Prevalence and Genetic Diversity of Parasites in Humans and Pet Dogs in Rural Areas of Los Ríos Region, Southern Chile
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Research Ethics Committee Approval
2.3. Collection and Processing Methodologies
2.4. Collection Methodology
2.5. Processing of Stool Samples
2.6. Sample Processing Using ELISA
2.7. Genetic Diversity of Giardia duodenalis and Blastocystis sp.
2.8. Statistical Analysis
3. Results
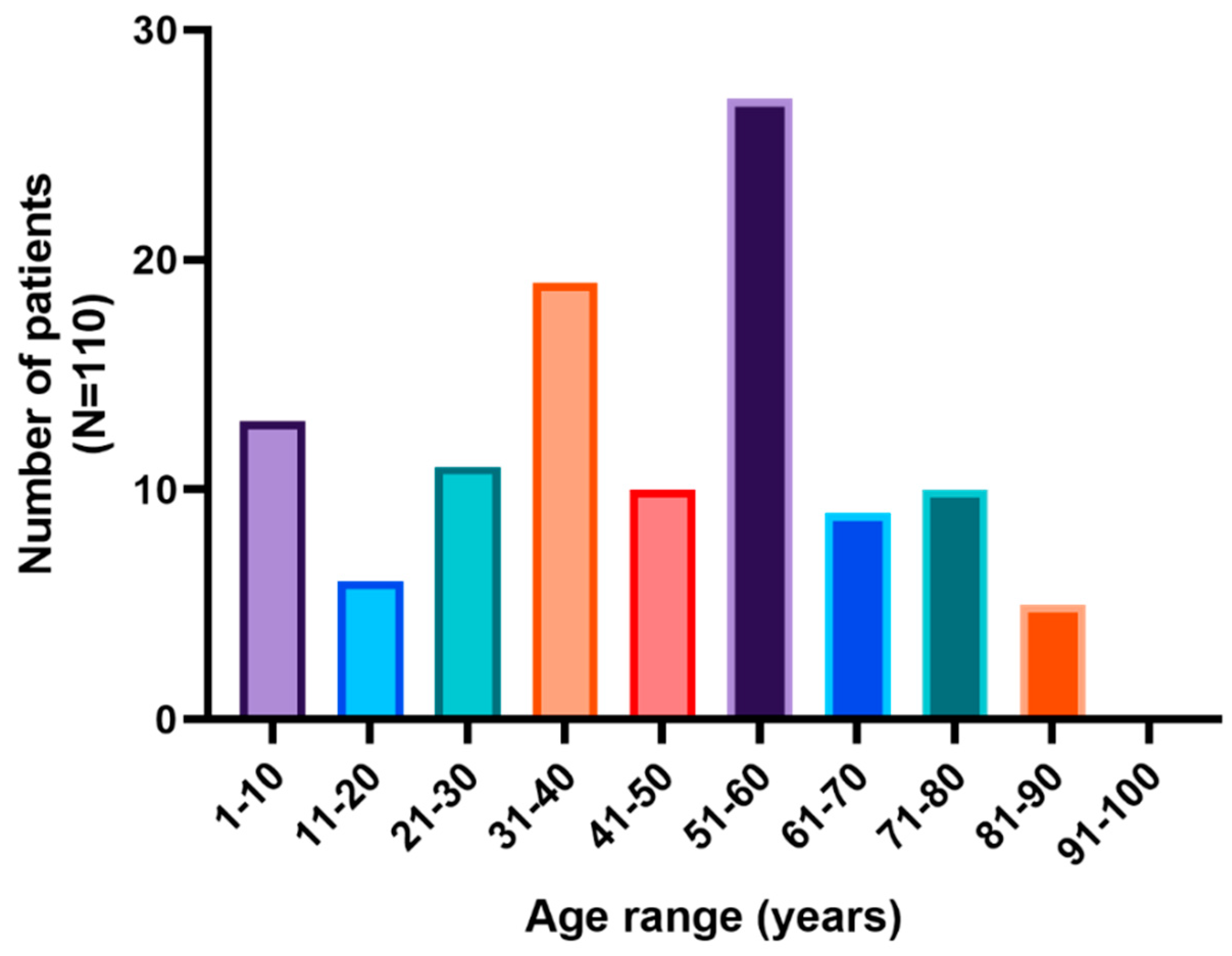
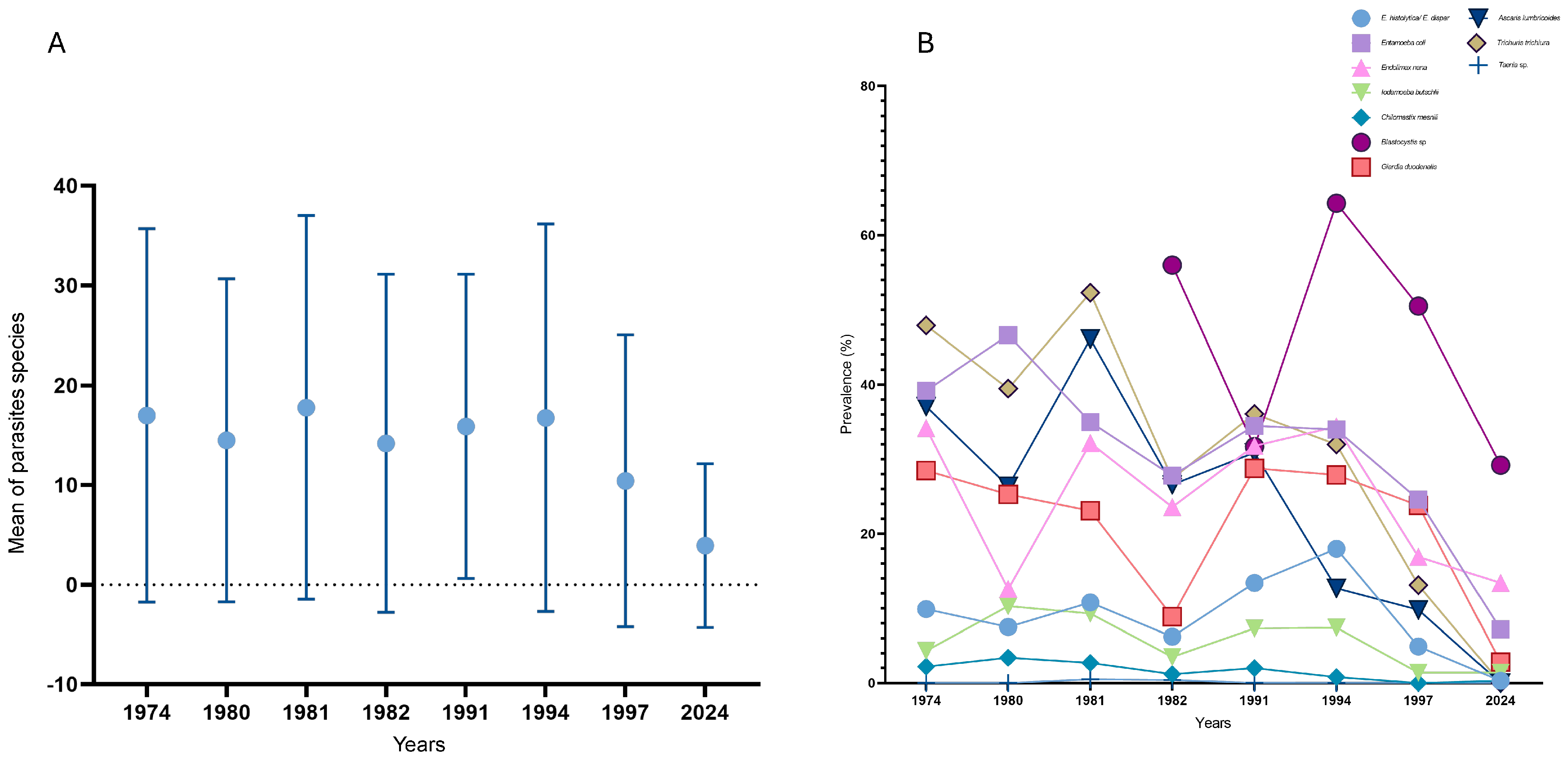
3.1. Results of Serial Stool Parasitological Analysis
Statistical Analysis of Serial Stool Parasitological Analysis
3.2. Serologic Study Results for IgG Anti-Toxocara canis and Anti-Echinococcus granulosus Antibodies
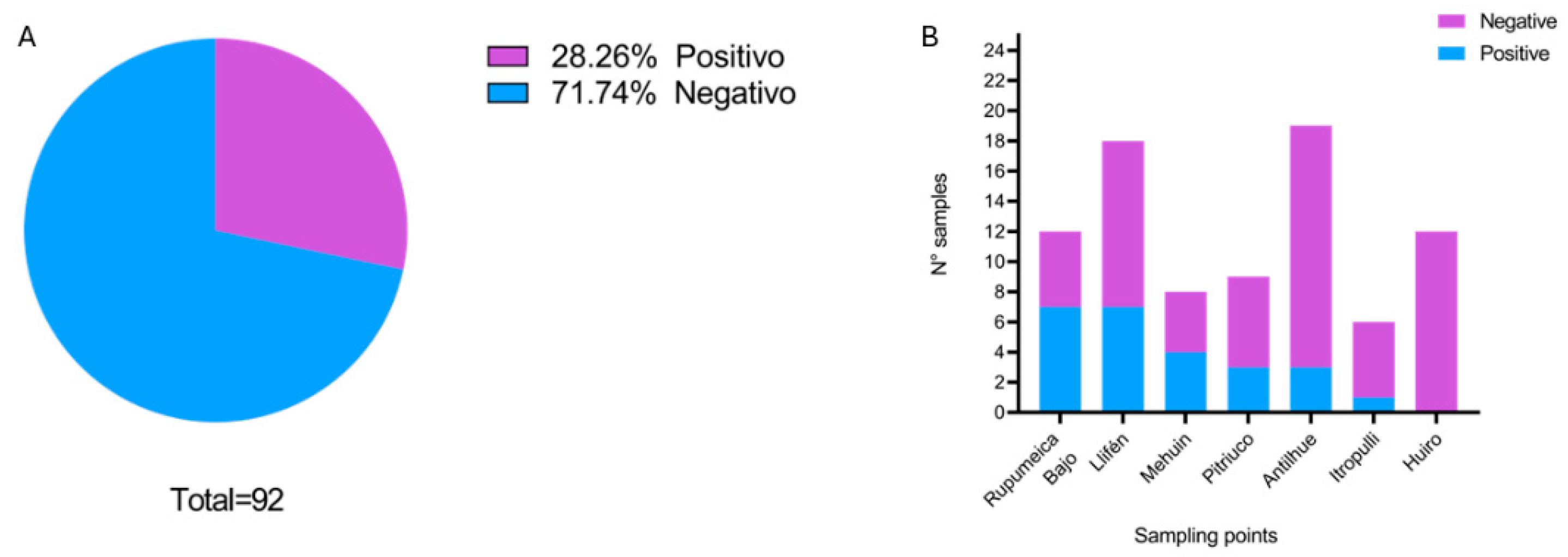
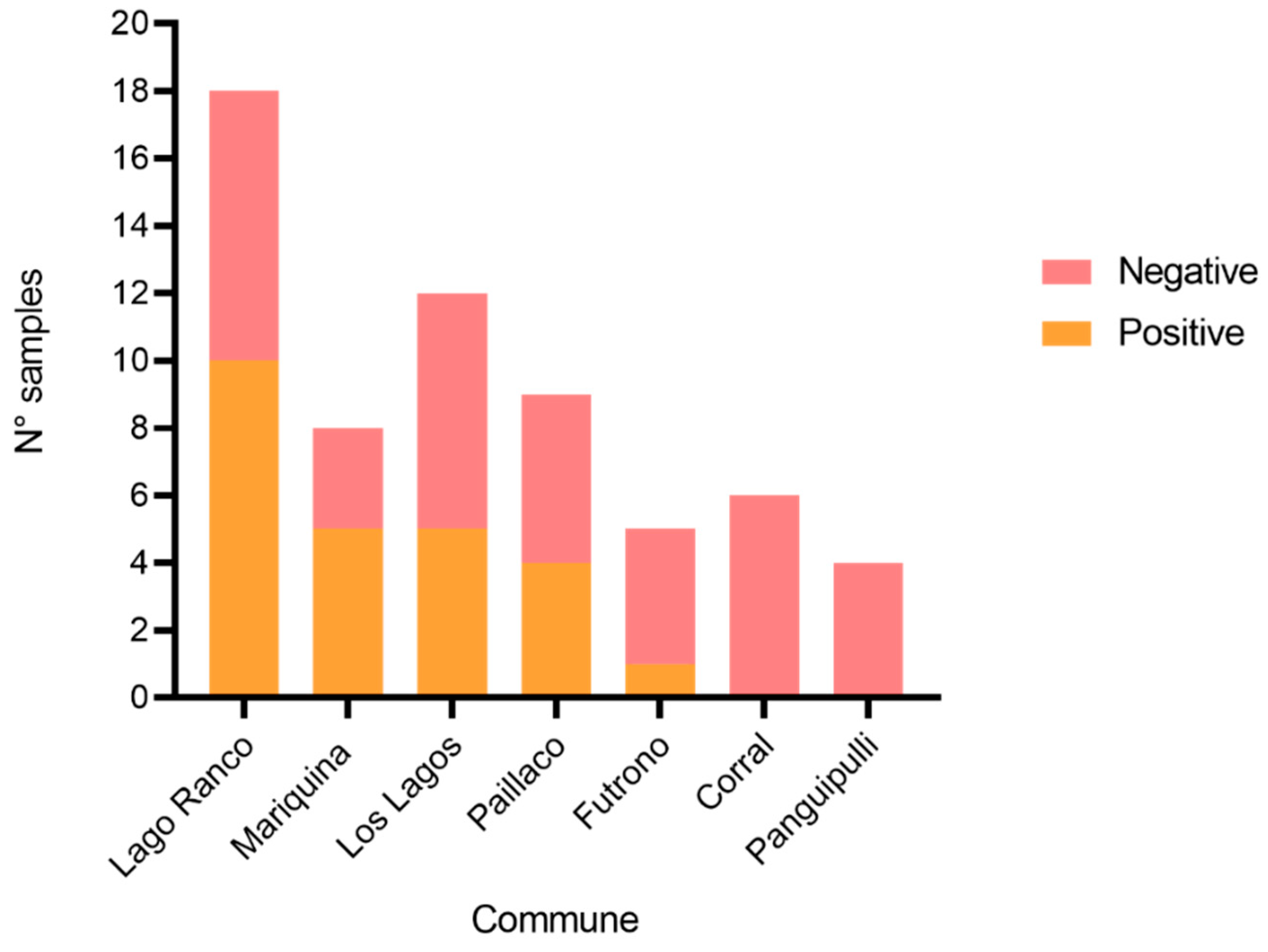
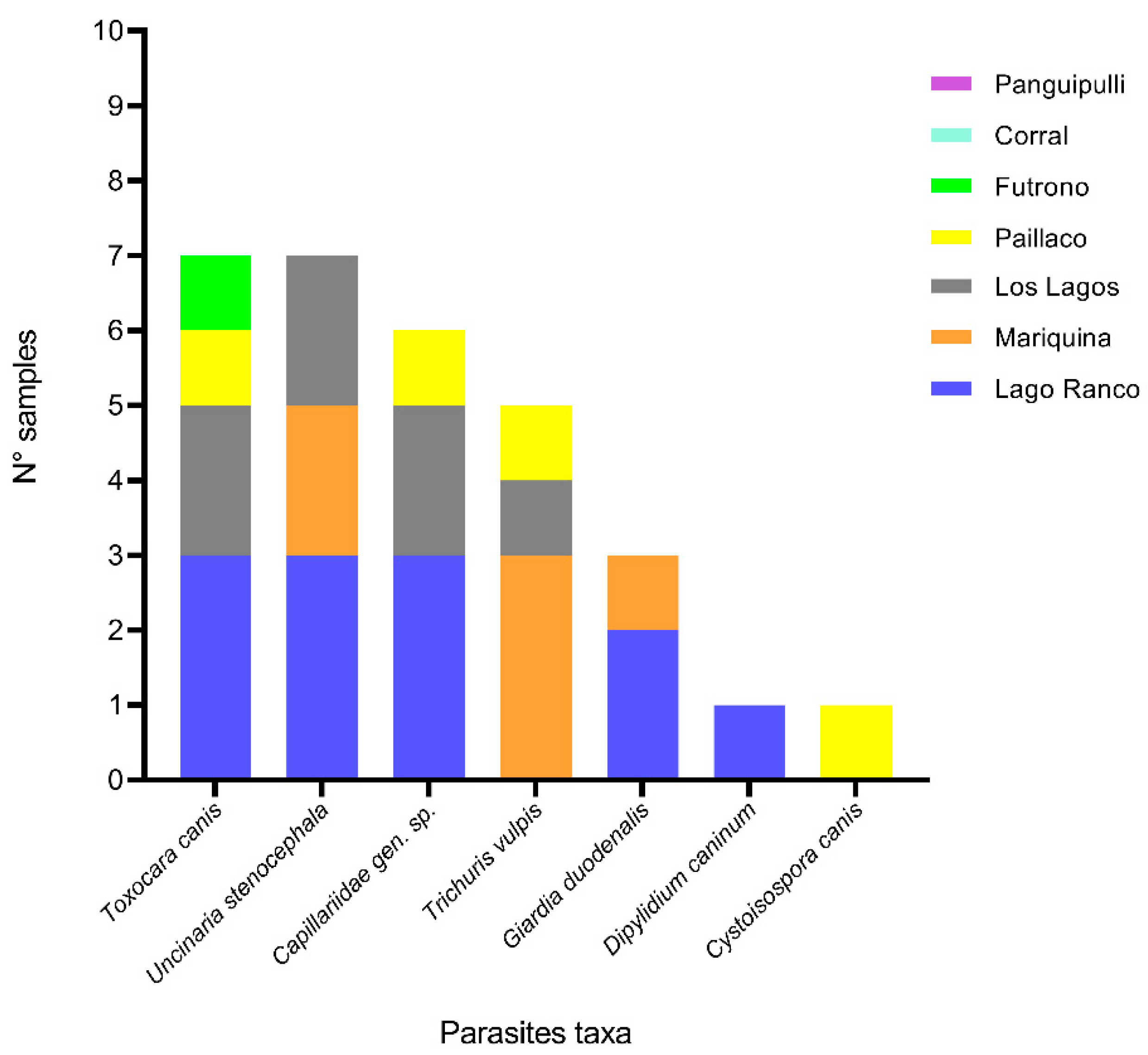
3.3. Results of Parasitological Examination of Dogs Feces
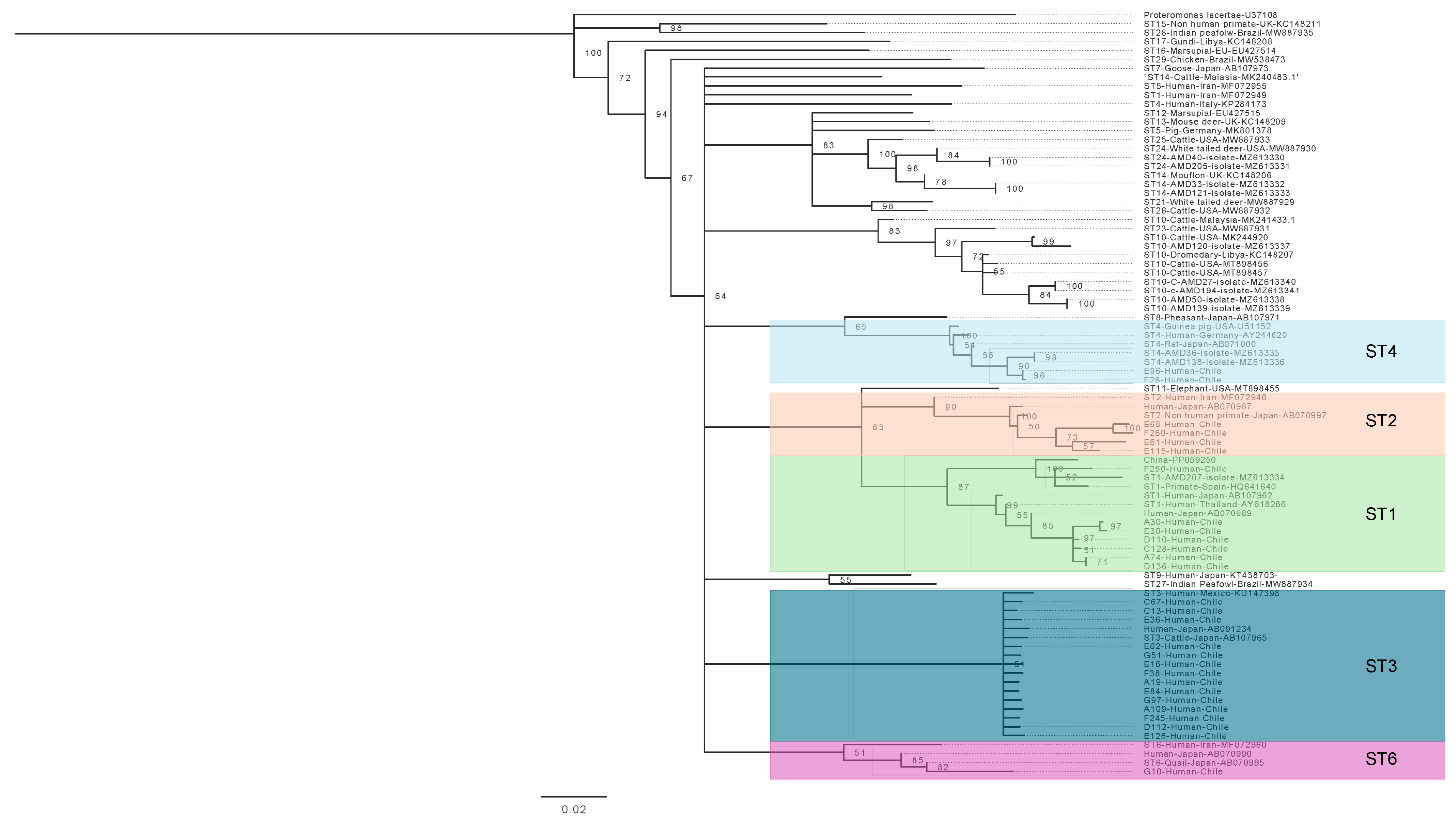
3.4. Phylogenetic Analysis of Blastocystis sp. and Giardia duodenalis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cox, F.E.G. History of Human Parasitology. Clin. Microbiol. Rev. 2002, 15, 595–612. [Google Scholar] [CrossRef]
