Rapid Detection of Panton–Valentine Leukocidin Production in Clinical Isolates of Staphylococcus aureus from Saxony and Brandenburg and Their Molecular Characterisation †
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolates
2.2. Antibodies and Lateral Flow Test Productions
2.3. Lateral Flow Test
2.4. Microarray Procedures
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CC | Clonal complex |
| MALDI-TOF | Matrix assisted laser desorption ionization (time-of-flight) |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| MSSA | Methicillin-susceptible Staphylococcus aureus |
| PVL | Panton–Valentine leukocidin |
| SSTI | Skin and soft tissue infection(s) |
| ST | Sequence type (as defined by Multilocus Sequence Typing) |
References
- Ito, T.; Katayama, Y.; Asada, K.; Mori, N.; Tsutsumimoto, K.; Tiensasitorn, C.; Hiramatsu, K. Structural comparison of three types of staphylococcal cassette chromosome mec integrated in the chromosome in methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2001, 45, 1323–1336. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Ma, X.X.; Takeuchi, F.; Okuma, K.; Yuzawa, H.; Hiramatsu, K. Novel type V staphylococcal cassette chromosome mec driven by a novel cassette chromosome recombinase, ccrC. Antimicrob. Agents Chemother. 2004, 48, 2637–2651. [Google Scholar] [CrossRef]
- Ito, T.; Okuma, K.; Ma, X.X.; Yuzawa, H.; Hiramatsu, K. Insights on antibiotic resistance of Staphylococcus aureus from its whole genome: Genomic island SCC. Drug Resist. Updat. 2003, 6, 41–52. [Google Scholar] [CrossRef]
- IWG-SCC. Classification of staphylococcal cassette chromosome mec (SCCmec): Guidelines for reporting novel SCCmec elements. Antimicrob. Agents Chemother. 2009, 53, 4961–4967. [Google Scholar] [CrossRef] [PubMed]
- Lina, G.; Piemont, Y.; Godail-Gamot, F.; Bes, M.; Peter, M.O.; Gauduchon, V.; Vandenesch, F.; Etienne, J. Involvement of Panton-Valentine leukocidin-producing Staphylococcus aureus in primary skin infections and pneumonia. Clin. Infect. Dis. 1999, 29, 1128–1132. [Google Scholar] [CrossRef]
- Panton, P.; Valentine, F. Staphylococcal toxin. Lancet 1932, 1, 506–508. [Google Scholar] [CrossRef]
- Labandeira-Rey, M.; Couzon, F.; Boisset, S.; Brown, E.L.; Bes, M.; Benito, Y.; Barbu, E.M.; Vazquez, V.; Hook, M.; Etienne, J.; et al. Staphylococcus aureus Panton-Valentine leukocidin causes necrotizing pneumonia. Science 2007, 315, 1130–1133. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, J.; Kamio, Y. Bacterial two-component and hetero-heptameric pore-forming cytolytic toxins: Structures, pore-forming mechanism, and organization of the genes. Biosci. Biotechnol. Biochem. 2004, 68, 981–1003. [Google Scholar] [CrossRef]
- Kaneko, J.; Kimura, T.; Kawakami, Y.; Tomita, T.; Kamio, Y. Panton-valentine leukocidin genes in a phage-like particle isolated from mitomycin C-treated Staphylococcus aureus V8 (ATCC 49775). Biosci. Biotechnol. Biochem. 1997, 61, 1960–1962. [Google Scholar] [CrossRef]
- Kaneko, J.; Kimura, T.; Narita, S.; Tomita, T.; Kamio, Y. Complete nucleotide sequence and molecular characterization of the temperate staphylococcal bacteriophage phiPVL carrying Panton-Valentine leukocidin genes. Gene 1998, 215, 57–67. [Google Scholar] [CrossRef]
- Coombs, G.; Baines, S.; Howden, B.; Swenson, K.; O’Brien, F. Diversity of bacteriophages encoding Panton-Valentine leukocidin in temporally and geographically related Staphylococcus aureus. PLoS ONE 2020, 15, e0228676. [Google Scholar] [CrossRef] [PubMed]
- Baba, T.; Takeuchi, F.; Kuroda, M.; Yuzawa, H.; Aoki, K.; Oguchi, A.; Nagai, Y.; Iwama, N.; Asano, K.; Naimi, T.; et al. Genome and virulence determinants of high virulence community-acquired MRSA. Lancet 2002, 359, 1819–1827. [Google Scholar] [CrossRef]
- Monecke, S.; Burgold-Voigt, S.; Braun, S.D.; Diezel, C.; Liebler-Tenorio, E.M.; Muller, E.; Nassar, R.; Reinicke, M.; Reissig, A.; Senok, A.; et al. Characterisation of PVL-Positive Staphylococcus argenteus from the United Arab Emirates. Antibiotics 2024, 13, 401. [Google Scholar] [CrossRef] [PubMed]
- Jayasinghe, L.; Bayley, H. The leukocidin pore: Evidence for an octamer with four LukF subunits and four LukS subunits alternating around a central axis. Protein Sci. 2005, 14, 2550–2561. [Google Scholar] [CrossRef]
- Löffler, B.; Hussain, M.; Grundmeier, M.; Bruck, M.; Holzinger, D.; Varga, G.; Roth, J.; Kahl, B.C.; Proctor, R.A.; Peters, G. Staphylococcus aureus panton-valentine leukocidin is a very potent cytotoxic factor for human neutrophils. PLoS Pathog. 2010, 6, e1000715. [Google Scholar] [CrossRef] [PubMed]
- Simpson, V.; Davison, N.; Hudson, L.; Whatmore, A.M. Staphylococcus aureus ST49 infection in red squirrels. Vet. Rec. 2010, 167, 69. [Google Scholar] [CrossRef]
- Simpson, V.R.; Davison, N.J.; Kearns, A.M.; Pichon, B.; Hudson, L.O.; Koylass, M.; Blackett, T.; Butler, H.; Rasigade, J.P.; Whatmore, A.M. Association of a lukM-positive clone of Staphylococcus aureus with fatal exudative dermatitis in red squirrels (Sciurus vulgaris). Vet. Microbiol. 2013, 162, 987–991. [Google Scholar] [CrossRef]
