For Better or Worse: Type I Interferon Responses in Bacterial Infection
Abstract
1. Introduction
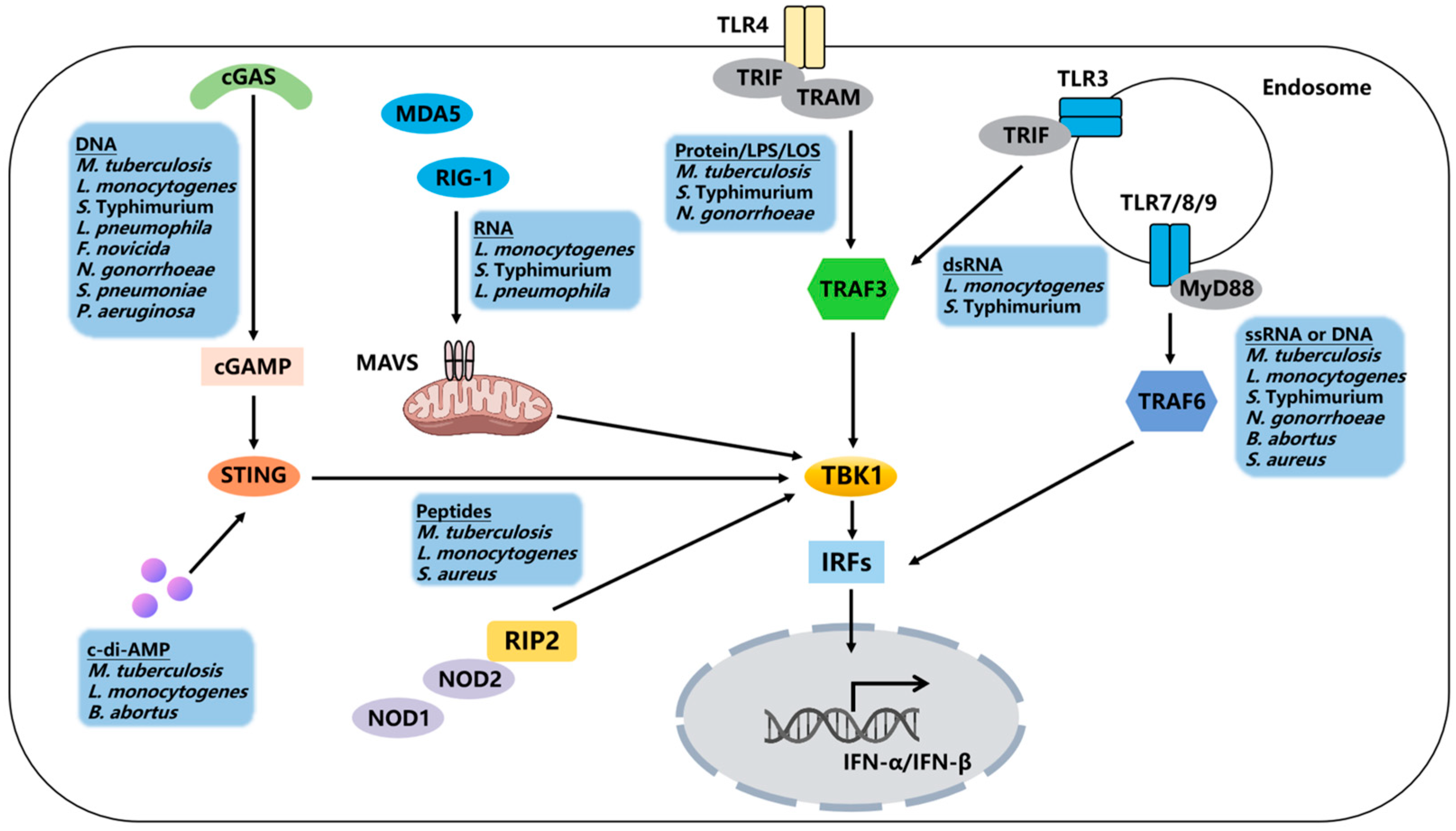
2. Type I IFNs Induction and Receptor Signaling
3. Type I IFNs in Bacterial Infection
3.1. M. tuberculosis
3.2. L. monocytogenes
3.3. S. Typhimurium
3.4. L. pneumophila
3.5. Francisella
3.6. Other Bacterial Infections
4. Discussion and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Yu, R.; Zhu, B.; Chen, D. Type I Interferon-Mediated Tumor Immunity and Its Role in Immunotherapy. Cell. Mol. Life Sci. 2022, 79, 191. [Google Scholar] [CrossRef] [PubMed]
- McNab, F.; Mayer-Barber, K.; Sher, A.; Wack, A.; O’Garra, A. Type I Interferons in Infectious Disease. Nat. Rev. Immunol. 2015, 15, 87–103. [Google Scholar] [CrossRef] [PubMed]
- Schoggins, J.W. Interferon-Stimulated Genes: What Do They All Do? Annu. Rev. Virol. 2019, 6, 567–584. [Google Scholar] [CrossRef] [PubMed]
- Schneider, W.M.; Chevillotte, M.D.; Rice, C.M. Interferon-Stimulated Genes: A Complex Web of Host Defenses. Annu. Rev. Immunol. 2014, 32, 513–545. [Google Scholar] [CrossRef]
- Ivashkiv, L.B.; Donlin, L.T. Regulation of Type I Interferon Responses. Nat. Rev. Immunol. 2014, 14, 36–49. [Google Scholar] [CrossRef]
- Novikov, A.; Cardone, M.; Thompson, R.; Shenderov, K.; Kirschman, K.D.; Mayer-Barber, K.D.; Myers, T.G.; Rabin, R.L.; Trinchieri, G.; Sher, A.; et al. Mycobacterium tuberculosis Triggers Host Type I IFN Signaling to Regulate IL-1β Production in Human Macrophages. J. Immunol. 2011, 187, 2540–2547. [Google Scholar] [CrossRef] [PubMed]
- Dorhoi, A.; Yeremeev, V.; Nouailles, G.; Weiner, J.; Jörg, S.; Heinemann, E.; Oberbeck-Müller, D.; Knaul, J.K.; Vogelzang, A.; Reece, S.T.; et al. Type I IFN Signaling Triggers Immunopathology in Tuberculosis-Susceptible Mice by Modulating Lung Phagocyte Dynamics. Eur. J. Immunol. 2014, 44, 2380–2393. [Google Scholar] [CrossRef]
- de Paus, R.A.; van Wengen, A.; Schmidt, I.; Visser, M.; Verdegaal, E.M.E.; van Dissel, J.T.; van de Vosse, E. Inhibition of the Type I Immune Responses of Human Monocytes by IFN-α and IFN-β. Cytokine 2013, 61, 645–655. [Google Scholar] [CrossRef]
- McNab, F.W.; Ewbank, J.; Howes, A.; Moreira-Teixeira, L.; Martirosyan, A.; Ghilardi, N.; Saraiva, M.; O’Garra, A. Type I IFN Induces IL-10 Production in an IL-27-Independent Manner and Blocks Responsiveness to IFN-γ for Production of IL-12 and Bacterial Killing in Mycobacterium tuberculosis-Infected Macrophages. J. Immunol. 2014, 193, 3600–3612. [Google Scholar] [CrossRef]
- Mayer-Barber, K.D.; Andrade, B.B.; Oland, S.D.; Amaral, E.P.; Barber, D.L.; Gonzales, J.; Derrick, S.C.; Shi, R.; Kumar, N.P.; Wei, W.; et al. Host-Directed Therapy of Tuberculosis Based on Interleukin-1 and Type I Interferon Crosstalk. Nature 2014, 511, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Zarogoulidis, P.; Kioumis, I.; Papanas, N.; Manika, K.; Kontakiotis, T.; Papagianis, A.; Zarogoulidis, K. The Effect of Combination IFN-Alpha-2a with Usual Antituberculosis Chemotherapy in Non-Responding Tuberculosis and Diabetes Mellitus: A Case Report and Review of the Literature. J. Chemother. 2012, 24, 173–177. [Google Scholar] [CrossRef]
- Rivas-Santiago, C.E.; Guerrero, G.G. IFN-α Boosting of Mycobacterium bovis Bacillus Calmette Güerin-Vaccine Promoted Th1 Type Cellular Response and Protection against M. tuberculosis Infection. BioMed Res. Int. 2017, 2017, 8796760. [Google Scholar] [CrossRef] [PubMed]
- Kernbauer, E.; Maier, V.; Rauch, I.; Müller, M.; Decker, T. Route of Infection Determines the Impact of Type I Interferons on Innate Immunity to Listeria monocytogenes. PLoS ONE 2013, 8, e65007. [Google Scholar] [CrossRef]
- Fujiki, T.; Tanaka, A. Antibacterial Activity of Recombinant Murine Beta Interferon. Infect. Immun. 1988, 56, 548–551. [Google Scholar] [CrossRef] [PubMed]