- Prieto-Pérez, L.; Pérez-Tanoira, R.; Cabello-Úbeda, A.; Petkova-Saiz, E.; Górgolas-Hernández-Mora, M. Geohelmintos. Enferm. Infect. Microbiol. Clin. 2016, 34, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Montoya, J.G.; Liesenfeld, O. Toxoplasmosis. Lancet 2004, 363, 1965–1976. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, S.M.; Stark, D.; Harkness, J.; Ellis, J. Enteric Protozoa in the Developed World: A Public Health Perspective. Clin. Microbiol. Rev. 2012, 25, 420–449. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M. Intestinal Parasitic Infections in 2023. Gastroenterol. Res. 2023, 16, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Mercado, R.; Castillo, D.; Muñoz, V.; Sandoval, L.; Jercic, I.; Gil, L.C.; Ueta, M.T.; Schenone, H. Intestinal protozoa and helminth infections in pre-school and elementary school-children from Colina county, Santiago, Chile, 2003. Parasitol. Latinoam. 2003, 58, 173–176. [Google Scholar] [CrossRef]
- Valenzuela, B.M.T.; Salinas, P.H.; Cárcamo, I.M.; Cerda, L.J.; Valdivia, C.G. Estrategias Para El Enfrentamiento Del Cólera. La Experiencia Chilena Desde Una Perspectiva de Salud Pública. Rev. Chil. Infect. 2010, 27, 407–410. [Google Scholar] [CrossRef]
