Abstract
Chagas disease, caused by Trypanosoma cruzi, is a neglected tropical disease with few options for treatment and no available vaccine. Deletion mutants for live attenuated vaccines, particularly deletions of proteins related to the cytoskeleton, have been widely tested in related parasites but candidates have not been tested in T. cruzi. Kharon is one such protein, identified as being associated with the cytoskeleton in Leishmania and essential for amastigote replication. Here we investigated the T. cruzi Kharon ortholog (TcKharon) to test if it has orthologous function and thus potential in generating a live attenuated vaccine. In silico analysis predicted TcKharon to be an intrinsically disordered protein, consistent with its ortholog feature, and GFP fusion protein revealed that TcKharon is associated with the cytoskeleton of epimastigotes. CRISPR-Cas9-mediated gene disruption impaired epimastigote proliferation and cytokinesis, resulting in altered nucleus-to-kinetoplast ratios and pronounced morphological defects, particularly in the posterior cell region. Despite these abnormalities, TcKharon−/− mutants retained the ability to differentiate into metacyclic trypomastigotes and exhibited in vitro infection rates comparable to wild-type parasites. Our data show that TcKharon is crucial for cell morphology. However, in contrast to close related parasites, TcKharon is not essential for in vitro infectivity.
1. Introduction
Chagas disease is a neglected tropical disease that affects 6–8 million people in Latin America [1]. Available treatments for the chronic stage of the disease have limited efficiency and significant side effects [2], while the development of vaccines is hindered by the fact that the causative agent, Trypanosoma cruzi, uses specialized mechanisms of immune escape. Thus far, few T. cruzi vaccine candidates have been investigated, yielding unsatisfactory results [3]. The use of live attenuated vaccines appears to be an option for diseases caused by trypanosomatids since it has been shown that inoculation with a living avirulent strain of a parasite protects against challenge with a respective pathogenic strain [4,5,6].
Currently, no approved vaccine exists for Chagas disease. Among the various vaccination strategies, live attenuated vaccines offer a promising alternative, although their development presents significant challenges. While some potential targets have been identified [7,8], and tested vaccines have reduced disease burden, the risk of relapse remains. An effective target for attenuation must prevent vector-borne parasite transmission, ensure stable attenuation (e.g., through gene knockout), exhibit limited replication to ensure safety, and elicit a robust immune response. Identifying novel targets for generating attenuated parasites is therefore crucial. Genes encoding proteins involved in replication, metabolism, and cell cycle progression are considered promising candidates [9,10]. Certain cytoskeleton-associated proteins, which play roles in cell division, motility, cytokinesis/mitosis, and organelle positioning, may fulfill these criteria [6,9,10].
Microtubules, which are key components of the cytoskeleton, are found in the flagellar axoneme, basal body, and subpellicular microtubules of T. cruzi. This last structure, located near the plasma membrane, maintains the parasite’s shape [11] and likely contributes to its survival in both insect and mammalian hosts. The subpellicular microtubules form a crosslinked network through microtubule-associated proteins (MAPs) [11]. Studies of T. brucei have identified over 70 proteins associated with the subpellicular array [12], with some localized throughout the array and others to specific subdomains, such as the cell body, reflecting potential local specializations (reviewed by Sinclair et al. [13]).
Subpellicular array-associated proteins are of interest as the deletion of genes encoding proteins that impair Leishmania division in the mammalian host. Notably, centrin-4 and Kharon-1 have been identified as candidates for parasite attenuation in vaccine development [6,14,15]. Interestingly, Kharon-1 was first identified in L. mexicana as a protein necessary for the specific localization of glucose transporter-1 (LmxGT1) to the flagellar membrane [16]. Similar function was later shown in T. brucei [17]. Endogenous tagging showed that Kharon localizes to the subpellicular microtubules, the basal body in L. mexicana [18], at the base of the flagellum, or subpellicular microtubules and mitotic spindle in bloodstream forms of T. brucei [17]. RNAi knockdown of TbKharon showed that it is necessary for normal cell division of the procyclic and bloodstream forms [17] while a deletion mutant showed that LmxKharon is necessary for normal cell division, but only in the amastigote form [18]. This mammalian stage-specific phenotype led to its gaining particular interest as a live attenuated vaccine candidate.
Kharon is one of a set of proteins that are spatially proximal, localized to the entire subpellicular microtubule array, and are necessary for normal division and morphogenesis. Two LmKharon-associated proteins, which localize only to the subpellicular microtubules, were identified by proximity labeling (BioID) [15]. The first (KHAP1, an ortholog of T. brucei MARP1) is necessary for normal Leishmania division [15], as would be expected given the T. brucei RNAi phenotype. MARP-1was previously identified as a repetitive-domain contained protein that binds to microtubules of the cytoskeleton in T. brucei [19]. The second (KHAP2) was also necessary for normal division [15]. In T. brucei CAP5.5 proximity labeling independently identified the KHAP2 T. brucei ortholog (which was named CAP50), along with CAP42 and CAP52, all of which were necessary for normal morphogenesis and cytokinesis [20]. Kharon is therefore one of a set of closely positioned subpellicular-associated proteins that are necessary for normal morphogenesis and cytokinesis (MARP, CAP5.5, Kharon, KHAP2, CAP52 and CAP42), but itself has an additional function in defining specialized plasma membrane domains.
While these and other subpellicular array-associated proteins have orthologs in many trypanosomatids, they often have high sequence divergence and do not necessarily have the same function. This can be illustrated with a protein in a different cytoskeletal structure. For example, FLA-1 in the flagellum attachment zone is essential for cytokinesis in T. brucei, however, its ortholog gp72 in T. cruzi can be deleted [21,22]. This highlights the need for studying these molecular players in each species.
Here, we analyze the cellular phenotype resulting from genetic disruption of the T. cruzi Kharon ortholog (TcKharon). Our aims were to: (1) determine TcKharon’s role in cell morphogenesis and division across different life stages, and (2) assess its potential as a target for parasite attenuation in vaccine development. Using markerless CRISPR-Cas9-mediated TcKharon disruption, we demonstrate that TcKharon is essential for normal morphogenesis but not for in vitro host cell infection.
2. Material and Methods
2.1. Bioinformatic Analysis
Kharon amino acid sequences from T. cruzi and related Trypanosomatids were retrieved from TriTrypDB (https://tritrypdb.org/tritrypdb/app accessed on 26 March 2021). The sequences were aligned using Clustal Omega and a phylogenetic tree was built in the SeaView v. 5.0.4 software [23]. pLDDT prediction and 3D structure prediction were performed using “ColabFold” (https://colab.research.google.com accessed on 18 May 2021). The colors in the 3D structure indicate the pLDDT values of each residue.
2.2. Parasite Maintenance and Growth Curve
T. cruzi strain Dm28c and LLC-MK2 cells were obtained from FIOCRUZ-PR (Carlos Chagas Institute—Curitiba/Brazil). T. cruzi epimastigotes of the Dm28c clone were cultured in a liver infusion tryptose (LIT) medium supplemented with heat-inactivated fetal bovine serum (FBS; 10%), hemin, and penicillin/streptomycin [24]. Metacyclic trypomastigotes (MTs) were obtained using the protocol described by Contreras et al. [25]. Briefly, epimastigotes were induced to MT differentiation by incubation on TAU media, and the MTs were purified on a DEAE-Column, and quantified. For growth curve analysis, epimastigotes were counted daily in a Neubauer chamber, and when the culture reached the stationary phase, the culture was diluted in 1× phosphate buffered saline (PBS).
