Toxoplasma gondii Type I TR and ROP16 Synergistically Downregulate IL-12 to Inhibit Host Reactive Oxygen Species Production
Abstract
1. Introduction
2. Materials and Methods
2.1. T. gondii and Cell Cultures
2.2. Construction of Transgenic Parasite Strains
2.3. Western Blot Analysis
2.4. Immunofluorescence Assay
2.5. Invasion and Proliferation Assays
2.6. Plaque Assay
2.7. Virulence Assay
2.8. Determination of Malondialdehyde (MDA), Total Antioxidant Capacity (T-AOC), and Reactive Oxygen Species (ROS) in Tachyzoites
2.9. Determination of ROS Levels in Macrophages
2.10. Quantitative Reverse Transcriptase PCR
2.11. Determination of Mouse Serum IL-12 Levels
2.12. Statistical Analysis
3. Results
3.1. Construction of the TR and ROP16 Double Gene Knockout Strain Using the CRISPR/Cas9 Method
3.2. Double Deletion of the TR and ROP16 Genes in T. gondii Decreases Growth Capacity In Vitro
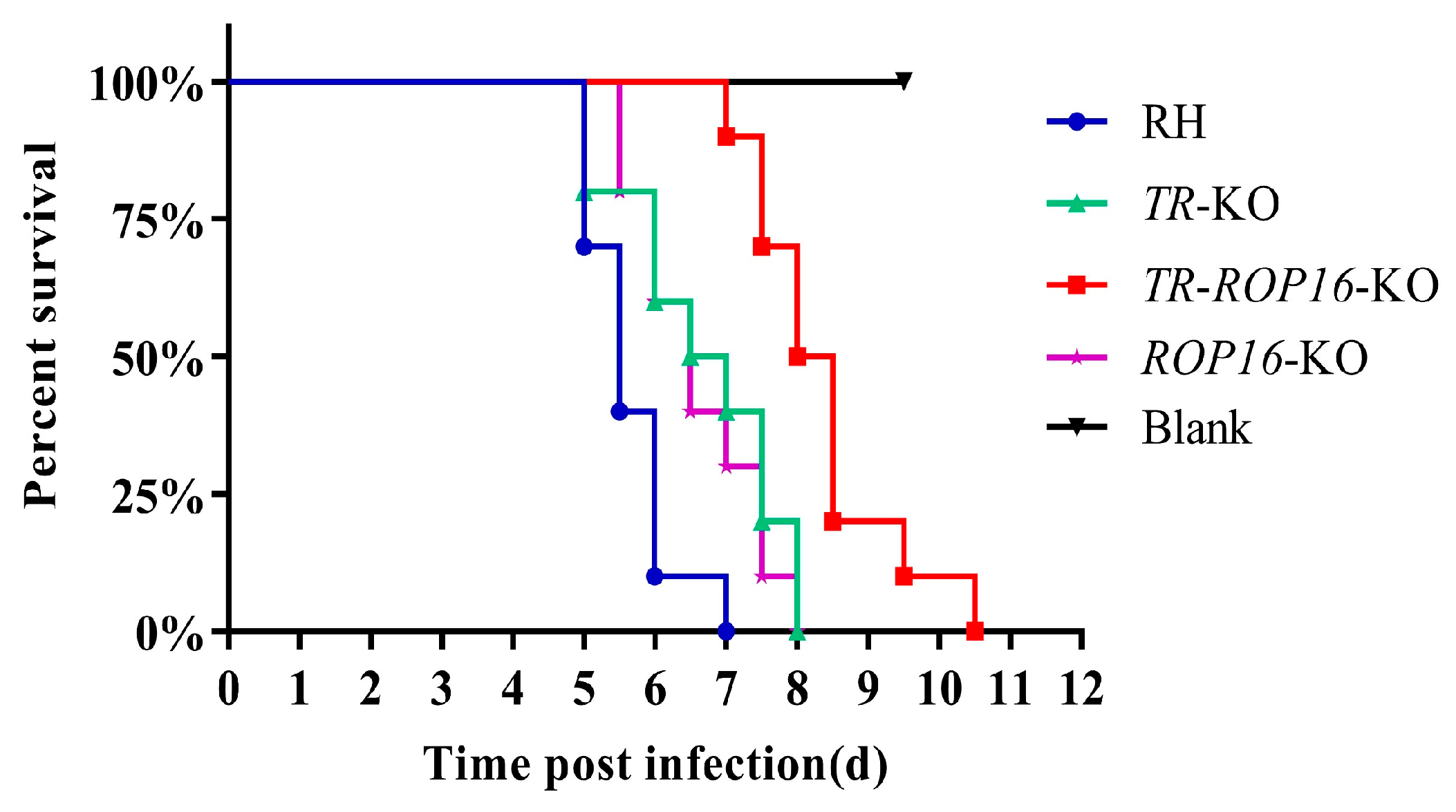
3.3. Double Deletion of the TR and ROP16 Genes Changes T. gondii Virulence in Mice
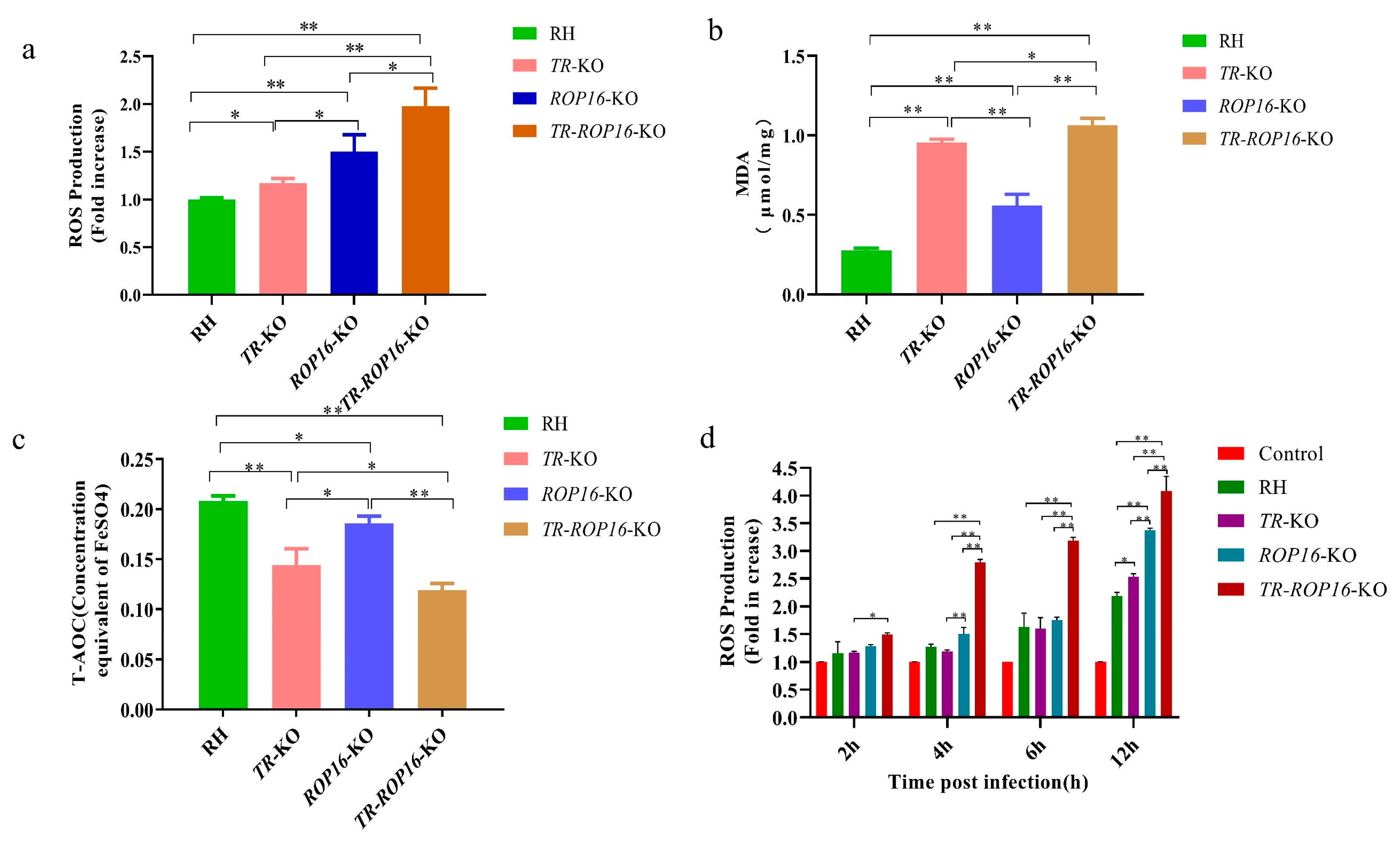
3.4. Double Deletion of the TR and ROP16 Genes in T. gondii Increases Intracellular Oxidative Stress Response
3.5. Cytokine Changes in the Host Are Affected by the Deletion of the TR and ROP16 Genes in T. gondii
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elmore, S.A.; Jones, J.L.; Conrad, P.A.; Patton, S.; Lindsay, D.S.; Dubey, J.P. Toxoplasma gondii: Epidemiology, feline clinical aspects, and prevention. Trends Parasitol. 2010, 26, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Mirza Alizadeh, A.; Jazaeri, S.; Shemshadi, B.; Hashempour-Baltork, F.; Sarlak, Z.; Pilevar, Z.; Hosseini, H. A review on inactivation methods of Toxoplasma gondii in foods. Pathog. Glob. Health 2018, 112, 306–319. [Google Scholar] [CrossRef]
- Montoya, J.G.; Liesenfeld, O. Toxoplasmosis. Lancet 2004, 363, 1965–1976. [Google Scholar] [CrossRef]
- Sanchez, S.G.; Besteiro, S. The pathogenicity and virulence of Toxoplasma gondii. Virulence 2021, 12, 3095–3114. [Google Scholar] [CrossRef]
