Google Scholar] [CrossRef] [PubMed]
- Basler, C.F.; Amarasinghe, G.K. Evasion of Interferon Responses by Ebola and Marburg Viruses. J. Interferon Cytokine Res. 2009, 29, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Ramanan, P.; Shabman, R.S.; Brown, C.S.; Amarasinghe, G.K.; Basler, C.F.; Leung, D.W. Filoviral Immune Evasion Mechanisms. Viruses 2011, 3, 1634–1649. [Google Scholar] [CrossRef]
- Ramanan, P.; Edwards, M.R.; Shabman, R.S.; Leung, D.W.; Endlich-Frazier, A.C.; Borek, D.M.; Otwinowski, Z.; Liu, G.; Huh, J.; Basler, C.F.; et al. Structural Basis for Marburg Virus VP35–Mediated Immune Evasion Mechanisms. Proc. Natl. Acad. Sci. USA 2012, 109, 20661–20666. [Google Scholar] [CrossRef]
- Sallusto, F.; Lanzavecchia, A. The Instructive Role of Dendritic Cells on T-Cell Responses. Arthritis Res. Ther. 2002, 4, S127. [Google Scholar] [CrossRef]
- Liu, K. Dendritic Cells. Encycl. Cell Biol. 2015, 741–749. [Google Scholar] [CrossRef]
- Bosio, C.M.; Aman, M.J.; Grogan, C.; Hogan, R.; Ruthel, G.; Negley, D.; Mohamadzadeh, M.; Bavari, S.; Schmaljohn, A. Ebola and Marburg Viruses Replicate in Monocyte-Derived Dendritic Cells without Inducing the Production of Cytokines and Full Maturation. J. Infect. Dis. 2003, 188, 1630–1638. [Google Scholar] [CrossRef]
- Neurath, A. Immune Response to Viruses: Antibody-Mediated Immunity. Encycl. Virol. 2008, 56–70. [Google Scholar]
- Kumar, A.; Tripathi, P.; Kumar, P.; Shekhar, R.; Pathak, R. From Detection to Protection: Antibodies and Their Crucial Role in Diagnosing and Combatting SARS-CoV-2. Vaccines 2024, 12, 459. [Google Scholar] [CrossRef]
- Wulff, H.; Johnson, K.M. Immunoglobulin M and G Responses Measured by Immunofluorescence in Patients with Lassa or Marburg Virus Infections. Bull. World Health Organ. 1979, 57, 631. [Google Scholar] [PubMed]
- Emperador, D.M.; Mazzola, L.T.; Trainor, B.W.; Chua, A.; Kelly-Cirino, C. Diagnostics for Filovirus Detection: Impact of Recent Outbreaks on the Diagnostic Landscape. BMJ Glob. Health 2019, 4, e001112. [Google Scholar] [CrossRef]
- Björkström, N.K.; Strunz, B.; Ljunggren, H.-G. Natural Killer Cells in Antiviral Immunity. Nat. Rev. Immunol. 2022, 22, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Fritz, E.A.; Geisbert, J.B.; Geisbert, T.W.; Hensley, L.E.; Reed, D.S. Cellular Immune Response to Marburg Virus Infection in Cynomolgus Macaques. Viral Immunol. 2008, 21, 355–364. [Google Scholar] [CrossRef]
- Hensley, L.E.; Alves, D.A.; Geisbert, J.B.; Fritz, E.A.; Reed, C.; Larsen, T.; Geisbert, T.W. Pathogenesis of Marburg Hemorrhagic Fever in Cynomolgus Macaques. J. Infect. Dis. 2011, 204, S1021–S1031. [Google Scholar] [CrossRef] [PubMed]
- Ströher, U.; West, E.; Bugany, H.; Klenk, H.-D.; Schnittler, H.-J.; Feldmann, H. Infection and Activation of Monocytes by Marburg and Ebola Viruses. J. Virol. 2001, 75, 11025–11033. [Google Scholar] [CrossRef]
- Alves, D.A.; Glynn, A.R.; Steele, K.E.; Lackemeyer, M.G.; Garza, N.L.; Buck, J.G.; Mech, C.; Reed, D.S. Aerosol Exposure to the Angola Strain of Marburg Virus Causes Lethal Viral Hemorrhagic Fever in Cynomolgus Macaques. Vet. Pathol. 2010, 47, 831–851. [Google Scholar] [CrossRef]
- Geisbert, T.W.; Daddario-DiCaprio, K.M.; Geisbert, J.B.; Young, H.A.; Formenty, P.; Fritz, E.A.; Larsen, T.; Hensley, L.E. Marburg Virus Angola Infection of Rhesus Macaques: Pathogenesis and Treatment with Recombinant Nematode Anticoagulant Protein C2. J. Infect. Dis. 2007, 196, S372–S381. [Google Scholar] [CrossRef]
- Barrientos, L.G.; Rollin, P.E. Release of Cellular Proteases into the Acidic Extracellular Milieu Exacerbates Ebola Virus-Induced Cell Damage. Virology 2007, 358, 1–9. [Google Scholar] [CrossRef]
- Sanchez, A.; Yang, Z.-Y.; Xu, L.; Nabel, G.J.; Crews, T.; Peters, C.J. Biochemical Analysis of the Secreted and Virion Glycoproteins of Ebola Virus. J. Virol. 1998, 72, 6442–6447. [Google Scholar] [CrossRef]
