Abstract
Type I interferons (IFNs) are pleiotropic cytokines, primarily comprising IFN-α and IFN-β, and their effect in host defense against viral infection has been extensively studied and well-established. However, in bacterial infection, the role of type I IFNs is more complex, exhibiting multifaceted effects that depend on several factors, such as the pathogen species, the specific cell populations, and the routes of infection. In this review, we summarize research progress on host type I interferon responses triggered by specific bacteria and their immune regulation function in order to better understand the role of type I IFNs in bacterial infection and provide insights for adjuvant therapies tailored to treat specific bacterial infections.
1. Introduction
Interferons (IFNs) are a class of proteins or glycoproteins with multiple biological activities, which are divided into type I, type II, and type III [1]. The type I IFN family includes IFN-β, IFN-ω, IFN-κ, IFN-ε, IFN-ζ, IFN-δ, IFN-τ, and 14 different subtypes of IFN-α. The type II IFN family consists only of IFN-γ, while the type III IFN family includes IFN-λ1 (IL-29), IFN-λ2 (IL-28A), IFN-λ3 (IL-28B), and IFN-λ4 [2]. Type I IFNs are generally considered to be immune regulatory factors with antiviral properties as they induce the expression of interferon-stimulated genes (ISGs) [3]. The products of these ISGs can inhibit various stages of viral replication, including blocking viral mRNA synthesis, suppressing viral protein translation, and disrupting virus assembly and release [4]. However, recent studies have shown that type I IFNs also play a critical role in combating bacterial infections, including those caused by Mycobacterium tuberculosis (M. tuberculosis), Listeria monocytogenes (L. monocytogenes), Legionella pneumophila (L. pneumophila), Salmonella typhimurium (S. Typhimurium), and Helicobacter pylori (H. pylori). In bacterial infections, the role of type I IFNs is variable, which may exert either beneficial or detrimental regulatory effects [5]. This article summarizes the induction and effects of type I IFNs in specific bacterial infections (Figure 1 and Table 1), and proposes potential intervention strategies to optimize their beneficial effects while minimizing adverse outcomes.
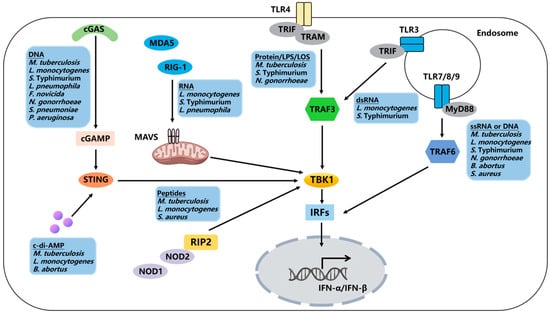
Figure 1.
Regulation of the host type I interferon signaling pathway by bacteria. Bacterial DNA/RNA can be recognized by nucleic acid sensors, including cGAS, RIG-I, MDA-5, and TLR3, 7, and 9. Additionally, bacterial components and products can activate type I interferon responses through STING, TLR4, NOD1, and NOD2.

Table 1.
Effects of type I IFNs during bacterial infection.
2. Type I IFNs Induction and Receptor Signaling
During bacterial infection, the expression of type I IFNs is driven by the interferon regulatory factor (IRF) family of transcription factors, particularly IRF3 and IRF7 [32]. In most cells, IRF3 serves as the primary transcription factor for early type I IFN expression. Subsequently, IRF7 is expressed as one of the ISGs, amplifying the transcription of type I IFNs [33]. Mammalian cells encode numerous pattern recognition receptors (PRRs) that sense invading pathogens and lead to type I interferon production (Figure 1). Upon stimulation of these PRRs, IRFs are activated in a phosphorylation-dependent manner, leading to the induction of type I IFN expression [34]. Bacterial surface components bind to Toll-like receptors (TLRs) 2 and 4 on the cell membrane, while TLR9 anchored in the endosome membrane is activated by bacterial DNA [35]. Single-stranded RNA is sensed by TLR7, TLR8, and TLR13 [36]. Except for TLR3, all TLRs recruit the downstream signal adaptor molecule MyD88 to induce type I IFN expression. TLR4 can recruit both MyD88 and TRIF, but it induces the expression of type I IFNs solely through the TRIF-dependent signaling pathway [37]. RNA is recognized by two RIG-I-like receptors (RLRs), RIG-I and MDA-5 [38]. Peptides from the bacterial cell wall are recognized by NOD1 [31] and NOD2 [39], thereby recruiting the signal adaptor molecule RIP2. DNA is recognized by the cytosolic sensor cGAS, which activates the STING-TBK1-IRF3 pathway through the production of the second messenger cGAMP [40]. In addition to cGAS, DNA can also be recognized by DDX41 and an AIM-like receptor (ALR), IFI16 [41].
Induced type I IFNs bind to their receptor IFNAR1–IFNAR2 heterodimer, activating Janus kinases JAK1 and TYK2. These kinases further phosphorylate STAT family members. Activated STAT1 and STAT2 dimerize and recruit IRF9 to form the ISGF3 complex. The complex subsequently translocates to the nucleus, where it binds to the interferon-stimulated response element (ISRE) in the gene promoter region, thereby initiating the transcription of ISGs [5]. Alternatively, activated STAT monomers form STAT1–STAT1 homodimers, which bind IFN-γ-activated sites (GAS) that promote gene transcription of ISGs [42]. These genes are essential for the activation, proliferation, differentiation, and regulation of inflammatory responses of immune cells [43].
3. Type I IFNs in Bacterial Infection
3.1. M. tuberculosis
M. tuberculosis is the bacterium that causes tuberculosis (TB), which is a leading cause of death from infectious diseases. M. tuberculosis can be recognized by host immune cells through surface and intracellular PRRs [44]. Sensing of M. tuberculosis infection by TLR4 and TLR9 has been found to induce the expression of type I IFNs [45,46]. Recognition of M. tuberculosis is not restricted to TLRs as it can also be detected by NOD2, which activates type I interferon responses in a TBK1- and IRF5-dependent manner in response to bacterial muramyl dipeptide (MDP) [39]. Additionally, M. tuberculosis infection triggers mitochondrial DNA release into the cytosol, which subsequently leads to cGAS/STING-dependent induction of type I IFNs [47]. M. tuberculosis-secreted cyclic di-AMP (c-di-AMP) can also directly activate STING independently of cGAS [48].