- Tonouhewa, A.B.N.; Akpo, Y.; Sherasiya, A.; Sessou, P.; Adinci, J.M.; Aplogan, G.L.; Youssao, I.; Assogba, M.N.; Farougou, S. A serological survey of Toxoplasma gondii infection in sheep and goat from Benin, West-Africa. J. Parasit. Dis. 2019, 43, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Lyu, C.; Zhao, J.; Shen, B. Sixty Years (1957–2017) of Research on Toxoplasmosis in China—An Overview. Front. Microbiol. 2017, 8, 1825. [Google Scholar] [CrossRef]
- Lima, T.S.; Lodoen, M.B. Mechanisms of Human Innate Immune Evasion by Toxoplasma gondii. Front. Cell Infect. Microbiol. 2019, 9, 103. [Google Scholar] [CrossRef]
- Hunter, C.A.; Sibley, L.D. Modulation of innate immunity by Toxoplasma gondii virulence effectors. Nat. Rev. Microbiol. 2012, 10, 766–778. [Google Scholar] [CrossRef] [PubMed]
- Lang, C.; Gross, U.; Lüder, C.G. Subversion of innate and adaptive immune responses by Toxoplasma gondii. Parasitol. Res. 2007, 100, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Ihara, F.; Yamamoto, M. The role of IFN-γ-mediated host immune responses in monitoring and the elimination of Toxoplasma gondii infection. Int. Immunol. 2024, 36, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Dubremetz, J.F.; Lebrun, M. Virulence factors of Toxoplasma gondii. Microbes Infect. 2012, 14, 1403–1410. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Kwok, L.Y.; Schlüter, D.; Clayton, C.; Soldati, D. The antioxidant systems in Toxoplasma gondii and the role of cytosolic catalase in defence against oxidative injury. Mol. Microbiol. 2004, 51, 47–61. [Google Scholar] [CrossRef] [PubMed]
- Li, T.T.; Zhao, D.Y.; Liang, Q.L.; Elsheikha, H.M.; Wang, M.; Sun, L.X.; Zhang, Z.W.; Chen, X.Q.; Zhu, X.Q.; Wang, J.L. The antioxidant protein glutaredoxin 1 is essential for oxidative stress response and pathogenicity of Toxoplasma gondii. FASEB J. 2023, 37, e22932. [Google Scholar] [CrossRef]
- Cheng, Z.; Arscott, L.D.; Ballou, D.P.; Williams, C.H., Jr. The relationship of the redox potentials of thioredoxin and thioredoxin reductase from Drosophila melanogaster to the enzymatic mechanism: Reduced thioredoxin is the reductant of glutathione in Drosophila. Biochemistry 2007, 46, 7875–7885. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Yu, S.; Zhao, G. Recent advances in the development of thioredoxin reductase inhibitors as anticancer agents. Curr. Drug Targets 2012, 13, 1432–1444. [Google Scholar] [CrossRef] [PubMed]
