Abstract
This quasi-experimental trial examined the relationship between Schistosoma haematobium infection and nutritional status, and the impact of single dose praziquantel (PZQ) therapy on undernutrition. A total of 353 children were examined, 112 of which were infected with S. haematobium and treated with PZQ. Children’s heights, weights, and mid-upper arm circumferences (MUAC) were measured at baseline and one month post-treatment. Infected children had significantly smaller mean BMI-for-age z-scores (BAZ) (−1.16 vs. 0.11, p < 0.01) and weight-for-age z-scores (WAZ) (−0.61 vs. −0.31, p = 0.03) than the uninfected ones at baseline. S. haematobium infection was associated with underweight (adjusted OR: 1.76, 95% CI: 1.63–1.90). One month after treatment, BAZ, WAZ, height for age z-scores (HAZ), and MUAC scores were comparable between treated and control children. However, there was a significant decrease in the prevalence of underweight among treated children, while no significant change was observed in the control group one month post-treatment. In conclusion, children infected with S. haematobium are likely to suffer from undernutrition; however, single dose PZQ therapy may not improve their nutritional status within one month of treatment. Future studies could have longer follow-up periods to better estimate the drug’s effect on nutrition.
1. Introduction
Urogenital schistosomiasis, caused by the helminth Schistosoma haematobium, remains a significant public health concern, particularly in sub-Saharan Africa. It is a major cause of morbidity, mortality, and disability worldwide [1], disproportionately affecting children due to their frequent contact with infected sources of water [2]. Indeed, the disease is infamous for the amount of disability it causes—undernutrition being one of the prime outcomes of interest. In addition to causing major damage to the urinary tract in 70% of infected children [3], it is well-known that the infection negatively impacts child growth by causing anemia and stunting [4] as both the helminths and their eggs consume the host’s (human’s) metabolites [5] and divert blood away from organs [3]. The World Health Organization claims that schistosomiasis-related undernutrition is “usually reversible with treatment” [4]. Moreover, some researchers have posited that treating the disease is “likely to improve child growth, appetite, physical fitness and activity levels” [6]. However, the evidence on this topic is surprisingly sparse and somewhat contradictory.
Despite the widespread use of praziquantel (PZQ) as an antischistosomal drug, the literature lacks comprehensive data on its impact on the nutritional status of infected children. A study reported thicker skin and higher hemoglobin levels among children infected with S. japonicum in the Philippines one month after PZQ treatment [7]. Another study in Tanzania documented improved hemoglobin levels and increased prevalence of wasting but lack of changes in the prevalence of stunting among children infected with S. mansoni eight months after PZQ treatment [8]. On the other hand, a study from Kenya showed improved appetite and physical fitness among children infected with S. haematobium and hookworms after treatment with praziquantel and/or metrifonate [9]. A clinical trial that involved Kenyan children discovered that, compared to a placebo, single dose praziquantel was successful at increasing weight, weight-for-age, weight-for-height, arm circumference, and skin thickness eight months post-treatment [10]. Likewise, PZQ treatment has been associated with increases in weight-for-age z-scores and BMI-for-age z-scores [11] and decreases in anemia [12] among S. mansoni-infected Ethiopian school children.
The preponderance of evidence suggests that praziquantel may have a beneficial effect on children’s nutritional status within the medium term (half a year to a year) [13]. To date, few studies have empirically and comprehensively measured the effect of PZQ therapy on the nutritional status of children infected with S. haematobium. Determining the effects of PZQ treatment on the nutritional status of S. haematobium-infected children is important given that the species primarily infects the urogenital tract and not the intestine, unlike all the other species. Thus, uncovering the effect of PZQ treatment on the nutritional status of S. haematobium-infected children would enable us to better gauge such species’ impact on children’s physical development. In addition, studies are scarce on this topic within the Ethiopian context, and none have examined the time needed to ameliorate S. haematobium-associated undernutrition. This study, therefore, provides evidence of how quickly undernutrition can be reversed among children with S. haematobium treated with PZQ. It is critical to understand how fast undernutrition can be addressed in children infected with S. haematobium as prolonged undernutrition increases the likelihood of permanent developmental deficits and reduces the chance of achieving developmental “catch-up” later in life [14,15]. Hence, it is necessary to fill this knowledge gap with real-world evidence. Therefore, to confirm past research on this topic and to replicate it on children specifically infected with S. haematobium, in Ethiopia, and within a smaller timeframe, this study assessed the nutritional status of S. haematobium-infected children and examined whether praziquantel therapy improves anthropometric nutritional parameters within a one month period of drug administration.