- Crespo, C.F. Chile: Nuevos Desafíos Sanitarios e Institucionales En Un País En Transición. Rev. Panam. Salud Publica 2018, 42, e137. [Google Scholar] [CrossRef]
- Bórquez, C.; Lobato, I.; Montalvo, M.T.; Marchant, P.; Martínez, P. Enteroparasitosis En Niños Escolares Del Valle de Lluta. Arica-Chile. Parasitol. Latinoam. 2004, 59, 175–178. [Google Scholar] [CrossRef]
- Instituto de Salud Publica de Chile. Vigilancia de Laboratorio de Echinococcosis Quística/Hidatidosis Chile, 2011–2021; Instituto de Salud Publica de Chile: Santiago, Chile, 2023. [Google Scholar]
- Torres, P.; Franjola, R.; Pérez, J.; Auad, S.; Uherek, F.; Miranda, J.C.; Flores, L.; Riquelme, J.; Salazar, S.; Hermosilla, C.; et al. Epidemiología de la difilobotriasis en la cuenca del río Valdivia, Chile. Rev. Saúde Publ. 1989, 23, 45–57. [Google Scholar] [CrossRef]
- Cabello, F.C. Acuicultura y Salud Pública: La Expansión de La Difilobotriasis En Chile y El Mundo. Rev. Med. Chil. 2007, 135, 1064–1071. [Google Scholar] [CrossRef]
- Torres, P.; Yera, H. Diphyllobothriidae. In Water and Sanitation for the 21st Century: Health and Microbiological Aspects of Excreta and Wastewater Management (Global Water Pathogen Project); Michigan State University: East Lansing, MI, USA, 2019. [Google Scholar]