- Auerbuch, V.; Brockstedt, D.G.; Meyer-Morse, N.; O’Riordan, M.; Portnoy, D.A. Mice Lacking the Type I Interferon Receptor Are Resistant to Listeria monocytogenes. J. Exp. Med. 2004, 200, 527–533. [Google Scholar] [CrossRef]
- O’Connell, R.M.; Saha, S.K.; Vaidya, S.A.; Bruhn, K.W.; Miranda, G.A.; Zarnegar, B.; Perry, A.K.; Nguyen, B.O.; Lane, T.F.; Taniguchi, T.; et al. Type I Interferon Production Enhances Susceptibility to Listeria monocytogenes Infection. J. Exp. Med. 2004, 200, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Kearney, S.J.; Delgado, C.; Eshleman, E.M.; Hill, K.K.; O’Connor, B.P.; Lenz, L.L. Type I IFNs Downregulate Myeloid Cell IFN-γ Receptor by Inducing Recruitment of an Early Growth Response 3/NGFI-A Binding Protein 1 Complex That Silences Ifngr1 Transcription. J. Immunol. 2013, 191, 3384–3392. [Google Scholar] [CrossRef]
- Archer, K.A.; Durack, J.; Portnoy, D.A. STING-Dependent Type I IFN Production Inhibits Cell-Mediated Immunity to Listeria monocytogenes. PLoS Pathog. 2014, 10, e1003861. [Google Scholar] [CrossRef]
- Perkins, D.J.; Rajaiah, R.; Tennant, S.M.; Ramachandran, G.; Higginson, E.E.; Dyson, T.N.; Vogel, S.N. Salmonella Typhimurium Co-Opts the Host Type I IFN System to Restrict Macrophage Innate Immune Transcriptional Responses Selectively. J. Immunol. 2015, 195, 2461–2471. [Google Scholar] [CrossRef]
- Robinson, N.; McComb, S.; Mulligan, R.; Dudani, R.; Krishnan, L.; Sad, S. Type I Interferon Induces Necroptosis in Macrophages during Infection with Salmonella enterica Serovar Typhimurium. Nat. Immunol. 2012, 13, 954–962. [Google Scholar] [CrossRef]
- Plumlee, C.R.; Lee, C.; Beg, A.A.; Decker, T.; Shuman, H.A.; Schindler, C. Interferons Direct an Effective Innate Response to Legionella pneumophila Infection. J. Biol. Chem. 2009, 284, 30058–30066. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Moreno, J.S.; Hamann, L.; Shah, J.A.; Verbon, A.; Mockenhaupt, F.P.; Puzianowska-Kuznicka, M.; Naujoks, J.; Sander, L.E.; Witzenrath, M.; Cambier, J.C.; et al. The Common HAQ STING Variant Impairs cGAS-Dependent Antibacterial Responses and Is Associated with Susceptibility to Legionnaires’ Disease in Humans. PLoS Pathog. 2018, 14, e1006829. [Google Scholar] [CrossRef] [PubMed]
- Henry, T.; Kirimanjeswara, G.S.; Ruby, T.; Jones, J.W.; Peng, K.; Perret, M.; Ho, L.; Sauer, J.-D.; Iwakura, Y.; Metzger, D.W.; et al. Type I IFN Signaling Constrains IL-17A/F Secretion by Gammadelta T Cells during Bacterial Infections. J. Immunol. 2010, 184, 3755–3767. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Man, S.M.; Karki, R.; Malireddi, R.K.S.; Kanneganti, T.-D. Detrimental Type I Interferon Signaling Dominates Protective AIM2 Inflammasome Responses during Francisella novicida Infection. Cell Rep. 2018, 22, 3168–3174. [Google Scholar] [CrossRef]
- Andrade, W.A.; Agarwal, S.; Mo, S.; Shaffer, S.A.; Dillard, J.P.; Schmidt, T.; Hornung, V.; Fitzgerald, K.A.; Kurt-Jones, E.A.; Golenbock, D.T. Type I Interferon Induction by Neisseria gonorrhoeae: Dual Requirement of Cyclic GMP-AMP Synthase and Toll-like Receptor 4. Cell Rep. 2016, 15, 2438–2448. [Google Scholar] [CrossRef]
- LeMessurier, K.S.; Häcker, H.; Chi, L.; Tuomanen, E.; Redecke, V. Type I Interferon Protects against Pneumococcal Invasive Disease by Inhibiting Bacterial Transmigration across the Lung. PLoS Pathog. 2013, 9, e1003727. [Google Scholar] [CrossRef]
- Zhou, C.-M.; Wang, B.; Wu, Q.; Lin, P.; Qin, S.-G.; Pu, Q.-Q.; Yu, X.-J.; Wu, M. Identification of cGAS as an Innate Immune Sensor of Extracellular Bacterium Pseudomonas aeruginosa. iScience 2021, 24, 101928. [Google Scholar] [CrossRef]
- de Almeida, L.A.; Carvalho, N.B.; Oliveira, F.S.; Lacerda, T.L.S.; Vasconcelos, A.C.; Nogueira, L.; Bafica, A.; Silva, A.M.; Oliveira, S.C. MyD88 and STING Signaling Pathways Are Required for IRF3-Mediated IFN-β Induction in Response to Brucella abortus Infection. PLoS ONE 2011, 6, e23135. [Google Scholar] [CrossRef] [PubMed]
