First Molecular Detection of Rickettsia conorii and Rickettsia helvetica in Ticks from Dogs in Luxembourg
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Site and Sample Collection
2.2. DNA Extraction and Molecular Characterization
2.3. Sequencing and Phylogenetic Analysis
2.4. Statistical Analysis
3. Results
3.1. Morphological Identification of Ticks
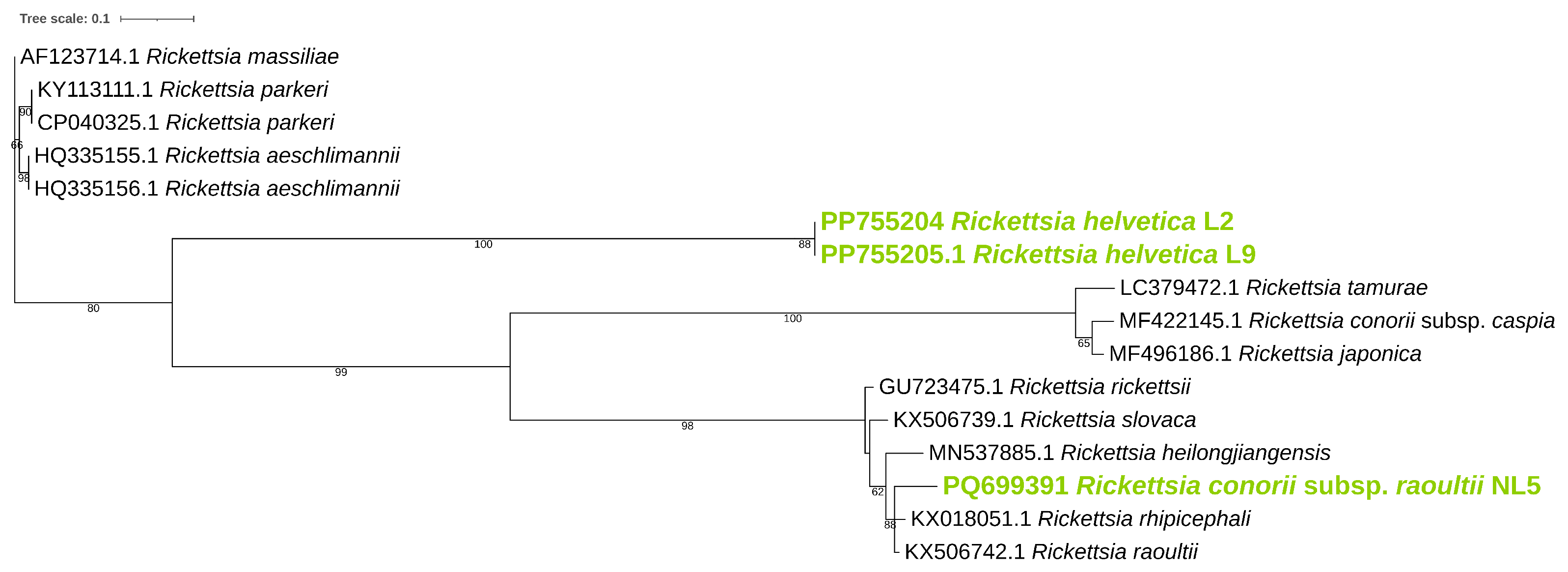