2. Materials and Methods
2.1. Study Design
To examine the relationship between S. haematobium infection and the nutritional status of children at baseline and one month post-treatment with PZQ, a cross-sectional study of baseline characteristics was conducted. To evaluate the effect of praziquantel therapy on children’s nutritional status, a quasi-experimental pilot study with a nonequivalent control group was performed among children residing in Afar and Gambella, Ethiopia. The research protocol was part of a larger trial conducted in the Afar, Gambella, and Benishangul-Gumuz regional states of the country to evaluate the use of pooled testing as a cost-effective strategy for diagnosing S. haematobium [16]. As such, the sample size was pre-determined.
2.2. Study Participants and Sites
In July 2023, 572 children between the ages of 5 and 15 years residing in villages located along the Middle Awash Valley of Afar Region, Ethiopia were recruited for this study; in July 2024, a further 405 children of the same age range residing in villages of the Gambella Region were recruited. Villages were selected based on prior studies [17,18] where a higher prevalence of S. haematobium infections was reported, in collaboration with local officials and guides who took accessibility into consideration. Children were only allowed to participate if they resided in a village that had not received mass praziquantel administration in the last ninety days and informed consent was obtained from them and their parents.
2.3. Data Collection
Upon admission, each child’s height (in centimeters), weight (in kilograms), mid-upper arm circumference (MUAC, in centimeters), sex, age, and village were recorded. The child was then directed to urinate at least 100 mL of urine into a plastic jar. To expedite treatment in light of the sheer volume of children being analyzed, those whose urine samples tested positive for hematuria were provided with praziquantel (Bermoxel by Medochemie, Limassol, Cyprus) at a single dose of 40 mg/kg. This was completed given that research has confirmed that the rapid testing of urine for hematuria has been found to be an accurate proxy for detecting S. haematobium infection [19]. The instructions from the manufacturer were followed to check for the presence of hematuria using Combur 10 Test reagent strips (Roche Diagnostics, Rotkreuz, Switzerland). In brief, the test strip was immersed in the urine sample for about two seconds, then removed and left on the bench for approximately one minute. The blood area on the test strip was then compared to the corresponding reference color fields. The strip test results were interpreted and recorded as zero (negative), ± (trace), + (weak), ++ (moderate), and +++ (strong), indicating the presence of erythrocytes per μL of urine.
All samples underwent confirmatory testing for S. haematobium eggs using standard urine filtration microscopy in accordance with the parent study [16], by which a 10 mL aliquot of each urine sample was filtered through a polycarbonate membrane and then analyzed for S. haematobium eggs under the microscope in the lab, the results of which were used to group children into treated (i.e., urine samples tested positive for S. haematobium egg and treated with PZQ) and control (i.e., urine samples tested negative for S. haematobium egg and untreated with PZQ) groups. Efforts were made to ensure that children who tested negative for hematuria but who tested positive using urine filtration microscopy were promptly treated with PZQ. Approximately one month after PZQ treatment of S. haematobium-infected children, the research team revisited the villages, recalled all the children, and had their height, weight, and mid-upper arm circumference re-measured. Of the 245 children who were confirmed positive for S. haematobium eggs at baseline, 52 were excluded from the study because they tested negative for hematuria in the field and were therefore not treated until later. Likewise, of the 721 children who were confirmed negative for S. haematobium eggs, 91 were excluded because they tested positive for hematuria in the field and were therefore given praziquantel. See Figure 1 for a description of how the sample sizes for treatment and control groups were reached.