- Costa Franco, M.M.; Marim, F.; Guimarães, E.S.; Assis, N.R.G.; Cerqueira, D.M.; Alves-Silva, J.; Harms, J.; Splitter, G.; Smith, J.; Kanneganti, T.-D.; et al. Brucella abortus Triggers a cGAS-Independent STING Pathway to Induce Host Protection That Involves Guanylate-Binding Proteins and Inflammasome Activation. J. Immunol. 2018, 200, 607–622. [Google Scholar] [CrossRef] [PubMed]
- Martin, F.J.; Gomez, M.I.; Wetzel, D.M.; Memmi, G.; O’Seaghdha, M.; Soong, G.; Schindler, C.; Prince, A. Staphylococcus aureus Activates Type I IFN Signaling in Mice and Humans through the Xr Repeated Sequences of Protein A. J. Clin. Investig. 2009, 119, 1931–1939. [Google Scholar] [CrossRef]
- Watanabe, T.; Asano, N.; Fichtner-Feigl, S.; Gorelick, P.L.; Tsuji, Y.; Matsumoto, Y.; Chiba, T.; Fuss, I.J.; Kitani, A.; Strober, W. NOD1 Contributes to Mouse Host Defense against Helicobacter Pylori via Induction of Type I IFN and Activation of the ISGF3 Signaling Pathway. J. Clin. Investig. 2010, 120, 1645–1662. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lei, X.; Jiang, Z.; Fitzgerald, K.A. Cellular Nucleic Acid-Binding Protein Is Essential for Type I Interferon-Mediated Immunity to RNA Virus Infection. Proc. Natl. Acad. Sci. USA 2021, 118, e2100383118. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Zhang, W.; Tian, L.; Brown, B.R.; Walters, M.S.; Metcalf, J.P. IRF7 Is Required for the Second Phase Interferon Induction during Influenza Virus Infection in Human Lung Epithelia. Viruses 2020, 12, 377. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Ji, Y.; Zeng, L.; Liu, Q.; Zhang, Z.; Guo, S.; Guo, X.; Tong, Y.; Zhao, X.; Li, C.-M.; et al. P200 Family Protein IFI204 Negatively Regulates Type I Interferon Responses by Targeting IRF7 in Nucleus. PLoS Pathog. 2019, 15, e1008079. [Google Scholar] [CrossRef]
- Perkins, D.J.; Vogel, S.N. Space and Time: New Considerations about the Relationship between Toll-like Receptors (TLRs) and Type I Interferons (IFNs). Cytokine 2015, 74, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Choo, M.-K.; Sano, Y.; Kim, C.; Yasuda, K.; Li, X.-D.; Lin, X.; Stenzel-Poore, M.; Alexopoulou, L.; Ghosh, S.; Latz, E.; et al. TLR Sensing of Bacterial Spore-Associated RNA Triggers Host Immune Responses with Detrimental Effects. J. Exp. Med. 2017, 214, 1297–1311. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. Toll-like Receptors and Their Crosstalk with Other Innate Receptors in Infection and Immunity. Immunity 2011, 34, 637–650. [Google Scholar] [CrossRef]
- Barral, P.M.; Sarkar, D.; Su, Z.; Barber, G.N.; DeSalle, R.; Racaniello, V.R.; Fisher, P.B. Functions of the Cytoplasmic RNA Sensors RIG-I and MDA-5: Key Regulators of Innate Immunity. Pharmacol. Ther. 2009, 124, 219–234. [Google Scholar] [CrossRef]
- Pandey, A.K.; Yang, Y.; Jiang, Z.; Fortune, S.M.; Coulombe, F.; Behr, M.A.; Fitzgerald, K.A.; Sassetti, C.M.; Kelliher, M.A. NOD2, RIP2 and IRF5 Play a Critical Role in the Type I Interferon Response to Mycobacterium tuberculosis. PLoS Pathog. 2009, 5, e1000500. [Google Scholar] [CrossRef]
- Kong, E.; Kim, H.D.; Kim, J. Deleting Key Autophagy Elongation Proteins Induces Acquirement of Tumor-Associated Phenotypes via ISG15. Cell Death Differ. 2020, 27, 2517–2530. [Google Scholar] [CrossRef]
- Roers, A.; Hiller, B.; Hornung, V. Recognition of Endogenous Nucleic Acids by the Innate Immune System. Immunity 2016, 44, 739–754. [Google Scholar] [CrossRef] [PubMed]
- Piaszyk-Borychowska, A.; Széles, L.; Csermely, A.; Chiang, H.-C.; Wesoły, J.; Lee, C.-K.; Nagy, L.; Bluyssen, H.A.R. Signal Integration of IFN-I and IFN-II with TLR4 Involves Sequential Recruitment of STAT1-Complexes and NFκB to Enhance Pro-Inflammatory Transcription. Front. Immunol. 2019, 10, 1253. [Google Scholar] [CrossRef]