- Koop, G.; Vrieling, M.; Storisteanu, D.M.; Lok, L.S.; Monie, T.; van Wigcheren, G.; Raisen, C.; Ba, X.; Gleadall, N.; Hadjirin, N.; et al. Identification of LukPQ, a novel, equid-adapted leukocidin of Staphylococcus aureus. Sci. Rep. 2017, 7, 40660. [Google Scholar] [CrossRef]
- Monecke, S.; Burgold-Voigt, S.; Feßler, A.T.; Krapf, M.; Loncaric, I.; Liebler-Tenorio, E.M.; Braun, S.D.; Diezel, C.; Müller, E.; Reinicke, M.; et al. Characterisation of Staphylococcus aureus Strains and Their Prophages That Carry Horse-Specific Leukocidin Genes lukP/Q. Toxins 2025, 17, 20. [Google Scholar] [CrossRef]
- Monecke, S.; Feßler, A.T.; Burgold-Voigt, S.; Krüger, H.; Mühldorfer, K.; Wibbelt, G.; Liebler-Tenorio, E.M.; Reinicke, M.; Braun, S.D.; Hanke, D.; et al. Staphylococcus aureus isolates from Eurasian Beavers (Castor fiber) carry a novel phage-borne bicomponent leukocidin related to the Panton-Valentine leukocidin. Sci. Rep. 2021, 11, 24394. [Google Scholar] [CrossRef]
- Gillet, Y.; Issartel, B.; Vanhems, P.; Fournet, J.C.; Lina, G.; Bes, M.; Vandenesch, F.; Piemont, Y.; Brousse, N.; Floret, D.; et al. Association between Staphylococcus aureus strains carrying gene for Panton-Valentine leukocidin and highly lethal necrotising pneumonia in young immunocompetent patients. Lancet 2002, 359, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Monecke, S.; Luedicke, C.; Slickers, P.; Ehricht, R. Molecular epidemiology of Staphylococcus aureus in asymptomatic carriers. Eur. J. Clin. Microbiol. Infect. Dis. 2009, 28, 1159–1165. [Google Scholar] [CrossRef]
- Monecke, S.; Slickers, P.; Ellington, M.; Kearns, A.; Ehricht, R. High diversity of Panton-Valentine leucocidin-positive, methicillin-susceptible isolates of Staphylococcus aureus and implications for the evolution of community-associated MRSA. Clin. Microbiol. Infect. 2007, 13, 1157–1164. [Google Scholar] [CrossRef]
- Darboe, S.; Dobreniecki, S.; Jarju, S.; Jallow, M.; Mohammed, N.I.; Wathuo, M.; Ceesay, B.; Tweed, S.; Basu Roy, R.; Okomo, U.; et al. Prevalence of Panton-Valentine Leukocidin (PVL) and Antimicrobial Resistance in Community-Acquired Clinical Staphylococcus aureus in an Urban Gambian Hospital: A 11-Year Period Retrospective Pilot Study. Front. Cell. Infect Microbiol. 2019, 9, 170. [Google Scholar] [CrossRef] [PubMed]
- Grebe, T.; Rudolf, V.; Gouleu, C.S.; Löffler, B.; Adegnika, A.A.; Shittu, A.O.; Deinhardt-Emmer, S.; Niemann, S.; Schaumburg, F. Neutralization of the Staphylococcus aureus Panton-Valentine leukocidin by African and Caucasian sera. BMC Microbiol. 2022, 22, 219. [Google Scholar] [CrossRef]
- Dunne, W.M., Jr. Panton-Valentine leukocidin genes in a laboratory quality control strain of Staphylococcus aureus. J. Clin. Microbiol. 2006, 44, 287. [Google Scholar] [CrossRef] [PubMed]
- Kearns, A.M.; Ganner, M.; Holmes, A. The ‘Oxford Staphylococcus’: A note of caution. J. Antimicrob. Chemother. 2006, 58, 480–481. [Google Scholar] [CrossRef]
- Frazee, B.W.; Lynn, J.; Charlebois, E.D.; Lambert, L.; Lowery, D.; Perdreau-Remington, F. High prevalence of methicillin-resistant Staphylococcus aureus in emergency department skin and soft tissue infections. Ann. Emerg. Med. 2005, 45, 311–320. [Google Scholar] [CrossRef]
- Moran, G.J.; Krishnadasan, A.; Gorwitz, R.J.; Fosheim, G.E.; McDougal, L.K.; Carey, R.B.; Talan, D.A.; Group, E.M.I.N.S. Methicillin-resistant S. aureus infections among patients in the emergency department. N. Engl. J. Med. 2006, 355, 666–674. [Google Scholar] [CrossRef]
- Monecke, S.; Baier, V.; Coombs, G.W.; Slickers, P.; Ziegler, A.; Ehricht, R. Genome sequencing and molecular characterisation of Staphylococcus aureus ST772-MRSA-V, “Bengal Bay Clone”. BMC Res. Notes 2013, 6, 1–7. [Google Scholar] [CrossRef]
- Steinig, E.J.; Duchene, S.; Robinson, D.A.; Monecke, S.; Yokoyama, M.; Laabei, M.; Slickers, P.; Andersson, P.; Williamson, D.; Kearns, A.; et al. Evolution and Global Transmission of a Multidrug-Resistant, Community-Associated Methicillin-Resistant Staphylococcus aureus Lineage from the Indian Subcontinent. mBio 2019, 10, e01105-19. [Google Scholar] [CrossRef]
- Prabhakara, S.; Khedkar, S.; Loganathan, R.M.; Chandana, S.; Gowda, M.; Arakere, G.; Seshasayee, A.S.N. Draft Genome Sequence of Staphylococcus aureus 118 (ST772), a Major Disease Clone from India. J. Bacteriol. 2012, 194, 3727–3728. [Google Scholar] [CrossRef]
- D’Souza, N.; Rodrigues, C.; Mehta, A. Molecular characterization of methicillin-resistant Staphylococcus aureus with emergence of epidemic clones of sequence type (ST) 22 and ST 772 in Mumbai, India. J. Clin. Microbiol. 2010, 48, 1806–1811. [Google Scholar] [CrossRef]
- Coombs, G.; Pearson, J.; Daley, D.; Robinson, O.; Nimmo, G.; Turnidge, J.D. Staphylococcus aureus Programme 2012 (SAP 2012) Community Survey, MRSA Epidemiology and Typing Report; Australian Group on Antimicrobial Resistance: East Perth, Australia, 2013; Available online: https://agargroup.org.au/wp-content/uploads/2022/09/FED-REPORT-SAP2012-MRSA-Typing-Report-FINAL.pdf (accessed on 26 January 2025).
