Changing Epidemiology of Candida spp. Causing Bloodstream Infections in a Tertiary Hospital in Northern Greece: Appearance of Candida auris
Abstract
1. Introduction
2. Materials
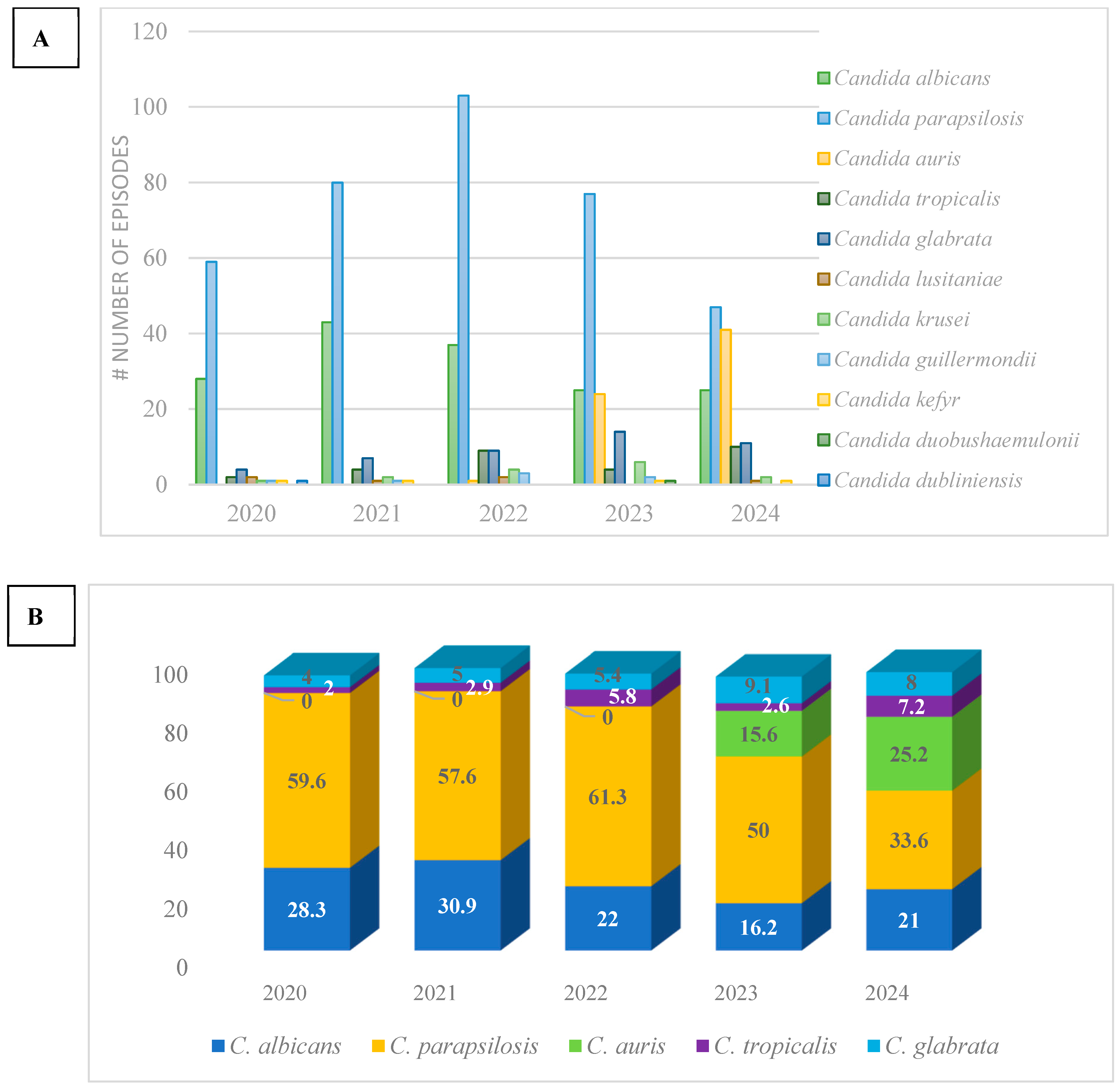
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Odoj, K.; Garlasco, J.; Pezzani, M.D.; Magnabosco, C.; Ortiz, D.; Manco, F.; Galia, L.; Foster, S.K.; Arieti, F.; Tacconelli, E. Tracking Candidemia Trends and Antifungal Resistance Patterns across Europe: An In-Depth Analysis of Surveillance Systems and Surveillance Studies. J. Fungi 2024, 10, 685. [Google Scholar] [CrossRef]
- Pappas, P.G.; Lionakis, M.S.; Arendrup, M.C.; Ostrosky-Zeichner, L.; Kullberg, B.J. Invasive Candidiasis. Nat. Rev. Dis. Prim. 2018, 4, 18026. [Google Scholar] [CrossRef] [PubMed]
- Magill, S.S.; Edwards, J.R.; Bamberg, W.; Beldavs, Z.G.; Dumyati, G.; Kainer, M.A.; Lynfield, R.; Maloney, M.; McAllister-Hollod, L.; Nadle, J.; et al. Emerging Infections Program Healthcare-Associated Infections and Antimicrobial Use Prevalence Survey Team Multistate point-prevalence survey of health care-associated infections. N. Engl. J. Med. 2014, 370, 1198–1208. [Google Scholar] [CrossRef] [PubMed]
- Meneghello, S.; Bernabè, G.; Di Pietra, G.; Di Sopra, S.; Del Vecchio, C.; Cattelan, A.M.; Castagliuolo, I.; Brun, P. Prevalence, Species Distribution and Resistance of Candidemia in Pediatric and Adult Patients in a Northeast Italy University Hospital. J. Fungi 2024, 10, 707. [Google Scholar] [CrossRef]
- Salmanton-García, J.; Cornely, O.A.; Stemler, J.; Barać, A.; Steinmann, J.; Siváková, A.; Akalin, E.H.; Arikan-Akdagli, S.; Loughlin, L.; Toscano, C.; et al. Attributable mortality of candidemia—Results from the ECMM Candida III multinational European Observational Cohort Study. J. Infect. 2024, 89, 106229. [Google Scholar] [CrossRef] [PubMed]