- Zhou, B.-X.; Li, J.; Liang, X.-L.; Pan, X.-P.; Hao, Y.-B.; Xie, P.-F.; Jiang, H.-M.; Yang, Z.-F.; Zhong, N.-S. β-Sitosterol Ameliorates Influenza A Virus-Induced Proinflammatory Response and Acute Lung Injury in Mice by Disrupting the Cross-Talk between RIG-I and IFN/STAT Signaling. Acta Pharmacol. Sin. 2020, 41, 1178–1196. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.H.; Liu, H.; Ge, B. Innate Immunity in Tuberculosis: Host Defense vs Pathogen Evasion. Cell Mol. Immunol. 2017, 14, 963–975. [Google Scholar] [CrossRef] [PubMed]
- Moreira-Teixeira, L.; Sousa, J.; McNab, F.W.; Torrado, E.; Cardoso, F.; Machado, H.; Castro, F.; Cardoso, V.; Gaifem, J.; Wu, X.; et al. Type I IFN Inhibits Alternative Macrophage Activation during Mycobacterium tuberculosis Infection and Leads to Enhanced Protection in the Absence of IFN-γ Signaling. J. Immunol. 2016, 197, 4714–4726. [Google Scholar] [CrossRef] [PubMed]
- Simmons, D.P.; Canaday, D.H.; Liu, Y.; Li, Q.; Huang, A.; Boom, W.H.; Harding, C.V. Mycobacterium tuberculosis and TLR2 Agonists Inhibit Induction of Type I IFN and Class I MHC Antigen Cross Processing by TLR9. J. Immunol. 2010, 185, 2405–2415. [Google Scholar] [CrossRef]
- Wiens, K.E.; Ernst, J.D. The Mechanism for Type I Interferon Induction by Mycobacterium tuberculosis Is Bacterial Strain-Dependent. PLoS Pathog. 2016, 12, e1005809. [Google Scholar] [CrossRef]
- Dey, B.; Dey, R.J.; Cheung, L.S.; Pokkali, S.; Guo, H.; Lee, J.-H.; Bishai, W.R. A Bacterial Cyclic Dinucleotide Activates the Cytosolic Surveillance Pathway and Mediates Innate Resistance to Tuberculosis. Nat. Med. 2015, 21, 401–406. [Google Scholar] [CrossRef]
- Manca, C.; Tsenova, L.; Freeman, S.; Barczak, A.K.; Tovey, M.; Murray, P.J.; Barry, C.; Kaplan, G. Hypervirulent M. tuberculosis W/Beijing Strains Upregulate Type I IFNs and Increase Expression of Negative Regulators of the Jak-Stat Pathway. J. Interferon Cytokine Res. 2005, 25, 694–701. [Google Scholar] [CrossRef]
- Zhang, G.; deWeerd, N.A.; Stifter, S.A.; Liu, L.; Zhou, B.; Wang, W.; Zhou, Y.; Ying, B.; Hu, X.; Matthews, A.Y.; et al. A Proline Deletion in IFNAR1 Impairs IFN-Signaling and Underlies Increased Resistance to Tuberculosis in Humans. Nat. Commun. 2018, 9, 85. [Google Scholar] [CrossRef]
- Mayer-Barber, K.D.; Barber, D.L.; Shenderov, K.; White, S.D.; Wilson, M.S.; Cheever, A.; Kugler, D.; Hieny, S.; Caspar, P.; Núñez, G.; et al. Caspase-1 Independent IL-1beta Production Is Critical for Host Resistance to Mycobacterium tuberculosis and Does Not Require TLR Signaling in Vivo. J. Immunol. 2010, 184, 3326–3330. [Google Scholar] [CrossRef] [PubMed]
- Teles, R.M.B.; Graeber, T.G.; Krutzik, S.R.; Montoya, D.; Schenk, M.; Lee, D.J.; Komisopoulou, E.; Kelly-Scumpia, K.; Chun, R.; Iyer, S.S.; et al. Type I Interferon Suppresses Type II Interferon-Triggered Human Anti-Mycobacterial Responses. Science 2013, 339, 1448–1453. [Google Scholar] [CrossRef]
- Mayer-Barber, K.D.; Andrade, B.B.; Barber, D.L.; Hieny, S.; Feng, C.G.; Caspar, P.; Oland, S.; Gordon, S.; Sher, A. Innate and Adaptive Interferons Suppress IL-1α and IL-1β Production by Distinct Pulmonary Myeloid Subsets during Mycobacterium tuberculosis Infection. Immunity 2011, 35, 1023–1034. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hussain, T.; Yue, R.; Liao, Y.; Li, Q.; Yao, J.; Song, Y.; Sun, X.; Wang, N.; Xu, L.; et al. MicroRNA-199a Inhibits Cellular Autophagy and Downregulates IFN-β Expression by Targeting TBK1 in Mycobacterium bovis Infected Cells. Front. Cell Infect. Microbiol. 2018, 8, 238. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Shen, H.; Lian, Q.; Jin, W.; Zhang, R.; Lin, X.; Gu, W.; Sun, X.; Meng, G.; Tian, Z.; et al. Deficiency of the AIM2-ASC Signal Uncovers the STING-Driven Overreactive Response of Type I IFN and Reciprocal Depression of Protective IFN-γ Immunity in Mycobacterial Infection. J. Immunol. 2018, 200, 1016–1026. [Google Scholar] [CrossRef]