- Torres, P.; Otth Rademacher, L.; Montefusco, A.; Wilson, G.; Ramírez, C.; Acuña, M.; Marín, F. Infección Por Protozoos y Helmintos Intestinales En Escolares de Sectores Ribereños, Con Distintos Niveles de Contaminación Fecal, Del Río Valdivia, Chile. Bol. Chil. Parasitol 1997, 52, 3–11. [Google Scholar]
- Torres, P.; Franjola, T.R.; Pérez, J.; Auad, S.; Hermosilla, C.; Flores, L.; Riquelme, J.; Salazar, S.; Miranda, J.C.; Montefusco, A. Geohelmintosis Intestinales En El Hombre y Animales Domésticos de Sectores Ribereños de La Cuenca Del Río Valdivia Chile. Bol. Chil. Parasitol 1995, 52, 57–66. [Google Scholar]
- Vargas, C.; Torres, P.; Jercic, M.I.; Lobos, M.; Oyarce, A.; Miranda, J.C.; Ayala, S. Frequency of Anti-Toxocara Spp. Antibodies in Individuals Attended by the Centro de Salud Familiar and Environmental Contamination with Toxocara Canis Eggs in Dog Feces, in the Coastal Niebla Town, Chile. Rev. Inst. Med. Trop. Sao Paulo 2016, 58, 62. [Google Scholar] [CrossRef] [PubMed]
- Subsecretaría de Desarrollo Regional y Administrativo (SUBDERE). Estimación de La Población Canina y Felina Del País y Diagnóstico de La Tenencia Responsable; Subsecretaría de Desarrollo Regional y Administrativo (SUBDERE): Santiago, Chile, 2022. [Google Scholar]