- Mete, B.; Zerdali, E.Y.; Aygun, G.; Saltoglu, N.; Balkan, I.I.; Karaali, R.; Kaya, S.Y.; Karaismailoglu, B.; Kaya, A.; Urkmez, S.; et al. Change in species distribution and antifungal susceptibility of candidemias in an intensive care unit of a university hospital (10-year experience). Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Lamoth, F.; Lockhart, S.R.; Berkow, E.L.; Calandra, T. Changes in the epidemiological landscape of invasive candidiasis. J. Antimicrob. Chemother. 2018, 73 (Suppl. S1), i4–i13. [Google Scholar] [CrossRef] [PubMed]
- Najafzadeh, M.J.; Shaban, T.; Zarrinfar, H.; Sedaghat, A.; Hosseinikargar, N.; Berenji, F.; Jalali, M.; Lackner, M.; James, J.E.; Ilkit, M.; et al. COVID-19 associated candidemia: From a shift in fungal epidemiology to a rise in azole drug resistance Multicenter Study. Med. Mycol. 2024, 62, myae031. [Google Scholar] [CrossRef] [PubMed]
- Lass-Flörl, C.; Kanj, S.S.; Govender, N.P.; Thompson, G.R., 3rd; Ostrosky-Zeichner, L.; Govrins, M.A. Invasive candidiasis. Nat. Rev. Dis. Primers. 2024, 10, 20. [Google Scholar] [CrossRef]
- Cristina, M.L.; Spagnolo, A.M.; Sartini, M.; Carbone, A.; Oliva, M.; Schinca, E.; Boni, S.; Pontali, E. An Overview on Candida auris in Healthcare Settings. J. Fungi 2023, 9, 913. [Google Scholar] [CrossRef]
- Berkow, E.L.; Lockhart, S.R.; Ostrosky-Zeichner, L. Antifungal Susceptibility Testing: Current Approaches. Clin. Microbiol. Rev. 2020, 33, e00069-19. [Google Scholar] [CrossRef]
- Chen, L.; Xie, Z.; Jian, J. Epidemiology and risk factors of candidemia a 8 year retrospective study from a teaching hospital in China. Infect. Drug Resist. 2024, 7, 3415–3423. [Google Scholar] [CrossRef] [PubMed]
- McCarty, T.P.; Pappas, P.G. Invasive Candidiasis. J. Infect. Dis. Clin. N. Am. 2016, 30, 103–124. [Google Scholar] [CrossRef] [PubMed]
- Kazancioglu, S.; Bodur, H.; Mumcuoglu, I.; Bastug, A.; Ozbay, B.O.; Aydos, O.; Dinc, B. Candidemia in critically ill COVID-19 patients: Risk factors and impact on mortality. Heliyon 2024, 10, e28033. [Google Scholar] [CrossRef] [PubMed]
- Aydın, S.; Mert, A.; Yılmaz, M.; Al Maslamani, M.; Rahimi, B.A.; Ayoade, F.; El-Kholy, A.; Belitova, M.; Sengel, B.E.; Jalal, S.; et al. Understanding clinical outcomes and factors influencing mortality in intensive care unit patients with COVID-19-associated candidemia. Mycoses 2024, 67, e13687. [Google Scholar] [CrossRef] [PubMed]