- Wang, J.; Liu, B.; Teng, Z.; Zhou, X.; Wang, X.; Zhang, B.; Lu, G.; Niu, X.; Yang, Y.; Deng, X. Phloretin Attenuates Listeria monocytogenes Virulence Both In Vitro and In Vivo by Simultaneously Targeting Listeriolysin O and Sortase A. Front. Cell Infect. Microbiol. 2017, 7, 9. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fernández-Martínez, N.F.; Ruiz-Montero, R.; Briones, E.; Baños, E.; García San Miguel Rodríguez-Alarcón, L.; Chaves, J.A.; Abad, R.; Varela, C.; LISMOAN team; Lorusso, N. Listeriosis Outbreak Caused by Contaminated Stuffed Pork, Andalusia, Spain, July to October 2019. Euro Surveill. 2022, 27, 2200279. [Google Scholar] [CrossRef]
- Abdullah, Z.; Schlee, M.; Roth, S.; Mraheil, M.A.; Barchet, W.; Böttcher, J.; Hain, T.; Geiger, S.; Hayakawa, Y.; Fritz, J.H.; et al. RIG-I Detects Infection with Live Listeria by Sensing Secreted Bacterial Nucleic Acids. EMBO J. 2012, 31, 4153–4164. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.; Prabakaran, T.; Laustsen, A.; Jørgensen, S.E.; Rahbæk, S.H.; Jensen, S.B.; Nielsen, R.; Leber, J.H.; Decker, T.; Horan, K.A.; et al. Listeria monocytogenes Induces IFNβ Expression through an IFI16-, cGAS- and STING-Dependent Pathway. EMBO J. 2014, 33, 1654–1666. [Google Scholar] [CrossRef]
- Nandakumar, R.; Tschismarov, R.; Meissner, F.; Prabakaran, T.; Krissanaprasit, A.; Farahani, E.; Zhang, B.-C.; Assil, S.; Martin, A.; Bertrams, W.; et al. Intracellular Bacteria Engage a STING-TBK1-MVB12b Pathway to Enable Paracrine cGAS-STING Signalling. Nat. Microbiol. 2019, 4, 701–713. [Google Scholar] [CrossRef] [PubMed]
- Aubry, C.; Corr, S.C.; Wienerroither, S.; Goulard, C.; Jones, R.; Jamieson, A.M.; Decker, T.; O’Neill, L.A.J.; Dussurget, O.; Cossart, P. Both TLR2 and TRIF Contribute to Interferon-β Production during Listeria Infection. PLoS ONE 2012, 7, e33299. [Google Scholar] [CrossRef] [PubMed]
- Stockinger, S.; Kastner, R.; Kernbauer, E.; Pilz, A.; Westermayer, S.; Reutterer, B.; Soulat, D.; Stengl, G.; Vogl, C.; Frenz, T.; et al. Characterization of the Interferon-Producing Cell in Mice Infected with Listeria monocytogenes. PLoS Pathog. 2009, 5, e1000355. [Google Scholar] [CrossRef]
- Leber, J.H.; Crimmins, G.T.; Raghavan, S.; Meyer-Morse, N.P.; Cox, J.S.; Portnoy, D.A. Distinct TLR- and NLR-Mediated Transcriptional Responses to an Intracellular Pathogen. PLoS Pathog. 2008, 4, e6. [Google Scholar] [CrossRef] [PubMed]
- Woodward, J.J.; Iavarone, A.T.; Portnoy, D.A. C-Di-AMP Secreted by Intracellular Listeria monocytogenes Activates a Host Type I Interferon Response. Science 2010, 328, 1703–1705. [Google Scholar] [CrossRef] [PubMed]
- Perelman, S.S.; Abrams, M.E.; Eitson, J.L.; Chen, D.; Jimenez, A.; Mettlen, M.; Schoggins, J.W.; Alto, N.M. Cell-Based Screen Identifies Human Interferon-Stimulated Regulators of Listeria monocytogenes Infection. PLoS Pathog. 2016, 12, e1006102. [Google Scholar] [CrossRef]
- Yoon, S.; Bogdanov, K.; Wallach, D. Site-Specific Ubiquitination of MLKL Targets It to Endosomes and Targets Listeria and Yersinia to the Lysosomes. Cell Death Differ. 2022, 29, 306–322. [Google Scholar] [CrossRef]
- Carrero, J.A.; Calderon, B.; Unanue, E.R. Type I Interferon Sensitizes Lymphocytes to Apoptosis and Reduces Resistance to Listeria Infection. J. Exp. Med. 2004, 200, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Amaya, F.A.; Blondel, C.J.; Barros-Infante, M.F.; Rivera, D.; Moreno-Switt, A.I.; Santiviago, C.A.; Pezoa, D. Identification of Type VI Secretion Systems Effector Proteins That Contribute to Interbacterial Competition in Salmonella dublin. Front. Microbiol. 2022, 13, 811932. [Google Scholar] [CrossRef] [PubMed]
- Schmolke, M.; Patel, J.R.; de Castro, E.; Sánchez-Aparicio, M.T.; Uccellini, M.B.; Miller, J.C.; Manicassamy, B.; Satoh, T.; Kawai, T.; Akira, S.; et al. RIG-I Detects mRNA of Intracellular Salmonella enterica Serovar Typhimurium during Bacterial Infection. mBio 2014, 5, e01006-14. [Google Scholar] [CrossRef]
- Owen, K.A.; Anderson, C.J.; Casanova, J.E. Salmonella Suppresses the TRIF-Dependent Type I Interferon Response in Macrophages. mBio 2016, 7, e02051-15. [Google Scholar] [CrossRef]