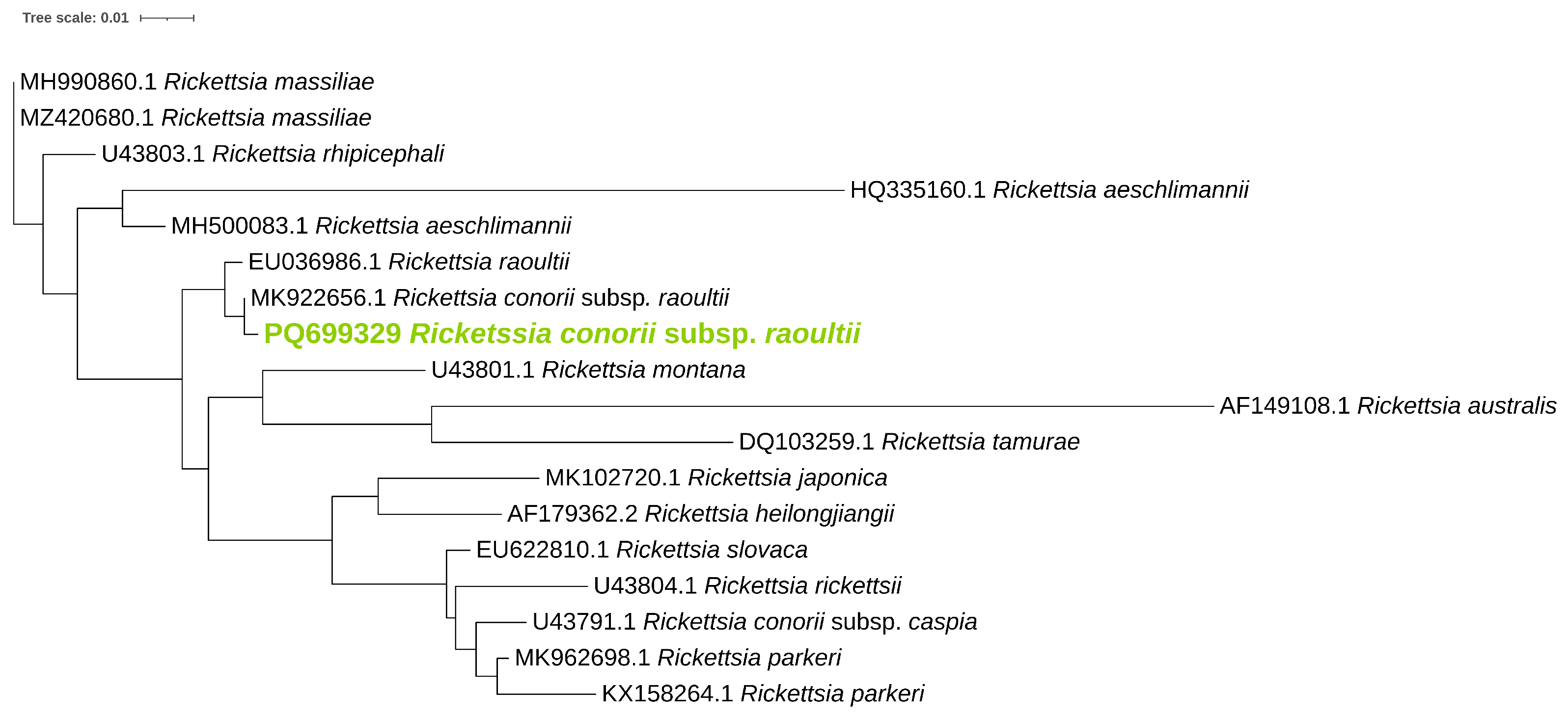
3.2. Identification of Rickettsiae in Examined Ticks
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vector-Borne Diseases. Available online: https://www.who.int/news-room/fact-sheets/detail/vector-borne-diseases (accessed on 28 November 2024).
- CDC. Vector-Borne Diseases. Available online: https://www.cdc.gov/climate-health/php/effects/vectors.html (accessed on 28 November 2024).