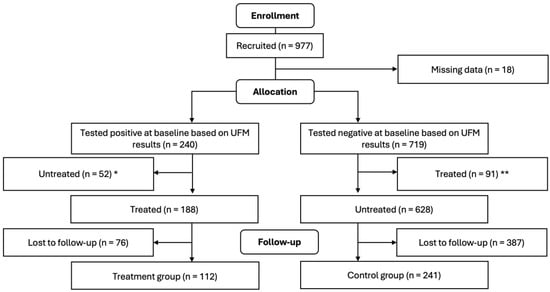
Figure 1.
Flow diagram of study participants (treatment was single dose PZQ 40 mg/kg). * These 52 children were untreated and excluded from this study because they tested negative for hematuria in the field using urine reagent strips but were eventually found positive in the lab using UFM. ** These 91 children were treated and excluded from this study because they tested positive for hematuria in the field using urine reagent strips but were eventually found negative in the lab using UFM.
2.4. Analysis
Of the 977 recruited children, 353 were successfully followed up for re-measurement. The results of this study were analyzed entirely on an intent-to-treat basis.
2.4.1. Determining Nutritional Status
The participants’ heights, weights, and ages were inputted into the World Health Organization’s AnthroPlus software (Version 1.0.4, Geneva, Switzerland) to categorize the children’s underweight, wasting, and stunting status [15]. Children whose BMI-for-age z-values (BAZ) were −2 or lower were categorized as “underweight;” those whose weight-for-age z-values (WAZ) were −2 or lower were categorized as “wasted;” and those whose height-for-age z-values (HAZ) were −2 or lower were categorized as “stunted”.
2.4.2. Association of S. haematobium Infection with Undernutrition at Baseline
Three generalized estimating equation models (GEE) were employed to examine the relationship between S. haematobium infection and undernutrition. In these three GEE models, the outcome variables were underweight (yes/no), stunting (yes/no), and wasting (yes/no), and the independent variable was S. haematobium infection status (yes/no). Furthermore, four linear regression models were used to estimate the changes in the mean MUAC, BAZ, WAZ, and HAZ values at baseline as S. haematobium infection status (yes/no) changes. In all seven models, the region (Afar vs. Gambella) where the children live was included/controlled as the cluster variable [20]. Age and sex were not included in the models, given that the nutritional parameters already took them into account. For all models, 95% confidence intervals were reported.
2.4.3. Effect of Praziquantel Treatment on Nutritional Status
Multiple statistical tests were employed to measure the effect of praziquantel treatment on nutritional status. First, paired samples t-tests were performed to compare the changes in the means of MUAC, BAZ, WAZ, and HAZ scores pre- and post-treatment between children who were infected with S. haematobium and treated with praziquantel and those who were uninfected with the parasite at baseline. Then generalized linear mixed models (GLMM) were employed to compare the individual level changes in the means of MUAC, BAZ, WAZ, and HAZ scores pre- and post-treatment between children who were infected with S. haematobium and treated with praziquantel and those who were uninfected with the parasite at baseline after controlling for the villages where they live. The GLMM model for MUAC also adjusted for age and sex given that such variables are not taken into account for MUAC, unlike BAZ, WAZ, and HAZ. In all four GLMM models, ID was used as the random variable.
Similarly, McNemar’s chi-square test was performed to compare the changes in the prevalence of underweight, wasting, and stunting before and after treatment between children infected and uninfected with S. haematobium. Then, GEE models were executed to estimate the population-level change in the prevalence of underweight, wasting, and stunting before and after treatment between children infected and uninfected with S. haematobium. Village was treated as a cluster variable. Statistical significance was set at an alpha level of 0.05. All statistical analyses were executed using SAS version 9.4 (SAS Institute, Cary, NC, USA).
2.5. Ethical Approval
Ethical approval was obtained from the Institutional Review Boards of the University of Nebraska Medical Center (IRB# 908-19-EP) and Aklilu Lemma Institute of Pathobiology, Addis Ababa University (Ref no. ALIPB-IRB/10/2012/20). This study included only children who agreed to participate and for whom parental written consent was obtained.