2.3. Molecular Cloning and In Vitro Transcription
For the generation of Kharon-null mutants (TcKharon−/−), first a single guide RNA (sgRNA) was designed and cloned as described by Saenz-Garcia et al. [26]. Briefly, the EuPaGDT v1.0 software (http://grna.ctegd.uga.edu/ accessed on 15 February 2018) was used to find sgRNA target sequences in the TcKharon coding sequence (ID = C4B63_14g70) from the Dm28c genome (TriTrypDB-28) considering the protospacer motif sequence (PAM sequence) of SaCas9 nuclease [27]. From the list that was returned, a sgRNA was selected by considering the score, position (located in the first half of the coding sequence), and whether the sgRNA target sequence was conserved among almost all available T. cruzi genomes.
The oligonucleotides corresponding to the target sequence (sgRNA-TcKharon608-Plus ATAGGCGCTTCCATGAACAAACCAGC and sgRNA-TcKharon608-Minus AAACGCTGGTTTGTTCATGGAAGCGC) were annealed and cloned into the pT7-SaCas9sgRNA-BsaI plasmid [26] that had been previously digested with BsaI, thus generating pT7-SaCas9-sgRNA-TcKharon608. This plasmid was used as a template for in vitro transcription, as suggested by the manufacturer’s protocol (MEGAscript™ T7 Transcription Kit—Thermo Fischer Scientific, Waltham, MA, USA).
To recover TcKharon expression in TcKharon−/− parasites or for overexpression in wild-type cells, the TcKharon coding sequence was fused to the GFP N-terminus, as follows. The primers, TcKharon-For-XbaI (5′-TCATCTAGAATGGCCACGGCAGCAGTTGAGC-3′) and TcKharon-Rev-BglII (5′-TTTAGATCTGGAAGATGTTGTTGATGCCGGTT-3′) were used for PCR amplification using genomic DNA from Dm28c clone. The PCR product was digested with XbaI/BglII, and cloned in the pTREX-Amastin::GFP-Neo plasmid [28] that had been previously digested with XbaI/BamHI. The new plasmid, named as pTREX-TcKharon::GFP-Neo, was transfected by electroporation in the wild-type (WT) and TcKharon−/− epimastigote cells, as previously described by Pacheco-Lugo et al. [29].
2.4. CRISPR/Cas9 Editing and Genotyping
Genome editing using CRISPR/Cas9 to disrupt TcKharon and genotyping of TcKharon−/− mutants were performed as described by Saenz-Garcia et al. [26]. Briefly, epimastigotes were transfected once or twice using the ribonucleoprotein (RNP) complex (SaCas9 plus TcKharon-specific sgRNA) in the presence of donor ssDNA (synthetic oligonucleotide) containing a sequence of three stop codons at different open reading frames to ensure coding sequence disruption after DNA repair and a BglII restriction site flanked by 25 nucleotides upstream and downstream of the SaCas9 cleavage site (AAAGCGCCGCTTCCATGAACAAACCTGAATGACTGAGATCTAAGCGTGAATCTCTTTGGACGTACAG). The parasites electroporated with the RNP and donor sequence were tested for gene disruption by PCR followed by digestion with the BglII enzyme.
2.5. Scanning and Transmission Electron Microscopy
For scanning electron microscopy (SEM) analyses, the parasite samples were processed as described by Souza-Melo et al. [30]. Briefly, epimastigotes were fixed in 2.5% glutaraldehyde diluted in cacodylate buffer (0.1 M, pH 7.2) for 1 h. The cells were adhered to poly-L-lysine coverslips, and treated with 1% osmium tetroxide diluted in cacodylate buffer for 1 h. Samples were dehydrated in a graded ethanol series (50%, 70%, 90%, and two exchanges of 100% ethanol for 10 min each step), and critical-point dried using CO2. The slides were coated with a 5 nm layer of platinum and then visualized in an EVO 10 and ZEISS FIB-SEM AURIGA 40 (Carl Zeiss Microscopy GmbH—Oberkochen, Baden-Württemberg, Germany) scanning electron microscope at the National Center for Structural Biology and Bioimaging (CENABIO) at UFRJ. Cell lengths were measured in the SEM images using the AxioVision4 program.
For transmission electron microscopy (TEM), log-phase epimastigotes were treated following the procedure described for scanning microscopy [31]. After treatment, the cells were washed with cacodylate buffer (0.1 M, pH 7.2) and fixed in cacodylate buffer (0.1 M, pH 7.2) containing paraformaldehyde (4%) and glutaraldehyde (2.5%). All samples were postfixed in osmium tetroxide (1%), dehydrated with acetone series, and embedded in EMbed 812® resin (Electron Microscopy Sciences—Hatfield, PA, USA.). Grids containing ultrathin sections (70 nm) were stained with uranyl acetate and lead citrate, and observed under a transmission electron microscope (TEM; JEOL JEM-1400Plus—JEOL Ltd., Akishima, Tokyo, Japan) at Carlos Chagas Institute (FIOCRUZ-PR, Curitiba, PR, Brazil).
2.6. Immunofluorescence Assay and Localization of GFP-Tagged TcKharon
Epimastigotes of WT, TcKharon−/−, and the addback cell lines, TcKharon-AB (TcKharon−/− carrying pTREX TcKharon::GFP) were fixed with 4% paraformaldehyde and coated on polylysine coverslips for 20 min. Coverslips were washed with PBS and the parasites were permeabilized with PBS containing 0.1% Triton X-100 and then blocked with 3% (m/v) bovine serum albumin (BSA) for 1 h at room temperature. The samples were incubated for 16 h at 4 °C with the monoclonal antibody 2F6 (mAb 2F6, 1:100) and the polyclonal anti-α-tubulin antibody (1:200), which recognize a flagellar protein of ~70 kDa [32] and α-tubulin polymers, respectively. The samples were washed three times with PBS containing 0.05% Tween-20, and incubated with the secondary antibody (anti-mouse conjugated with Alexa-488—Thermo Fisher Scientific, Waltham, MA, USA), 1:500. The coverslips were washed three times, mounted on a microscope slide, and analyzed by confocal microscopy (Nikon A1R Multiphoton confocal microscope—Nikon Corporation, Tokyo, Japan). Parasites of TcKharon-AB, WT overexpressing TcKharon::GFP, and WT overexpressing GFP alone (pTREX-GFP) [33] were also analyzed for confocal microscopy as detailed above. Cytoskeletons from these parasites were obtained by incubating the parasites with 40 μL of cold PEME (100 mM PIPES, 1 mM MgSO4, 0.1 mM EDTA, 2 mM EGTA, pH 6.9) containing Triton X-100 (1% v/v), and then fixed. The images from confocal microscopy were analyzed with the Fiji 2.3.0 (Image J2) software.
2.7. Western Blot
Epimastigotes (5 × 107 cells) of TcKharon-AB and WT expressing TcKharon::GFP were harvested, washed once with PBS, resuspended in Laemmli buffer, sonicated for 3 min, then electrophoresed on a 12% SDS-PAGE gel. The proteins were transferred to a nitrocellulose membrane, then the membrane was washed with PBS 1× and blocked with 5% non-fat milk in PBS. The membranes were incubated with a polyclonal anti-GFP antibody (1:1000), washed, and then incubated with a secondary anti-rabbit antibody conjugated with peroxidase (1:1000). The antibody recognition was detected using the ECL Chemiluminescence Kit (Thermo Fisher Scientific, Waltham, MA, USA), and revealed on an X-ray film. Images were acquired by exposing X-ray films on the UVP Bioimaging system.