- Tursi, S.A.; Lee, E.Y.; Medeiros, N.J.; Lee, M.H.; Nicastro, L.K.; Buttaro, B.; Gallucci, S.; Wilson, R.P.; Wong, G.C.L.; Tükel, Ç. Bacterial Amyloid Curli Acts as a Carrier for DNA to Elicit an Autoimmune Response via TLR2 and TLR9. PLoS Pathog. 2017, 13, e1006315. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Li, M.; Yang, Y.; Zhang, C.; Xie, Z.; Tang, J.; Shi, Z.; Chen, S.; Li, G.; Gu, Y.; et al. Salmonella Induces the cGAS-STING-Dependent Type I Interferon Response in Murine Macrophages by Triggering mtDNA Release. mBio 2022, 13, e0363221. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Qin, X. Critical Role of Type I Interferon-Induced Macrophage Necroptosis during Infection with Salmonella enterica Serovar Typhimurium. Cell Mol. Immunol. 2013, 10, 99–100. [Google Scholar] [CrossRef]
- Broz, P.; Ruby, T.; Belhocine, K.; Bouley, D.M.; Kayagaki, N.; Dixit, V.M.; Monack, D.M. Caspase-11 Increases Susceptibility to Salmonella Infection in the Absence of Caspase-1. Nature 2012, 490, 288–291. [Google Scholar] [CrossRef]
- Song, L.; Luo, J.; Wang, H.; Huang, D.; Tan, Y.; Liu, Y.; Wang, Y.; Yu, K.; Zhang, Y.; Liu, X.; et al. Legionella pneumophila Regulates Host Cell Motility by Targeting Phldb2 with a 14-3-3ζ-Dependent Protease Effector. Elife 2022, 11, e73220. [Google Scholar] [CrossRef]
- Fu, J.; Zhou, M.; Gritsenko, M.A.; Nakayasu, E.S.; Song, L.; Luo, Z.-Q. Legionella pneumophila Modulates Host Energy Metabolism by ADP-Ribosylation of ADP/ATP Translocases. Elife 2022, 11, e73611. [Google Scholar] [CrossRef]
- Lippmann, J.; Müller, H.C.; Naujoks, J.; Tabeling, C.; Shin, S.; Witzenrath, M.; Hellwig, K.; Kirschning, C.J.; Taylor, G.A.; Barchet, W.; et al. Dissection of a Type I Interferon Pathway in Controlling Bacterial Intracellular Infection in Mice. Cell. Microbiol. 2011, 13, 1668–1682. [Google Scholar] [CrossRef]
- Kim, H.; Kubori, T.; Yamazaki, K.; Kwak, M.-J.; Park, S.-Y.; Nagai, H.; Vogel, J.P.; Oh, B.-H. Structural Basis for Effector Protein Recognition by the Dot/Icm Type IVB Coupling Protein Complex. Nat. Commun. 2020, 11, 2623. [Google Scholar] [CrossRef] [PubMed]
- Monroe, K.M.; McWhirter, S.M.; Vance, R.E. Identification of Host Cytosolic Sensors and Bacterial Factors Regulating the Type I Interferon Response to Legionella pneumophila. PLoS Pathog. 2009, 5, e1000665. [Google Scholar] [CrossRef] [PubMed]
- Opitz, B.; Vinzing, M.; van Laak, V.; Schmeck, B.; Heine, G.; Günther, S.; Preissner, R.; Slevogt, H.; N’Guessan, P.D.; Eitel, J.; et al. Legionella pneumophila Induces IFNbeta in Lung Epithelial Cells via IPS-1 and IRF3, Which Also Control Bacterial Replication. J. Biol. Chem. 2006, 281, 36173–36179. [Google Scholar] [CrossRef]
- Naujoks, J.; Tabeling, C.; Dill, B.D.; Hoffmann, C.; Brown, A.S.; Kunze, M.; Kempa, S.; Peter, A.; Mollenkopf, H.-J.; Dorhoi, A.; et al. IFNs Modify the Proteome of Legionella-Containing Vacuoles and Restrict Infection Via IRG1-Derived Itaconic Acid. PLoS Pathog. 2016, 12, e1005408. [Google Scholar] [CrossRef] [PubMed]
- Suresh, R.V.; Bradley, E.W.; Higgs, M.; Russo, V.C.; Alqahtani, M.; Huang, W.; Bakshi, C.S.; Malik, M. Nlrp3 Increases the Host’s Susceptibility to Tularemia. Front. Microbiol. 2021, 12, 725572. [Google Scholar] [CrossRef] [PubMed]
- Grossman, T.H.; Anderson, M.S.; Christ, D.; Gooldy, M.; Henning, L.N.; Heine, H.S.; Kindt, M.V.; Lin, W.; Siefkas-Patterson, K.; Radcliff, A.K.; et al. The Fluorocycline TP-271 Is Efficacious in Models of Aerosolized Francisella Tularensis SCHU S4 Infection in BALB/c Mice and Cynomolgus Macaques. Antimicrob. Agents Chemother. 2017, 61, e00448-17. [Google Scholar] [CrossRef] [PubMed]
- Santic, M.; Molmeret, M.; Klose, K.E.; Jones, S.; Kwaik, Y.A. The Francisella tularensis Pathogenicity Island Protein IglC and Its Regulator MglA Are Essential for Modulating Phagosome Biogenesis and Subsequent Bacterial Escape into the Cytoplasm. Cell. Microbiol. 2005, 7, 969–979. [Google Scholar] [CrossRef]