3. Results
3.1. Baseline Demographic and Nutritional Characteristics of Study Participants
Of the 353 children included in the study, 32% were S. haematobium positive and treated with praziquantel (Table 1). The participants were almost evenly divided between females and males. Females and males were also evenly divided between treatment and control groups. A majority of the study participants (79%) were young (aged from five to ten years). Out of the 112 children in the treatment group, 35 (31%) were aged from 11 to 15 years, whilst 38 (16%) out of the 241 children in the control group were in this age group. Of all the children included in the study, 72% of the ones from Gambella tested positive for S. haematobium infection compared to 18% of those from Afar (p < 0.01).

Table 1.
Baseline demographic and nutritional characteristics of the study participants.
3.2. Relationship Between S. haematobium Infection and Undernutrition
At baseline, children infected with S. haematobium had a lower BAZ (−1.16 vs. 0.11, p < 0.01), and WAZ (−0.61 vs. −0.31, p = 0.03) score compared to uninfected children (Table 1). The prevalence of underweight (25% vs. 4%, p < 0.01) and wasting (13% vs. 3%, p < 0.01) was also greater among the infected than the uninfected children at baseline. However, the infected children had greater mean MUAC scores (17.18 vs. 16.55, p < 0.01) and HAZ scores (−0.35 vs. −0.87, p < 0.01) than the uninfected ones. There was no statistically significant difference in the prevalence of stunting between infected and uninfected children (p = 0.07).
After controlling for region, children who were infected with S. haematobium had 76% greater odds of being underweight compared to uninfected children (aOR: 1.76, 95% CI: 1.63–1.90) (Table 2). Infection status did not appear to have a significant effect on any of the other nutritional parameters at an alpha level of 0.05 in the adjusted model. Nonetheless, a child living in Afar was less likely to be underweight (aOR: 0.03, 95% CI: 0.03–0.03). Likewise, a child living in Afar was also less likely to be wasted (aOR: 0.17, 95% CI: 0.07–0.38).

Table 2.
Predictors of baseline nutritional status among children residing in Afar and Gambella, July 2023 & July 2024.
3.3. Impact of Praziquantel Therapy on Nutritional Status
Crude analysis showed an increase in mean WAZ scores among children infected with S. haematobium after being treated with praziquantel (+14.8%, p = 0.03) (Table 3). On the other hand, children who were not treated experienced a decrease in mean BAZ scores (−181.8%, p < 0.01) but an increased mean HAZ scores (+11.5%, p = 0.04). Though the difference was not significant, the prevalence of wasting decreased among treated children one month after PZQ therapy (−40%, p = 0.10) but increased among the controls (+42.9%, p = 0.08). The decrease in the prevalence of underweight among children treated with praziquantel compared to the control children was marginally significant (p = 0.05) after clustering for village. None of the other changes in the nutritional parameters showed significant change one month after praziquantel treatment in the adjusted/clustered models.

Table 3.
Nutritional status among children before and after praziquantel treatment.
Children who received praziquantel had 47% lower odds of being underweight one month following treatment compared to the non-treated (OR = 0.53, 95% CI: 0.28–1.00) (Table 4). Likewise, the treated children had 34% lower odds of being stunted one month following treatment compared to the controls (OR = 0.66, 95% CI: 0.35–1.24), although this was not statistically significant (p = 0.20). Paradoxically, treated children appeared to have greater odds of being wasted one month after treatment compared to the control children (OR = 5.89, 95% CIL 0.28–124.81), however, the difference was not statistically significant (p = 0.26) and the confidence interval was too wide to draw any conclusion from this estimate.

Table 4.
The odds ratios for dichotomous nutritional parameters in treated versus non-treated children residing in Afar and Gambella one month following praziquantel therapy, July 2023 & July 2024.
4. Discussion
This study evaluated the relationship between S. haematobium infection and undernutrition in children and the changes in nutritional status following PZQ treatment. It demonstrated that children infected with S. haematobium have significantly lower BAZ and WAZ scores than uninfected children at baseline. After adjusting for region, S. haematobium infection was also significantly associated with increased odds of being underweight. PZQ therapy had a marginally statistically significant effect on reducing the prevalence of underweight. However, the changes in the mean MUAC, BAZ, WAZ, HAZ, and the prevalences of wasting and stunting were non-significant one month after PZQ therapy.