2.8. Cell Infection Assays and Tissue-Cultured Derived Trypomastigote Counts
LLC-MK2 monolayers cultured to 100% cell confluence were treated with trypsin (0.05%) (Thermo Fisher Scientific, Waltham, MA, USA) and washed twice with PBS. Cells (4 × 104 cells/well) were placed in a 24-well plate containing coverslips and cultured in RPMI 1640 (Thermo Fisher) supplemented with 5% FBS in 5% CO2 at 37 °C. Tissue culture-derived trypomastigotes (TCTs) of WT, TcKharon−/−, and TcKharon-AB obtained from a previous infection of LLC-MK2 cells with metacyclic trypomastigotes were used at a ratio of 10 parasites per LLC-MK2 cell (MOI of 10:1) for 5 h. After the infection period, cells were washed with PBS three times to remove extracellular forms, and fresh RPMI media supplemented with 5% FBS was added. The samples were washed, fixed, and analyzed on an Operetta CLS High Content Analysis System (PerkinElmer, Waltham, MA, USA).
2.9. Statistical Analysis
Each experiment was conducted in triplicate before analysis. A paired t-test was employed to compare two groups. One-way ANOVA with Tukey’s post-hoc test was employed for the statistical analysis among several groups. A p-value lower than 0.05 was considered statistically significant. GraphPad version 8.0.2 was used for statistical analysis and graphs.
3. Results
3.1. Kharon Is an Intrinsically Disordered Protein Based on the 3D Structure Prediction
The multiple sequence alignment of Kharon using Clustal Omega showed low conservation among the Trypanosomatids, which included T. cruzi, Trypanosoma congolense, Trypanosoma vivax, T. b. brucei, Paratrypanosoma. confusum, Endotrypanum monterogeii, Leishmania major, Leishmania donovani, Leishmania infantum, Crithidia fasciculata, Leptomonas seymouri, Leishmania mexicana, Leishmania amazonensis, Angomonas deanei, and Leishmania braziliensis, as indicated in Supplementary Figure S1. A phylogenetic tree showed that Kharon sequence similarity mirrors the known species phylogeny (Figure 1A). The highest identity to TcKharon was the ortholog from T. congolense (47.38% identity), while the lowest was the ortholog from A. deanei (25.99% identity). Notably, we were unable to identify Kharon orthologs in Bodo saltans or outside the kinetoplastid lineage.
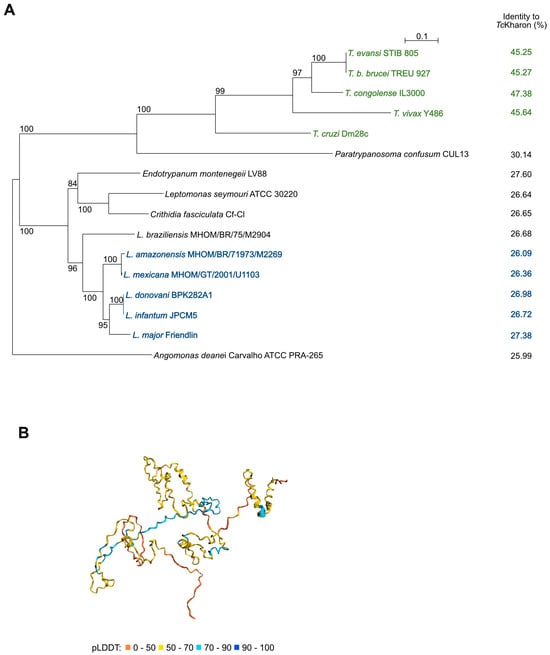
Figure 1.
Phylogenetic tree from protein sequences of related trypanosomatids, and TcKharon structure prediction using AlphaFold2. (A) Amino acid sequences from TriTrypDB (https://tritrypdb.org/tritrypdb/app accessed on 26 March 2021) were aligned using the SeaView software. The aligned sequences were used to generate a phylogenetic tree (SeaView 5.4). (B) Prediction model from https://colab.research.google.com/github/sokrypton/ColabFold/blob/main/AlphaFold2 (accessed on 26 March 2021). The colors indicate the predicted pLDDT values for TcKharon as given in the key.
Recently, DeepMind developed AlphaFold, marking a significant advancement in predicting protein tertiary structures from primary sequences [34]. In collaboration with EMBL-EBI, AlphaFold was used to model the structure of a curated subset of UniProt, including TcKharon (accession V5B3K7). The TcKharon prediction yielded a low predicted local distance difference test (pLDDT) score, which is often associated with intrinsically disordered regions (IDRs) [35]. However, AlphaFold generates its most confident predictions from deep multiple sequence alignments, and the DeepMind/EMBL-EBI pipeline is not well-optimized for divergent lineages that are underrepresented in UniProt, such as trypanosomatids [36]. Therefore, low pLDDT scores may reflect genuine structural uncertainty rather than IDRs. To improve accuracy, a version of ColabFold [37] optimized for trypanosomatids [36] was used for structure prediction. The resulting models for Lmx, Tb, and TcKharon exhibited similar structural features, including consistently low pLDDT scores (see Supplementary Figure S2). Furthermore, PONDR, a traditional IDR prediction tool indicates that the overall disordered residues range from 48.65 to 74.32%, aligning with high proline content (>10%), and charged amino acids (>28.5%) observed in Lmx, Tb and TcKharon (see Supplementary Figure S3).
3.2. TcKharon::GFP Is Localized at the Subpellicular Cytoskeleton
To determine the cellular localization of TcKharon and whether it is associated with the T. cruzi cytoskeleton, Dm28c WT epimastigotes were transfected with pTREX-GFP or pTREX-TcKharon::GFP. Parasites overexpressing GFP or TcKharon::GFP were subjected to confocal microscopy analysis (Figure 2A,B), wherein both parasites showed GFP fluorescence throughout the whole cell body. When the epimastigotes were treated with detergent for cytoskeleton extraction, as previously described [38], GFP signal was lost (Figure 2B), while TcKharon::GFP retained fluorescence signal in the detergent-insoluble cortical cytoskeleton. This distribution seems similar to the distribution of LmxKharon or TbKharon to the subpellicular microtubules [17,18].
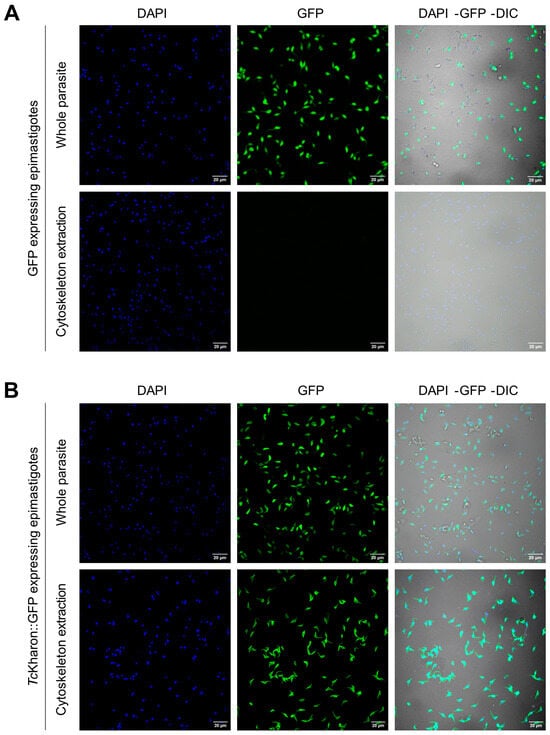
Figure 2.
Localization of TcKharon tagged with GFP. WT parasites transfected with the plasmid pTREX-GFP (control plasmid—epimastigotes expressing GFP) (A), or pTREX-TcKharon::GFP (epimastigotes expressing TcKharon::GFP) (B). These cells were either treated or not treated with PEME buffer containing TritonX-100 for cytoskeleton extraction as indicated (see Section 2). Left images, DAPI staining (blue); center images, GFP fluorescence (green); and right images, overlay of DAPI, GFP fluorescence, and DIC images.