- Papadimitriou-Olivgeris, M.; Kolonitsiou, F.; Kefala, S.; Spiliopoulou, A.; Aretha, D.; Bartzavali, C.; Siapika, A.; Marangos, M.; Fligou, F. Increased incidence of candidemia in critically ill patients during the Coronavirus Disease 2019 (COVID-19) pandemic. Braz. J. Infect. Dis. 2022, 26, 102353. [Google Scholar] [CrossRef] [PubMed]
- Siopi, M.; Tarpatzi, A.; Kalogeropoulou, E.; Damianidou, S.; Vasilakopoulou, A.; Vourli, S.; Pournaras, S.; Meletiadis, J. Epidemiological Trends of Fungemia in Greece with a Focus on Candidemia during the Recent Financial Crisis: A 10-Year Survey in a Tertiary Care Academic Hospital and Review of Literature. Antimicrob. Agents Chemother. 2020, 64, e01516-19. [Google Scholar] [CrossRef] [PubMed]
- Charisi, K.; Galanis, I.; Zarras, C.; Totikidis, G.; Kouroupis, D.; Massa, E.; Michailidou, C.; Goumperi, S.; Kosmidou, E.; Alektoridou, C.; et al. Impact of a Polyhexanide-based Antiseptic Skin Solution on Candida auris Colonization and Invasive Fungemia. J. Hosp. Infect. 2024, 156, 61–63. [Google Scholar] [CrossRef] [PubMed]
- Meletiadis, J.; Siopi, M.; Spruijtenburg, B.; Georgiou, P.-C.; Kostoula, M.; Vourli, S.; Frantzeskaki, F.; Paramythiotou, E.; F Meis, J.F.; Tsangaris, I.; et al. Candida auris fungaemia outbreak in a tertiary care academic hospital and emergence of a pan-echinocandin resistant isolate, Greece, 2021 to 2023. Euro Surveill. 2024, 45, 2400128. [Google Scholar] [CrossRef]
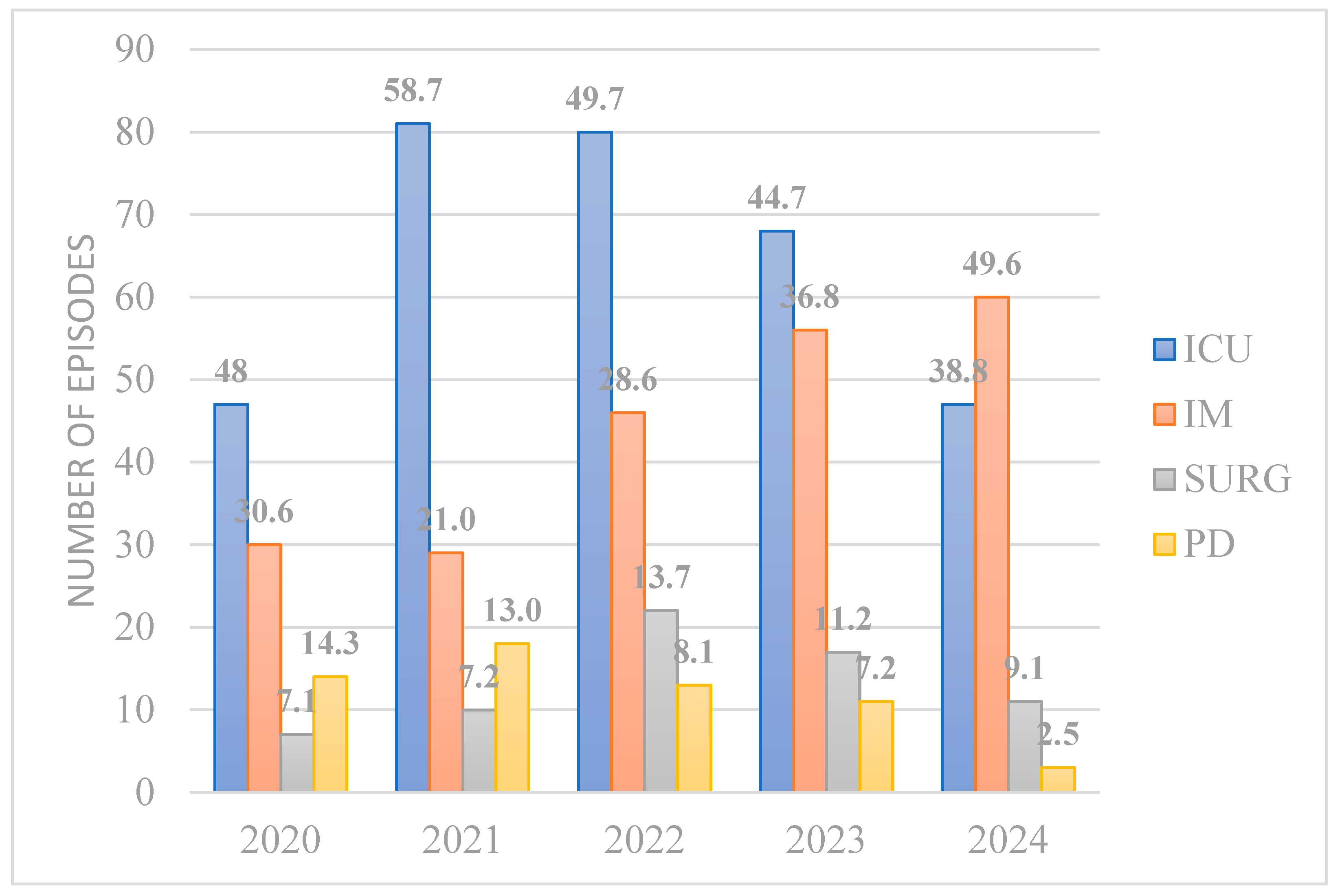
| C. albicans (79) | C. glabrata (32) | C. parapsilosis (249) | C. kefyr (2) | C. tropicalis (26) | C. krusei (11) | C. lusitaniae (4) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R (%) | Total AST | R (s%) | Total AST | R (%) | Total AST | R (%) | Total AST | R (%) | Total AST | R (%) | Total AST | R (%) | Total AST | |
| Fluconazole | 1% | 79 | 52% | 249 | 4% | 26 | 100% | 11 | ||||||