- Coombs, G.W.; Goering, R.V.; Chua, K.Y.; Monecke, S.; Howden, B.P.; Stinear, T.P.; Ehricht, R.; O’Brien, F.G.; Christiansen, K.J. The Molecular Epidemiology of the Highly Virulent ST93 Australian Community Staphylococcus aureus Strain. PLoS ONE 2012, 7, e43037. [Google Scholar] [CrossRef] [PubMed]
- Coombs, G.W.; Nimmo, G.R.; Bell, J.M.; Huygens, F.; O’Brien, F.G.; Malkowski, M.J.; Pearson, J.C.; Stephens, A.J.; Giffard, P.M.; Resistance, A.G.A. Genetic diversity among community methicillin-resistant Staphylococcus aureus strains causing outpatient infections in Australia. J. Clin. Microbiol. 2004, 42, 4735–4743. [Google Scholar] [CrossRef]
- Coombs, G.W.; Pearson, J.C.; O’Brien, F.G.; Murray, R.J.; Grubb, W.B.; Christiansen, K.J. Methicillin-resistant Staphylococcus aureus clones, Western Australia. Emerg. Infect. Dis. 2006, 12, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Chroboczek, T.; Boisset, S.; Rasigade, J.P.; Meugnier, H.; Akpaka, P.E.; Nicholson, A.; Nicolas, M.; Olive, C.; Bes, M.; Vandenesch, F.; et al. Major West Indies MRSA clones in human beings: Do they travel with their hosts? J. Travel Med. 2013, 20, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Helgason, K.O.; Jones, M.E.; Edwards, G. Panton-valentine leukocidin-positive Staphylococcus aureus and foreign travel. J. Clin. Microbiol. 2008, 46, 832–833. [Google Scholar] [CrossRef]
- Maier, J.; Melzl, H.; Reischl, U.; Drubel, I.; Witte, W.; Lehn, N.; Linde, H. Panton-Valentine leukocidin-positive methicillin-resistant Staphylococcus aureus in Germany associated with travel or foreign family origin. Eur. J. Clin. Microbiol. Infect. Dis. 2005, 24, 637–639. [Google Scholar] [CrossRef]
- Nurjadi, D.; Friedrich-Janicke, B.; Schafer, J.; Van Genderen, P.J.; Goorhuis, A.; Perignon, A.; Neumayr, A.; Mueller, A.; Kantele, A.; Schunk, M.; et al. Skin and soft tissue infections in intercontinental travellers and the import of multi-resistant Staphylococcus aureus to Europe. Clin. Microbiol. Infect. 2015, 21, 567.e1–567.e10. [Google Scholar] [CrossRef]
- Zanger, P.; Nurjadi, D.; Schleucher, R.; Scherbaum, H.; Wolz, C.; Kremsner, P.G.; Schulte, B. Import and spread of Panton-Valentine Leukocidin-positive Staphylococcus aureus through nasal carriage and skin infections in travelers returning from the tropics and subtropics. Clin. Infect. Dis. 2012, 54, 483–492. [Google Scholar] [CrossRef]
- PVL Subgroup of the Steering Group on Healthcare Associated Infection. Guidance on the Diagnosis and Management of PVL-Associated Staphylococcus aureus Infections (PVL-SA) in England; Health Protection Agency: London, UK, 2008. [Google Scholar]
- Leistner, R.; Hanitsch, L.G.; Krüger, R.; Lindner, A.K.; Stegemann, M.S.; Nurjadi, D. Skin Infections Due to Panton-Valentine Leukocidin–Producing S. aureus. Dtsch Arztebl. Int. 2022, 119, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Merbecks, S.S. Neue bundesweit geltende Meldepflichten: Bedeutung für Sachsen. Ärzteblatt Sachs. 2016, 235–236. Available online: https://www.slaek.de/media/dokumente/ueber-uns/presse/aerzteblatt/archiv/2011-2020/2016/aebl0616.pdf (accessed on 26 January 2025).
- Sächsische Infektionsschutz-Meldeverordnung vom 19. Juli 2024 (SächsGVBl. S. 745, Fsn-Nr.: 250-6/3 ). SächsGVBl 2024, 745.