3.3. TcKharon Disruption Affects Cell Growth and Cytokinesis of Epimastigotes
To analyze TcKharon function, genome editing with the CRISPR/Cas9 system was applied to the central part of the TcKharon gene, as shown in Figure 3A. Similar to our previous work [26], two rounds of electroporation were required to improve genome editing, since the TcKharon PCR product of the edited population is more sensitive to BglII digestion (since this restriction site is present on the oligonucleotide donor) compared to one round of transfection (Figure 3B). For identification of homozygous edited clones, limiting dilutions were carried out followed by genotyping. Both alleles were edited in three out of 12 of the clones, as detected by BglII digestion that produced the two expected fragments of 602 and 633 bp, which were not resolved in these electrophoresis conditions (Figure 3C). The efficiency in generating homozygous clones was also similar to that observed in our previous work [26].
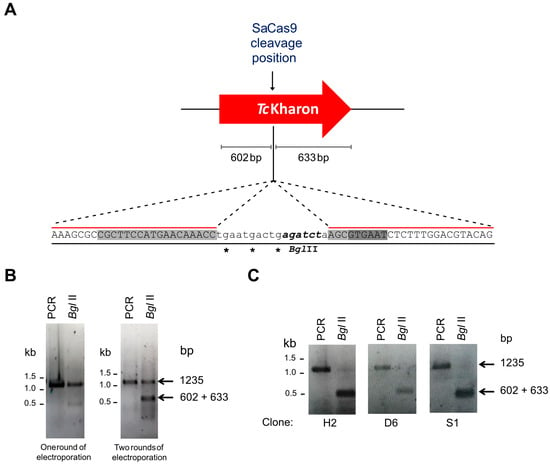
Figure 3.
Genome editing of TcKharon using CRISPR/Cas9. (A) Schematic representation of the sgRNA targeting site by the SaCas9 ribonucleoprotein (RNP) complex in the TcKharon gene (C4B63_14g70). The RNP complex cleaves right after nucleotide 585 of the TcKharon coding sequence (CDS length: 1218 bp). The insertion point of a BglII restriction site (italics) is represented in order to easily track parasite editing through DNA digestion. Stop codons (asterisks) ensure CDS disruption by homologous recombination using a donor DNA sequence. The sequence highlighted in light gray in the donor sequence corresponds to the sgRNA target site, and the dark gray sequence is the PAM sequence. (B) Genotyping of TcKharon showing genome editing of wild-type (WT) parasites. The gels show undigested PCR product (amplicon sizes: WT TcKharon = 1218 bp and TcKharon−/− = 1235 bp) and BglII-digested (BglII) PCR product of the full-length open reading frame (ORF) of TcKharon of two cultures. The left image corresponds to the PCR product and BglII-digested PCR derived from a mixed population of parasites transfected once with SaCas9 RNP plus donor sequence. The right gel corresponds to the genotyping of a culture transfected twice with RNP complex plus donor DNA. (C) Genotyping of individual clones containing both TcKharon edited alleles.
The disruption of TcKharon affected the cellular multiplication of epimastigotes in vitro. The number of parasites was markedly reduced from day 5 to 7 when compared to the WT cells. At day 6, WT cells achieved a number of 1.5 × 107 cells/mL ± 5.8 × 105 while TcKharon−/− reached 8.9 × 106 cells/mL ± 1.73 × 105. As expected, adding back TcKharon in the deficient line (TcKharon-AB) recovered the epimastigote growth fitness, reaching 1.25 × 107 cells/mL ± 5.66 × 105 (Figure 4A).
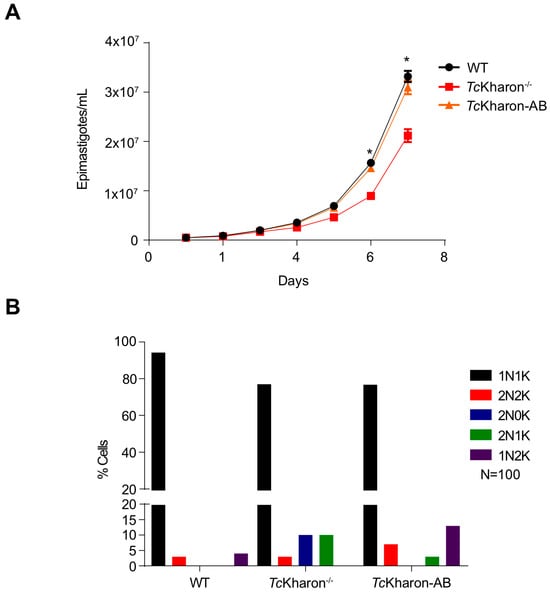
Figure 4.
Growth of TcKharon−/− is impaired. (A) Parasites were cultured for 7 days in LIT medium and counted daily. Asterisks indicate statistically significant differences between WT and TcKharon−/− at p < 0.05. TcKharon-AB corresponds to TcKharon−/− overexpressing TcKharon::GFP. These experiments were performed in triplicate. (B) Quantification of nuclei and kinetoplast numbers through DAPI staining of cells.
To evaluate abnormalities in cell division affecting the number of nuclei (N) and kinetoplasts (K) per cell, WT and the mutant clone were DAPI stained and the DNA positive organelles were counted. After counting at least 100 cells of a log phase population, some alterations in the percentage of the N:K cell types were observed (Figure 4B, and Supplementary Figure S4). For WT, 93% of cells were 1N1K, 3% were 1N2K, and 4% were 2N2K. On the other hand, 77% of the TcKharon−/− cells were 1N1K, 3% were 2N2K, 10% were 2N1K, and 10% were 2N0K. The data show that TcKharon disruption affected epimastigote division. Despite completely recovering the epimastigote cell growth, the TcKharon-AB cell population only partially recovered the percentage N:K phenotype of WT cells (Figure 4B). This may represent a lower rate of aberrant division, and persistence of the aberrant cells in the population.
3.4. Morphology of TcKharon-Null Epimastigotes Strongly Affects the Posterior Cell Region
The TcKharon−/− mutant exhibited a distinct morphological abnormality, with most epimastigotes adopting a rounded shape that deviated significantly from the typical T. cruzi epimastigote form (Figure 5A). Images acquired from SEM and TEM of the epimastigotes enabled analysis of the cell body area, wherein the WT parasites had an average area of 15.36 ± 3.19 µm2, TcKharon−/− parasites were 8.77 ± 2.19 µm2, and TcKharon-AB parasites were 12.68 ± 2.87 µm2 (Figure 5B). The difference in cell body area between WT and TcKharon−/− parasites was statistically significant (p < 0.0005), and although TcKharon-AB appeared to have a greater cell body size than TcKharon−/−, this was still significantly smaller than WT (p < 0.005).
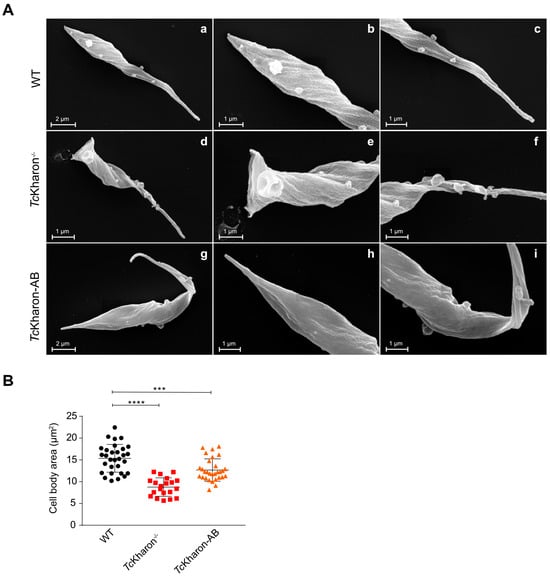
Figure 5.