Type I IFNs play a dual role in M. tuberculosis infection, promoting both infection and immune defense. The expression levels of type I IFNs induced by M. tuberculosis are closely related to the virulence of strains. Compared to M. tuberculosis CDC1551, the more virulent clinical isolates HN878 and W4 induce higher levels of IFN-α [49]. This enhanced virulence may be partially attributed to the expression of type I IFNs, which suppresses the production of IL-1β [6]. Interestingly, this effect can be observed in macrophages but not in human monocytes [6]. A mouse infection model indicated that the absence of IFNAR1 enhances the survival rates of mice infected with M. tuberculosis [7]. Similarly, Zhang et al. [50] found that genetic variations in the human IFNAR1 gene impair type I IFN signaling and reduce susceptibility to M. tuberculosis. In addition, some studies reported that sustained and high levels of type I interferon responses exacerbate M. tuberculosis infections. Researchers explained the phenomenon by suggesting that type I IFNs can suppress the expression of pro-inflammatory cytokines, such as IL-1α, IL-1β, TNF-α, and IL-12 [8,9,51], while promoting the expression of the immunosuppressive cytokine IL-10 [9], which favors the intracellular survival of M. tuberculosis. Type I IFNs can also enhance the spread of M. tuberculosis within the host by promoting cell necrosis through the suppression of PGE2 [10]. High levels of type I IFNs not only inhibit the expression of inflammatory cytokines but also impair the host Th1 response, thereby exacerbating the M. tuberculosis pathogenic process [52,53]. Although numerous studies support the negative effects of type I IFNs on M. tuberculosis infection, there are also reports indicating that type I IFNs may facilitate anti-TB immune responses. A clinical case report suggested that a combination of IFN-α and anti-mycobacterial chemotherapy improves clinical symptoms and reduces bacterial load in patients with active pulmonary tuberculosis who are resistant to conventional treatment or experience disease relapse [11]. Mice lacking both type I IFNs and IFN-γ receptors (IFNGR1−/−/IFNAR1−/−) exhibited more severe lung pathology and higher mortality rates compared to mice with a single IFNGR deficiency (IFNGR1−/−) [45]. Further, type I IFNs can enhance the protective efficacy of BCG vaccines by promoting the production of protective cytokines, such as IFN-γ, TNF-α, and IL-12, and increasing resistance to M. tuberculosis infection [12]. Generally, researchers agree that sustained high levels of type I IFNs exacerbate TB infection, while early induction of low levels of type I IFNs plays a beneficial role in combating M. tuberculosis infection [54,55].
3.2. L. monocytogenes
L. monocytogenes is a significant foodborne zoonotic pathogen that can penetrate the host intestinal barrier, fetal–placental barrier, and blood–brain barrier, leading to host infection [56]. Clinical symptoms following infection primarily manifest as gastroenteritis, meningitis, sepsis, and miscarriage [57]. After infection of macrophages by L. monocytogenes, bacterial RNA is recognized by the cytosolic sensors RIG-I and MDA5 [58], while bacterial DNA activates the DNA sensors IFI16 and cGAS to induce the production of type I IFNs [59]. Cells infected with L. monocytogenes secrete extracellular vesicles (EVs) containing bacterial DNA, which fuse with surrounding cells to release the DNA into the cytosol of uninfected cells, thereby activating the cGAS-STING pathway [60]. Type I IFN induction in response to L. monocytogenes infection also relies on TLR3 and TLR9 [61,62]. Notably, IFN-β mRNA levels decrease in TLR9-deficient dendritic cells (pDCs) infected with L. monocytogenes, while the expression of IFN-α or IFN-β is TLR9-independent in L. monocytogenes-infected mice [62]. Furthermore, L. monocytogenes MDP can be sensed by NOD2 [63] and L. monocytogenes-secreted c-di-AMP can directly activate STING independently of cGAS [64], resulting in the production of type I IFNs.
Early research by T. Fujiki et al. [14] demonstrated that intravenous injection of IFN-β improved the survival rate of mice challenged with L. monocytogenes via intravenous injection. Similarly, Elisabeth Kernbauer et al. [13] found that IFNAR1 is essential for mice to resist L. monocytogenes through intragastric infection. However, it is worth noting that bacterial survival increased in IFNAR1−/− mice after infection intravenously with L. monocytogenes [16], indicating that the route of infection affects the effect of type I IFNs. Recently, more and more studies showed that type I IFNs may exacerbate L. monocytogenes infections [65]. The cholesterol-dependent cytolysin, listeriolysin O (LLO), secreted by L. monocytogenes, disrupts the integrity of host cellular lysosomes, enabling the bacteria to escape into the cytoplasm [66]. This exposure allows for the recognition of bacterial DNA and RNA by cytosolic sensors. These sensors are subsequently activated, leading to type I IFN-dependent induction of pro-apoptotic genes, such as DAXX, PKR, and TRAIL, which promote apoptosis in macrophages and lymphocytes, thereby facilitating bacterial dissemination and proliferation [16,67]. Consequently, IRF3−/− or IFNAR1−/− mice exhibit enhanced resistance to L. monocytogenes infection, which is attributed to reduced apoptotic cell death, particularly in lymphocytes [16]. During L. monocytogenes infection, IFN-α/β also plays a regulatory role in the expression of cytokines. In L. monocytogenes infection models, serum levels of IL-12p70 and TNF-α were higher in IFN-α/βR−/− mice than in wild-type mice [15]. Type I IFNs can exert immunosuppressive effects through an alternative mechanism by downregulating myeloid cell IFN-γ receptor expression. This occurs via the recruitment of an Egr3/Nab1 complex that silences IFNGR1 transcription. As a consequence, myeloid cells become more susceptible to L. monocytogenes infection [17]. In addition, type I IFNs have been found to suppress the host adaptive immune response in L. monocytogenes-infected mice. Compared to normal mice, STING-deficient mice exhibited restricted bacterial growth and displayed a greater number of cytotoxic lymphocytes upon re-infection [18]
3.3. S. Typhimurium
Salmonellosis is an important zoonotic infectious disease caused by Salmonella spp., primarily characterized by septicemia and enteritis, posing a substantial threat to livestock farming and public health [68]. The host innate immune system senses Salmonella infection through different PRRs to initiate type I interferon responses. In non-phagocytic cells, Salmonella mRNA is recognized by RIG-1, leading to the induction of type I IFN expression [69]. In phagocytic cells, Salmonella dsRNA, LPS, and Curli-DNA are detected by TLR3 [70], TLR4 [69], and TLR9 [71], respectively, resulting in the production of type I IFNs. Salmonella can also trigger host cell mitochondrial damage, resulting in the release of mitochondrial DNA, which activates the cGAS-STING signaling pathway and induces the expression of type I IFNs [72].
To investigate the effects of type I IFNs on S. Typhimurium infection, peritoneal macrophages were pre-incubated with IFN-β, followed by infection with S. Typhimurium SL1344. IFN-β was found to significantly suppress the expression of cytokines IL-1β and IL-18, and the chemokines CXCL1, CXCL2, and CXCL5 [19]. Further, IFN-β-deficient mice exhibit enhanced resistance to S. Typhimurium infection, with a slower spread of S. Typhimurium to sterile sites. Concurrently, higher transcript levels of cytokines and chemokines were observed in IFN-β-deficient mice, which facilitate the recruitment and activation of immune cells to more effectively eliminate pathogens [19]. Similarly, Nirmal Robinson et al. found that the survival rate of S. Typhimurium was significantly higher in IFNAR1−/− mice compared to wild-type mice, and S. Typhimurium-induced cell death was inhibited [20]. Induction of cell death by type I IFNs is a crucial pathogenic mechanism employed by S. Typhimurium [73]. TLR4/TRIF-dependent IFN-β production is essential for caspase-11 activation, which contributes to macrophage death during S. Typhimurium infection [74]. Although cytokine expression and inflammasome activation are not impaired in IFNAR1−/− macrophages, they exhibit high resistance to S. Typhimurium-induced cell death [20]. Specific inhibition of RIP1 or knockdown of RIP3 gene expression can suppress S. Typhimurium-induced macrophage death, indicating that S. Typhimurium induces cell death via necroptosis [20]. Additionally, the survival ability of S. Typhimurium was significantly reduced in RIP3−/− macrophages [20]. Thus, S. Typhimurium induces macrophage necroptosis by promoting type I IFN expression, allowing the pathogen to evade host immune responses.