- Luzio, A.; Espejo, S.; Troncoso, I.; Fernández, I.; Fischer, C. Determinación Coproscópica de Formas Parasitarias En Heces de “Canis Lupus Familiaris” Diseminadas En Playas de La Comuna de Tomé, Región Del Bío Bío, Chile. Rev. Ibero-Latinoam. De Parasitol. 2013, 72, 88–94. [Google Scholar]
- Correa Robles, M.F. Fauna Parasitaria Gastrointestinal En Perros de Criaderos En La Región Metropolitana de Santiago, Chile. 2014. Available online: https://repositorio.uchile.cl/handle/2250/143088 (accessed on 1 March 2022).
- Quilodrán González, D. Parasitismo Gastrointestinal En Perros (Canis Lupus Familiaris) Con Propietario, Mediante Análisis Coproparasitario, En La Comuna de Cabrero, Región Bío Bío, Chile. Bachelor’s Thesis, Universidad de Concepción, Concpción, Chile, 2014. Available online: https://repositorio.udec.cl/items/b3699cc0-9db5-4e37-bfca-ecc49f581121 (accessed on 1 March 2022).
- Troncoso Sanchez, C.J.; Muñoz Alvarado, P.d.P. Determinación de parásitos gastrointestinales en perros de la del rey, región de Los Ríos, Chile. Bachelor’s Thesis, Universidad Austral de Chile, Valdivia, Chile, 2017. Available online: http://cybertesis.uach.cl/tesis/uach/2017/fvt853d/doc/fvt853d.pdf (accessed on 1 March 2022).