TcKharon−/− epimastigotes are smaller in size. (A) SEM images of T. cruzi WT (a–c), TcKharon−/− (d–f), and addback (TcKharon-AB) (g–i) cell lines. Central (b,e,h), and right (c,f,i) images correspond to zoom of both ends of the parasites. (B) Scatter plot of cell body area. At least 25 cells were analyzed using Fiji 2.3.0 software (N = 25). Asterisks represent statistically significant differences between groups at *** p < 0.001 and **** p < 0.0001.
Next, the distance between the nucleus (N) and posterior cell tip (P) was analyzed in cells stained with DAPI and the α-tubulin antibody (Figure 6A). The distance between N and P in WT epimastigotes was 3.14 ± 1.36 µm, in TcKharon−/− mutants the distance was 1.86 ± 0.78 µm, and 3.05 ± 0.99 µm for TcKharon-AB cells. The N–P distance was found to be statistically different when comparing WT to TcKharon−/− cells (p < 0.0005), but not with TcKharon-AB cells (Figure 6B). This indicates that TcKharon disruption impacts parasite morphology, and this can be complemented by reintroducing TcKharon expression.
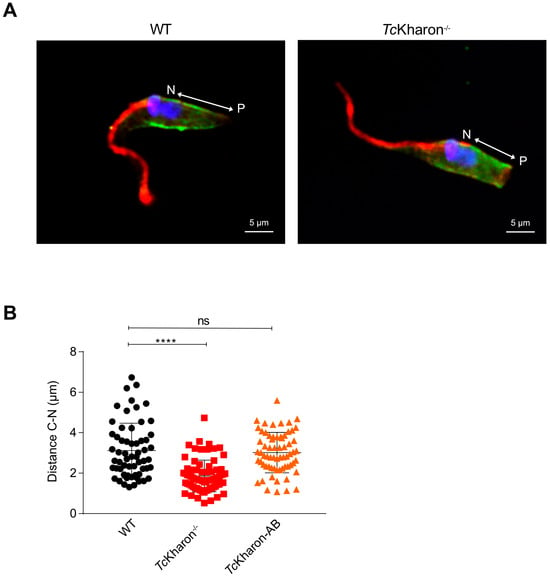
Figure 6.
The posterior end of TcKharon−/− epimastigotes is shortened. (A) Immunofluorescence of TcKharon−/− and WT cells stained with DAPI (blue) and the antibodies to 2F7 (flagellum; red) and α-tubulin (green). (B) Scatter plot showing data from the measures of the distance between the N (Nucleus) and P (Posterior Cortical Tip). The asterisk represents a statistically significant difference at **** p < 0.0001; ns represents a non-statistical difference.
3.5. Metacyclogenesis and Cell Invasion of the TcKharon−/− Mutant Are Not Affected
Epimastigotes were induced to differentiate into metacyclic trypomastigotes (MTs) as described by Contreras [25]. Since TcKharon−/− presented abnormal epimastigotes (Figure 5A), the MTs were separated from epimastigotes by chromatography using the DEAE-Cellulose column [39], and the MTs yield determined by cell counting. The purified forms from null mutants (i.e., probable MTs) showed morphological alterations (Figure 7A) The TcKharon−/− mutant showed a higher percentage of metacyclic cells (48.50 ± 2.12%) compared to TcKharon-AB (36.00 ± 5.65%) and WT (29.50 ± 3.53%) (Figure 7B). The positive identification of metacyclic cells was challenging as almost 90% of the cells in the TcKharon −/− cell line did not exhibit a clear kinetoplast transition from the anterior to the posterior region of the cell (Figure 7C). Besides cell morphology alterations, 12% of binucleated cells were observed in null mutants (Figure 7D).
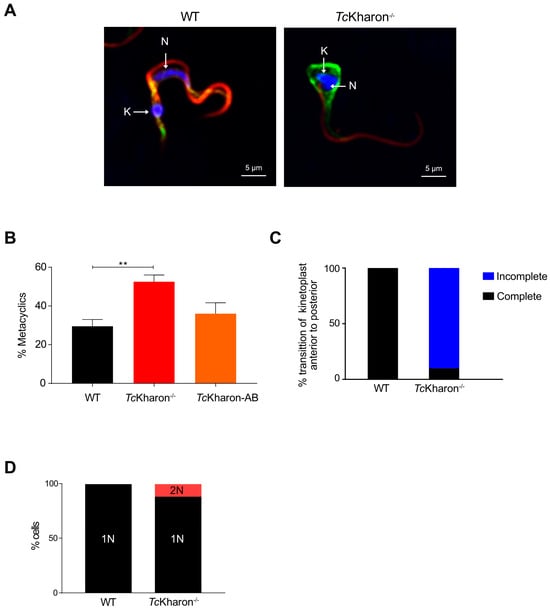
Figure 7.
Metacyclogenesis of TcKharon−/− mutants. (A) Immunofluorescence of metacyclic trypomastigotes (MTs) from WT and TcKharon−/− cultures showing the defect in morphology. Antibodies to 2F7 (flagellum; red) and actin (green) were used. White arrows indicate the nucleus and kinetoplast. (B) Percentage of metacyclic cells. Asterisk indicates statistically significant difference at p < 0.05. (C) Bar graphs showing the transition of the kinetoplast from the anterior to posterior region of the cell body. (D) Bar graphs showing the number of binucleated cells in the total cell population.
Despite the morphological changes of TcKharon−/− MTs this mutant was capable of infecting cell monolayers. Thus, the released tissue culture trypomastigotes (TCTs) could be used to perform infection assays. When LLC-MK2 cells were infected with WT, TcKharon−/−, or TcKharon-AB TCTs all three presented similar infection rates and numbers of amastigotes per host cell (Figure 8A,B). These data indicate that, despite the morphological alterations caused by TcKharon disruption, the parasites are able to achieve similar infection rates in vitro.
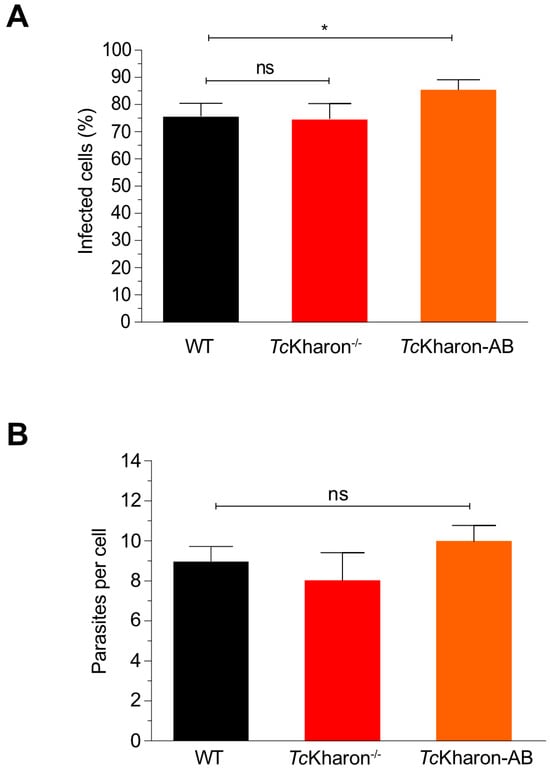
Figure 8.