- Buey, R.M.; Schmitz, R.A.; Buchanan, B.B.; Balsera, M. Crystal Structure of the Apo-Form of NADPH-Dependent Thioredoxin Reductase from a Methane-Producing Archaeon. Antioxidants 2018, 7, 166. [Google Scholar] [CrossRef]
- Xue, J.; Jiang, W.; Chen, Y.; Gong, F.; Wang, M.; Zeng, P.; Xia, C.; Wang, Q.; Huang, K. Thioredoxin reductase from Toxoplasma gondii: An essential virulence effector with antioxidant function. Faseb. J. 2017, 31, 4447–4457. [Google Scholar] [CrossRef]
- Saeij, J.P.; Boyle, J.P.; Coller, S.; Taylor, S.; Sibley, L.D.; Brooke-Powell, E.T.; Ajioka, J.W.; Boothroyd, J.C. Polymorphic secreted kinases are key virulence factors in toxoplasmosis. Science 2006, 314, 1780–1783. [Google Scholar] [CrossRef]
- Butcher, B.A.; Fox, B.A.; Rommereim, L.M.; Kim, S.G.; Maurer, K.J.; Yarovinsky, F.; Herbert, D.R.; Bzik, D.J.; Denkers, E.Y. Toxoplasma gondii rhoptry kinase ROP16 activates STAT3 and STAT6 resulting in cytokine inhibition and arginase-1-dependent growth control. PLoS Pathog. 2011, 7, e1002236. [Google Scholar] [CrossRef]
- Yamamoto, M.; Standley, D.M.; Takashima, S.; Saiga, H.; Okuyama, M.; Kayama, H.; Kubo, E.; Ito, H.; Takaura, M.; Matsuda, T.; et al. A single polymorphic amino acid on Toxoplasma gondii kinase ROP16 determines the direct and strain-specific activation of Stat3. J. Exp. Med. 2009, 206, 2747–2760. [Google Scholar] [CrossRef]
- Ong, Y.C.; Reese, M.L.; Boothroyd, J.C. Toxoplasma rhoptry protein 16 (ROP16) subverts host function by direct tyrosine phosphorylation of STAT6. J. Biol. Chem. 2010, 285, 28731–28740. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Wang, C.; Luo, Q.; Xing, T.; Shen, J.; Wang, W. Toxoplasma gondii ROP16(I) Deletion: The Exacerbated Impact on Adverse Pregnant Outcomes in Mice. Front. Microbiol. 2019, 10, 3151. [Google Scholar] [CrossRef]