- Acosta-Jamett, G.; Cleaveland, S.; Cunningham, A.A.; Bronsvoort, B.D. Demography of Domestic Dogs in Rural and Urban Areas of the Coquimbo Region of Chile and Implications for Disease Transmission. Prev. Vet. Med. 2010, 94, 272–281. [Google Scholar] [CrossRef]
- Yaoyu, F.; Xiao, L. Zoonotic Potential and Molecular Epidemiology of Giardia Species and Giardiasis. Clin. Microbiol. Rev. 2011, 24, 110–140. [Google Scholar] [CrossRef]
- Koehler, A.V.; Herath, H.M.P.D.; Hall, R.S.; Wilcox, S.; Gasser, R.B. Marked Genetic Diversity within Blastocystis in Australian Wildlife Revealed Using a next Generation Sequencing–Phylogenetic Approach. Int. J. Parasitol. Parasites Wildl. 2024, 23, 100902. [Google Scholar] [CrossRef] [PubMed]
- Popruk, S.; Adao, D.E.V.; Rivera, W.L. Epidemiology and Subtype Distribution of Blastocystis in Humans: A Review. Infect. Genet. Evol. 2021, 95, 105085. [Google Scholar] [CrossRef] [PubMed]
- Skotarczak, B. Genetic Diversity and Pathogenicity of Blastocystis. Ann. Agric. Environ. Med. 2018, 25, 411–416. [Google Scholar] [CrossRef]
- Biblioteca del Congreso Nacional Region de Los Rios. Available online: https://www.bcn.cl/siit/nuestropais/nuestropais/region14 (accessed on 1 March 2022).
- Torres, P.; Navarrete, N. Comparación Entre Los Métodos Del Fijador PAFS y Del Telemann Modificado En El Diagnóstico de Protozoos Intestinales Del Hombre. Bol. Chil. De Parasitol. 1972, 27, 90–95. [Google Scholar]
- Dos Reis, L.L.; de Souza, L.S.S.; Braga, F.C.D.O.; Lima, D.C.D.S.; Lima, N.A.D.S.; Padinha, J.D.S.; Nava, A.F.D.; Vicente, A.C.P. Zoonotic Giardia Duodenalis Assemblage A in Northern Sloth from Brazilian Amazon. Mem. Inst. Oswaldo Cruz 2023, 118, e230088. [Google Scholar] [CrossRef]
- Chen, S.H.; Codoceo, A.; Carrasco, O.; Torres, M. Enteroparasitosis en la poblacion de la tercera edad consultante en centros medicos de la Pontificia Universidad Catolica De Chile, 1997. Parasitol. Al Día 1998, 22, 114–116. [Google Scholar] [CrossRef]
- Calchi, M.; Acurero, E.; Uribe, I.; Villalobos, R.; Fuenmayor, A.; Roo, J. Protozoarios y Helmintos Intestinales En Adultos Asintomáticos Del Estado Zulia, Venezuela. Kasmera 2012, 40, 186–194. [Google Scholar]
- Ytalia, B.; Madyoris, C.; Jonairbeth, H.; Iván, A.; Rodolfo, D. Parasitos Intestinales En Adultos Mayores Del Instituto Nacional De Servicios Sociales (Inass), Ciudad Bolívar, Estado Bolívar, Venezuela. Salud Arte Cuid. 2013, 6, 5–19. [Google Scholar]
- Ortiz, V.M.; Chacón, P.V.L.; Limache, G.; Matias, D.D.C. Blastocistosis y Otras Parasitosis Intestinales En Adultos Mayores Del Hogar San Ramón, Ciudad de La Paz, Bolivia Blastocistosis and Other Intestinal Parasitosis in Elderlyresidents from San Ramón Home, La Paz, Bolivia. Biofarbo 2008, 16, 9. [Google Scholar]