- Storek, K.M.; Gertsvolf, N.A.; Ohlson, M.B.; Monack, D.M. cGAS and Ifi204 Cooperate to Produce Type I IFNs in Response to Francisella Infection. J. Immunol. 2015, 194, 3236–3245. [Google Scholar] [CrossRef] [PubMed]
- Smatlik, N.; Drexler, S.K.; Burian, M.; Röcken, M.; Yazdi, A.S. ASC Speck Formation after Inflammasome Activation in Primary Human Keratinocytes. Oxid. Med. Cell Longev. 2021, 2021, 7914829. [Google Scholar] [CrossRef]
- Huang, B.; Qian, Y.; Xie, S.; Ye, X.; Chen, H.; Chen, Z.; Zhang, L.; Xu, J.; Hu, H.; Ma, S.; et al. Ticagrelor Inhibits the NLRP3 Inflammasome to Protect against Inflammatory Disease Independent of the P2Y12 Signaling Pathway. Cell Mol. Immunol. 2021, 18, 1278–1289. [Google Scholar] [CrossRef]
- Lamkanfi, M.; Dixit, V.M. Mechanisms and Functions of Inflammasomes. Cell 2014, 157, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
- Fernandes-Alnemri, T.; Yu, J.-W.; Juliana, C.; Solorzano, L.; Kang, S.; Wu, J.; Datta, P.; McCormick, M.; Huang, L.; McDermott, E.; et al. The AIM2 Inflammasome Is Critical for Innate Immunity to Francisella tularensis. Nat. Immunol. 2010, 11, 385–393. [Google Scholar] [CrossRef]
- Schroder, K.; Tschopp, J. The Inflammasomes. Cell 2010, 140, 821–832. [Google Scholar] [CrossRef] [PubMed]
- Henry, T.; Brotcke, A.; Weiss, D.S.; Thompson, L.J.; Monack, D.M. Type I Interferon Signaling Is Required for Activation of the Inflammasome during Francisella Infection. J. Exp. Med. 2007, 204, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Château, A.; Seifert, H.S. Neisseria Gonorrhoeae Survives within and Modulates Apoptosis and Inflammatory Cytokine Production of Human Macrophages. Cell. Microbiol. 2016, 18, 546–560. [Google Scholar] [CrossRef]
- Dobson-Belaire, W.N.; Rebbapragada, A.; Malott, R.J.; Yue, F.Y.; Kovacs, C.; Kaul, R.; Ostrowski, M.A.; Gray-Owen, S.D. Neisseria gonorrhoeae Effectively Blocks HIV-1 Replication by Eliciting a Potent TLR9-Dependent Interferon-α Response from Plasmacytoid Dendritic Cells. Cell. Microbiol. 2010, 12, 1703–1717. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Peng, X.; Lu, C.; Zhang, X.; Gan, L.; Gao, Y.; Yang, S.; Xu, W.; Wang, J.; Yin, Y.; et al. Type I IFN Expression Is Stimulated by Cytosolic MtDNA Released from Pneumolysin-Damaged Mitochondria via the STING Signaling Pathway in Macrophages. FEBS J. 2019, 286, 4754–4768. [Google Scholar] [CrossRef]
- Guimarães, E.S.; Gomes, M.T.R.; Campos, P.C.; Mansur, D.S.; Dos Santos, A.A.; Harms, J.; Splitter, G.; Smith, J.A.; Barber, G.N.; Oliveira, S.C. Brucella abortus Cyclic Dinucleotides Trigger STING-Dependent Unfolded Protein Response That Favors Bacterial Replication. J. Immunol. 2019, 202, 2671–2681. [Google Scholar] [CrossRef]
- Parker, D.; Planet, P.J.; Soong, G.; Narechania, A.; Prince, A. Induction of Type I Interferon Signaling Determines the Relative Pathogenicity of Staphylococcus Aureus Strains. PLoS Pathog. 2014, 10, e1003951. [Google Scholar] [CrossRef] [PubMed]
- Gratz, N.; Hartweger, H.; Matt, U.; Kratochvill, F.; Janos, M.; Sigel, S.; Drobits, B.; Li, X.-D.; Knapp, S.; Kovarik, P. Type I Interferon Production Induced by Streptococcus pyogenes-Derived Nucleic Acids Is Required for Host Protection. PLoS Pathog. 2011, 7, e1001345. [Google Scholar] [CrossRef] [PubMed]
- Viala, J.; Chaput, C.; Boneca, I.G.; Cardona, A.; Girardin, S.E.; Moran, A.P.; Athman, R.; Mémet, S.; Huerre, M.R.; Coyle, A.J.; et al. Nod1 Responds to Peptidoglycan Delivered by the Helicobacter pylori Cag Pathogenicity Island. Nat. Immunol. 2004, 5, 1166–1174. [Google Scholar] [CrossRef]
| Pathogen | Location | Type I IFN Signaling PRRs | Mechanisms/Outcome | Effects of Type I IFNs | Reference |
|---|---|---|---|---|---|
| M. tuberculosis | Intracellular | cGAS, STING, NOD2, TLR4, TLR9 | Limiting the production of IL-1β (macrophages); no effect (monocytes) | Detrimental | [6] |