TcKharon disruption does not impair LLC-MK2 cell infection. (A) Tissue culture-derived trypomastigotes (TCTs) of T. cruzi WT, TcKharon−/− and TcKharon-AB were incubated with LLC-MK2 cells for 5 h. (B) Quantification of amastigotes per cell. The asterisk represents a statistically significant difference at p < 0.05); ns represents a non-statistical difference.
4. Discussion
Previously, through Western blot and immunofluorescence analysis, LmxKharon and TbKharon have been identified as being associated with the cytoskeleton, mainly in the subpellicular microtubules [17]. Here, we show that TcKharon is associated with the T. cruzi subpellicular microtubule cytoskeleton, like its L. mexicana and T. brucei orthologs. TbKharon is also associated with the spindle [17]. Due to the low level of mitotic cells in the unsynchronized T. cruzi epimastigote culture and strong subpellicular microtubule signal, we could not identify whether TcKharon is associated with the mitotic spindle.
Understanding Kharon deletion and knockdown phenotypes is complicated by Kharon’s multifunctional nature. It was originally discovered from its role in the correct localization of a protein (LmxGT1) to the flagellar or pellicular plasma membrane in L. mexicana [16] and similarly in T. brucei TbKharon is necessary for the correct localization of the TbCaCh protein to the flagellar membrane. However, not all flagellar membrane proteins require Kharon for correct localization, including FCaBP and SMP-1 [16,17]. Furthermore, RNAi knockdown in T. brucei caused flagellum detachment then disruption of mitotic spindles, although only at later time points [17].
The lethality of Kharon deletion or knockdown arises through cell division defects. In L. mexicana, the deletion of LmxKharon did not impair promastigote proliferation, but in amastigotes it led to an increased number of multinucleated cells through cell division failure, leading to a collapse in host macrophage infection [18]. Similar defects were observed in in vivo experiments [18]. RNAi knockdown of TbKharon in T. brucei led to a lethal phenotype, with the presence of multinucleated cells in both bloodstream and procyclic form trypomastigotes, suggesting a failure of cytokinesis [17]. Here, we show that TcKharon can be deleted and is not essential for T. cruzi epimastigotes, trypomastigotes or amastigotes in vitro. Nonetheless, it did cause a mild defect in growth rate and a tendency for cells with an abnormal nucleus and kinetoplast number.
In the cell cycle of T. cruzi, the kinetoplast (K) replicates before the nucleus, meaning that epimastigote cells containing one nucleus and one kinetoplast (1N1K) become 1N2K cells, and then 2N2K cells, followed by cytokinesis [40]. In this work, TcKharon−/− epimastigote culture has an increase in 2N1K and 2N0K cells, which does not match the normal separation of organelles during the cell cycle [41]. This change may explain the mild growth defect of TcKharon−/− culture. Furthermore, we observed that TcKharon−/− cells are smaller compared to WT, and show a clear reduction in the distance from the nucleus to the posterior tip. The cells incorporate new tubulin units into old microtubules and regulate microtubule elongation at the posterior end to maintain cell shape and size, so the shrinkage of TcKharon−/− cells likely indicates defective pellicular array growth [42,43].
Another noticeable feature of the TcKharon−/− parasites is their larger and rounded posterior end, and the functional analysis of MAPs from T. brucei revealed similar features. The silencing of the WCB protein through RNAi caused this kind of phenotype since the cells became more rounded, with a slower growth rate, zoids (non-nucleated cells), and polynucleated cells, all of which show that cytokinesis fails in both procyclic and bloodstream forms [44]. Protein distribution and regulation play a crucial role in maintaining the shape and organization of subpellicular microtubules. In T. brucei, the overexpression of two MAPs (CAP15/CAP17) leads to aberrant cells that have an abnormal number of kinetoplasts and nuclei. This means that there is a problem with segregation coordination during cytokinesis, which causes mislocalization of organelles [45].
Why Kharon deletion causes cytokinesis defects is unclear. Given its prominent localization to the subpellicular array, the most straightforward conclusion is that it directly affects the stability of subpellicular microtubules or the regulation of morphogenesis [17]. However, the possibility of cytokinesis defects arising indirectly as a result of altered membrane composition has been proposed [17]. Our data support the idea that TcKharon plays a key, but not essential, role as an MAP in organizing the subpellicular array. However, we did not analyze whether flagellar membrane targeting of proteins is affected by Kharon deletion in T. cruzi, and thus cannot fully address this possibility.
TcKharon has no detectable orthologs outside of parasitic kinetoplastids, and our multiple sequence alignment analysis shows a low primary sequence conservation when compared to its orthologs in other kinetoplastids. However, Kharon has intriguing similarities to human Tau, a microtubule-binding protein highly expressed in neurons. Like Kharon, Tau is predicted to be almost entirely an IDP (intrinsically disordered protein) and binds to a parallel array/bundle of microtubules. Tau is very rich in proline and charged amino acids, similar to Kharon: 12.2 and 11.4% proline, 24.5 and 30.1% charged amino acids for Tau and TcKharon, respectively. This suggests similarity in IDP MAPs across extremely large evolutionary distances.
Kharon-deficient L. infantum has been demonstrated as a candidate for a live attenuated vaccine for leishmaniasis, since the gene deletion impairs amastigote replication [14]. Here, we were able to obtain trypomastigote T. cruzi from monolayers infected with MTs, providing strong but indirect evidence that TcKharon deletion is not lethal in intracellular amastigotes. It is possible that there are morphological changes to the amastigotes which would detrimentally affect in vivo infection. However, our data suggest TcKharon deletion is not a good route to generate a live attenuated T. cruzi vaccine.
Our analysis of the Kharon deletion phenotype in T. cruzi completes the picture of Kharon function in the different life cycle stages of all three human-infective trypanosomatid parasites. This revealed surprisingly large species to species and life stage to stage differences in how important Kharon is for cell division. This highlights the need to dissect the key players in cytoskeletal morphogenesis to explore possibilities for live attenuated vaccine development.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pathogens14040312/s1, Figure S1: Multiple alignment of TcKharon orthologs and identity matrix; Figure S2: Structure prediction and protein sequence alignment of Kharon orthologs; Figure S3: PONDR® prediction (A–E), proline and charged amino acids compositions (F) of Kharon orthologs.; Figure S4: TEM images of TcKharon−/− mutant cells with 2N1K configuration; Figure S5: Analysis of fluorescence of the TcKharon-AB cells [46,47].
Author Contributions
Conceptualization, J.L.S.-G., N.S.-M., L.A.P.-L., N.M., L.C.S.-M. and W.D.D.; Methodology, J.L.S.-G., N.S.-M., J.S.M., J.M.Q.-S., B.B., R.W., L.C.S.-M. and W.D.D.; Formal analysis, J.L.S.-G. and N.S.-M.; Investigation, J.L.S.-G., N.S.-M., J.M.Q.-S. and L.C.S.-M.; Data curation, N.S.-M.; Writing—original draft, J.L.S.-G. and W.D.D.; Writing—review & editing, N.M., R.W. and W.D.D.; Supervision, W.D.D.; Project administration, W.D.D.; Funding acquisition, L.C.S.-M. and W.D.D. All authors have read and agreed to the published version of the manuscript.