- Puga, S. Infeccion Humana Por Protozoos y Helmintos Intestinales En Dos Poblaciones Costaneras de La Provincia de Valdivia. [Human Infection by Protozoa and Helminths in Two Small Towns of the Province of Valdivia]. Rev. Med. Chil. 1980, 108, 608–611. [Google Scholar] [PubMed]
- Torres, P.; Gallegos, O.; Santibáñez, J.; Háuser, M. Infecciones Por Protozoos y Helmintos Intestinales En Grupos Familiares de Cinco Distritos Rurales Del Sur de Chile. Bol. Chil. Parasitol 1982, 37, 69–71. [Google Scholar] [PubMed]
- Figueroa, L.; Navarrete, N.; Franjola, R.; Puga, S. Enteroparasitosis En La Población Escolar Rural de La Provincia de Valdivia, Chile. Bol. Chil. Parasitol 1981, 36, 66–67. [Google Scholar]
- Navarrete, N.; Torres, P. Prevalencia de Infección Por Protozoos y Helmintos Intestinales En Escolares de Un Sector Costero de La Provincia de Valdivia, Chile. Bol. Chil. Parasitol 1994, 49, 79–80. [Google Scholar] [PubMed]
- Puga, S.; Figueroa, L.; Navarrete, N. Protozoos y Helmintos Intestinales En La Población Preescolar y Escolar de La Ciudad de Valdivia, Chile. Parasitol. Día 1991, 15, 57–58. [Google Scholar]
- Torres, P.; Figueroa, L.; Puga, S.; Franjola, R.; Navarrete, N.; Momberg, J.; Valdivia, L.; Filipick, L.; Mohr, P.; Cabezas, P. Intestinal Protozoans and Helminths in School Children from Valdivia, Chile. Bol. Chil. Parasitol. 1974, 29, 112–114. [Google Scholar]
- Bourli, P.; Eslahi, A.V.; Tzoraki, O.; Karanis, P. Waterborne Transmission of Protozoan Parasites: A Review of Worldwide Outbreaks–an Update 2017–2022. J. Water Health 2023, 21, 1421–1447. [Google Scholar] [CrossRef]
- Block, S.; Emerson, J.W.; Esty, D.C.; de Sherbinin, A.; Wendling, Z.A. Environmental Performance Index; Yale Center for Environmental Law: New Haven, CT, USA, 2024. [Google Scholar]
- Quilodrán-González, D.; Gadicke, P.; Junod, T.; Villaguala-Pacheco, C.; Landaeta-Aqueveque, C. Factores de Riesgo Asociados Con Parásitos Gastrointestinales Zoonóticos En Perros de Cabrero, Región Del Biobío, Chile. Chil. J. Agric. Anim. Sci. 2018, 34, 118–125. [Google Scholar] [CrossRef]
- Macpherson, C.N.L. The Epidemiology and Public Health Importance of Toxocariasis: A Zoonosis of Global Importance. Int. J. Parasitol. 2013, 43, 999–1008. [Google Scholar] [CrossRef]
- Baneth, G.; Thamsborg, S.M.; Otranto, D.; Guillot, J.; Blaga, R.; Deplazes, P.; Solano-Gallego, L. Major Parasitic Zoonoses Associated with Dogs and Cats in Europe. J. Comp. Pathol. 2016, 155, S54–S74. [Google Scholar] [CrossRef] [PubMed]
- Pisarski, K. The Global Burden of Disease of Zoonotic Parasitic Diseases: Top 5 Contenders for Priority Consideration. Trop. Med. Infect. Dis. 2019, 4, 44. [Google Scholar] [CrossRef] [PubMed]