- Blanton, L.S. The Rickettsioses: A Practical Update. Infect. Dis. Clin. N. Am. 2019, 33, 213–229. [Google Scholar] [CrossRef] [PubMed]
- Parte, A.C. LPSN—List of prokaryotic names with standing in nomenclature. Nucleic Acids Res. 2014, 42, D613–D616. [Google Scholar] [CrossRef]
- Vikentjeva, M.; Geller, J.; Remm, J.; Golovljova, I. Rickettsia spp. in rodent-attached ticks in Estonia and first evidence of spotted fever group Rickettsia species Candidatus Rickettsia uralica in Europe. Parasites Vectors 2021, 14, 65. [Google Scholar] [CrossRef] [PubMed]
- Ivan, T.; Matei, I.; Novac, C.; Kalmár, Z.; Borșan, S.D.; Panait, L.; Gherman, C.; Ionică, A.; Papuc, I.; Mihalca, A. Spotted Fever Group Rickettsia spp. Diversity in Ticks and the First Report of Rickettsia hoogstraalii in Romania. Vet. Sci. 2022, 9, 343. [Google Scholar] [CrossRef]
- Špitalská, E.; Sparagano, O.; Stanko, M.; Schwarzová, K.; Špitalský, Z.; Škultéty, L.; Havlíková, S.F. Diversity of Coxiella-like and Francisella-like endosymbionts, and Rickettsia spp., Coxiella burnetii as pathogens in the tick populations of Slovakia, Central Europe. Ticks Tick-Borne Dis. 2018, 9, 1207–1211. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, S.I.; Paul, R.E.L.; Bischoff, E.; Cote, M.; Naour, E.L. First identification of Rickettsia helvetica in questing ticks from a French Northern Brittany Forest. PLoS Neglected Trop. Dis. 2017, 11, e0005416. [Google Scholar] [CrossRef] [PubMed]
- Matulaitytė, V.; Paulauskas, A.; Bratchikov, M.; Radzijevskaja, J. New record of Rickettsia vini in Ixodes lividus ticks from Lithuania. Ticks Tick-Borne Dis. 2020, 11, 101372. [Google Scholar] [CrossRef] [PubMed]
- Stańczak, J.; Biernat, B.; Racewicz, M.; Zalewska, M.; Matyjasek, A. Prevalence of different Rickettsia spp. in Ixodes ricinus and Dermacentor reticulatus ticks (Acari: Ixodidae) in north-eastern Poland. Ticks Tick-Borne Dis. 2017, 9, 427–434. [Google Scholar] [CrossRef]
- Blanco, J.; Oteo, J. Rickettsiosis in Europe. Ann. N. Y. Acad. Sci. 2006, 1078, 26–33. [Google Scholar] [CrossRef]
- Oteo, J.A.; Portillo, A. Tick-borne rickettsioses in Europe. Ticks Tick-Borne Dis. 2012, 3, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Portillo, A.; Santibáñez, S.; García-Álvarez, L.; Palomar, A.; Oteo, J. Rickettsioses in Europe. Microbes Infect. 2015, 17, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, A.; Krämer, A.; Sachse, S.; Straube, E. Detection of Rickettsia spp. and Anaplasma phagocytophilum in Ixodes ricinus ticks in a region of Middle Germany (Thuringia). Ticks Tick-Borne Dis. 2010, 1, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Key to Genera | ESCCAP UK & Ireland. Available online: https://www.esccapuk.org.uk/page/Key+to+Genera/49/ (accessed on 5 December 2024).
- Bugmyrin, S.; Belova, O.; Bespyatova, L.A.; Ieshko, E.P.; Karganova, G. Morphological features of Ixodes persulcatus and I. ricinus hybrids: Nymphs and adults. Exp. Appl. Acarol. 2016, 69, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Saracho-Bottero, M.N.; Venzal, J.; Tarragona, E.L.; Thompson, C.; Mangold, A.; Beati, L.; Guglielmone, A.; Nava, S. The Ixodes ricinus complex (Acari: Ixodidae) in the Southern Cone of America: Ixodes pararicinus, Ixodes aragaoi, and Ixodes sp. cf. I. affinis. Parasitol. Res. 2019, 119, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Sonenshine, D.E.; Roe, R.M. Biology of Ticks. Volume 1, 2nd ed.; Oxford University Press: Oxford, UK, 2014. [Google Scholar]