- Saeij, J.P.; Coller, S.; Boyle, J.P.; Jerome, M.E.; White, M.W.; Boothroyd, J.C. Toxoplasma co-opts host gene expression by injection of a polymorphic kinase homologue. Nature 2007, 445, 324–327. [Google Scholar] [CrossRef] [PubMed]
- Rochet, E.; Argy, N.; Greigert, V.; Brunet, J.; Sabou, M.; Marcellin, L.; de-la-Torre, A.; Sauer, A.; Candolfi, E.; Pfaff, A.W. Type I ROP16 regulates retinal inflammatory responses during ocular toxoplasmosis. PLoS ONE 2019, 14, e0214310. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Cheng, W.; Yu, Q.; Xing, T.; Chen, S.; Liu, L.; Yu, L.; Du, J.; Luo, Q.; Shen, J.; et al. Toxoplasma Chinese 1 Strain of WH3Δrop16(I/III)/gra15(II) Genetic Background Contributes to Abnormal Pregnant Outcomes in Murine Model. Front. Immunol. 2018, 9, 1222. [Google Scholar] [CrossRef]
- Xia, L.; Nordman, T.; Olsson, J.M.; Damdimopoulos, A.; Björkhem-Bergman, L.; Nalvarte, I.; Eriksson, L.C.; Arnér, E.S.; Spyrou, G.; Björnstedt, M. The mammalian cytosolic selenoenzyme thioredoxin reductase reduces ubiquinone. A novel mechanism for defense against oxidative stress. J. Biol. Chem. 2003, 278, 2141–2146. [Google Scholar] [CrossRef] [PubMed]
- Rundlöf, A.K.; Arnér, E.S. Regulation of the mammalian selenoprotein thioredoxin reductase 1 in relation to cellular phenotype, growth, and signaling events. Antioxid. Redox. Signal 2004, 6, 41–52. [Google Scholar] [CrossRef]
- Cinato, M.; Andersson, L.; Miljanovic, A.; Laudette, M.; Kunduzova, O.; Borén, J.; Levin, M.C. Role of Perilipins in Oxidative Stress-Implications for Cardiovascular Disease. Antioxidants 2024, 13, 209. [Google Scholar] [CrossRef]
- Canli, E.G.; Baykose, A.; Uslu, L.H.; Canli, M. Changes in energy reserves and responses of some biomarkers in freshwater mussels exposed to metal-oxide nanoparticles. Environ. Toxicol. Pharmacol. 2023, 98, 104077. [Google Scholar] [CrossRef] [PubMed]
- Pratiwi, D.A.; Haryanto, B. Effect of particulate matter 2.5 exposure to urinary malondialdehyde levels of public transport drivers in Jakarta. Rev. Environ. Health 2020, 35, 295–300. [Google Scholar] [CrossRef] [PubMed]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, A.V.; Javadov, S.; Sommer, N. Cellular ROS and Antioxidants: Physiological and Pathological Role. Antioxidants 2024, 13, 602. [Google Scholar] [CrossRef]