| Voriconazole | 4% | 79 | 0% | 1 | 17% | 248 | 0% | 26 | ||||||
| Caspofungin | 0% | 79 | 0% | 31 | 0% | 247 | 0% | 2 | 0% | 26 | 0% | 11 | 0% | 3 |
| Micafungin | 1% | 79 | 3% | 32 | 1% | 249 | 0% | 1 | ||||||
| Amphotericin B | 3% | 79 | 0% | 32 | 2% | 248 | 0% | 26 | 18% | 11 | ||||
| Flucytosine | 3% | 76 | 0% | 31 | 1% | 242 | 0% | 2 | 4% | 26 | 0% | 11 | 25% | 4 |
| Total | 2% | 471 | 1% | 127 | 12% | 1243 | 0% | 4 | 2% | 131 | 30% | 44 | 14% | 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pyrpasopoulou, A.; Zarras, C.; Mouloudi, E.; Vakalis, G.; Ftergioti, A.; Kouroupis, D.; Papathanasiou, A.-I.; Iosifidis, E.; Goumperi, S.; Lampada, C.; et al. Changing Epidemiology of Candida spp. Causing Bloodstream Infections in a Tertiary Hospital in Northern Greece: Appearance of Candida auris. Pathogens 2025, 14, 161. https://doi.org/10.3390/pathogens14020161
Pyrpasopoulou A, Zarras C, Mouloudi E, Vakalis G, Ftergioti A, Kouroupis D, Papathanasiou A-I, Iosifidis E, Goumperi S, Lampada C, et al. Changing Epidemiology of Candida spp. Causing Bloodstream Infections in a Tertiary Hospital in Northern Greece: Appearance of Candida auris. Pathogens. 2025; 14(2):161. https://doi.org/10.3390/pathogens14020161
Chicago/Turabian StylePyrpasopoulou, Athina, Charalampos Zarras, Eleni Mouloudi, Georgios Vakalis, Argyro Ftergioti, Dimitrios Kouroupis, Anastasia-Izampella Papathanasiou, Elias Iosifidis, Stella Goumperi, Charis Lampada, and et al. 2025. "Changing Epidemiology of Candida spp. Causing Bloodstream Infections in a Tertiary Hospital in Northern Greece: Appearance of Candida auris" Pathogens 14, no. 2: 161. https://doi.org/10.3390/pathogens14020161
APA StylePyrpasopoulou, A., Zarras, C., Mouloudi, E., Vakalis, G., Ftergioti, A., Kouroupis, D., Papathanasiou, A.-I., Iosifidis, E., Goumperi, S., Lampada, C., Terzaki, M., & Roilides, E. (2025). Changing Epidemiology of Candida spp. Causing Bloodstream Infections in a Tertiary Hospital in Northern Greece: Appearance of Candida auris. Pathogens, 14(2), 161. https://doi.org/10.3390/pathogens14020161