- Dolnik, O.; Volchkova, V.; Garten, W.; Carbonnelle, C.; Becker, S.; Kahnt, J.; Ströher, U.; Klenk, H.; Volchkov, V. Ectodomain Shedding of the Glycoprotein GP of Ebola Virus. EMBO J. 2004, 23, 2175–2184. [Google Scholar] [CrossRef] [PubMed]
- Hawiger, D.; Inaba, K.; Dorsett, Y.; Guo, M.; Mahnke, K.; Rivera, M.; Ravetch, J.V.; Steinman, R.M.; Nussenzweig, M.C. Dendritic Cells Induce Peripheral T Cell Unresponsiveness under Steady State Conditions in Vivo. J. Exp. Med. 2001, 194, 769–780. [Google Scholar] [CrossRef] [PubMed]
- Mahanty, S.; Hutchinson, K.; Agarwal, S.; Mcrae, M.; Rollin, P.E.; Pulendran, B. Cutting Edge: Impairment of Dendritic Cells and Adaptive Immunity by Ebola and Lassa Viruses. J. Immunol. 2003, 170, 2797–2801. [Google Scholar] [CrossRef]
- Chaplin, D.D. Overview of the Immune Response. J. Allergy Clin. Immunol. 2010, 125, S3–S23. [Google Scholar] [CrossRef]
- Fernando, L.; Qiu, X.; Melito, P.L.; Williams, K.J.; Feldmann, F.; Feldmann, H.; Jones, S.M.; Alimonti, J.B. Immune Response to Marburg Virus Angola Infection in Nonhuman Primates. J. Infect. Dis. 2015, 212, S234–S241. [Google Scholar] [CrossRef]
- Guito, J.C.; Kirejczyk, S.G.M.; Schuh, A.J.; Amman, B.R.; Sealy, T.K.; Graziano, J.; Spengler, J.R.; Harmon, J.R.; Wozniak, D.M.; Prescott, J.B.; et al. Coordinated Inflammatory Responses Dictate Marburg Virus Control by Reservoir Bats. Nat. Commun. 2024, 15, 1826. [Google Scholar] [CrossRef] [PubMed]
- Warfield, K.L.; Bradfute, S.B.; Wells, J.; Lofts, L.; Cooper, M.T.; Alves, D.A.; Reed, D.K.; VanTongeren, S.A.; Mech, C.A.; Bavari, S. Development and Characterization of a Mouse Model for Marburg Hemorrhagic Fever. J. Virol. 2009, 83, 6404–6415. [Google Scholar] [CrossRef]
- Ciesielska-Figlon, K.; Lisowska, K.A. The Role of the CD28 Family Receptors in T-Cell Immunomodulation. Int. J. Mol. Sci. 2024, 25, 1274. [Google Scholar] [CrossRef]
- Schildberg, F.A.; Klein, S.R.; Freeman, G.J.; Sharpe, A.H. Coinhibitory Pathways in the B7-CD28 Ligand-Receptor Family. Immunity 2016, 44, 955–972. [Google Scholar] [CrossRef]
- Keir, M.E.; Butte, M.J.; Freeman, G.J.; Sharpe, A.H. PD-1 and Its Ligands in Tolerance and Immunity. Annu. Rev. Immunol. 2008, 26, 677–704. [Google Scholar] [CrossRef] [PubMed]
- Arasanz, H.; Gato-Cañas, M.; Zuazo, M.; Ibañez-Vea, M.; Breckpot, K.; Kochan, G.; Escors, D. PD1 Signal Transduction Pathways in T Cells. Oncotarget 2017, 8, 51936. [Google Scholar] [CrossRef] [PubMed]
- Blank, C.; Mackensen, A. Contribution of the PD-L1/PD-1 Pathway to T-Cell Exhaustion: An Update on Implications for Chronic Infections and Tumor Evasion. Cancer Immunol. Immunother. 2007, 56, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Bixler, S.L.; Goff, A.J. The Role of Cytokines and Chemokines in Filovirus Infection. Viruses 2015, 7, 5489–5507. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siddig, E.E.; Ndembi, N.; Ahmed, A.; Muvunyi, C.M. Immunogenicity, Pathogenesis, and Host’s Immuno-Responses to Marburg Virus Infection. Pathogens 2025, 14, 323. https://doi.org/10.3390/pathogens14040323
Siddig EE, Ndembi N, Ahmed A, Muvunyi CM. Immunogenicity, Pathogenesis, and Host’s Immuno-Responses to Marburg Virus Infection. Pathogens. 2025; 14(4):323. https://doi.org/10.3390/pathogens14040323
Chicago/Turabian StyleSiddig, Emmanuel Edwar, Nicaise Ndembi, Ayman Ahmed, and Claude Mambo Muvunyi. 2025. "Immunogenicity, Pathogenesis, and Host’s Immuno-Responses to Marburg Virus Infection" Pathogens 14, no. 4: 323. https://doi.org/10.3390/pathogens14040323
APA StyleSiddig, E. E., Ndembi, N., Ahmed, A., & Muvunyi, C. M. (2025). Immunogenicity, Pathogenesis, and Host’s Immuno-Responses to Marburg Virus Infection. Pathogens, 14(4), 323. https://doi.org/10.3390/pathogens14040323