This study confirmed the findings from past research that demonstrated an association between S. haematobium infection and undernutrition [4,21]. At baseline, infected children had lower mean BAZ and WAZ scores than uninfected children. Moreover, infected children had a greater prevalence of wasting and underweight than uninfected children, and infection is a statistically significant predictor of being underweight. These findings lend credence to the notion that undernutrition may be a major symptom of S. haematobium infection, whether it be caused by the parasite consuming the host’s metabolites [5], through the inducement of abdominal pain and diarrhea [4], or the alteration of the child’s metabolism [21] (or some combination thereof).
Although the difference was not statistically significant, the treated children benefitted from a 14.8% increase in their mean weight-for-age z-scores, whilst the control children suffered a decline of 22.6%. Moreover, the treated children experienced a 40% decrease in wasting prevalence whilst control children saw a 42.9% increase. These results align with a meta-analysis that found schistosomiasis to be associated with wasting [22]. Yet, our cross-sectional analysis found no statistically significant relationship between S. haematobium infection and wasting, which may have been due to the relatively small sample size. Future research should seek to clarify the interplay between schistosomiasis, praziquantel therapy, and wasting status.
In terms of public health, this study suggests that single-dose praziquantel monotherapy is unlikely to significantly reverse S. haematobium-associated undernutrition in the short term among infected children in an endemic setting. Whilst these results may not align with our hypothesis, it should be cautioned that they only present the effect of praziquantel treatment one month after treatment. One month of follow-up time is likely insufficient to detect any significant change in the children’s physical measurements and falls well short of the follow-up time that was used in previous trials on the use of praziquantel to improve nutritional status [7,23]. This underscores the need to conduct future studies with longer follow-up periods and may be with repeated PZQ treatment. After all, the effect that nutritional intake has on the body develops over the course of longer time frames [24].
The main advantage of this study was the presence of a control group to evaluate the changes in the nutrition status due to PZQ treatment. Nonetheless, because of the context of this research setting as well as resource limitations, we were unable to collect richer data that would have enabled us to control for other biasing factors, including a history of past infection, co-infection with other parasites and soil-transmitted helminths, exposure to bodies of water during the follow-up period which may cause rapid reinfection, and other social determinants of undernutrition, such as food insecurity, availability and access to health foods, and financial insecurity. Likewise, we were unable to measure more facets of nutritional status, including those that can only be measured using clinical/laboratory testing, including blood iron levels, blood glucose levels, proteinuria, and skin thickness, among others, as were done in other studies [7,23]. Given that the praziquantel was provided to parents with instructions for administration to their infected children and that during the follow-up survey, the researchers were unable to ascertain drug consumption due to logistical constraints, only an intent-to-treat analysis was performed. As such, the nondifferential misclassification that may have resulted would have likely biased the results of this study toward the null.
Moreover, it should be noted that this study experienced a large loss to follow-up as 42% of the experimental group and 62% of the control group failed to show up in the follow-up survey. As the participants of this study consisted primarily of nomadic Afar and displaced persons living in the war-torn region of Gambella, successfully following up with the children was challenging. Nonetheless, there was a major difference in follow-up rates between the two groups, which poses a serious problem for this study’s internal validity [25,26]. Prior studies have identified various factors that are strongly associated with loss of follow-up among Ethiopian children, including living in rural areas—which describes this study population well—and poor drug adherence [27]. A comparison of those children who were and were not lost to follow-up is summarized in Supplementary Table S1.
In addition, it must be noted that different personnel performed pre- and post-treatment measurements. This might have introduced bias in measurements at the two time points due to differing techniques and may partially account for why some post-treatment measurements were implausibly different from the pre-treatment ones (in particular, the MUAC scores for the control group). It should be noted that since an analog scale was used, the children’s weights were read in increments of 1 kg. This study was, therefore, likely insensitive to minute changes in the children’s weights. Furthermore, because of the cross-sectional nature of this baseline survey, it cannot be definitively proven that the undernutrition observed was caused by S. haematobium infection. Finally, this study’s sample size was 353, which, despite being larger than that of a previous trial [7], is still relatively small and lacks statistical power. Preliminary calculations estimated that the ideal sample size would be 684. However, because this study was a spinoff of a larger research venture, our recruitment fell well short of that estimate. Future studies should be performed with a greater number of participants.
