Virome of Terrestrial Mammals and Bats from Southern Brazil: Circulation of New Putative Members of the Togaviridae Family and Other Findings
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Area
2.2. Animal Sampling
2.3. Viral Nucleic Acids Isolation, Enrichment and Sequencing
2.4. Viral Metagenomic Data Assembly and Sequence Analyses
3. Results
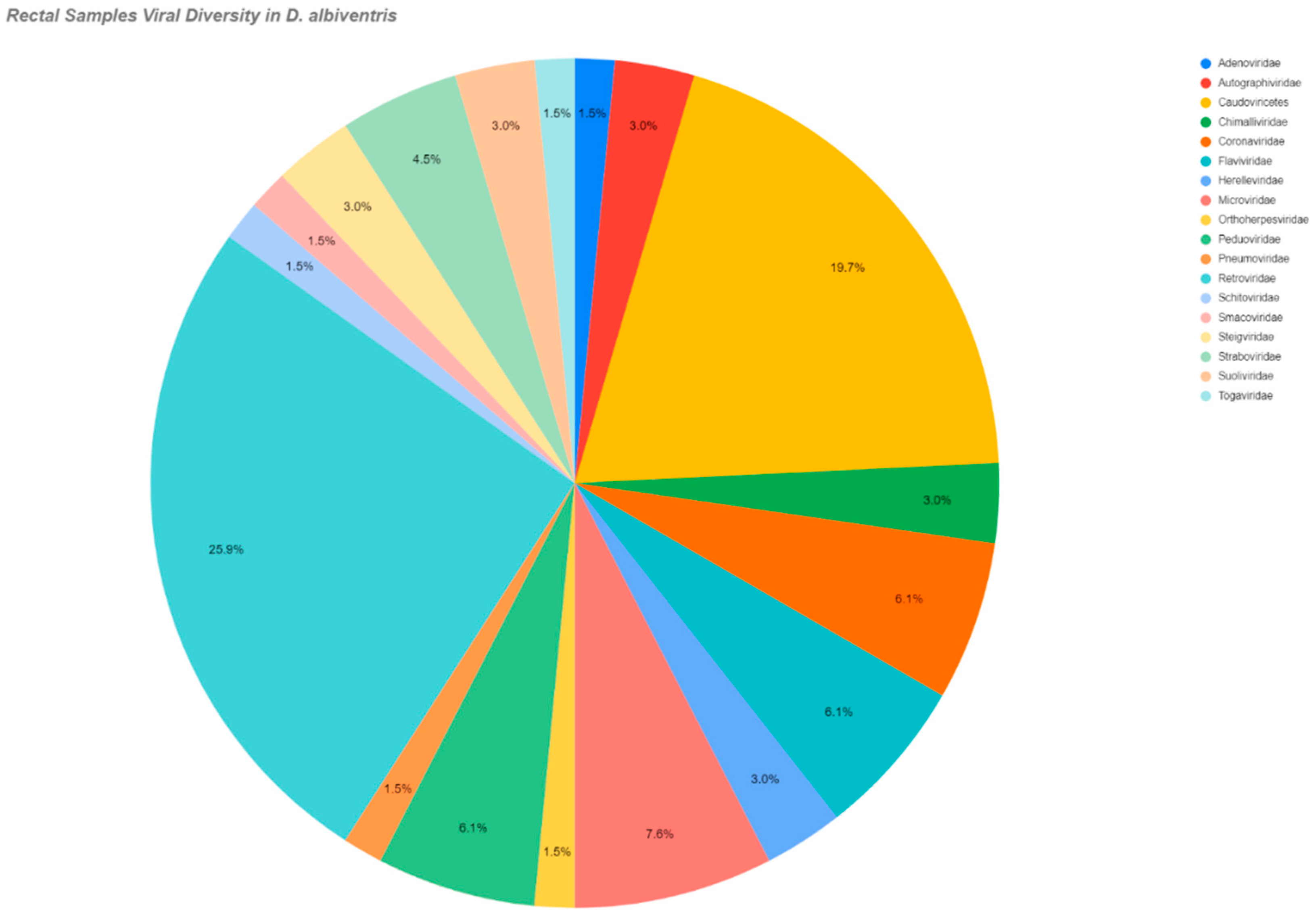
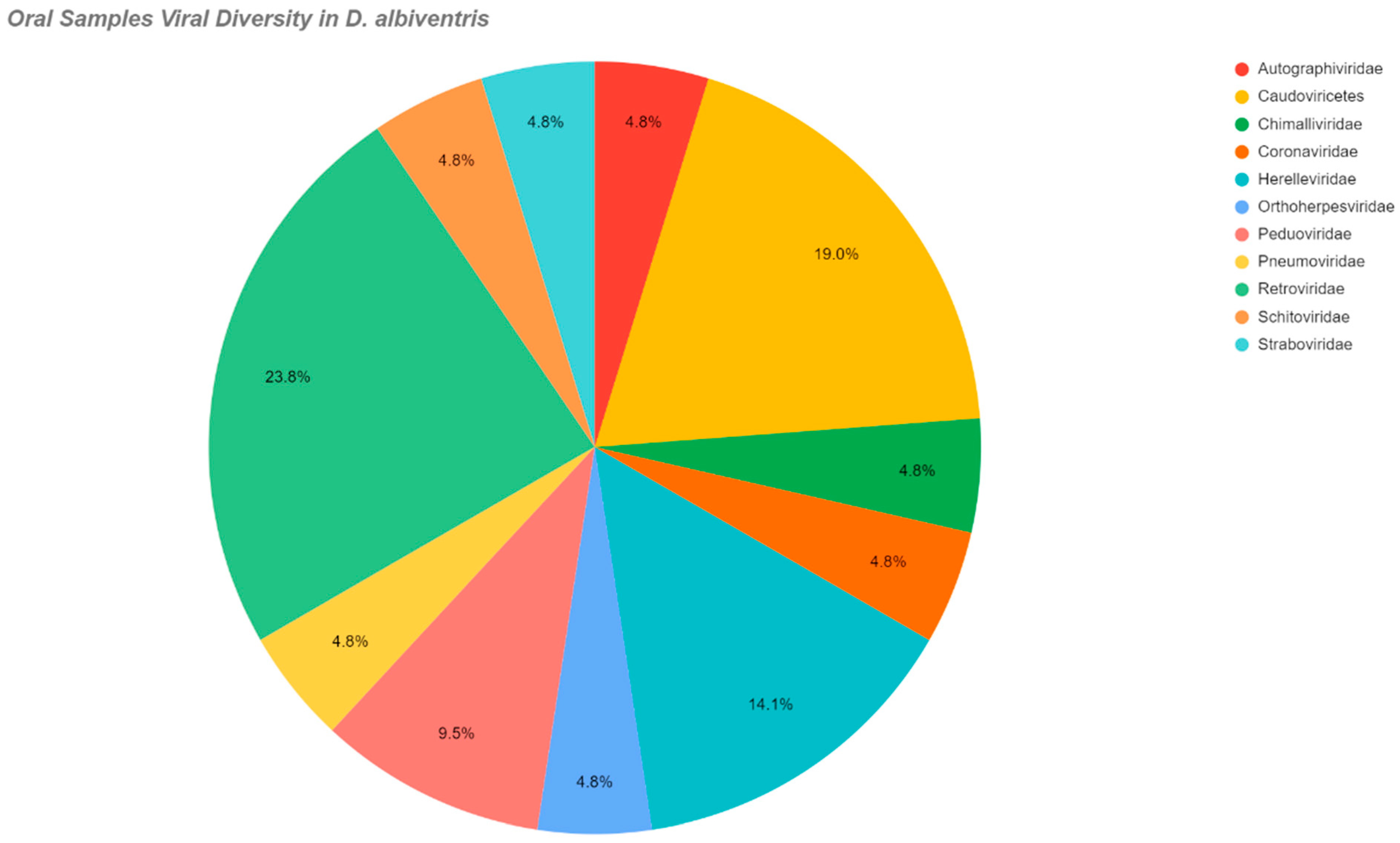
3.1. Overview
3.2. Viruses in the Studied Species
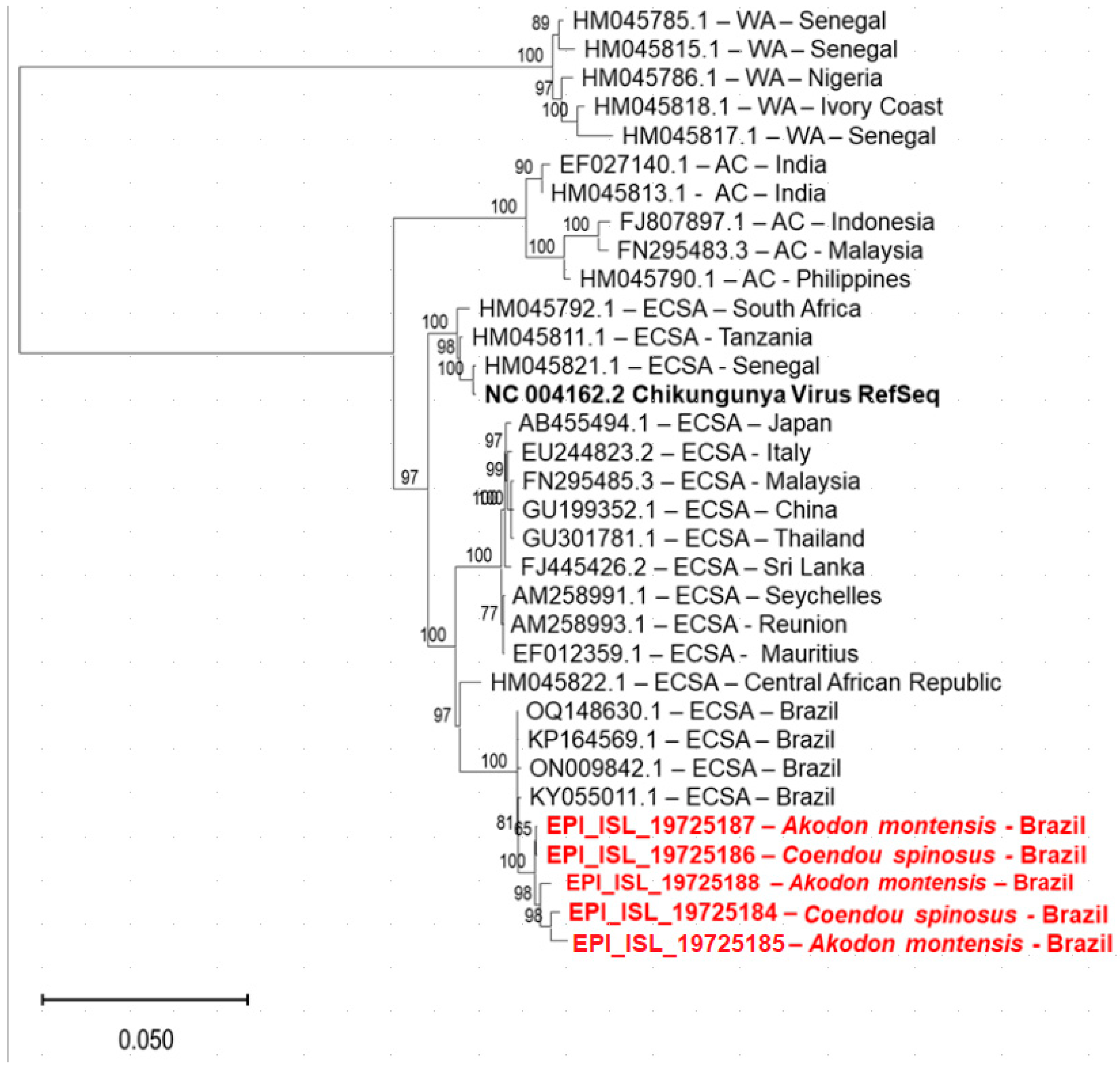
3.3. Chikungunya Virus (ChikV)
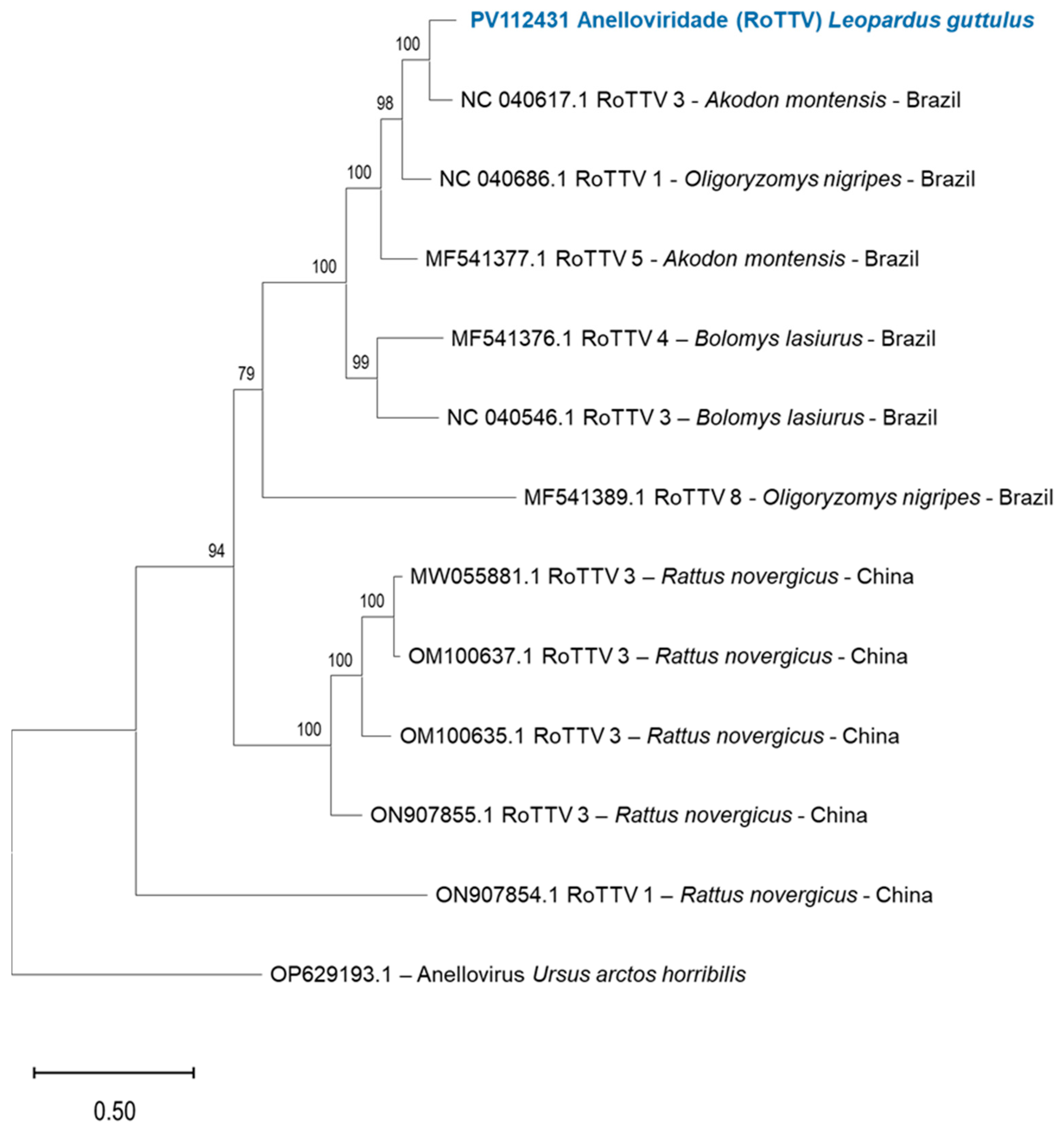
3.4. Anellovirus in L. guttulus
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CAPES | Coordination for the Improvement of Higher Education Personnel |
| CNPQ | Brazilian National Research Council |
References