| Promoting intracellular M. tuberculosis replication, diminishing alveolar macrophage numbers, and driving tissue damage (aerogenic infection) | Detrimental | [7] | |||
| Reducing TNF, IL-1β, and IL-12 production, and CD54 expression (monocytes); promoting the expression of IL-10 (macrophages) | Detrimental | [8,9] | |||
| Promoting cell necrosis through the suppression of PGE2 (aerosol infection) | Detrimental | [10] | |||
| Combination IFN-alpha-2a with antituberculosis chemotherapy improving a patient’s clinical symptoms | Protective | [11] | |||
| Enhancing BCG-promoted Th1 response (aerosol infection) | Protective | [12] | |||
| L. monocytogenes | Intracellular | cGAS, STING, NOD2, RIG, TLR3, TLR9 | Increasing the mRNA levels of pro-inflammatory cytokines (intragastric gavage infection) | Protective | [13] |
| Intravenous injection of IFN-β enhancing host resistance | Protective | [14] | |||
| Inhibiting the production of IL-12p70 and TNF-α (intravenous injection) | Detrimental | [15] | |||
| Enhancing the expression of pro-apoptotic genes and inducing splenic apoptosis (intravenous injection) | Detrimental | [16] | |||
| Downregulating myeloid cell IFN-γ receptor expression (macrophages) | Detrimental | [17] | |||
| Repressing the host adaptive immune response (intravenous injection) | Detrimental | [18] | |||
| S. Typhimurium | Intracellular | cGAS, RIG, TLR4, TLR3, TLR9 | Suppressing the expression of pro-inflammatory cytokines and chemokines, inhibiting the recruitment and activation of immune cells (oral gavage) | Detrimental | [19] |
| Inducing macrophage necroptosis (macrophage) | Detrimental | [20] | |||
| L. pneumophila | Intracellular | cGAS, RIG | Activating M1 macrophages (macrophages) | Protective | [21] |
| Promoting pro-inflammatory cytokines expression (intranasal infection) | Protective | [22] | |||
| F. tularensis/F. novicida | Intracellular | cGAS | Inhibiting the expression of IL-17A in γδT cells (intranasal infection) | Detrimental | [23] |
| Activating apoptotic caspases and cell death (subcutaneous infection) | Detrimental | [24] | |||
| N. gonorrhoeae | Extracellular | cGAS, TLR4, TLR9 | Enhancing the intracellular iron pool (macrophages) | Detrimental | [25] |
| S. pneumoniae | Extracellular | cGAS | Reducing the invasion of epithelial and endothelial cells (intranasal infection) | Protective | [26] |
| P. aeruginosa | Extracellular | cGAS | Activating the unfolded protein response (intranasal infection) | Protective | [27] |
| B. abortus | Intracellular | TLR9, STING | Suppressing the production of IFN-γ and NO, and inducing apoptosis (intraperitoneal injection) | Detrimental | [28] |
| Inducing pro-inflammatory cytokine production (macrophages) | Protective | [29] | |||
| S. aureus | Extracellular | TLR8, NOD2 | Enhancing immune cells recruitment (respiratory infection) | Detrimental | [30] |
| H. pylori | Extracellular | NOD1 | Inducing the production of CXCL10 (HT-29 cell) | Protective | [31] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, A.; Li, X.; Zhao, C.; Meng, X.; Kari, G.; Wang, Y. For Better or Worse: Type I Interferon Responses in Bacterial Infection. Pathogens 2025, 14, 229. https://doi.org/10.3390/pathogens14030229
Xia A, Li X, Zhao C, Meng X, Kari G, Wang Y. For Better or Worse: Type I Interferon Responses in Bacterial Infection. Pathogens. 2025; 14(3):229. https://doi.org/10.3390/pathogens14030229
Chicago/Turabian StyleXia, Aihong, Xin Li, Changjing Zhao, Xiaojing Meng, Gulmela Kari, and Yongjuan Wang. 2025. "For Better or Worse: Type I Interferon Responses in Bacterial Infection" Pathogens 14, no. 3: 229. https://doi.org/10.3390/pathogens14030229
APA StyleXia, A., Li, X., Zhao, C., Meng, X., Kari, G., & Wang, Y. (2025). For Better or Worse: Type I Interferon Responses in Bacterial Infection. Pathogens, 14(3), 229. https://doi.org/10.3390/pathogens14030229