3.4. L. pneumophila
L. pneumophila is a Gram-negative intracellular pathogen that primarily infects amoebae and other protozoa in aquatic environments [75]. However, it can also opportunistically infect humans, particularly those with weakened immune systems, leading to Legionnaires’ disease, a severe form of pneumonia [76]. L. pneumophila DNA serves as a primary ligand for inducing host type I interferon responses. Transfection of macrophages with L. pneumophila DNA leads to the production of significant amounts of IFN-β [77]. When L. pneumophila extract was pre-incubated with DNase I, RNase A, RNase H, and Proteinase K prior to transfection, DNase I could significantly inhibit the secretion of IFN-β induced by Legionella extract [77]. During the infection process, L. pneumophila DNA is translocated into the host cell cytoplasm through the Dot/Icm-encoded type IV secretion system (T4SS) [78]. Subsequently, L. pneumophila DNA is recognized by the intracellular DNA sensor, leading to the production of IFN-β in a STING- and IRF3-dependent manner [21,77]. Meanwhile, L. pneumophila RNA can be recognized by RIG-I and MDA5, inducing the expression of type I IFNs [79].
Numerous studies have shown that type I IFNs can protect against L. pneumophila infection. Pre-treating mouse bone marrow-derived macrophages (BMDMs) with IFN-α, followed by infection with L. pneumophila, demonstrated that IFN-α significantly inhibited the replication of intracellular L. pneumophila [21]. Additionally, pre-treatment with IFN-β before L. pneumophila infection significantly reduced the intracellular survival of bacteria in BMDMs [77]. Type I IFNs also protect lung epithelial cells from L. pneumophila infection in vitro as treatment with IFN-α/β limits the growth of intracellular bacteria [80]. In mouse infection models, IRF3−/−- and IFNAR−/−-deficient mice exhibited increased susceptibility to L. pneumophila [77], and bacterial loads increased in cGAS−/− and TMEM173−/− mice intranasally infected with L. pneumophila [22] compared to wild-type mice. The molecular mechanisms by which type I IFNs protect the host from L. pneumophila infection remain unclear, but they may be related to the promotion of pro-inflammatory cytokine expression [22], the activation of M1 macrophages, and the induction of NO production [21]. Research by Naujoks et al. [81] also suggests that type I IFNs, in conjunction with type II IFNs, alter the composition of bacterial vacuoles by inducing the production of itaconate through IRG1, thereby limiting the replication of L. pneumophila in alveolar macrophages and the lungs.
3.5. Francisella
Francisella is a Gram-negative bacterium, and the most notable species within this genus is Francisella tularensis, which causes the disease known as tularemia [82]. This zoonotic pathogen can be transmitted to humans primarily through the bite of an infected arthropod vector, such as ticks, deer flies, or mosquitoes, but also through direct contact with infected animals, inhalation of contaminated dust or aerosols, or ingestion of contaminated water or food [83]. Francisella escapes from phagosomes to the cytosol with the assistance of Francisella pathogenicity island (FPI) protein IglC and regulator MglA [84]. Once inside the cytosol, Francisella dsDNA triggers a type I interferon response through a cGAS- and IFI204-dependent pathway [85], which in turn drives the expression of effector proteins, leading to bacteriolysis and the release of bacterial DNA.
The expression of type I IFNs can promote the expression of absent in melanoma 2 (AIM2) [85], a protein that can bind cytosolic DNA, during Francisella infection. Upon recognizing and binding cytosolic dsDNA, AIM2 oligomerizes and recruits ASC [86]. ASC acts as a bridging molecule, further recruiting and activating pro-caspase-1, leading to its self-cleavage into the active form of caspase-1 [87]. Activated caspase-1 can cleave precursor forms of pro-inflammatory cytokines, such as pro-IL-1β and pro-IL-18, processing them into mature, bioactive forms that facilitate their secretion [88]. Additionally, the active AIM2 inflammasome leads to caspase-1-dependent cell death, which helps to restrict pathogen spread and enhance the host immune response [89,90]. At the same time, the IFN-β produced by infected cells serves as a paracrine signal, enhancing the activation of inflammasomes in neighboring cells, thereby facilitating the clearance of Francisella before widespread bacterial replication [91]. Paradoxically, type I IFN exacerbates Francisella infections. The absence of cGAS, STING, IFNAR1, IFNAR2, or IRF3 has been shown to enhance the resistance of mice to Francisella infections [24,85,91]. Qifan Zhu et al. explained that the detrimental effects of type I IFN signaling override the protective responses of the AIM2 inflammasome [24]. In their study, IFNAR2−/− mice and IFNAR2−/−AIM2−/− mice were resistant to the Francisella infection, whereas all AIM2−/− mice succumbed to the infection after 12 days. In addition, type I IFNs can suppress host antibacterial responses by inhibiting the expression of IL-17A in γδT cells. In a mouse infection model, IFNAR1−/− mice infected with Francisella exhibited increased expression of IL-17A, enhanced neutrophil recruitment in the spleen, and improved bacterial clearance and survival rates [23]. Similar to L. monocytogenes and Brucella abortus (B. abortus), type I IFNs also have been shown to participate in TRAIL-mediated apoptosis [24], which increases host susceptibility to Francisella infection.
3.6. Other Bacterial Infections
The induction of type I IFNs by Neisseria gonorrhoeae (N. gonorrhoeae) infection is mainly mediated by the TLR4-TRIF-IRF3 and cGAS-STING-TBK-1-IRF3 signaling pathways, with TLR4 recognizing N. gonorrhoeae lipooligosaccharides (LOS) and cGAS detecting dsDNA [25,92]. In pDC cells, blockade of the interaction between CpG and TLR9 significantly inhibits N. gonorrhoeae-induced type I IFN production, indicating that type I interferon responses depend on TLR9 signaling [93]. Activation of the type I IFN pathway hampers the clearance of N. gonorrhoeae as the type I interferon responses can increase iron retention within host cells, creating a microenvironment that favors bacterial survival [25]. Pneumolysin produced by Streptococcus pneumoniae (S. pneumoniae) causes mitochondrial damage and the subsequent leakage of mitochondrial DNA into the cytoplasm, which activates the cGAS-STING signaling pathway, triggering the production of IFN-β [94]. IFNAR1−/− mice exhibited increased bacterial loads following intranasal infection with S. pneumoniae [26]. During infection with Pseudomonas aeruginosa (P. aeruginosa), the cGAS-STING pathway is activated by P. aeruginosa DNA to induce type I IFNs [27]. The absence of cGAS or STING reduced type I IFN production and increased the mortality rate in mice infected with P. aeruginosa [27]. B. abortus DNA serves as the primary bacterial component that triggers the expression of type I IFNs in a TLR9- and STING-dependent manner, increasing susceptibility to B. abortus infection through the suppression of IFN-γ and NO production and the induction of apoptosis [28,95]. However, type I interferon responses were also found to be essential for pro-inflammatory cytokine production, thereby enhancing the resistance of macrophages to B. abortus [29]. Staphylococcus aureus (S. aureus) was sensed by TLR8 and NOD2, leading to the activation of IRF5 and production of IFN-β [96]. Martin et al. [30] demonstrated that a higher survival rate was observed in IFNAR1−/−-deficient mice infected with S. aureus via intranasal administration, which may be partly dependent on the enhanced recruitment of CD11c+ DCs. NOD1 can recognize a peptide derived from H. pylori peptidoglycan, leading to the induction of type I IFN expression and the subsequent production of CXCL10 [31]. Mice lacking type I IFN receptor or NOD1 are unable to effectively restrict the proliferation of H. pylori [97,98].