- Köhler, G.; Milstein, C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 1975, 256, 495–497. [Google Scholar] [CrossRef]
- Monecke, S.; Slickers, P.; Ehricht, R. Assignment of Staphylococcus aureus isolates to clonal complexes based on microarray analysis and pattern recognition. FEMS Immunol. Med. Microbiol. 2008, 53, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Monecke, S.; Coombs, G.; Shore, A.C.; Coleman, D.C.; Akpaka, P.; Borg, M.; Chow, H.; Ip, M.; Jatzwauk, L.; Jonas, D.; et al. A field guide to pandemic, epidemic and sporadic clones of methicillin-resistant Staphylococcus aureus. PLoS ONE 2011, 6, e17936. [Google Scholar] [CrossRef] [PubMed]
- Monecke, S.; Gavier-Widen, D.; Mattsson, R.; Rangstrup-Christensen, L.; Lazaris, A.; Coleman, D.C.; Shore, A.C.; Ehricht, R. Detection of mecC-positive Staphylococcus aureus (CC130-MRSA-XI) in diseased European hedgehogs (Erinaceus europaeus) in Sweden. PLoS ONE 2013, 8, e66166. [Google Scholar] [CrossRef]
- Stieber, B.; Monecke, S.; Müller, E.; Ehricht, R. Measuring of S. aureus Panton-Valentine leukocidin expression and regulation using protein microarrays. Int. J. Med. Microbiol. 2012, 302, 66–67. [Google Scholar]
- Stieber, B.; Sabat, A.; Monecke, S.; Slickers, P.; Akkerboom, V.; Müller, E.; Friedrich, A.W.; Ehricht, R. PVL overexpression due to genomic rearrangements and mutations in the S. aureus reference strain ATCC25923. BMC Res. Notes 2017, 10, 576. [Google Scholar] [CrossRef]
- Noda, M.; Kato, I. Purification and crystallization of staphylococcal leukocidin. Methods Enzymol. 1988, 165, 22–32. [Google Scholar] [CrossRef]
- Monecke, S.; Akpaka, P.E.; Smith, M.R.; Unakal, C.G.; Thoms Rodriguez, C.A.; Ashraph, K.; Müller, E.; Braun, S.D.; Diezel, C.; Reinicke, M.; et al. Clonal Complexes Distribution of Staphylococcus aureus Isolates from Clinical Samples from the Caribbean Islands. Antibiotics 2023, 12, 1050. [Google Scholar] [CrossRef]
- Monecke, S.; Müller, E.; Buechler, J.; Rejman, J.; Stieber, B.; Akpaka, P.E.; Bandt, D.; Burris, R.; Coombs, G.; Hidalgo-Arroyo, G.A.; et al. Rapid detection of Panton-Valentine leukocidin in Staphylococcus aureus cultures by use of a lateral flow assay based on monoclonal antibodies. J. Clin. Microbiol. 2013, 51, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Linde, H.; Wagenlehner, F.; Strommenger, B.; Drubel, I.; Tanzer, J.; Reischl, U.; Raab, U.; Holler, C.; Naber, K.G.; Witte, W.; et al. Healthcare-associated outbreaks and community-acquired infections due to MRSA carrying the Panton-Valentine leucocidin gene in southeastern Germany. Eur. J. Clin. Microbiol. Infect. Dis. 2005, 24, 419–422. [Google Scholar] [CrossRef] [PubMed]
- Monecke, S.; Jatzwauk, L.; Müller, E.; Nitschke, H.; Pfohl, K.; Slickers, P.; Reissig, A.; Ruppelt-Lorz, A.; Ehricht, R. Diversity of SCCmec elements in Staphylococcus aureus as observed in South-Eastern Germany. PLoS ONE 2016, 11, e0162654. [Google Scholar] [CrossRef]
- Monecke, S.; Jatzwauk, L.; Ruppelt, A.; Albrecht, N.; Stieber, B.; Ehricht, R. The rise of the Barnim epidemic strain (CC22-MRSA-IV)—Changes affecting the MRSA population structure in a German university hospital during fourteen years. Int. J. Med. Microbiol. 2013, 303, 84. [Google Scholar]
- Monecke, S.; Jatzwauk, L.; Slickers, P.; Ehricht, R. Surveillance of emerging community acquired MRSA: The situation in Dresden/Saxony. Int. J. Med. Microbiol. 2008, 298, 59. [Google Scholar]
- Monecke, S.; Jatzwauk, L.; Weber, S.; Slickers, P.; Ehricht, R. DNA microarray-based genotyping of methicillin-resistant Staphylococcus aureus strains from Eastern Saxony. Clin. Microbiol. Infect. 2008, 14, 534–545. [Google Scholar] [CrossRef] [PubMed]
- Egyir, B.; Guardabassi, L.; Sorum, M.; Nielsen, S.S.; Kolekang, A.; Frimpong, E.; Addo, K.K.; Newman, M.J.; Larsen, A.R. Molecular epidemiology and antimicrobial susceptibility of clinical Staphylococcus aureus from healthcare institutions in Ghana. PLoS ONE 2014, 9, e89716. [Google Scholar] [CrossRef]