- Kotsafti, A.; Scarpa, M.; Castagliuolo, I.; Scarpa, M. Reactive Oxygen Species and Antitumor Immunity—From Surveillance to Evasion. Cancers 2020, 12, 1748. [Google Scholar] [CrossRef]
- Fokam, D.; Hoskin, D. Instrumental role for reactive oxygen species in the inflammatory response. Front. Biosci. 2020, 25, 1110–1119. [Google Scholar] [CrossRef] [PubMed]
- Duwe, A.K.; Werkmeister, J.; Roder, J.C.; Lauzon, R.; Payne, U. Natural killer cell-mediated lysis involves an hydroxyl radical-dependent step. J. Immunol. 1985, 134, 2637–2644. [Google Scholar] [CrossRef] [PubMed]
- Sasai, M.; Pradipta, A.; Yamamoto, M. Host immune responses to Toxoplasma gondii. Int. Immunol. 2018, 30, 113–119. [Google Scholar] [CrossRef]
- Scanga, C.A.; Aliberti, J.; Jankovic, D.; Tilloy, F.; Bennouna, S.; Denkers, E.Y.; Medzhitov, R.; Sher, A. Cutting edge: MyD88 is required for resistance to Toxoplasma gondii infection and regulates parasite-induced IL-12 production by dendritic cells. J. Immunol. 2002, 168, 5997–6001. [Google Scholar] [CrossRef] [PubMed]
- Lüder, C.G.K. IFNs in host defence and parasite immune evasion during Toxoplasma gondii infections. Front. Immunol. 2024, 15, 1356216. [Google Scholar] [CrossRef]
- Yap, G.; Pesin, M.; Sher, A. Cutting edge: IL-12 is required for the maintenance of IFN-gamma production in T cells mediating chronic resistance to the intracellular pathogen, Toxoplasma gondii. J. Immunol. 2000, 165, 628–631. [Google Scholar] [CrossRef] [PubMed]
- Sasai, M.; Yamamoto, M. Innate, adaptive, and cell-autonomous immunity against Toxoplasma gondii infection. Exp. Mol. Med. 2019, 51, 1–10. [Google Scholar] [CrossRef]
- Hernández-de-Los-Ríos, A.; Murillo-Leon, M.; Mantilla-Muriel, L.E.; Arenas, A.F.; Vargas-Montes, M.; Cardona, N.; de-la-Torre, A.; Sepúlveda-Arias, J.C.; Gómez-Marín, J.E. Influence of Two Major Toxoplasma gondii Virulence Factors (ROP16 and ROP18) on the Immune Response of Peripheral Blood Mononuclear Cells to Human Toxoplasmosis Infection. Front. Cell Infect. Microbiol. 2019, 9, 413. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Zou, L.; Lu, J.; Holmgren, A. Selenocysteine in mammalian thioredoxin reductase and application of ebselen as a therapeutic. Free Radic Biol. Med. 2018, 127, 238–247. [Google Scholar] [CrossRef]