4. Discussion and Future Directions
Type I IFNs induce a powerful antiviral response by upregulating a series of ISGs. In recent years, numerous studies revealed that type I IFNs also play an important role in the host defense against bacterial infection. Unlike their well-defined functions in viral infection, the functions of type I IFNs in bacterial infection are often unpredictable. On one hand, type I IFNs may enhance the host antibacterial immune response by upregulating pro-inflammatory cytokine production, activating the unfolded protein response, reducing bacterial invasion, and inducing M1 macrophage polarization. On the other hand, type I IFNs may also suppress the antibacterial immune response by inducing IL-10 production, inhibiting the expression of pro-inflammatory cytokines and chemokines, and promoting immune cell death (Table 1). The difference in the function of type I IFNs may not only be due to the different preferences of different cell types for the expression of IFN-α or IFN-β and the different affinities for IFNAR, but also due to different infection models, routes, and sites of infection. In the future, we need more in vitro and in vivo experiments to decipher the molecular, cellular, and organismal physiology of type I IFNs in specific bacterial infections, so as to provide new insights for the identification of antibacterial drug targets and vaccine development.
Author Contributions
Writing—original draft preparation, A.X., X.L., C.Z. and X.M.; writing—review and editing, A.X., X.L., G.K. and Y.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Science and Technology Program of Jiangsu (BK20230298), the Natural Science Foundation of Jiangsu Higher Education Institutions (23KJB230003), the Science and Technology Innovation Team Project of Jiangsu Agri-Animal Husbandry Vocational College (NSF2024TC01), Jiangsu Agri-Animal Husbandry Vocational College Project (NSF2023CB03), the Science and Technology Program of Kizilsu Kirgiz Autonomous Prefecture (22), Taizhou Sixth Phase “311 High-level Talent Training Special Program”, and Taizhou “Feng Cheng Ying Cai Program” youth science and technology talent.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Yu, R.; Zhu, B.; Chen, D. Type I Interferon-Mediated Tumor Immunity and Its Role in Immunotherapy. Cell. Mol. Life Sci. 2022, 79, 191. [Google Scholar] [CrossRef] [PubMed]
- McNab, F.; Mayer-Barber, K.; Sher, A.; Wack, A.; O’Garra, A. Type I Interferons in Infectious Disease. Nat. Rev. Immunol. 2015, 15, 87–103. [Google Scholar] [CrossRef] [PubMed]
- Schoggins, J.W. Interferon-Stimulated Genes: What Do They All Do? Annu. Rev. Virol. 2019, 6, 567–584. [Google Scholar] [CrossRef] [PubMed]
- Schneider, W.M.; Chevillotte, M.D.; Rice, C.M. Interferon-Stimulated Genes: A Complex Web of Host Defenses. Annu. Rev. Immunol. 2014, 32, 513–545. [Google Scholar] [CrossRef]
- Ivashkiv, L.B.; Donlin, L.T. Regulation of Type I Interferon Responses. Nat. Rev. Immunol. 2014, 14, 36–49. [Google Scholar] [CrossRef]
- Novikov, A.; Cardone, M.; Thompson, R.; Shenderov, K.; Kirschman, K.D.; Mayer-Barber, K.D.; Myers, T.G.; Rabin, R.L.; Trinchieri, G.; Sher, A.; et al. Mycobacterium tuberculosis Triggers Host Type I IFN Signaling to Regulate IL-1β Production in Human Macrophages. J. Immunol. 2011, 187, 2540–2547. [Google Scholar] [CrossRef] [PubMed]
- Dorhoi, A.; Yeremeev, V.; Nouailles, G.; Weiner, J.; Jörg, S.; Heinemann, E.; Oberbeck-Müller, D.; Knaul, J.K.; Vogelzang, A.; Reece, S.T.; et al. Type I IFN Signaling Triggers Immunopathology in Tuberculosis-Susceptible Mice by Modulating Lung Phagocyte Dynamics. Eur. J. Immunol. 2014, 44, 2380–2393. [Google Scholar] [CrossRef]
- de Paus, R.A.; van Wengen, A.; Schmidt, I.; Visser, M.; Verdegaal, E.M.E.; van Dissel, J.T.; van de Vosse, E. Inhibition of the Type I Immune Responses of Human Monocytes by IFN-α and IFN-β. Cytokine 2013, 61, 645–655. [Google Scholar] [CrossRef]
- McNab, F.W.; Ewbank, J.; Howes, A.; Moreira-Teixeira, L.; Martirosyan, A.; Ghilardi, N.; Saraiva, M.; O’Garra, A. Type I IFN Induces IL-10 Production in an IL-27-Independent Manner and Blocks Responsiveness to IFN-γ for Production of IL-12 and Bacterial Killing in Mycobacterium tuberculosis-Infected Macrophages. J. Immunol. 2014, 193, 3600–3612. [Google Scholar] [CrossRef]
- Mayer-Barber, K.D.; Andrade, B.B.; Oland, S.D.; Amaral, E.P.; Barber, D.L.; Gonzales, J.; Derrick, S.C.; Shi, R.; Kumar, N.P.; Wei, W.; et al. Host-Directed Therapy of Tuberculosis Based on Interleukin-1 and Type I Interferon Crosstalk. Nature 2014, 511, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Zarogoulidis, P.; Kioumis, I.; Papanas, N.; Manika, K.; Kontakiotis, T.; Papagianis, A.; Zarogoulidis, K. The Effect of Combination IFN-Alpha-2a with Usual Antituberculosis Chemotherapy in Non-Responding Tuberculosis and Diabetes Mellitus: A Case Report and Review of the Literature. J. Chemother. 2012, 24, 173–177. [Google Scholar] [CrossRef]
- Rivas-Santiago, C.E.; Guerrero, G.G. IFN-α Boosting of Mycobacterium bovis Bacillus Calmette Güerin-Vaccine Promoted Th1 Type Cellular Response and Protection against M. tuberculosis Infection. BioMed Res. Int. 2017, 2017, 8796760. [Google Scholar] [CrossRef] [PubMed]
- Kernbauer, E.; Maier, V.; Rauch, I.; Müller, M.; Decker, T. Route of Infection Determines the Impact of Type I Interferons on Innate Immunity to Listeria monocytogenes. PLoS ONE 2013, 8, e65007. [Google Scholar] [CrossRef]
- Fujiki, T.; Tanaka, A. Antibacterial Activity of Recombinant Murine Beta Interferon. Infect. Immun. 1988, 56, 548–551. [Google Scholar] [CrossRef] [PubMed]
- Auerbuch, V.; Brockstedt, D.G.; Meyer-Morse, N.; O’Riordan, M.; Portnoy, D.A. Mice Lacking the Type I Interferon Receptor Are Resistant to Listeria monocytogenes. J. Exp. Med. 2004, 200, 527–533. [Google Scholar] [CrossRef]