Funding
The authors were supported by the Fundação Araucária (FIOCRUZ/ICC/Fundação Araucária—grant #2020221000012), National Counsel of Technological and Scientific Development (CNPq), and CAPES agency (CT-INFRA FINEP, PROEX, and PROAP programs). JS-G and NS-M had scholarships from CNPq. This study was financed, in part, by the São Paulo Research Foundation (FAPESP—Brazil) process #2018/09948-0, #2020/07870-4 and #2022/03075-0. We also thank the Wellcome Trust (211075/Z/18/Z) for funding RW’s research group.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
We thank the Center of Advanced Technologies using Fluorescence from the Federal University of Paraná (CTAF/UFPR, Brazil), and the Program for Technological Development in Tools for Health (PDTIS) for use of the flow cytometry facility (RPT08L) at the Carlos Chagas Institute-FIOCRUZ/PR, Brazil. This work was supported by the Fundação Araucária (FIOCRUZ/ICC/Fundação Araucária—grant #2020221000012), the National Counsel of Technological and Scientific Development (CNPq) Brazil, and the CAPES agency (FINEP, PROEX PROAP programs), Brazil. JLSG and WD have scholarships from CNPq.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gómez-Ochoa, S.A.; Rojas, L.Z.; Echeverría, L.E.; Muka, T.; Franco, O.H. Global, Regional, and National Trends of Chagas Disease from 1990 to 2019: Comprehensive Analysis of the Global Burden of Disease Study. Glob. Heart 2022, 17, 59. [Google Scholar] [CrossRef]
- Kratz, J.M.; Garcia Bournissen, F.; Forsyth, C.J.; Sosa-Estani, S. Clinical and pharmacological profile of benznidazole for treatment of Chagas disease. Expert Rev. Clin. Pharmacol. 2018, 11, 943–957. [Google Scholar] [CrossRef]
- Rios, L.; Campos, E.E.; Menon, R.; Zago, M.P.; Garg, N.J. Epidemiology and pathogenesis of maternal-fetal transmission of Trypanosoma cruzi and a case for vaccine development against congenital Chagas disease. Biochim. Biophys. Acta Mol. Basis. Dis. 2020, 1866, 165591. [Google Scholar] [CrossRef]
- Junqueira, C.; Santos, L.I.; Galvão-Filho, B.; Teixeira, S.M.; Rodrigues, F.G.; DaRocha, W.D.; Chiari, E.; Jungbluth, A.A.; Ritter, G.; Gnjatic, S.; et al. Trypanosoma cruzi as an effective cancer antigen delivery vector. Proc. Natl. Acad. Sci. USA 2011, 108, 19695–19700. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.P.M.M.; Machado, L.F.M.; Doro, D.; Nascimento, F.C.; Damasceno, L.; Gazzinelli, R.T.; Fernandes, A.P.; Junqueira, C. New vaccine formulations containing a modified version of the Amastigote 2 antigen and the non-virulent Trypanosoma cruzi CL-14 strain are highly antigenic and protective against Leishmania infantum challenge. Front. Immunol. 2018, 9, 465. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.W.; Karmakar, S.; Gannavaram, S.; Dey, R.; Lypaczewski, P.; Ismail, N.; Siddiqui, A.; Simonyan, V.; Oliveira, F.; Coutinho-Abreu, I.V.; et al. A second generation leishmanization vaccine with a markerless attenuated Leishmania major strain using CRISPR gene editing. Nat. Commun. 2020, 11, 3461. [Google Scholar] [CrossRef]
- Burle-Caldas, G.A.; Dos Santos, N.S.A.; de Castro, J.T.; Mugge, F.L.B.; Grazielle-Silva, V.; Oliveira, A.E.R.; Pereira, M.C.A.; Reis-Cunha, J.L.; dos Santos, A.C.; Gomes, D.A.; et al. Disruption of active Trans-sialidase genes impairs egress from mammalian host cells and generates highly attenuated Trypanosoma cruzi parasites. mBio 2022, 13, e0347821. [Google Scholar] [CrossRef]
- Perez Brandan, C.; Padilla, A.M.; Xu, D.; Tarleton, R.L.; Basombrio, M.A. Knockout of the DHFR-TS gene in Trypanosoma cruzi generates attenuated parasites able to confer protection against a virulent challenge. PLoS Negl. Trop. Dis. 2011, 5, e1418. [Google Scholar] [CrossRef]
- Rooholamin, Z.; Dianat-Moghadam, H.; Esmaeilifallah, M.; Khanahmad, H. From classical approaches to new developments in genetic engineering of live attenuated vaccine against cutaneous leishmaniasis: Potential and immunization. Front. Public Health 2024, 12, 1382996. [Google Scholar] [CrossRef]
- Moreira, P.O.L.; Nogueira, P.M.; Monte-Neto, R.L. Next-generation leishmanization: Revisiting molecular targets for selecting genetically engineered live-attenuated Leishmania. Microorganisms 2023, 11, 1043. [Google Scholar] [CrossRef]
- Souza, W. Structural organization of Trypanosoma cruzi. Mem. Inst. Oswaldo Cruz. 2009, 104 (Suppl. 1), 89–100. [Google Scholar] [CrossRef]
- Billington, K.; Halliday, C.; Madden, R.; Dyer, P.; Barker, A.R.; Moreira-Leite, F.F.; Carrington, M.; Vaughan, S.; Hertz-Fowler, C.; Dean, S.; et al. Genome-wide subcellular protein map for the flagellate parasite Trypanosoma brucei. Nat. Microbiol. 2023, 8, 533–547. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, A.N.; Huynh, C.T.; Sladewski, T.E.; Zuromski, J.L.; Ruiz, A.E.; de Graffenried, C.L. The Trypanosoma brucei subpellicular microtubule array is organized into functionally discrete subdomains defined by microtubule associated proteins. PLoS Pathog. 2021, 17, e1009588. [Google Scholar] [CrossRef] [PubMed]
- Santi, A.M.M.; Lanza, J.S.; Tunes, L.G.; Fiuza, J.A.; Roy, G.; Orfanó, A.D.S.; De Carvalho, A.T.; Frézard, F.; De Barros, A.L.B.; Murta, S.M.F.; et al. Growth arrested live-attenuated Leishmania infantum KHARON1 null mutants display cytokinesis defect and protective immunity in mice. Sci. Rep. 2018, 8, 11627. [Google Scholar] [CrossRef]
- Kelly, F.D.; Tran, K.D.; Hatfield, J.; Schmidt, K.; Sanchez, M.A.; Landfear, S.M. A cytoskeletal protein complex is essential for division of intracellular amastigotes of Leishmania mexicana. J. Biol. Chem. 2020, 295, 13106–13122. [Google Scholar] [CrossRef] [PubMed]
- Tran, K.D.; Rodriguez-Contreras, D.; Vieira, D.P.; Yates, P.A.; David, L.; Beatty, W.; Elferich, J.; Landfear, S.M. KHARON1 mediates flagellar targeting of a glucose transporter in Leishmania mexicana and is critical for viability of infectious intracellular amastigotes. J. Biol. Chem. 2013, 288, 22721–22733. [Google Scholar] [CrossRef]
- Sanchez, M.A.; Tran, K.D.; Valli, J.; Hobbs, S.; Johnson, E.; Gluenz, E.; Landfear, S.M. KHARON is an essential cytoskeletal protein involved in the trafficking of flagellar membrane proteins and cell division in african trypanosomes. J. Biol. Chem. 2016, 291, 19760–19773. [Google Scholar] [CrossRef]
- Tran, K.D.; Vieira, D.P.; Sanchez, M.A.; Valli, J.; Gluenz, E.; Landfear, S.M. Kharon1 null mutants of Leishmania mexicana are avirulent in mice and exhibit a cytokinesis defect within macrophages. PLoS ONE 2015, 10, e0134432. [Google Scholar] [CrossRef]