- Roux, S.; Páez-Espino, D.; Chen, I.M.A.; Palaniappan, K.; Ratner, A.; Chu, K.; Reddy, T.B.K.; Nayfach, S.; Schulz, F.; Call, L.; et al. IMG/VR v3: An integrated ecological and evolutionary framework for interrogating genomes of uncultivated viruses. Nucleic Acids Res. 2021, 49, D764–D775. [Google Scholar] [CrossRef] [PubMed]
- Tisza, M.J.; Pastrana, D.V.; Welch, N.L.; Stewart, B.; Peretti, A.; Starrett, G.J.; Pang, Y.Y.S.; Krishnamurthy, S.R.; Pesavento, P.A.; McDermott, D.H.; et al. Discovery of several thousand highly diverse circular DNA viruses. eLife 2020, 9, e51971. [Google Scholar] [CrossRef] [PubMed]
- Sommers, P.; Chatterjee, A.; Varsani, A.; Tubll, G. Integrating Viral Metagenomics into an Ecological Framework. Annu. Rev. Microbiol. 2021, 8, 133–158. [Google Scholar] [CrossRef]
- He, W.T.; Hou, X.; Zhao, J.; Sun, J.; He, H.; Si, W.; Wang, J.; Jiang, Z.; Yan, Z.; Xing, G.; et al. Virome characterization of game animals in China reveals a spectrum of emerging pathogens. Cell 2022, 185, 1117–1129. [Google Scholar] [CrossRef]
- Mendes, T.; Silva, H. Genomic data in the adaptive development of vaccines for emerging viral threats. Vaccine Res. 2022, 15, 75–88. [Google Scholar]
- Kim, S.; Zhang, Y.; Chan, J. The impact of climate change on the spread of arboviral diseases. Clim. Health J. 2023, 12, 219–230. [Google Scholar]
- Duarte, M.A.; Silva, J.M.F.; Brito, C.R.; Teixeira, D.S.; Melo, F.L.; Ribeiro, B.M.; Nagata, T.S.; Campos, F. Faecal Virome Analysis of Wild Animals from Brazil. Viruses 2019, 11, 803. [Google Scholar] [CrossRef]
- Dean, K.R.; Dirzo, R.; Jakobsen, K.S.; Khan, I.; Leirs, H.; Shi, Z.L.; Wolfe, N.D.; Yang, R.; Stenseth, N.C. The Animal Origin of Major Human Infectious Diseases: What Can Past Epidemics Teach Us About Preventing the Next Pandemic? Zoonoses 2022, 2. [Google Scholar] [CrossRef]
- Chukwudozie, K.I.; Wang, H.; Wang, X.; Lu, C.; Xue, J.; Zhang, W.; Shan, T. Viral metagenomic analysis reveals diverse viruses and a novel bocaparvovirus in the enteric virome of snow leopard (Panthera uncia). Heliyon 2024, 10, e29799. [Google Scholar] [CrossRef]
- Pinheiro, L.R.S.; Rodrigues, É.D.L.; Paiva, F.A.d.S.; Cruz, A.C.R.; Medeiros, D.B.d.A.; Casseb, A.d.R.; Silva, S.P.d.; Casseb, L.M.N. Identification of Viruses in Molossus Bats from the Brazilian Amazon: A Descriptive Metagenomic Analysis. Microorganisms 2024, 12, 593. [Google Scholar] [CrossRef]
- Comitesinos. Sinos River Watershed Management Committee. Characterization of the Sinos River Watershed. 2023. Available online: https://www.comitesinos.com.br/caracterizacao (accessed on 31 March 2022).