- O’Connell, R.M.; Saha, S.K.; Vaidya, S.A.; Bruhn, K.W.; Miranda, G.A.; Zarnegar, B.; Perry, A.K.; Nguyen, B.O.; Lane, T.F.; Taniguchi, T.; et al. Type I Interferon Production Enhances Susceptibility to Listeria monocytogenes Infection. J. Exp. Med. 2004, 200, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Kearney, S.J.; Delgado, C.; Eshleman, E.M.; Hill, K.K.; O’Connor, B.P.; Lenz, L.L. Type I IFNs Downregulate Myeloid Cell IFN-γ Receptor by Inducing Recruitment of an Early Growth Response 3/NGFI-A Binding Protein 1 Complex That Silences Ifngr1 Transcription. J. Immunol. 2013, 191, 3384–3392. [Google Scholar] [CrossRef]
- Archer, K.A.; Durack, J.; Portnoy, D.A. STING-Dependent Type I IFN Production Inhibits Cell-Mediated Immunity to Listeria monocytogenes. PLoS Pathog. 2014, 10, e1003861. [Google Scholar] [CrossRef]
- Perkins, D.J.; Rajaiah, R.; Tennant, S.M.; Ramachandran, G.; Higginson, E.E.; Dyson, T.N.; Vogel, S.N. Salmonella Typhimurium Co-Opts the Host Type I IFN System to Restrict Macrophage Innate Immune Transcriptional Responses Selectively. J. Immunol. 2015, 195, 2461–2471. [Google Scholar] [CrossRef]
- Robinson, N.; McComb, S.; Mulligan, R.; Dudani, R.; Krishnan, L.; Sad, S. Type I Interferon Induces Necroptosis in Macrophages during Infection with Salmonella enterica Serovar Typhimurium. Nat. Immunol. 2012, 13, 954–962. [Google Scholar] [CrossRef]
- Plumlee, C.R.; Lee, C.; Beg, A.A.; Decker, T.; Shuman, H.A.; Schindler, C. Interferons Direct an Effective Innate Response to Legionella pneumophila Infection. J. Biol. Chem. 2009, 284, 30058–30066. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Moreno, J.S.; Hamann, L.; Shah, J.A.; Verbon, A.; Mockenhaupt, F.P.; Puzianowska-Kuznicka, M.; Naujoks, J.; Sander, L.E.; Witzenrath, M.; Cambier, J.C.; et al. The Common HAQ STING Variant Impairs cGAS-Dependent Antibacterial Responses and Is Associated with Susceptibility to Legionnaires’ Disease in Humans. PLoS Pathog. 2018, 14, e1006829. [Google Scholar] [CrossRef] [PubMed]
- Henry, T.; Kirimanjeswara, G.S.; Ruby, T.; Jones, J.W.; Peng, K.; Perret, M.; Ho, L.; Sauer, J.-D.; Iwakura, Y.; Metzger, D.W.; et al. Type I IFN Signaling Constrains IL-17A/F Secretion by Gammadelta T Cells during Bacterial Infections. J. Immunol. 2010, 184, 3755–3767. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Man, S.M.; Karki, R.; Malireddi, R.K.S.; Kanneganti, T.-D. Detrimental Type I Interferon Signaling Dominates Protective AIM2 Inflammasome Responses during Francisella novicida Infection. Cell Rep. 2018, 22, 3168–3174. [Google Scholar] [CrossRef]
- Andrade, W.A.; Agarwal, S.; Mo, S.; Shaffer, S.A.; Dillard, J.P.; Schmidt, T.; Hornung, V.; Fitzgerald, K.A.; Kurt-Jones, E.A.; Golenbock, D.T. Type I Interferon Induction by Neisseria gonorrhoeae: Dual Requirement of Cyclic GMP-AMP Synthase and Toll-like Receptor 4. Cell Rep. 2016, 15, 2438–2448. [Google Scholar] [CrossRef]
- LeMessurier, K.S.; Häcker, H.; Chi, L.; Tuomanen, E.; Redecke, V. Type I Interferon Protects against Pneumococcal Invasive Disease by Inhibiting Bacterial Transmigration across the Lung. PLoS Pathog. 2013, 9, e1003727. [Google Scholar] [CrossRef]
- Zhou, C.-M.; Wang, B.; Wu, Q.; Lin, P.; Qin, S.-G.; Pu, Q.-Q.; Yu, X.-J.; Wu, M. Identification of cGAS as an Innate Immune Sensor of Extracellular Bacterium Pseudomonas aeruginosa. iScience 2021, 24, 101928. [Google Scholar] [CrossRef]
- de Almeida, L.A.; Carvalho, N.B.; Oliveira, F.S.; Lacerda, T.L.S.; Vasconcelos, A.C.; Nogueira, L.; Bafica, A.; Silva, A.M.; Oliveira, S.C. MyD88 and STING Signaling Pathways Are Required for IRF3-Mediated IFN-β Induction in Response to Brucella abortus Infection. PLoS ONE 2011, 6, e23135. [Google Scholar] [CrossRef] [PubMed]
- Costa Franco, M.M.; Marim, F.; Guimarães, E.S.; Assis, N.R.G.; Cerqueira, D.M.; Alves-Silva, J.; Harms, J.; Splitter, G.; Smith, J.; Kanneganti, T.-D.; et al. Brucella abortus Triggers a cGAS-Independent STING Pathway to Induce Host Protection That Involves Guanylate-Binding Proteins and Inflammasome Activation. J. Immunol. 2018, 200, 607–622. [Google Scholar] [CrossRef] [PubMed]
- Martin, F.J.; Gomez, M.I.; Wetzel, D.M.; Memmi, G.; O’Seaghdha, M.; Soong, G.; Schindler, C.; Prince, A. Staphylococcus aureus Activates Type I IFN Signaling in Mice and Humans through the Xr Repeated Sequences of Protein A. J. Clin. Investig. 2009, 119, 1931–1939. [Google Scholar] [CrossRef]
- Watanabe, T.; Asano, N.; Fichtner-Feigl, S.; Gorelick, P.L.; Tsuji, Y.; Matsumoto, Y.; Chiba, T.; Fuss, I.J.; Kitani, A.; Strober, W. NOD1 Contributes to Mouse Host Defense against Helicobacter Pylori via Induction of Type I IFN and Activation of the ISGF3 Signaling Pathway. J. Clin. Investig. 2010, 120, 1645–1662. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lei, X.; Jiang, Z.; Fitzgerald, K.A. Cellular Nucleic Acid-Binding Protein Is Essential for Type I Interferon-Mediated Immunity to RNA Virus Infection. Proc. Natl. Acad. Sci. USA 2021, 118, e2100383118. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Zhang, W.; Tian, L.; Brown, B.R.; Walters, M.S.; Metcalf, J.P. IRF7 Is Required for the Second Phase Interferon Induction during Influenza Virus Infection in Human Lung Epithelia. Viruses 2020, 12, 377. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Ji, Y.; Zeng, L.; Liu, Q.; Zhang, Z.; Guo, S.; Guo, X.; Tong, Y.; Zhao, X.; Li, C.-M.; et al. P200 Family Protein IFI204 Negatively Regulates Type I Interferon Responses by Targeting IRF7 in Nucleus. PLoS Pathog. 2019, 15, e1008079. [Google Scholar] [CrossRef]