- Sayler, K.A.; Wamsley, H.L.; Pate, M.; Barbet, A.F.; Alleman, A.R. Cultivation of Rickettsia amblyommii in tick cells, prevalence in Florida lone star ticks (Amblyomma americanum). Parasites Vectors 2014, 7, 270. [Google Scholar] [CrossRef] [PubMed]
- Crowder, C.D.; Rounds, M.A.; Phillipson, C.A.; Picuri, J.M.; Matthews, H.E.; Halverson, J.; Schutzer, S.E.; Ecker, D.J.; Eshoo, M.W. Extraction of total nucleic acids from ticks for the detection of bacterial and viral pathogens. J. Med. Entomol. 2010, 47, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Lee, S.H.; Park, K.H.; Koh, Y.S.; Lee, K.H.; Baik, H.S.; Choi, M.S.; Kim, I.S.; Jang, W.J. Evaluation of PCR-Based Assay for Diagnosis of Spotted Fever Group Rickettsiosis in Human Serum Samples. Clin. Diagn. Lab. Immunol. 2005, 12, 759–763. [Google Scholar] [CrossRef]
- Regnery, R.L.; Spruill, C.L.; Plikaytis, B.D. Genotypic identification of rickettsiae and estimation of intraspecies sequence divergence for portions of two rickettsial genes. J. Bacteriol. 1991, 173, 1576–1589. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, R.; Ismail, N.; Dória-Nóbrega, S.; Costa, P.; Abreu, T.; França, A.; Amaro, M.; Proença, P.; Brito, P.; Poças, J.; et al. The Presence of Eschars, but Not Greater Severity, in Portuguese Patients Infected with Israeli Spotted Fever. Ann. N. Y. Acad. Sci. 2005, 1063, 197–202. [Google Scholar] [CrossRef]
- Santos-Silva, S.; Santos, N.; Boratyński, Z.; Mesquita, J.R.; Barradas, P.F. Diversity of Rickettsia spp. in ticks from wild mammals of Morocco and Mauritania. Ticks Tick-Borne Dis. 2023, 14, 102235. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; von Haeseler, A.; Lanfear, R. IQ-TREE 2: New Models and Efficient Methods for Phylogenetic Inference in the Genomic Era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v4: Recent updates and new developments. Nucleic Acids Res. 2019, 47, W256–W259. [Google Scholar] [CrossRef]
- Sprong, H.; Wielinga, P.; Fonville, M.; Reusken, C.; Brandenburg, A.; Borgsteede, F.; Gaasenbeek, C.; Giessen, J.V.D.v.d. I. ricinus ticks are reservoir hosts for Rickettsia helvetica and potentially carry flea-borne Rickettsia species. Parasites Vectors 2009, 2, 41. [Google Scholar] [CrossRef]
- Rudolf, I.; Venclíková, K.; Blažejová, H.; Betášová, L.; Mendel, J.; Hubálek, Z.; Parola, P. First report of Rickettsia raoultii and Rickettsia helvetica in Dermacentor reticulatus ticks from the Czech Republic. Ticks Tick-Borne Dis. 2016, 7, 1222–1224. [Google Scholar] [CrossRef]
- Biernat, B.; Stańczak, J.; Michalik, J.; Sikora, B.; Wierzbicka, A. Prevalence of infection with Rickettsia helvetica in Ixodes ricinus ticks feeding on non-rickettsiemic rodent hosts in sylvatic habitats of west-central Poland. Ticks Tick-Borne Dis. 2016, 7, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Mediannikov, O.; Matsumoto, K.; Samoylenko, I.; Drancourt, M.; Roux, V.; Rydkina, E.; Davoust, B.; Tarasevich, I.; Brouqui, P.; Fournier, P. Rickettsia raoultii sp. nov., a spotted fever group rickettsia associated with Dermacentor ticks in Europe and Russia. Int. J. Syst. Evol. Microbiol. 2008, 58 Pt 7, 1635–1639. [Google Scholar] [CrossRef]
- Jahfari, S.; Ruyts, S.C.; Frazer-Mendelewska, E.; Jaarsma, R.; Verheyen, K.; Sprong, H. Melting pot of tick-borne zoonoses: The European hedgehog contributes to the maintenance of various tick-borne diseases in natural cycles urban and suburban areas. Parasites Vectors 2017, 10, 134. [Google Scholar] [CrossRef]