- Demoliner, M.; Filippi, M.; Gularte, J.; Silva, M.; de Almeida, P.M.; Pereira, V.M.A.G.; Heldt, F.; Spilki, F.R.S. Genome of a husavirus from Southern Brazil. Rev. Inst. Med. Trop. 2023, 65, e5. [Google Scholar] [CrossRef]
- Vital, O.V.; Massardi, N.T.; Brasileiro, S.L.S.; Côrrea, T.C.V.; Gjorup, D.F.; Jerusalinsky, L.; de Melo, F.R. New records for Callithrix aurita and Callithrix hybrids in the region of Viçosa, Minas Gerais, Brazil. Neotrop. Primates 2020, 26, 104–109. [Google Scholar] [CrossRef]
- Bezerra, B.; Bicca-Marques, J.; Miranda, J.; Mittermeier, R.A.; Oliveira, L.; Pereira, D.; Ruiz-Miranda, C.; Valença Montenegro, M.; da Cruz, M.; do Valle, R.R. Callithrix jacchus. In The IUCN Red List of Threatened Species; International Union for Conservation of Nature: Gland, Switzerland, 2018. [Google Scholar] [CrossRef]
- Vilsker, M.; Moosa, Y.; Nooij, S.; Fonseca, V.; Ghysens, Y.; Dumon, K.; Pauwels, R.; Alcantara, L.C.; Vanden Eynden, E.; Vandamme, A.M.; et al. Genome Detective: An automated system for virus identification from high-throughput sequencing data. Bioinformatics 2019, 35, 871–873. [Google Scholar] [CrossRef]
- Kane, Y.; Tendu, A.; Li, R.; Chen, Y.; Mastriani, E.; Lan, J.; Hughes, A.C.; Berthet, N.; Wong, G. Viral diversity in wild and urban rodents of Yunnan Province, China. Emerg. Microbes Infect. 2024, 13, 2290842. [Google Scholar] [CrossRef]
- Fan, Y.; Hou, Y.; Li, Q.; Dian, Z.; Wang, B.; Xia, X. RNA virus diversity in rodents. Arch Microbiol 2024, 206, 9. [Google Scholar] [CrossRef]
- Caserta, L.C.; do Nascimento, G.M.; Joshi, L.R.; Simão, R.M.; Miller, M.E.; Felippe, P.A.N.; Diel, D.G.; Arns, C.W. Bacterial and Viral Diversity of Didelphid Opossums from Brazil. EcoHealth 2023, 20, 362–369. [Google Scholar] [CrossRef]
- Dias, C.A.R.; Perini, F.A. Biogeography and early emergence of the genus Didelphis (Didelphimorphia, Mammalia). Zool Scr. 2018, 47, 645–654. [Google Scholar] [CrossRef]
- Strauss, J.H.; Strauss, E.G. Viruses Whose Life Cycle Uses Reverse Transcriptase. In Viruses and Human Disease; Academic Press: Cambridge, MA, USA, 2008; pp. 211–259. [Google Scholar]
- Bitencourt, M.M.; Bezerra, A.M.R. Infection agents of Didelphidae (Didelphimorphia) of Brazilian underestimated matter in zoonoses research. Mammalia 2022, 86, 105–122. [Google Scholar] [CrossRef]
- Matusali, G.; Colavita, F.; Bordi, L.; Lalle, E.; Ippolito, G.; Capobianchi, M.R.; Castilletti, C. Tropism of the Chikungunya Virus. Viruses 2019, 11, 175. [Google Scholar] [CrossRef]
- Petitdemange, C.; Wauquier, N.; Vieillard, V. Control of immunopathology during Chikungunya virus infection. J. Allergy Control. Clin. Immunol. 2015, 135, 846–855. [Google Scholar]
- Plowright, R.K.; Parrish, C.R.; McCallum, H.; Hudson, P.J.; Ko, A.I.; Graham, A.L.; Lloyd-Smith, J.O. Pathways to zoonotic spillover. Nat. Rev. Microbiol. 2017, 15, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, W.P.; Moizéis, R.N.C.; Salmeron, A.C.A.; Pereira, H.W.B.; de Araújo, J.M.G.; Guedes, P.M.M.; Fernandes, J.V.; Nascimento, M.S.L. Innate immune response in patients with acute Chikungunya disease. Med. Microbiol. Immunol. 2023, 212, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Cavalcanti, T.Y.V.L.; Pereira, M.R.; de Paula, S.O.; Franca, R.F.d.O. A Review on Chikungunya Virus Epidemiology, Pathogenesis and Current Vaccine Development. Viruses 2022, 14, 969. [Google Scholar] [CrossRef]