- Perkins, D.J.; Vogel, S.N. Space and Time: New Considerations about the Relationship between Toll-like Receptors (TLRs) and Type I Interferons (IFNs). Cytokine 2015, 74, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Choo, M.-K.; Sano, Y.; Kim, C.; Yasuda, K.; Li, X.-D.; Lin, X.; Stenzel-Poore, M.; Alexopoulou, L.; Ghosh, S.; Latz, E.; et al. TLR Sensing of Bacterial Spore-Associated RNA Triggers Host Immune Responses with Detrimental Effects. J. Exp. Med. 2017, 214, 1297–1311. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. Toll-like Receptors and Their Crosstalk with Other Innate Receptors in Infection and Immunity. Immunity 2011, 34, 637–650. [Google Scholar] [CrossRef]
- Barral, P.M.; Sarkar, D.; Su, Z.; Barber, G.N.; DeSalle, R.; Racaniello, V.R.; Fisher, P.B. Functions of the Cytoplasmic RNA Sensors RIG-I and MDA-5: Key Regulators of Innate Immunity. Pharmacol. Ther. 2009, 124, 219–234. [Google Scholar] [CrossRef]
- Pandey, A.K.; Yang, Y.; Jiang, Z.; Fortune, S.M.; Coulombe, F.; Behr, M.A.; Fitzgerald, K.A.; Sassetti, C.M.; Kelliher, M.A. NOD2, RIP2 and IRF5 Play a Critical Role in the Type I Interferon Response to Mycobacterium tuberculosis. PLoS Pathog. 2009, 5, e1000500. [Google Scholar] [CrossRef]
- Kong, E.; Kim, H.D.; Kim, J. Deleting Key Autophagy Elongation Proteins Induces Acquirement of Tumor-Associated Phenotypes via ISG15. Cell Death Differ. 2020, 27, 2517–2530. [Google Scholar] [CrossRef]
- Roers, A.; Hiller, B.; Hornung, V. Recognition of Endogenous Nucleic Acids by the Innate Immune System. Immunity 2016, 44, 739–754. [Google Scholar] [CrossRef] [PubMed]
- Piaszyk-Borychowska, A.; Széles, L.; Csermely, A.; Chiang, H.-C.; Wesoły, J.; Lee, C.-K.; Nagy, L.; Bluyssen, H.A.R. Signal Integration of IFN-I and IFN-II with TLR4 Involves Sequential Recruitment of STAT1-Complexes and NFκB to Enhance Pro-Inflammatory Transcription. Front. Immunol. 2019, 10, 1253. [Google Scholar] [CrossRef]
- Zhou, B.-X.; Li, J.; Liang, X.-L.; Pan, X.-P.; Hao, Y.-B.; Xie, P.-F.; Jiang, H.-M.; Yang, Z.-F.; Zhong, N.-S. β-Sitosterol Ameliorates Influenza A Virus-Induced Proinflammatory Response and Acute Lung Injury in Mice by Disrupting the Cross-Talk between RIG-I and IFN/STAT Signaling. Acta Pharmacol. Sin. 2020, 41, 1178–1196. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.H.; Liu, H.; Ge, B. Innate Immunity in Tuberculosis: Host Defense vs Pathogen Evasion. Cell Mol. Immunol. 2017, 14, 963–975. [Google Scholar] [CrossRef] [PubMed]
- Moreira-Teixeira, L.; Sousa, J.; McNab, F.W.; Torrado, E.; Cardoso, F.; Machado, H.; Castro, F.; Cardoso, V.; Gaifem, J.; Wu, X.; et al. Type I IFN Inhibits Alternative Macrophage Activation during Mycobacterium tuberculosis Infection and Leads to Enhanced Protection in the Absence of IFN-γ Signaling. J. Immunol. 2016, 197, 4714–4726. [Google Scholar] [CrossRef] [PubMed]
- Simmons, D.P.; Canaday, D.H.; Liu, Y.; Li, Q.; Huang, A.; Boom, W.H.; Harding, C.V. Mycobacterium tuberculosis and TLR2 Agonists Inhibit Induction of Type I IFN and Class I MHC Antigen Cross Processing by TLR9. J. Immunol. 2010, 185, 2405–2415. [Google Scholar] [CrossRef]
- Wiens, K.E.; Ernst, J.D. The Mechanism for Type I Interferon Induction by Mycobacterium tuberculosis Is Bacterial Strain-Dependent. PLoS Pathog. 2016, 12, e1005809. [Google Scholar] [CrossRef]
- Dey, B.; Dey, R.J.; Cheung, L.S.; Pokkali, S.; Guo, H.; Lee, J.-H.; Bishai, W.R. A Bacterial Cyclic Dinucleotide Activates the Cytosolic Surveillance Pathway and Mediates Innate Resistance to Tuberculosis. Nat. Med. 2015, 21, 401–406. [Google Scholar] [CrossRef]
- Manca, C.; Tsenova, L.; Freeman, S.; Barczak, A.K.; Tovey, M.; Murray, P.J.; Barry, C.; Kaplan, G. Hypervirulent M. tuberculosis W/Beijing Strains Upregulate Type I IFNs and Increase Expression of Negative Regulators of the Jak-Stat Pathway. J. Interferon Cytokine Res. 2005, 25, 694–701. [Google Scholar] [CrossRef]
- Zhang, G.; deWeerd, N.A.; Stifter, S.A.; Liu, L.; Zhou, B.; Wang, W.; Zhou, Y.; Ying, B.; Hu, X.; Matthews, A.Y.; et al. A Proline Deletion in IFNAR1 Impairs IFN-Signaling and Underlies Increased Resistance to Tuberculosis in Humans. Nat. Commun. 2018, 9, 85. [Google Scholar] [CrossRef]
- Mayer-Barber, K.D.; Barber, D.L.; Shenderov, K.; White, S.D.; Wilson, M.S.; Cheever, A.; Kugler, D.; Hieny, S.; Caspar, P.; Núñez, G.; et al. Caspase-1 Independent IL-1beta Production Is Critical for Host Resistance to Mycobacterium tuberculosis and Does Not Require TLR Signaling in Vivo. J. Immunol. 2010, 184, 3326–3330. [Google Scholar] [CrossRef] [PubMed]
- Teles, R.M.B.; Graeber, T.G.; Krutzik, S.R.; Montoya, D.; Schenk, M.; Lee, D.J.; Komisopoulou, E.; Kelly-Scumpia, K.; Chun, R.; Iyer, S.S.; et al. Type I Interferon Suppresses Type II Interferon-Triggered Human Anti-Mycobacterial Responses. Science 2013, 339, 1448–1453. [Google Scholar] [CrossRef]
- Mayer-Barber, K.D.; Andrade, B.B.; Barber, D.L.; Hieny, S.; Feng, C.G.; Caspar, P.; Oland, S.; Gordon, S.; Sher, A. Innate and Adaptive Interferons Suppress IL-1α and IL-1β Production by Distinct Pulmonary Myeloid Subsets during Mycobacterium tuberculosis Infection. Immunity 2011, 35, 1023–1034. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hussain, T.; Yue, R.; Liao, Y.; Li, Q.; Yao, J.; Song, Y.; Sun, X.; Wang, N.; Xu, L.; et al. MicroRNA-199a Inhibits Cellular Autophagy and Downregulates IFN-β Expression by Targeting TBK1 in Mycobacterium bovis Infected Cells. Front. Cell Infect. Microbiol. 2018, 8, 238. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Shen, H.; Lian, Q.; Jin, W.; Zhang, R.; Lin, X.; Gu, W.; Sun, X.; Meng, G.; Tian, Z.; et al. Deficiency of the AIM2-ASC Signal Uncovers the STING-Driven Overreactive Response of Type I IFN and Reciprocal Depression of Protective IFN-γ Immunity in Mycobacterial Infection. J. Immunol. 2018, 200, 1016–1026. [Google Scholar] [CrossRef]