- Ruimy, R.; Maiga, A.; Armand-Lefevre, L.; Maiga, I.; Diallo, A.; Koumare, A.K.; Ouattara, K.; Soumare, S.; Gaillard, K.; Lucet, J.C.; et al. The carriage population of Staphylococcus aureus from Mali is composed of a combination of pandemic clones and the divergent Panton-Valentine leukocidin-positive genotype ST152. J. Bacteriol. 2008, 190, 3962–3968. [Google Scholar] [CrossRef]
- Shittu, A.; Oyedara, O.; Abegunrin, F.; Okon, K.; Raji, A.; Taiwo, S.; Ogunsola, F.; Onyedibe, K.; Elisha, G. Characterization of methicillin-susceptible and -resistant staphylococci in the clinical setting: A multicentre study in Nigeria. BMC Infect. Dis. 2012, 12, 286. [Google Scholar] [CrossRef]
- Shittu, A.O.; Okon, K.; Adesida, S.; Oyedara, O.; Witte, W.; Strommenger, B.; Layer, F.; Nubel, U. Antibiotic resistance and molecular epidemiology of Staphylococcus aureus in Nigeria. BMC Microbiol. 2011, 11, 92. [Google Scholar] [CrossRef] [PubMed]
- Breurec, S.; Fall, C.; Pouillot, R.; Boisier, P.; Brisse, S.; Diene-Sarr, F.; Djibo, S.; Etienne, J.; Fonkoua, M.C.; Perrier-Gros-Claude, J.D.; et al. Epidemiology of methicillin-susceptible Staphylococcus aureus lineages in five major African towns: High prevalence of Panton-Valentine leukocidin genes. Clin. Microbiol. Infect. 2011, 17, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Strauß, L.; Ruffing, U.; Abdulla, S.; Alabi, A.; Akulenko, R.; Garrine, M.; Germann, A.; Grobusch, M.P.; Helms, V.; Herrmann, M.; et al. Detecting Staphylococcus aureus Virulence and Resistance Genes: A Comparison of Whole-Genome Sequencing and DNA Microarray Technology. J. Clin. Microbiol. 2016, 54, 1008–1016. [Google Scholar] [CrossRef]
- Monecke, S.; Bedewy, A.K.; Müller, E.; Braun, S.D.; Diezel, C.; Elsheredy, A.; Kader, O.; Reinicke, M.; Ghazal, A.; Rezk, S.; et al. Characterisation of Methicillin-Resistant Staphylococcus aureus from Alexandria, Egypt. Antibiotics 2023, 12, 78. [Google Scholar] [CrossRef]
- Montelongo, C.; Mores, C.R.; Putonti, C.; Wolfe, A.J.; Abouelfetouh, A.; Mkrtchyan, H.V. Whole-Genome Sequencing of Staphylococcus aureus and Staphylococcus haemolyticus Clinical Isolates from Egypt. Microbiol. Spectr. 2022, 10, e02413-21. [Google Scholar] [CrossRef]
- Goudarzi, M.; Goudarzi, H.; Sá Figueiredo, A.M.; Udo, E.E.; Fazeli, M.; Asadzadeh, M.; Seyedjavadi, S.S. Molecular Characterization of Methicillin Resistant Staphylococcus aureus Strains Isolated from Intensive Care Units in Iran: ST22-SCCmec IV/t790 Emerges as the Major Clone. PLoS ONE 2016, 11, e0155529. [Google Scholar] [CrossRef]
- Senok, A.; Monecke, S.; Nassar, R.; Celiloglu, H.; Thyagarajan, S.; Müller, E.; Ehricht, R. Lateral Flow Immunoassay for the Detection of Panton-Valentine Leukocidin in Staphylococcus aureus from skin and soft tissue infections in the United Arab Emirates. Front. Microbiol. 2021, 11, 754523. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, X.; Wang, B.; Shen, L.; Rao, L.; Wang, X.; Zhang, J.; Xiao, Y.; Xu, Y.; Yu, J.; et al. Phenotypic and genomic analysis of the hypervirulent ST22 methicillin-resistant Staphylococcus aureus in China. mSystems 2023, 8, e0124222. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.C.; Joshi, P.R.; Greninger, A.L.; Melendez, D.; Paudel, S.; Acharya, M.; Bimali, N.K.; Koju, N.P.; No, D.; Chalise, M.; et al. The human clone ST22 SCCmec IV methicillin-resistant Staphylococcus aureus isolated from swine herds and wild primates in Nepal: Is man the common source? FEMS Microbiol. Ecol. 2018, 94, fiy052. [Google Scholar] [CrossRef]
- Reyes, J.; Rincon, S.; Diaz, L.; Panesso, D.; Contreras, G.A.; Zurita, J.; Carrillo, C.; Rizzi, A.; Guzman, M.; Adachi, J.; et al. Dissemination of methicillin-resistant Staphylococcus aureus USA300 sequence type 8 lineage in Latin America. Clin. Infect. Dis. 2009, 49, 1861–1867. [Google Scholar] [CrossRef]