- Diallo, D.; Sall, A.A.; Buenemann, M.; Chen, R.; Faye, O.; Diagne, C.T.; Faye, O.; Ba, Y.; Dia, I.; Watts, D.; et al. Landscape ecology of sylvatic chikungunya virus and mosquito vectors in southeastern Senegal. PLoS Negl Trop Dis. 2012, 6, e1649. [Google Scholar] [CrossRef]
- Migné, C.V.; Moutailler, S.; Attoui, H. Strategies for Assessing Arbovirus Genetic Variability in Vectors and/or Mammals. Pathogens 2020, 9, 915. [Google Scholar] [CrossRef]
- Gibb, R.; Redding, D.W.; Chin, K.Q.; Donnelly, C.A.; Blackburn, T.M.; Newbold, T.; Jones, K.E. Zoonotic host diversity increases in human-dominated ecosystems. Nature 2020, 584, 398–402. [Google Scholar] [CrossRef]
- García-Romero, C.; Carrillo Bilbao, G.A.; Navarro, J.-C.; Martin-Solano, S.; Saegerman, C. Arboviruses in Mammals in the Neotropics: A Systematic Review to Strengthen Epidemiological Monitoring Strategies and Conservation Medicine. Viruses 2023, 15, 417. [Google Scholar] [CrossRef]
- Dobson, A.P.; Pimm, S.L.; Hannah, L.; Kaufman, L.; Ahumada, J.A.; Ando, A.W.; Bernstein, A.; Busch, J.; Daszak, P.; Engelmann, J.; et al. Ecology and economics for pandemic prevention. Science 2020, 369, 379–381. [Google Scholar] [CrossRef]
- Adewale, V.O.; Adekunle, A.J.; Tahiru, I.K.; David, O.O. Influence of Meteorological Variables on Diversity and Abundance of Mosquito Vectors in Two Livestock Farms in Ibadan, Nigeria: Public Health Implications. J. Mosq. Res. 2017, 7, 70–78. [Google Scholar] [CrossRef]
- Chaves, B.A.; Orfano, A.S.; Nogueira, P.M.; Rodrigues, N.B.; Campolina, T.B.; Nacif-Pimenta, R.; Pires, A.C.A.M.; Júnior, A.B.V.; Paz, A.D.C.; Vaz, E.B.D.C.; et al. Coinfection with Zika Virus (ZIKV) and Dengue Virus Results in Preferential ZIKV Transmission by Vector Bite to Vertebrate Host. J. Infect. Dis. 2018, 13, 563–571. [Google Scholar] [CrossRef]
- Fraiture, M.-A.; Coucke, W.; Pol, M.; Rousset, D.; Gourinat, A.-C.; Biron, A.; Broeders, S.; Vandermassen, E.; Dupont-Rouzeyrol, M.; Roosens, N.H. Non-Invasive versus Invasive Samples for Zika Virus Surveillance: A Comparative Study in New Caledonia and French Guiana in 2015–2016. Microorganisms 2021, 9, 1312. [Google Scholar] [CrossRef] [PubMed]
- dos Santos Fonseca, L.M.; Carvalho, R.H.; Bandeira, A.C.; Sardi, S.I.; Campos, G.S. Oropouche Virus Detection in Febrile Patients’Saliva and Urine Samples in Salvador, Bahia, Brazil. Jn. J. Infect. Dis. 2020, 73, 164–165. [Google Scholar]
- Milich, K.M.; Koestler, B.J.; Simmons, J.H.; Nehete, P.N.; Di Fiore, A.; Williams, L.E.; Dudley, J.P.; Vanchiere, J.; Payne, S.M. Methods for detecting Zika virus in feces: A case study in captive squirrel monkeys (Saimiri boliviensis boliviensis). PLoS ONE 2018, 13, e0209391. [Google Scholar] [CrossRef]
- Oliveira, T.; Trigo, T.; Tortato, M.; Paviolo, A.; Bianchi, R.; Leite-Pitman, M.R.P. Leopardus guttulus. In The IUCN Red List of Threatened Species; International Union for Conservation of Nature: Gland, Switzerland, 2016; p. 1. [Google Scholar]
- Oliveira, T.G. Ecologia e Conservação de Pequenos Felinos no Brasil e suas Implicações para o Manejo. Ph.D. Thesis, Universidade Federal de Minas Gerais, Belo Horizonte, Brazil, 2011; 204p. [Google Scholar]
- Kitchener, A.; Breitenmoser, C.; Eizirik, E.; Gentry, A.; Werdelin, L.; Wilting, A.; Yamaguchi, N.; Abramov, A.; Christiansen, P.; Driscoll, C.; et al. A revised taxonomy of the Felidae. In Cat News; The Final Report of the Cat Classification Task Force of the IUCN/SSC Cat Specialist Group; IUCN SSC Cat Specialist Group (CSG): Vermillion, SD, USA, 2017; 80p. [Google Scholar]