- Wang, J.; Liu, B.; Teng, Z.; Zhou, X.; Wang, X.; Zhang, B.; Lu, G.; Niu, X.; Yang, Y.; Deng, X. Phloretin Attenuates Listeria monocytogenes Virulence Both In Vitro and In Vivo by Simultaneously Targeting Listeriolysin O and Sortase A. Front. Cell Infect. Microbiol. 2017, 7, 9. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fernández-Martínez, N.F.; Ruiz-Montero, R.; Briones, E.; Baños, E.; García San Miguel Rodríguez-Alarcón, L.; Chaves, J.A.; Abad, R.; Varela, C.; LISMOAN team; Lorusso, N. Listeriosis Outbreak Caused by Contaminated Stuffed Pork, Andalusia, Spain, July to October 2019. Euro Surveill. 2022, 27, 2200279. [Google Scholar] [CrossRef]
- Abdullah, Z.; Schlee, M.; Roth, S.; Mraheil, M.A.; Barchet, W.; Böttcher, J.; Hain, T.; Geiger, S.; Hayakawa, Y.; Fritz, J.H.; et al. RIG-I Detects Infection with Live Listeria by Sensing Secreted Bacterial Nucleic Acids. EMBO J. 2012, 31, 4153–4164. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.; Prabakaran, T.; Laustsen, A.; Jørgensen, S.E.; Rahbæk, S.H.; Jensen, S.B.; Nielsen, R.; Leber, J.H.; Decker, T.; Horan, K.A.; et al. Listeria monocytogenes Induces IFNβ Expression through an IFI16-, cGAS- and STING-Dependent Pathway. EMBO J. 2014, 33, 1654–1666. [Google Scholar] [CrossRef]
- Nandakumar, R.; Tschismarov, R.; Meissner, F.; Prabakaran, T.; Krissanaprasit, A.; Farahani, E.; Zhang, B.-C.; Assil, S.; Martin, A.; Bertrams, W.; et al. Intracellular Bacteria Engage a STING-TBK1-MVB12b Pathway to Enable Paracrine cGAS-STING Signalling. Nat. Microbiol. 2019, 4, 701–713. [Google Scholar] [CrossRef] [PubMed]
- Aubry, C.; Corr, S.C.; Wienerroither, S.; Goulard, C.; Jones, R.; Jamieson, A.M.; Decker, T.; O’Neill, L.A.J.; Dussurget, O.; Cossart, P. Both TLR2 and TRIF Contribute to Interferon-β Production during Listeria Infection. PLoS ONE 2012, 7, e33299. [Google Scholar] [CrossRef] [PubMed]
- Stockinger, S.; Kastner, R.; Kernbauer, E.; Pilz, A.; Westermayer, S.; Reutterer, B.; Soulat, D.; Stengl, G.; Vogl, C.; Frenz, T.; et al. Characterization of the Interferon-Producing Cell in Mice Infected with Listeria monocytogenes. PLoS Pathog. 2009, 5, e1000355. [Google Scholar] [CrossRef]
- Leber, J.H.; Crimmins, G.T.; Raghavan, S.; Meyer-Morse, N.P.; Cox, J.S.; Portnoy, D.A. Distinct TLR- and NLR-Mediated Transcriptional Responses to an Intracellular Pathogen. PLoS Pathog. 2008, 4, e6. [Google Scholar] [CrossRef] [PubMed]
- Woodward, J.J.; Iavarone, A.T.; Portnoy, D.A. C-Di-AMP Secreted by Intracellular Listeria monocytogenes Activates a Host Type I Interferon Response. Science 2010, 328, 1703–1705. [Google Scholar] [CrossRef] [PubMed]
- Perelman, S.S.; Abrams, M.E.; Eitson, J.L.; Chen, D.; Jimenez, A.; Mettlen, M.; Schoggins, J.W.; Alto, N.M. Cell-Based Screen Identifies Human Interferon-Stimulated Regulators of Listeria monocytogenes Infection. PLoS Pathog. 2016, 12, e1006102. [Google Scholar] [CrossRef]
- Yoon, S.; Bogdanov, K.; Wallach, D. Site-Specific Ubiquitination of MLKL Targets It to Endosomes and Targets Listeria and Yersinia to the Lysosomes. Cell Death Differ. 2022, 29, 306–322. [Google Scholar] [CrossRef]
- Carrero, J.A.; Calderon, B.; Unanue, E.R. Type I Interferon Sensitizes Lymphocytes to Apoptosis and Reduces Resistance to Listeria Infection. J. Exp. Med. 2004, 200, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Amaya, F.A.; Blondel, C.J.; Barros-Infante, M.F.; Rivera, D.; Moreno-Switt, A.I.; Santiviago, C.A.; Pezoa, D. Identification of Type VI Secretion Systems Effector Proteins That Contribute to Interbacterial Competition in Salmonella dublin. Front. Microbiol. 2022, 13, 811932. [Google Scholar] [CrossRef] [PubMed]
- Schmolke, M.; Patel, J.R.; de Castro, E.; Sánchez-Aparicio, M.T.; Uccellini, M.B.; Miller, J.C.; Manicassamy, B.; Satoh, T.; Kawai, T.; Akira, S.; et al. RIG-I Detects mRNA of Intracellular Salmonella enterica Serovar Typhimurium during Bacterial Infection. mBio 2014, 5, e01006-14. [Google Scholar] [CrossRef]
- Owen, K.A.; Anderson, C.J.; Casanova, J.E. Salmonella Suppresses the TRIF-Dependent Type I Interferon Response in Macrophages. mBio 2016, 7, e02051-15. [Google Scholar] [CrossRef]
- Tursi, S.A.; Lee, E.Y.; Medeiros, N.J.; Lee, M.H.; Nicastro, L.K.; Buttaro, B.; Gallucci, S.; Wilson, R.P.; Wong, G.C.L.; Tükel, Ç. Bacterial Amyloid Curli Acts as a Carrier for DNA to Elicit an Autoimmune Response via TLR2 and TLR9. PLoS Pathog. 2017, 13, e1006315. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Li, M.; Yang, Y.; Zhang, C.; Xie, Z.; Tang, J.; Shi, Z.; Chen, S.; Li, G.; Gu, Y.; et al. Salmonella Induces the cGAS-STING-Dependent Type I Interferon Response in Murine Macrophages by Triggering mtDNA Release. mBio 2022, 13, e0363221. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Qin, X. Critical Role of Type I Interferon-Induced Macrophage Necroptosis during Infection with Salmonella enterica Serovar Typhimurium. Cell Mol. Immunol. 2013, 10, 99–100. [Google Scholar] [CrossRef]
- Broz, P.; Ruby, T.; Belhocine, K.; Bouley, D.M.; Kayagaki, N.; Dixit, V.M.; Monack, D.M. Caspase-11 Increases Susceptibility to Salmonella Infection in the Absence of Caspase-1. Nature 2012, 490, 288–291. [Google Scholar] [CrossRef]
- Song, L.; Luo, J.; Wang, H.; Huang, D.; Tan, Y.; Liu, Y.; Wang, Y.; Yu, K.; Zhang, Y.; Liu, X.; et al. Legionella pneumophila Regulates Host Cell Motility by Targeting Phldb2 with a 14-3-3ζ-Dependent Protease Effector. Elife 2022, 11, e73220. [Google Scholar] [CrossRef]
- Fu, J.; Zhou, M.; Gritsenko, M.A.; Nakayasu, E.S.; Song, L.; Luo, Z.-Q. Legionella pneumophila Modulates Host Energy Metabolism by ADP-Ribosylation of ADP/ATP Translocases. Elife 2022, 11, e73611. [Google Scholar] [CrossRef]
- Lippmann, J.; Müller, H.C.; Naujoks, J.; Tabeling, C.; Shin, S.; Witzenrath, M.; Hellwig, K.; Kirschning, C.J.; Taylor, G.A.; Barchet, W.; et al. Dissection of a Type I Interferon Pathway in Controlling Bacterial Intracellular Infection in Mice. Cell. Microbiol. 2011, 13, 1668–1682. [Google Scholar] [CrossRef]