- Planet, P.J.; Diaz, L.; Rios, R.; Arias, C.A. Global Spread of the Community-Associated Methicillin-Resistant Staphylococcus aureus USA300 Latin American Variant. J. Infect. Dis. 2016, 214, 1609–1610. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, R.P.; Cook, G.M.; Lamont, I.; Lang, S.; Heffernan, H.; Smith, J.M. Phenotypic and molecular characterization of community occurring, Western Samoan phage pattern methicillin-resistant Staphylococcus aureus. J. Antimicrob. Chemother. 2002, 50, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.M.; Cook, G.M. A decade of community MRSA in New Zealand. Epidemiol. Infect. 2005, 133, 899–904. [Google Scholar] [CrossRef] [PubMed]
- Aloba, B.K.; Kinnevey, P.M.; Monecke, S.; Brennan, G.I.; O’Connell, B.; Blomfeldt, A.; McManus, B.A.; Schneider-Brachert, W.; Tkadlec, J.; Ehricht, R.; et al. An emerging Panton-Valentine leukocidin-positive CC5-meticillin-resistant Staphylococcus aureus-IVc clone recovered from hospital and community settings over a 17-year period from 12 countries investigated by whole-genome sequencing. J. Hosp. Infect. 2023, 132, 8–19. [Google Scholar] [CrossRef]
- McManus, B.A.; Aloba, B.K.; Earls, M.R.; Brennan, G.I.; O’Connell, B.; Monecke, S.; Ehricht, R.; Shore, A.C.; Coleman, D.C. Multiple distinct outbreaks of Panton-Valentine leukocidin (PVL)-positive community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) in Ireland investigated by whole-genome sequencing. J. Hosp. Infect. 2020, 108, 72–80. [Google Scholar] [CrossRef]
- Witte, W.; Enright, M.; Schmitz, F.J.; Cuny, C.; Braulke, C.; Heuck, D. Characteristics of a new epidemic MRSA in Germany ancestral to United Kingdom EMRSA 15. Int. J. Med. Microbiol. 2001, 290, 677–682. [Google Scholar] [CrossRef]
- Earls, M.R.; Kinnevey, P.M.; Brennan, G.I.; Lazaris, A.; Skally, M.; O’Connell, B.; Humphreys, H.; Shore, A.C.; Coleman, D.C. The recent emergence in hospitals of multidrug-resistant community-associated sequence type 1 and spa type t127 methicillin-resistant Staphylococcus aureus investigated by whole-genome sequencing: Implications for screening. PLoS ONE 2017, 12, e0175542. [Google Scholar] [CrossRef]
- Monecke, S.; Konig, E.; Earls, M.R.; Leitner, E.; Müller, E.; Wagner, G.E.; Poitz, D.M.; Jatzwauk, L.; Vremera, T.; Dorneanu, O.S.; et al. An epidemic CC1-MRSA-IV clone yields false-negative test results in molecular MRSA identification assays: A note of caution, Austria, Germany, Ireland, 2020. Eurosurveillance 2020, 25, 2000929. [Google Scholar] [CrossRef]
- Earls, M.R.; Steinig, E.J.; Monecke, S.; Samaniego Castruita, J.A.; Simbeck, A.; Schneider-Brachert, W.; Vremerǎ, T.; Dorneanu, O.S.; Loncaric, I.; Bes, M.; et al. Exploring the evolution and epidemiology of European CC1-MRSA-IV: Tracking a multidrug-resistant community-associated meticillin-resistant Staphylococcus aureus clone. Microb. Genom. 2021, 7, 000601. [Google Scholar] [CrossRef]
- Scicluna, E.A.; Shore, A.C.; Thurmer, A.; Ehricht, R.; Slickers, P.; Borg, M.A.; Coleman, D.C.; Monecke, S. Characterisation of MRSA from Malta and the description of a Maltese epidemic MRSA strain. Eur. J. Clin. Microbiol. Infect. Dis. 2010, 29, 163–170. [Google Scholar] [CrossRef]
- Monecke, S.; Skakni, L.; Hasan, R.; Ruppelt, A.; Ghazal, S.S.; Hakawi, A.; Slickers, P.; Ehricht, R. Characterisation of MRSA strains isolated from patients in a hospital in Riyadh, Kingdom of Saudi Arabia. BMC Microbiol. 2012, 12, 146. [Google Scholar] [CrossRef] [PubMed]
- Senok, A.; Ehricht, R.; Monecke, S.; Al-Saedan, R.; Somily, A. Molecular characterization of methicillin-resistant Staphylococcus aureus in nosocomial infections in a tertiary-care facility: Emergence of new clonal complexes in Saudi Arabia. New Microbes New Infect. 2016, 14, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Senok, A.; Nassar, R.; Kaklamanos, E.G.; Belhoul, K.; Abu Fanas, S.; Nassar, M.; Azar, A.J.; Muller, E.; Reissig, A.; Gawlik, D.; et al. Molecular characterization of Staphylococcus aureus isolates associated with nasal colonization and environmental contamination in academic dental clinics. Microb. Drug Resist. 2020, 26, 661–669. [Google Scholar] [CrossRef]