- Trigo, T.C.; Tirelli, F.P.; Machado, L.F.; Peters, F.B.; Indrusiak, C.B.; Mazim, F.D.; Sana, D.; Eizirik, E.; de Freitas, T.R.O. Geographic distribution and food habits of Leopardus tigrinus and L. geoffroyi (Carnivora, Felidae) at their geographic contact zone in southern Brazil. Stud. Neotrop. Fauna Environ. 2013, 48, 56–67. [Google Scholar] [CrossRef]
- Rinaldi, A.R.; Rodriguez, F.H.; Carvalho, A.L.; Passos, F.C. Alimentação de pequenos felídeos neotropicais (Felidae: Carnivora) e sobreposição de nicho trófico na paisagem mosaico antropizada no sul do Brasil. Biotemas 2015, 28, 155–168. [Google Scholar] [CrossRef]
- Seibert, J.B.; Moreira, D.; Mendes, S.L.; Gatti, A. Diet of two sympatric felids (Leopardus guttulus and Leopardus wiedii) in a remnant of Atlantic forest, in the montane region of Espírito Santo, southeastern Brazil. Bol. Do Mus. Biol. Mello Leitão 2015, 37, 193–200. [Google Scholar]
- de Souza, W.M.; Fumagalli, M.J.; de Araujo, J.; Sabino-Santos, G.; Maia, F.G.M.; Farignoli Romeiro, M.F.; Modha, S.; Nardi, M.S.; Queiroz, L.H.; Durigon, E.L.; et al. Discovery of novel anelloviruses in small mammals expands the host range and diversity of the Anelloviridae. Virology 2018, 514, 9–17. [Google Scholar] [CrossRef]
- Kraberger, S.; Opriessnig, T.; Celer, V.; Maggi, F.; Okamoto, H.; Blomström, A.-L.; Cadar, D.; Harrach, B.; Biagini, P.; Varsani, A. Taxonomic updates for the genus Gyrovirus (family Anelloviridae): Recognition of several new members and establishment of species demarcation criteria. Arch. Virol. 2021, 166, 2937–2942. [Google Scholar] [CrossRef]
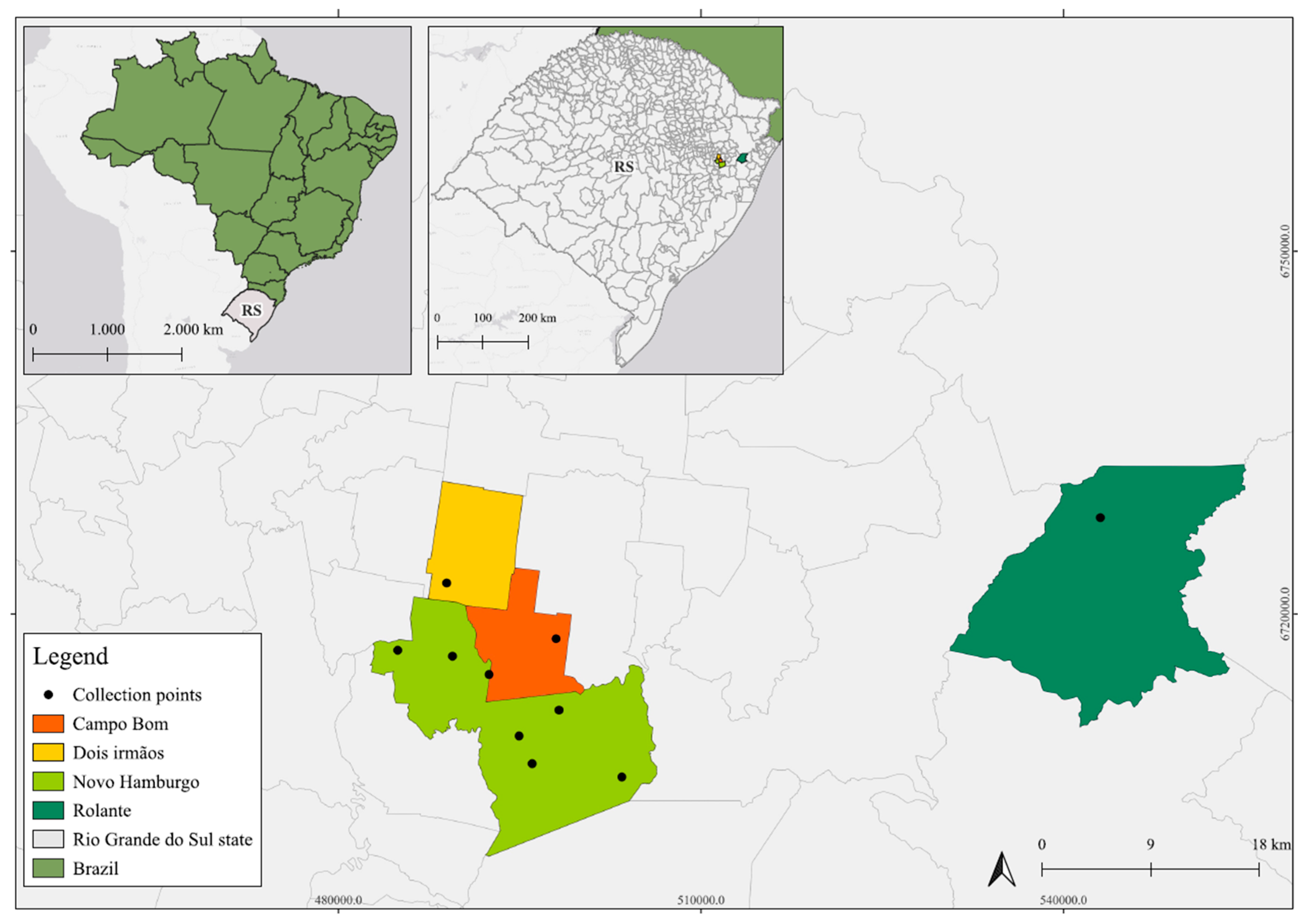


| Total Number of Samples | Animals Collected | Common Name | Species | Geographic Coordinates | Location | |
|---|---|---|---|---|---|---|
| Oral Swabs | Rectal Swabs | |||||
| - | 01/01 | 01 | Nutria | Myocastor coypus | −29.761097 −51.042227 | Novo Hamburgo |
| - | 01/01 | 01 | Atlantic Forest tiger-cat | Leopardus guttulus | −29.6273186 −51.1128742 | Dois Irmãos |
| 01/01 | 01/01 | 01 | White-tufted-ear Marmoset | Callithrix jacchus | −29.688729 −51.108325 | Novo Hamburgo |
| 01/01 | 01/01 | 01 | Ihering’s Three-striped Opossum | Monodelphis iheringi | −29.652316 −50.576316 | Rolante |
| 01/01 | 01/01 | 01 | Small wild rodent | Brucepattersonius iheringi | −29.652316 −50.576316 | Rolante |
| 01/01 | 01/01 | 01 | Small wild rodent | Thaptomys nigrita | −29.652316 −50.576316 | Rolante |
| 01/01 | 01/01 | 01 | Small wild rodent | Sooretamys angouya | −29.652316 −50.576316 | Rolante |
| 01/01 | 01/01 | 01 | Bat | Eptesicus furinalis | −29.769097 −50.964491 | Novo Hamburgo |
| 01/01 | 01/01 | 01 | Bat | Lasiurus blossevillii | −29.769097 −50.964491 | Novo Hamburgo |
| 04/17 | 17/17 | 17 | Bat | Molossus molossus | −29.7215628 −51.0180754 | Novo Hamburgo |
| 15/15 | 15/15 | 15 | Small wild rodent | Akodon montensis | −29.7407319 −51.0523446 | Novo Hamburgo |
| 01/04 | 04/04 | 04 | Porcupine | Coendou spinosus |
|
|
| 06/43 | 43/43 | 43 | Opossum | Didelphis Albiventris | −29.688729 −51.108325 | Novo Hamburgo |
| Species | Viral Class/Family | Swab |
|---|---|---|
| Didelphis albiventris | Autographiviridae | Rectal/Oral |
| Togaviridae | Rectal | |
| Coronaviridae | Rectal/Oral | |
| Retroviridae | ||
| Flaviviridae | Rectal | |
| Orthoherpesviridae | Rectal/Oral | |
| Pneumoviridae | ||
| Adenoviridae | Rectal | |
| Smacoviridae | Rectal | |