- Fusaro, C.; Bernal, J.E.; Baldiris-Ávila, R.; González-Cuello, R.; Cisneros-Lorduy, J.; Reales-Ruiz, A.; Castro-Orozco, R.; Sarria-Guzmán, Y. Molecular Prevalence and Subtypes Distribution of Blastocystis spp. in Humans of Latin America: A Systematic Review. Trop. Med. Infect. Dis. 2024, 9, 38. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, P.; Muñoz, M.; Ramírez, J.D. An Update on the Distribution of Blastocystis Subtypes in the Americas. Heliyon 2022, 8, e12592. [Google Scholar] [CrossRef]
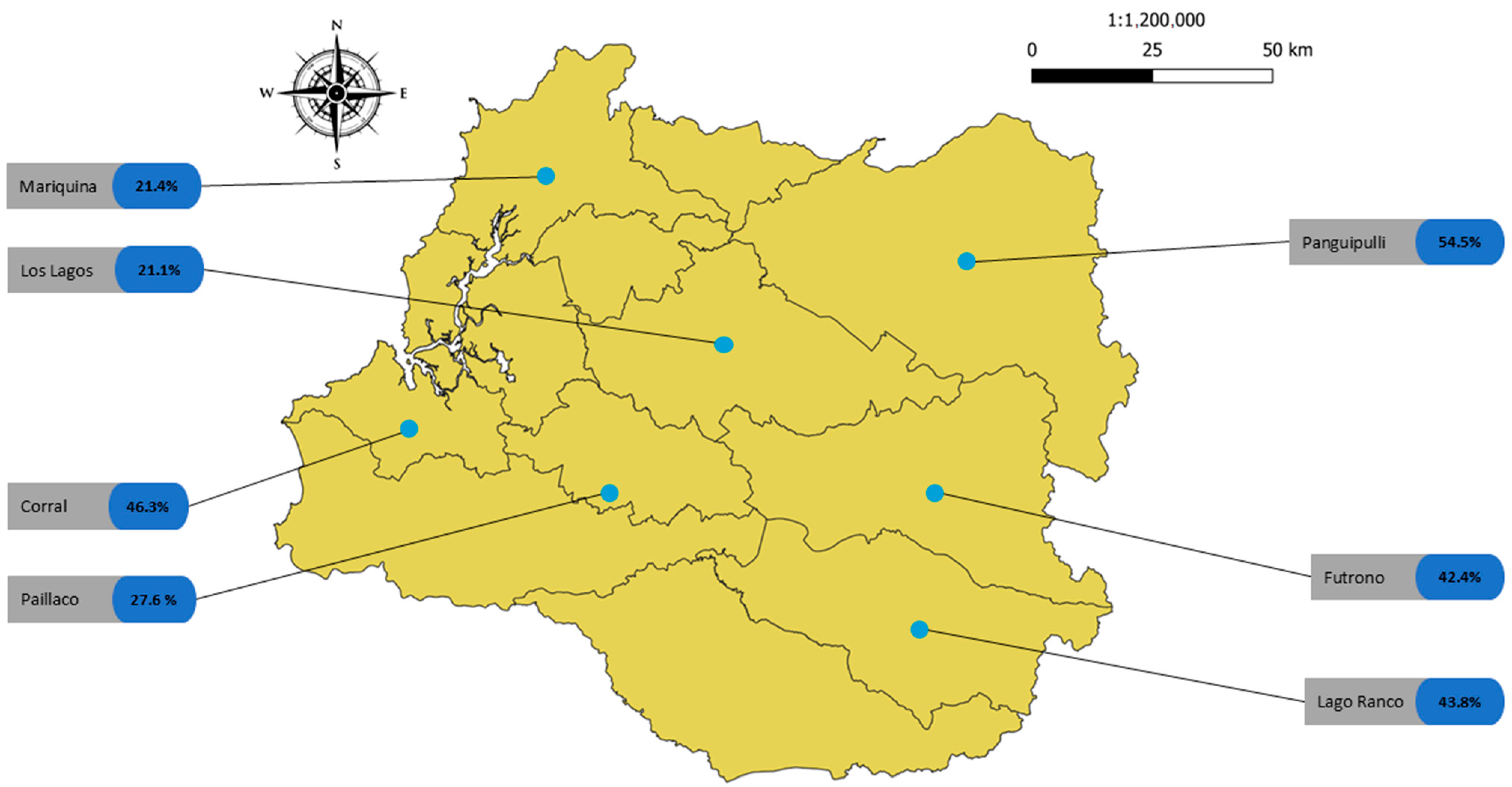


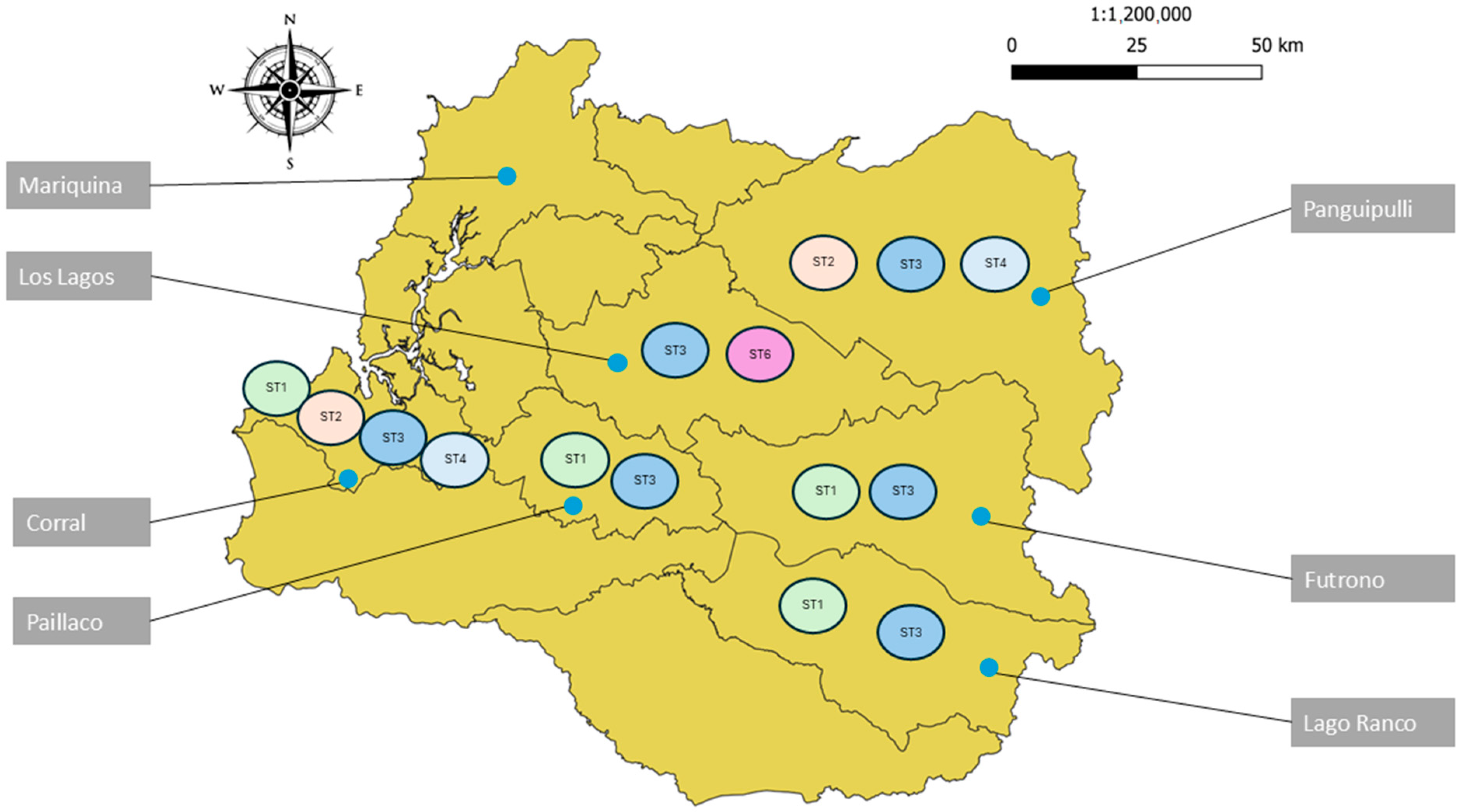
| Sampling Points in Los Rios Region, Chile | ||||||||
|---|---|---|---|---|---|---|---|---|
| Commune | Mariquina | Corral | Paillaco | Los Lagos | Lago Ranco | Futrono | Pangupulli | |
| ID of Sampling Points | Mehuin | Isla del Rey | Pichirropulli | El Salto | Pitriuco | Maihue | Panguipulli | Cayumapu |
| Missisipi | Chaihuin | Reumén | Pellinada | Riñinahue | Curriñe | Coñaripe | Huitag | |
| Chan chan | Huape | Santa Filomena | Malihue | Illahuapi | Chabranco | Choshuenco | Lago Neltume | |
| Iñipulli | San Juan | Aguas Negras | Antilhue | Calcurrupe | Arquilhue | Neltume | Puerto Fuy | |
| Las Coloradas | Santa Rosa | Riñihue | Pocura | Llifén | Liquiñe | |||
| Huiro | Itropulli | Las Huellas | Rupumeica bajo | Loncopán | Melefquen | |||
| El Llolly | Huapi | Bocatoma | ||||||
| La Luma | Pocura | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanhueza Teneo, D.; Venegas, T.; Videla, F.; Chesnais, C.B.; Loncoman, C.; Valenzuela-Nieto, G. Prevalence and Genetic Diversity of Parasites in Humans and Pet Dogs in Rural Areas of Los Ríos Region, Southern Chile. Pathogens 2025, 14, 186. https://doi.org/10.3390/pathogens14020186
Sanhueza Teneo D, Venegas T, Videla F, Chesnais CB, Loncoman C, Valenzuela-Nieto G. Prevalence and Genetic Diversity of Parasites in Humans and Pet Dogs in Rural Areas of Los Ríos Region, Southern Chile. Pathogens. 2025; 14(2):186. https://doi.org/10.3390/pathogens14020186
Chicago/Turabian StyleSanhueza Teneo, Daniel, Tamara Venegas, Francisca Videla, Cedric B. Chesnais, Carlos Loncoman, and Guillermo Valenzuela-Nieto. 2025. "Prevalence and Genetic Diversity of Parasites in Humans and Pet Dogs in Rural Areas of Los Ríos Region, Southern Chile" Pathogens 14, no. 2: 186. https://doi.org/10.3390/pathogens14020186
APA StyleSanhueza Teneo, D., Venegas, T., Videla, F., Chesnais, C. B., Loncoman, C., & Valenzuela-Nieto, G. (2025). Prevalence and Genetic Diversity of Parasites in Humans and Pet Dogs in Rural Areas of Los Ríos Region, Southern Chile. Pathogens, 14(2), 186. https://doi.org/10.3390/pathogens14020186