- Kim, H.; Kubori, T.; Yamazaki, K.; Kwak, M.-J.; Park, S.-Y.; Nagai, H.; Vogel, J.P.; Oh, B.-H. Structural Basis for Effector Protein Recognition by the Dot/Icm Type IVB Coupling Protein Complex. Nat. Commun. 2020, 11, 2623. [Google Scholar] [CrossRef] [PubMed]
- Monroe, K.M.; McWhirter, S.M.; Vance, R.E. Identification of Host Cytosolic Sensors and Bacterial Factors Regulating the Type I Interferon Response to Legionella pneumophila. PLoS Pathog. 2009, 5, e1000665. [Google Scholar] [CrossRef] [PubMed]
- Opitz, B.; Vinzing, M.; van Laak, V.; Schmeck, B.; Heine, G.; Günther, S.; Preissner, R.; Slevogt, H.; N’Guessan, P.D.; Eitel, J.; et al. Legionella pneumophila Induces IFNbeta in Lung Epithelial Cells via IPS-1 and IRF3, Which Also Control Bacterial Replication. J. Biol. Chem. 2006, 281, 36173–36179. [Google Scholar] [CrossRef]
- Naujoks, J.; Tabeling, C.; Dill, B.D.; Hoffmann, C.; Brown, A.S.; Kunze, M.; Kempa, S.; Peter, A.; Mollenkopf, H.-J.; Dorhoi, A.; et al. IFNs Modify the Proteome of Legionella-Containing Vacuoles and Restrict Infection Via IRG1-Derived Itaconic Acid. PLoS Pathog. 2016, 12, e1005408. [Google Scholar] [CrossRef] [PubMed]
- Suresh, R.V.; Bradley, E.W.; Higgs, M.; Russo, V.C.; Alqahtani, M.; Huang, W.; Bakshi, C.S.; Malik, M. Nlrp3 Increases the Host’s Susceptibility to Tularemia. Front. Microbiol. 2021, 12, 725572. [Google Scholar] [CrossRef] [PubMed]
- Grossman, T.H.; Anderson, M.S.; Christ, D.; Gooldy, M.; Henning, L.N.; Heine, H.S.; Kindt, M.V.; Lin, W.; Siefkas-Patterson, K.; Radcliff, A.K.; et al. The Fluorocycline TP-271 Is Efficacious in Models of Aerosolized Francisella Tularensis SCHU S4 Infection in BALB/c Mice and Cynomolgus Macaques. Antimicrob. Agents Chemother. 2017, 61, e00448-17. [Google Scholar] [CrossRef] [PubMed]
- Santic, M.; Molmeret, M.; Klose, K.E.; Jones, S.; Kwaik, Y.A. The Francisella tularensis Pathogenicity Island Protein IglC and Its Regulator MglA Are Essential for Modulating Phagosome Biogenesis and Subsequent Bacterial Escape into the Cytoplasm. Cell. Microbiol. 2005, 7, 969–979. [Google Scholar] [CrossRef]
- Storek, K.M.; Gertsvolf, N.A.; Ohlson, M.B.; Monack, D.M. cGAS and Ifi204 Cooperate to Produce Type I IFNs in Response to Francisella Infection. J. Immunol. 2015, 194, 3236–3245. [Google Scholar] [CrossRef] [PubMed]
- Smatlik, N.; Drexler, S.K.; Burian, M.; Röcken, M.; Yazdi, A.S. ASC Speck Formation after Inflammasome Activation in Primary Human Keratinocytes. Oxid. Med. Cell Longev. 2021, 2021, 7914829. [Google Scholar] [CrossRef]
- Huang, B.; Qian, Y.; Xie, S.; Ye, X.; Chen, H.; Chen, Z.; Zhang, L.; Xu, J.; Hu, H.; Ma, S.; et al. Ticagrelor Inhibits the NLRP3 Inflammasome to Protect against Inflammatory Disease Independent of the P2Y12 Signaling Pathway. Cell Mol. Immunol. 2021, 18, 1278–1289. [Google Scholar] [CrossRef]
- Lamkanfi, M.; Dixit, V.M. Mechanisms and Functions of Inflammasomes. Cell 2014, 157, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
- Fernandes-Alnemri, T.; Yu, J.-W.; Juliana, C.; Solorzano, L.; Kang, S.; Wu, J.; Datta, P.; McCormick, M.; Huang, L.; McDermott, E.; et al. The AIM2 Inflammasome Is Critical for Innate Immunity to Francisella tularensis. Nat. Immunol. 2010, 11, 385–393. [Google Scholar] [CrossRef]
- Schroder, K.; Tschopp, J. The Inflammasomes. Cell 2010, 140, 821–832. [Google Scholar] [CrossRef] [PubMed]
- Henry, T.; Brotcke, A.; Weiss, D.S.; Thompson, L.J.; Monack, D.M. Type I Interferon Signaling Is Required for Activation of the Inflammasome during Francisella Infection. J. Exp. Med. 2007, 204, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Château, A.; Seifert, H.S. Neisseria Gonorrhoeae Survives within and Modulates Apoptosis and Inflammatory Cytokine Production of Human Macrophages. Cell. Microbiol. 2016, 18, 546–560. [Google Scholar] [CrossRef]
- Dobson-Belaire, W.N.; Rebbapragada, A.; Malott, R.J.; Yue, F.Y.; Kovacs, C.; Kaul, R.; Ostrowski, M.A.; Gray-Owen, S.D. Neisseria gonorrhoeae Effectively Blocks HIV-1 Replication by Eliciting a Potent TLR9-Dependent Interferon-α Response from Plasmacytoid Dendritic Cells. Cell. Microbiol. 2010, 12, 1703–1717. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Peng, X.; Lu, C.; Zhang, X.; Gan, L.; Gao, Y.; Yang, S.; Xu, W.; Wang, J.; Yin, Y.; et al. Type I IFN Expression Is Stimulated by Cytosolic MtDNA Released from Pneumolysin-Damaged Mitochondria via the STING Signaling Pathway in Macrophages. FEBS J. 2019, 286, 4754–4768. [Google Scholar] [CrossRef]
- Guimarães, E.S.; Gomes, M.T.R.; Campos, P.C.; Mansur, D.S.; Dos Santos, A.A.; Harms, J.; Splitter, G.; Smith, J.A.; Barber, G.N.; Oliveira, S.C. Brucella abortus Cyclic Dinucleotides Trigger STING-Dependent Unfolded Protein Response That Favors Bacterial Replication. J. Immunol. 2019, 202, 2671–2681. [Google Scholar] [CrossRef]
- Parker, D.; Planet, P.J.; Soong, G.; Narechania, A.; Prince, A. Induction of Type I Interferon Signaling Determines the Relative Pathogenicity of Staphylococcus Aureus Strains. PLoS Pathog. 2014, 10, e1003951. [Google Scholar] [CrossRef] [PubMed]
- Gratz, N.; Hartweger, H.; Matt, U.; Kratochvill, F.; Janos, M.; Sigel, S.; Drobits, B.; Li, X.-D.; Knapp, S.; Kovarik, P. Type I Interferon Production Induced by Streptococcus pyogenes-Derived Nucleic Acids Is Required for Host Protection. PLoS Pathog. 2011, 7, e1001345. [Google Scholar] [CrossRef] [PubMed]
- Viala, J.; Chaput, C.; Boneca, I.G.; Cardona, A.; Girardin, S.E.; Moran, A.P.; Athman, R.; Mémet, S.; Huerre, M.R.; Coyle, A.J.; et al. Nod1 Responds to Peptidoglycan Delivered by the Helicobacter pylori Cag Pathogenicity Island. Nat. Immunol. 2004, 5, 1166–1174. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).