| Coendou spinosus | Togaviridae | Rectal/Oral |
| Retroviridae | ||
| Leopardus guttulus | Anelloviridae | Rectal |
| Dicistoviridae | ||
| Parvoviridae | ||
| Eptesicus furinalis | Retroviridae | Oral |
| Sooretamys angouya | Retroviridae | Oral |
| Brucepattersonius iheringi | Retroviridae | Oral |
| Monodelphis iheringi | Roboviria | Rectal |
| Orthomyxoviridae | Oral | |
| Akodon montensis | Togaviridae | Rectal/Oral |
| Retroviridae | ||
| Marseilleviridae | ||
| Autographiviridae | Oral | |
| Adenoviridae | Oral | |
| Molossus molossus | Orthoherpesviridae | Rectal |
| Retroviridae | ||
| Coronaviridae | ||
| Lasiurus blossevillii | Coronaviridae | Oral |
| Species | Phage Family or Class | Swab |
| Didelphis albiventris | Microviridae | Rectal |
| Caudovirecetes | Rectal/Oral | |
| Steigviridae | Rectal | |
| Schitoviridae | Rectal/Oral | |
| Herelleviridae | ||
| Peduoviridae | ||
| Chimalliviridae | ||
| Straboviridae | ||
| Suoliviridae | Rectal | |
| Coendou spinosus | Caudovirecetes | Rectal/Oral |
| Leopardus guttulus | Microviridae | Rectal |
| Casjensviridae | ||
| Sooretamys angouya | Caudovirecetes | Oral |
| Thaptomys nigrita | Caudovirecetes | Rectal |
| Peduoviridae | ||
| Callithrix jacchus | Caudovirecetes | Rectal/Oral |
| Monodelphis iheringi | Caudovirecetes | Rectal/Oral |
| Akodon montensis | Straboviridae | Oral |
| Peduoviridae | Rectal/Oral | |
| Caudovirecetes | ||
| Molossus molossus | Caudovirecetes | Rectal/Oral |
| Microviridae |
| Depth Coverge | Coverage (%) | Reads | Contigs | Swab | Specie |
|---|---|---|---|---|---|
| 9.7 | 98.92 | 1214 | 2 | Rectal | Coendou spinosus |
| 8.6 | 98.48 | 1097 | 1 | Rectal | Coendou spinosus |
| 74.8 | 99.3 | 10,084 | 1 | Rectal | Akodon montensis |
| 11.5 | 98.7 | 1473 | 1 | Oral | Akodon montensis |
| 15.5 | 98.64 | 2054 | 1 | Rectal | Akodon montensis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matos, J.S.S.; Demoliner, M.; Gularte, J.S.; Filippi, M.; de Abreu Góes Pereira, V.M.; da Silva, M.S.; Weber, M.N.; de Barros, M.P.; Spilki, F.R. Virome of Terrestrial Mammals and Bats from Southern Brazil: Circulation of New Putative Members of the Togaviridae Family and Other Findings. Pathogens 2025, 14, 310. https://doi.org/10.3390/pathogens14040310
Matos JSS, Demoliner M, Gularte JS, Filippi M, de Abreu Góes Pereira VM, da Silva MS, Weber MN, de Barros MP, Spilki FR. Virome of Terrestrial Mammals and Bats from Southern Brazil: Circulation of New Putative Members of the Togaviridae Family and Other Findings. Pathogens. 2025; 14(4):310. https://doi.org/10.3390/pathogens14040310
Chicago/Turabian StyleMatos, Julyana Sthéfanie Simões, Meriane Demoliner, Juliana Schons Gularte, Micheli Filippi, Vyctoria Malayhka de Abreu Góes Pereira, Mariana Soares da Silva, Matheus Nunes Weber, Marcelo Pereira de Barros, and Fernando Rosado Spilki. 2025. "Virome of Terrestrial Mammals and Bats from Southern Brazil: Circulation of New Putative Members of the Togaviridae Family and Other Findings" Pathogens 14, no. 4: 310. https://doi.org/10.3390/pathogens14040310
APA StyleMatos, J. S. S., Demoliner, M., Gularte, J. S., Filippi, M., de Abreu Góes Pereira, V. M., da Silva, M. S., Weber, M. N., de Barros, M. P., & Spilki, F. R. (2025). Virome of Terrestrial Mammals and Bats from Southern Brazil: Circulation of New Putative Members of the Togaviridae Family and Other Findings. Pathogens, 14(4), 310. https://doi.org/10.3390/pathogens14040310