- Szekeres, S.; Docters van Leeuwen, A.; Tóth, E.; Majoros, G.; Sprong, H.; Földvári, G. Road-killed mammals provide insight into tick-borne bacterial pathogen communities within urban habitats. Transbound. Emerg. Dis. 2019, 66, 277–286. [Google Scholar] [CrossRef]
- Miranda, J.; Mattar, S. Molecular detection of Rickettsia bellii and Rickettsia sp. strain Colombianensi in ticks from Cordoba, Colombia. Ticks Tick-Borne Dis. 2014, 5, 208–212. [Google Scholar] [CrossRef]
- Parola, P. Tick-borne rickettsial diseases: Emerging risks in Europe. Comp. Immunol. Microbiol. Infect. Dis. 2004, 27, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Grassi, L.; Menandro, M.L.; Cassini, R.; Mondin, A.; Pasotto, D.; Grillini, M.; Rocca, G.; Drigo, M. High Prevalence of Tick-Borne Zoonotic Rickettsia slovaca in Ticks from Wild Boars, Northeastern Italy. Animals 2022, 12, 967. [Google Scholar] [CrossRef] [PubMed]
- Speck, S.; Perseke, L.; Petney, T.; Skuballa, J.; Pfäffle, M.; Taraschewski, H.; Bunnell, T.; Essbauer, S.; Dobler, G. Detection of Rickettsia helvetica in ticks collected from European hedgehogs (Erinaceus europaeus, Linnaeus, 1758). Ticks Tick-Borne Dis. 2013, 4, 222–226. [Google Scholar] [CrossRef]
- Sréter, T.; Lancz, Z.S.; Széll, Z.; Egyed, L. [Rickettsia helvetica: An emerging tick-borne pathogen in Hungary and Europe]. Orvosi Hetil. 2005, 146, 2547–2552. [Google Scholar]
- Zhang, L.; Ma, D.; Li, C.; Zhou, R.; Wang, J.; Liu, Q. Projecting the Potential Distribution Areas of Ixodes scapularis (Acari: Ixodidae) Driven by Climate Change. Biology 2022, 11, 107. [Google Scholar] [CrossRef]
- Yang, X.; Gao, Z.; Wang, L.; Xiao, L.; Dong, N.; Wu, H.; Li, S. Projecting the potential distribution of ticks in China under climate and land use change. Int. J. Parasitol. 2021, 51, 749–759. [Google Scholar] [CrossRef]
- Alkishe, A.; Raghavan, R.; Peterson, A. Likely Geographic Distributional Shifts among Medically Important Tick Species and Tick-Associated Diseases under Climate Change in North America: A Review. Insects 2021, 12, 225. [Google Scholar] [CrossRef]
- Voyiatzaki, C.; Papailia, S.I.; Venetikou, M.; Pouris, J.; Tsoumani, M.; Papageorgiou, E. Climate Changes Exacerbate the Spread of Ixodes ricinus and the Occurrence of Lyme Borreliosis and Tick-Borne Encephalitis in Europe—How Climate Models Are Used as a Risk Assessment Approach for Tick-Borne Diseases. Int. J. Environ. Res. Public Health 2022, 19, 6516. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Chung, S.Y. The threat of climate change on tick-borne infections: Rising trend of infections and geographical distribution of climate risk factors associated with ticks. J. Infect. Dis. 2022, 227, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, E.; Talleklint, L.; Polfeldt, T. Impact of climatic change on the northern latitude limit and population density of the disease-transmitting European tick Ixodes ricinus. Environ. Health Perspect. 1999, 108, 119–123. [Google Scholar] [CrossRef]
- Porretta, D.; Mastrantonio, V.; Amendolia, S.; Gaiarsa, S.; Epis, S.; Genchi, C.; Bandi, C.; Otranto, D.; Urbanelli, S. Effects of global changes on the climatic niche of the tick Ixodes ricinus inferred by species distribution modelling. Parasites Vectors 2013, 6, 271. [Google Scholar] [CrossRef]