- Hasan, A.A.; Kalinina, E.; Tatarskiy, V.; Shtil, A. The Thioredoxin System of Mammalian Cells and Its Modulators. Biomedicines 2022, 10, 1757. [Google Scholar] [CrossRef] [PubMed]
- Heilman, J.M.; Burke, T.J.; McClain, C.J.; Watson, W.H. Transactivation of gene expression by NF-κB is dependent on thioredoxin reductase activity. Free Radic Biol. Med. 2011, 51, 1533–1542. [Google Scholar] [CrossRef][Green Version]
- Kelleher, Z.T.; Sha, Y.; Foster, M.W.; Foster, W.M.; Forrester, M.T.; Marshall, H.E. Thioredoxin-mediated denitrosylation regulates cytokine-induced nuclear factor κB (NF-κB) activation. J. Biol. Chem. 2014, 289, 3066–3072. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geng, X.; Li, R.; Du, J.; Zhang, M.; Jiang, W.; Sun, Q.; Mi, R.; Qin, S.; Wang, Q. Toxoplasma gondii Type I TR and ROP16 Synergistically Downregulate IL-12 to Inhibit Host Reactive Oxygen Species Production. Pathogens 2025, 14, 171. https://doi.org/10.3390/pathogens14020171
Geng X, Li R, Du J, Zhang M, Jiang W, Sun Q, Mi R, Qin S, Wang Q. Toxoplasma gondii Type I TR and ROP16 Synergistically Downregulate IL-12 to Inhibit Host Reactive Oxygen Species Production. Pathogens. 2025; 14(2):171. https://doi.org/10.3390/pathogens14020171
Chicago/Turabian StyleGeng, Xiaoling, Ruifang Li, Jingying Du, Manyu Zhang, Wei Jiang, Qing Sun, Rongsheng Mi, Shuang Qin, and Quan Wang. 2025. "Toxoplasma gondii Type I TR and ROP16 Synergistically Downregulate IL-12 to Inhibit Host Reactive Oxygen Species Production" Pathogens 14, no. 2: 171. https://doi.org/10.3390/pathogens14020171
APA StyleGeng, X., Li, R., Du, J., Zhang, M., Jiang, W., Sun, Q., Mi, R., Qin, S., & Wang, Q. (2025). Toxoplasma gondii Type I TR and ROP16 Synergistically Downregulate IL-12 to Inhibit Host Reactive Oxygen Species Production. Pathogens, 14(2), 171. https://doi.org/10.3390/pathogens14020171