- Monecke, S.; Müller, E.; Braun, S.D.; Armengol-Porta, M.; Bes, M.; Boswihi, S.; El-Ashker, M.; Engelmann, I.; Gawlik, D.; Gwida, M.; et al. Characterisation of S. aureus/MRSA CC1153 and review of mobile genetic elements carrying the fusidic acid resistance gene fusC. Sci. Rep. 2021, 11, 8128. [Google Scholar] [CrossRef]
- Senok, A.; Somily, A.M.; Nassar, R.; Garaween, G.; Kim Sing, G.; Müller, E.; Reissig, A.; Gawlik, D.; Ehricht, R.; Monecke, S. Emergence of novel methicillin-resistant Staphylococcus aureus strains in a tertiary care facility in Riyadh, Saudi Arabia. Infect. Drug Resist. 2019, 12, 2739–2746. [Google Scholar] [CrossRef] [PubMed]
- Senok, A.; Somily, A.; Raji, M.; Garaween, G.; Kabil, M.; Shibl, A.; Monecke, S.; Ehricht, R. Genotyping of Staphylococcus aureus associated with nasal colonization among healthcare workers using DNA microarray. J. Infect. Dev. Ctries 2018, 12, 321–325. [Google Scholar] [CrossRef]
- Senok, A.; Nassar, R.; Celiloglu, H.; Nabi, A.; Alfaresi, M.; Weber, S.; Rizvi, I.; Müller, E.; Reissig, A.; Gawlik, D.; et al. Genotyping of methicillin resistant Staphylococcus aureus from the United Arab Emirates. Sci. Rep. 2020, 10, 18551. [Google Scholar] [CrossRef]
- Boswihi, S.S.; Udo, E.E.; Monecke, S.; Mathew, B.; Noronha, B.; Verghese, T.; Tappa, S.B. Emerging variants of methicillin-resistant Staphylococcus aureus genotypes in Kuwait hospitals. PLoS ONE 2018, 13, e0195933. [Google Scholar] [CrossRef]
- Müller, E.; Monecke, S.; Armengol Porta, M.; Narvaez Encalada, M.V.; Schröttner, P.; Schwede, I.; Söffing, H.-H.; Thürmer, A.; Ehricht, R. Characterisation of PVL-positive Staphylococcus aureus isolates from Saxony and Brandenburg. In Proceedings of the Annual Conference of the German Society for Microbiology and Hygiene, Würzburg, Germany, 2–5 June 2024; p. 132. [Google Scholar]
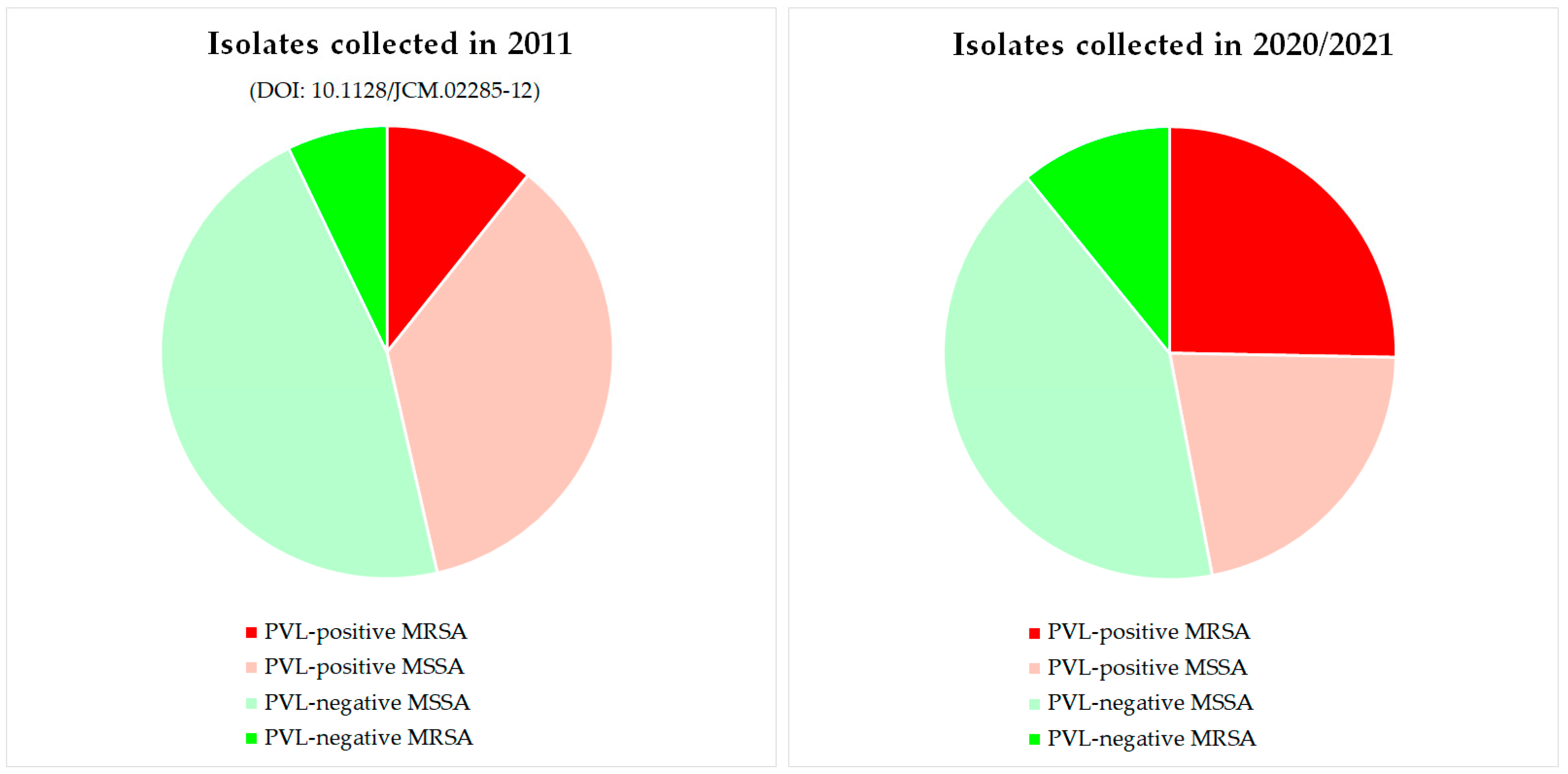
| Category | CC | Strain | N | Percent |
|---|---|---|---|---|
| PVL-negative MSSA | 35 | 42.2% | ||
| CC5 | CC5-MSSA | 1 | 1.2% | |
| CC7 | CC7-MSSA | 7 | 8.4% | |
| CC10 | CC10-MSSA | 2 | 2.4% | |
| CC15 | CC15-MSSA | 4 | 4.8% | |
| CC15 (ST199)-MSSA | 1 | 1.2% | ||
| CC15 (ST582)-MSSA | 2 | 2.4% | ||
| CC22 | CC22-MSSA | 4 | 4.8% | |
| CC22-MSSA-[ccrAB4] | 2 | 2.4% | ||
| CC25 | CC25-MSSA | 1 | 1.2% | |
| CC30 | CC30-MSSA | 4 | 4.8% | |
| CC45 | CC45-MSSA | 4 | 4.8% | |
| CC398 | CC398-MSSA | 2 | 2.4% | |
| ST2867 | ST2867-MSSA | 1 | 1.2% | |
| PVL-negative MRSA | 9 | 10.8% | ||
| CC1 | CC1-MRSA-IV (aphA3/sat-positive), “European CC1-MRSA-IV strain” | 1 | 1.2% | |
| CC1-MRSA-[IV+fus+ccrAB1], “WA MRSA-1/45” | 1 | 1.2% | ||
| CC5 | CC5-MRSA-[IV+fus+ccrAB], “Maltese Clone” | 3 | 3.6% | |
| CC5-MRSA-[VI+fus] | 1 | 1.2% | ||
| CC22 | CC22-MRSA-IV, “UK-EMRSA-15/Barnim EMRSA” | 1 | 1.2% | |
| CC22-MRSA-[V/VT+fus] | 1 | 1.2% | ||
| CC59 | CC59-MRSA-[V/VT+fus] | 1 | 1.2% | |
| PVL-positive MSSA | 18 | 21.7% | ||
| CC5 | CC5-MSSA (PVL+) | 2 | 2.4% | |
| CC6 | CC6-MSSA (PVL+) | 1 | 1.2% | |
| CC15 | CC15-MSSA (PVL+) | 1 | 1.2% | |
| CC30 | CC30-MSSA (PVL+) | 2 | 2.4% | |
| CC88 | CC88-MSSA (PVL+) | 1 | 1.2% | |
| CC121 | CC121-MSSA (PVL+) | 4 | 4.8% | |
| CC152 | CC152-MSSA (PVL+) | 6 | 7.2% | |
| CC772 | CC772-MSSA (PVL+) | 1 | 1.2% | |
| PVL-positive MRSA | 21 | 25.3% | ||
| CC1 | CC1-MRSA-[V/VT+fus+ccrAB1] (PVL+) | 1 | 1.2% | |
| CC5 | CC5-MRSA-IV (sed/j/r+, PVL+), “Sri Lanka Clone” | 4 | 4.8% | |
| CC8 | CC8-MRSA-[IV+Hg] (PVL+), “Spanish/Latin American “USA300” | 4 | 4.8% | |
| CC8-MRSA-[IV+ACME] (PVL+), „USA300“ | 1 | 1.2% | ||
| CC22 | CC22-MRSA-IV (PVL+/tst+) | 2 | 2.4% | |
| CC30 | CC30-MRSA-IV (PVL+), “WSPP/Southwest Pacific Clone” | 1 | 1.2% | |