- Affolter, M.; Hemphill, A.; Roditi, I.; Müller, N.; Seebeck, T. The repetitive microtubule-associated proteins MARP-1 and MARP-2 of Trypanosoma brucei. J. Struct. Biol. 1994, 112, 241–251. [Google Scholar] [CrossRef]
- Schock, M.; Schmidt, S.; Ersfeld, K. Novel cytoskeleton-associated proteins in Trypanosoma brucei are essential for cell morphogenesis and cytokinesis. Microorganisms 2021, 9, 2234. [Google Scholar] [CrossRef]
- Cooper, R.; de Jesus, A.R.; Cross, G.A. Deletion of an immunodominant Trypanosoma cruzi surface glycoprotein disrupts flagellum-cell adhesion. J. Cell Biol. 1993, 122, 149–156. [Google Scholar] [PubMed]
- LaCount, D.J.; Barrett, B.; Donelson, J.E. Trypanosoma brucei FLA1 is required for flagellum attachment and cytokinesis. J. Biol. Chem. 2002, 277, 17580–17588. [Google Scholar] [CrossRef]
- Gouy, M.; Guindon, S.; Gascuel, O. SeaView version 4: A multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol. Biol. Evol. 2010, 27, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Camargo, E.P. Growth and differentiation in Trypanosoma cruzi. I. Origin of metacyclic trypanosomes in liquid media. Rev. Inst. Med. Trop. Sao Paulo 1964, 6, 93–100. [Google Scholar] [PubMed]
- Contreras, V.T.; Salles, J.M.; Thomas, N.; Morel, C.M.; Goldenberg, S. In vitro differentiation of Trypanosoma cruzi under chemically defined conditions. Mol. Biochem. Parasitol. 1985, 16, 315–327. [Google Scholar] [CrossRef]
- Saenz-Garcia, J.L.; Borges, B.S.; Souza-Melo, N.; Machado, L.V.; Miranda, J.S.; Pacheco-Lugo, L.A.; Moretti, N.S.; Wheleer, R.; Medeiros, L.C.S.; DaRocha, W.D. Trypanin disruption affects the motility and infectivity of the protozoan Trypanosoma cruzi. Front. Cell. Infect. Microbiol. 2022, 11, 807236. [Google Scholar] [CrossRef]
- Peng, D.; Tarleton, R. EuPaGDT: A web tool tailored to design CRISPR guide RNAs for eukaryotic pathogens. Microb Genom. 2015, 1, e000033. [Google Scholar] [CrossRef]
- Kangussu-Marcolino, M.M.; Cunha, A.P.; Avila, A.R.; Herman, J.P.; DaRocha, W.D. Conditional removal of selectable markers in Trypanosoma cruzi using a site-specific recombination tool: Proof of concept. Mol. Biochem. Parasitol. 2014, 198, 71–74. [Google Scholar] [CrossRef]
- Pacheco-Lugo, L.; Díaz-Olmos, Y.; Sáenz-García, J.; Probst, C.M.; DaRocha, W.D. Effective gene delivery to Trypanosoma cruzi epimastigotes through nucleofection. Parasitol. Int. 2017, 66, 236–239. [Google Scholar] [CrossRef]
- Souza-Melo, N.; Alcantara, C.L.; Vidal, J.C.; Rocha, G.M.; de Souza, W. Implications of flagellar attachment zone proteins TcGP72 and TcFLA-1BP in morphology, proliferation, and intracellular dynamics in Trypanosoma cruzi. Pathogens 2023, 12, 1367. [Google Scholar] [CrossRef]
- de Almeida, J.M.; Nunes, F.O.; Ceole, L.F.; Klimeck, T.D.F.; da Cruz, L.A.; Tófoli, D.; Borges, B.S.; Garcez, W.S.; Tozetti, I.A.; Medeiros, L.C.S.; et al. Synergistic effect and ultrastructural changes in Trypanosoma cruzi caused by isoobtusilactone A in short exposure of time. PLoS ONE 2021, 16, e0245882. [Google Scholar] [CrossRef] [PubMed]
- Ramos, T.C.P.; Freymüller-Haapalainen, E.; Schenkman, S. Three-dimensional reconstruction of Trypanosoma cruzi epimastigotes and organelle distribution along the cell division cycle: 3D Electron Microscopy of Trypanosoma cruzi. Cytom. Part A 2011, 79A, 538–544. [Google Scholar] [CrossRef]
- DaRocha, W.D.; Silva, R.A.; Bartholomeu, D.C.; Pires, S.F.; Freitas, J.M.; Macedo, A.M.; Vazquez, M.P.; Levin, M.J.; Teixeira, S.M.R. Expression of exogenous genes in Trypanosoma cruzi: Improving vectors and electroporation protocols. Parasitol. Res. 2004, 92, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Ruff, K.M.; Pappu, R.V. AlphaFold and implications for intrinsically disordered proteins. J. Mol. Biol. 2021, 433, 167208. [Google Scholar] [CrossRef]
- Wheeler, R.J. A resource for improved predictions of Trypanosoma and Leishmania protein three-dimensional structure. PLoS ONE 2021, 16, e0259871. [Google Scholar] [CrossRef]
- Mirdita, M.; Schütze, K.; Moriwaki, Y.; Heo, L.; Ovchinnikov, S.; Steinegger, M. ColabFold: Making protein folding accessible to all. Nat. Methods 2022, 19, 679–682. [Google Scholar] [CrossRef]
- Gadelha, C.; Wickstead, B.; de Souza, W.; Gull, K.; Cunha-e-Silva, N. Cryptic paraflagellar rod in endosymbiont-containing kinetoplastid protozoa. Eukaryot. Cell. 2005, 4, 516–525. [Google Scholar] [CrossRef]
- Sousa, M.A.D. A simple method to purify biologically and antigenically preserved bloodstream trypomastigotes of Trypanosoma cruzi using Deae-cellulose columns. Mem. Inst. Oswaldo Cruz 1983, 78, 317–333. [Google Scholar] [CrossRef]
- Elias, M.C.; da Cunha, J.P.; de Faria, F.P.; Mortara, R.A.; Freymüller, E.; Schenkman, S.; de Faria, F.P. Morphological events during the Trypanosoma cruzi cell cycle. Protist 2007, 158, 147–157. [Google Scholar] [CrossRef]
- Wheeler, R.J.; Gull, K.; Sunter, J.D. Coordination of the cell cycle in Trypanosomes. Annu. Rev. Microbiol. 2019, 73, 133–154. [Google Scholar] [CrossRef]
- Robinson, D.R.; Sherwin, T.; Ploubidou, A.; Byard, E.H.; Gull, K. Microtubule polarity and dynamics in the control of organelle positioning, segregation, and cytokinesis in the trypanosome cell cycle. J. Cell Biol. 1995, 128, 1163–1172. [Google Scholar] [CrossRef]
- Sinclair, A.N.; de Graffenried, C.L. More than microtubules: The structure and function of the subpellicular array in Trypanosomatids. Trends Parasitol. 2019, 35, 760–777. [Google Scholar] [CrossRef] [PubMed]
- Baines, A.; Gull, K. WCB is a C2 domain protein defining the plasma membrane-sub-pellicular microtubule corset of kinetoplastid parasites. Protist 2008, 159, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Vedrenne, C.; Giroud, C.; Robinson, D.R.; Besteiro, S.; Bosc, C.; Bringaud, F.; Baltz, T. Two related subpellicular cytoskeleton-associated proteins in Trypanosoma brucei stabilize microtubules. Mol. Biol. Cell 2002, 13, 1058–1070. [Google Scholar] [CrossRef] [PubMed]
- Abramson, J.; Adler, J.; Dunger, J.; Evans, R.; Green, T.; Pritzel, A.; Ronneberger, O.; Willmore, L.; Ballard, A.J.; Bambrick, J.; et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature 2024, 630, 493–500. [Google Scholar] [CrossRef]
- Robert, X.; Gouet, P. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res. 2014, 42, W320–W324. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).