- Estrada-Peña, A.; Ayllón, N.; Fuente, J.D.L. Impact of Climate Trends on Tick-Borne Pathogen Transmission. Front. Physiol. 2012, 3. [Google Scholar] [CrossRef]
- Gilbert, L. Altitudinal patterns of tick and host abundance: A potential role for climate change in regulating tick-borne diseases? Oecologia 2009, 162, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Ogden, N.; Radojević, M.; Wu, X.; Duvvuri, V.; Leighton, P.; Wu, J. Estimated Effects of Projected Climate Change on the Basic Reproductive Number of the Lyme Disease Vector Ixodes scapularis. Environ. Health Perspect. 2014, 122, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Semenza, J.; Suk, J.E. Vector-borne diseases and climate change: A European perspective. FEMS Microbiol. Lett. 2017, 365. [Google Scholar] [CrossRef] [PubMed]
- Ogden, N.; Ogden, N.; Beard, C.; Ginsberg, H.; Tsao, J. Possible Effects of Climate Change on Ixodid Ticks and the Pathogens They Transmit: Predictions and Observations. J. Med. Entomol. 2020, 58, 1536–1545. [Google Scholar] [CrossRef]
- Ma, R.; Li, C.; Gao, A.; Jiang, N.; Li, J.; Hu, W.; Feng, X. Tick species diversity and potential distribution alternation of dominant ticks under different climate scenarios in Xinjiang, China. PLoS Neglected Trop. Dis. 2024, 18, e0012108. [Google Scholar] [CrossRef] [PubMed]

| Gene | Primer | Nucleotide Sequence (5′–3′) |
|---|---|---|
| ompB | ompB F | GTAACCGGAAGTAATCGTTTCGTAA |
| ompB R | GCTTTATAACCAGCTAAACCACC | |
| ompA | ompA F | ATGGCGAATATTTCTCCAAAA |
| ompA R | AGTGCAGCATTCGCTCCCCCT | |
| gltA | gltA F | GCTATTATGCTTGCGGCTGT |
| gltA R | TGCATTTCTTTCCATTGTGC |
| Gene | Accession Number | Citation |
|---|---|---|
| ompA | MZ420682 | [24] |
| gltA | MZ420697 | [24] |
| ompB | MZ420689 | [24] |
| Gene | Sample | Accession Number | Closest Match | Identity (%) |
|---|---|---|---|---|
| ompB | L2 | PP755204 | R. helvetica (JX683116) | 99.8% |
| L9 | PP755205 | R. helvetica (AF123725) | 99.8% | |
| NL5 | PQ699391 | R. conorii (KU310592) | 98.4% | |
| ompA | NL5 | PQ699392 | R. conorii subsp. raoultii (MK922656) | 100% |
| gltA | L2 | PQ66306 | R. helvetica (OQ866615) | 100% |
| L9 | PQ66306 | R. helvetica (U59723) | 100% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreira, G.; Moreira, R.S.S.; Neves, F.A.d.; Swiontek, V.; Barradas, P.F.; Gomes-Gonçalves, S.; Mesquita, J.R. First Molecular Detection of Rickettsia conorii and Rickettsia helvetica in Ticks from Dogs in Luxembourg. Pathogens 2025, 14, 204. https://doi.org/10.3390/pathogens14020204
Moreira G, Moreira RSS, Neves FAd, Swiontek V, Barradas PF, Gomes-Gonçalves S, Mesquita JR. First Molecular Detection of Rickettsia conorii and Rickettsia helvetica in Ticks from Dogs in Luxembourg. Pathogens. 2025; 14(2):204. https://doi.org/10.3390/pathogens14020204
Chicago/Turabian StyleMoreira, Guilherme, Rafaela S. S. Moreira, Floriane André das Neves, Vanessa Swiontek, Patrícia F. Barradas, Sara Gomes-Gonçalves, and João R. Mesquita. 2025. "First Molecular Detection of Rickettsia conorii and Rickettsia helvetica in Ticks from Dogs in Luxembourg" Pathogens 14, no. 2: 204. https://doi.org/10.3390/pathogens14020204
APA StyleMoreira, G., Moreira, R. S. S., Neves, F. A. d., Swiontek, V., Barradas, P. F., Gomes-Gonçalves, S., & Mesquita, J. R. (2025). First Molecular Detection of Rickettsia conorii and Rickettsia helvetica in Ticks from Dogs in Luxembourg. Pathogens, 14(2), 204. https://doi.org/10.3390/pathogens14020204