| CC88 | CC88-MRSA-IV (PVL+) | 1 | 1.2% | |
| CC152 | CC152-MRSA-IV (PVL+) | 1 | 1.2% | |
| CC152-MRSA-[V/VT+fus] (PVL+) | 1 | 1.2% | ||
| CC398 | CC398-MRSA-V/VT (PVL+) | 5 | 6.0% | |
| Gene | Explanation/Corresponding Resistance Phenotype | Total (n = 83) | PVL-Negat. MSSA (n = 35) | PVL-Negat. MRSA (n = 9) | PVL-Posit. MSSA (n = 18) | PVL-Posit. MRSA (n = 23) |
|---|---|---|---|---|---|---|
| mecA | MRSA, methicillin/β-lactam | 30 (36.1%) | 0 | 9 (100%) | 0 | 21 (100%) |
| mecC | MRSA, methicillin/β-lactam | 0 | 0 | 0 | 0 | 0 |
| blaZ | β-lactamase/penicillin | 65 (78.3%) | 23 (65.7%) | 9 (100%) | 13 (72.2%) | 20 (95.2%) |
| fusC | Fusidic acid (SCC-borne) | 9 (10.8%) | 0 | 7 (77.8%) | 0 | 2 (9.5%) |
| far1 | Fusidic acid (plasmid-borne) | 0 | 0 | 0 | 0 | 0 |
| erm(A) | Macrolides, clindamycin | 5 (6%) | 0 | 0 | 0 | 5 (23.8%) |
| erm(B) | Macrolides, clindamycin | 2 (2.4%) | 1 (2.9%) | 0 | 0 | 1 (4.8%) |
| erm(C) | Macrolides, clindamycin | 7 (8.4%) | 0 | 2 (22.2%) | 0 | 5 (23.8%) |
| linA/lnu(A) | Lincosamides, clindamycin | 0 | 0 | 0 | 0 | 0 |
| msr(A) | Macrolides | 4 (4.8%) | 1 (2.9%) | 0 | 1 (5.6%) | 2 (9.5%) |
| mef(A) | Macrolides | 0 | 0 | 0 | 0 | 0 |
| mph(C) | Macrolides | 3 (3.6%) | 0 | 0 | 1 (5.6%) | 2 (9.5%) |
| aacA-aphD | Gentamicin, tobramycin | 7 (8.4%) | 2 (5.7%) | 0 | 0 | 5 (23.8%) |
| aadD | Tobramycin | 3 (3.6%) | 2 (5.7%) | 0 | 0 | 1 (4.8%) |
| aphA3 | Kanamycin | 5 (6%) | 0 | 1 (11.1%) | 1 (5.6%) | 3 (14.3%) |
| dfrA | Trimethoprim | 5 (6%) | 0 | 1 (11.1%) | 0 | 4 (19%) |
| mupA | Mupirocin (high level) | 2 (2.4%) | 1 (2.9%) | 0 | 0 | 1 (4.8%) |
| tet(K) | Tetracyclines | 13 (15.7%) | 0 | 1 (11.1%) | 2 (11.1%) | 10 (47.6%) |
| tet(M) | Tetracyclines | 2 (2.4%) | 0 | 1 (11.1%) | 1 (5.6%) | 0 |
| cat (all variants) * | Chloramphenicol | 4 (4.8%) | 1 (2.9%) | 0 | 2 (11.1%) | 1 (4.8%) |
| cfr | “PhlopsA” resistance phenotype ** | 0 | 0 | 0 | 0 | 0 |
| fexA | Chloramphenicol/florfenicol | 1 (1.2%) | 0 | 1 (11.1%) | 0 | 0 |
| vanA | Vancomycin, teicoplanin | 0 | 0 | 0 | 0 | 0 |
| qacA/B | Biozide resistance | 0 | 0 | 0 | 0 | 0 |
| qacC=smr | Biozide resistance | 3 (3.6%) | 1 (2.9%) | 0 | 1 (5.6%) | 1 (4.8%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Müller, E.; Monecke, S.; Armengol Porta, M.; Narvaez Encalada, M.V.; Reissig, A.; Rüttiger, L.; Schröttner, P.; Schwede, I.; Söffing, H.-H.; Thürmer, A.; et al. Rapid Detection of Panton–Valentine Leukocidin Production in Clinical Isolates of Staphylococcus aureus from Saxony and Brandenburg and Their Molecular Characterisation. Pathogens 2025, 14, 238. https://doi.org/10.3390/pathogens14030238
Müller E, Monecke S, Armengol Porta M, Narvaez Encalada MV, Reissig A, Rüttiger L, Schröttner P, Schwede I, Söffing H-H, Thürmer A, et al. Rapid Detection of Panton–Valentine Leukocidin Production in Clinical Isolates of Staphylococcus aureus from Saxony and Brandenburg and Their Molecular Characterisation. Pathogens. 2025; 14(3):238. https://doi.org/10.3390/pathogens14030238
Chicago/Turabian StyleMüller, Elke, Stefan Monecke, Marc Armengol Porta, Marco Vinicio Narvaez Encalada, Annett Reissig, Lukas Rüttiger, Percy Schröttner, Ilona Schwede, Hans-Herman Söffing, Alexander Thürmer, and et al. 2025. "Rapid Detection of Panton–Valentine Leukocidin Production in Clinical Isolates of Staphylococcus aureus from Saxony and Brandenburg and Their Molecular Characterisation" Pathogens 14, no. 3: 238. https://doi.org/10.3390/pathogens14030238
APA StyleMüller, E., Monecke, S., Armengol Porta, M., Narvaez Encalada, M. V., Reissig, A., Rüttiger, L., Schröttner, P., Schwede, I., Söffing, H.-H., Thürmer, A., & Ehricht, R. (2025). Rapid Detection of Panton–Valentine Leukocidin Production in Clinical Isolates of Staphylococcus aureus from Saxony and Brandenburg and Their Molecular Characterisation. Pathogens, 14(3), 238. https://doi.org/10.3390/pathogens14030238