Prior research has uncovered those other variables, like village, age, and sex, are significantly associated with undernutrition [28,29]. Echoing those findings, this study has uncovered that region is significantly associated with underweight, stunting, and wasting status, as well as MUAC and BAZ. It could thus be posited that the environment in which a child grows up may have a stronger effect on his or her nutritional status than the consumption of an anthelminthic. After all, previous research has found that administering antimicrobials to African children does not improve growth [30,31]. Public health efforts that aim to improve childhood nutrition in Africa may be put to better use by focusing on interventions that seek to affect how social and environmental dynamics impact a child’s nutrition rather than employing pharmaceutical interventions.
5. Conclusions
Urogenital schistosomiasis may cause underweight and wasting in school-aged children. However, treating the infection with a single dose of praziquantel does not appear to significantly improve anthropometric nutritional parameters within one month of treatment. Future studies should consider monitoring children over longer periods, incorporating repeated praziquantel treatments to better understand the potential for reversing S. haematobium-related undernutrition. Controlling for other social and environmental factors influencing nutrition will also be essential during such studies.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pathogens14020123/s1, Table S1: Baseline demographic and nutritional characteristics of the viable study participants who were lost to follow-up.
Author Contributions
Conceptualization, L.F. and A.D.; data curation, L.F., Y.N., A.A. and A.D.; formal analysis, L.F.; funding acquisition, B.E., D.M.B.-M., A.A. and A.D.; investigation, L.F., B.E., A.A. and A.D.; methodology, L.F., B.E., D.M.B.-M. and A.D.; project administration, A.D.; resources, B.E., A.A. and A.D.; software, L.F. and A.D.; supervision, B.E., A.A., H.D.D., J.L. and A.D.; validation, D.M.B.-M., H.D.D., J.L. and A.D.; visualization, L.F.; writing—original draft, L.F.; writing—review and editing, L.F., B.E., D.M.B.-M., A.A., H.D.D., J.L., Y.N. and A.D. All authors have read and agreed to the published version of the manuscript.
Funding
The research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number R21AI164376. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Boards of the University of Nebraska Medical Center (IRB # 908-19-EP) and the Aklilu Lemma Institute of Pathobiology (Ref. No. ALIPB-IRB/10/2012/20).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy/ethical issues.
Acknowledgments
Foremost, the authors would like to thank the study participants of this project, the children of Afar and Gambella, Ethiopia, and their parents, for their cooperation and participation. Without them, this research would not be possible. Much gratitude is owed to the laboratory staff of the Aklilu Lemma Institute of Pathobiology for their hard work and dedication in processing and analyzing the urine samples as per the study protocol. Finally, the authors would like to acknowledge the help of the local guides and the ancillary staff of Addis Ababa University in enabling and facilitating the data collection.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Siddiqui, A.J.; Bhardwaj, J.; Saxena, J.; Jahan, S.; Snoussi, M.; Bardakci, F.; Badraoui, R.; Adnan, M. A Critical Review on Human Malaria and Schistosomiasis Vaccines: Current State, Recent Advancements, and Developments. Vaccines 2023, 11, 792. [Google Scholar] [CrossRef] [PubMed]
- Bustinduy, A.L.; King, C.H. 52—Schistosomiasis. In Manson’s Tropical Infectious Diseases, 23rd ed.; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- WHO Expert Committee on the Control of Schistosomiasis. Public health impact of schistosomiasis: Disease and mortality. Bull. World Health Organ. 1993, 71, 657–662. [Google Scholar]
- WHO, “Schistosomiasis”. World Health Organization. Available online: https://www.who.int/news-room/fact-sheets/detail/schistosomiasis (accessed on 8 January 2022).
- Warren, K.S. Schistosomiasis: Host-pathogen biology. Rev. Infect. Dis. 1982, 4, 771–775. [Google Scholar] [CrossRef]
- Stephenson, L. The impact of schistosomiasis on human nutrition. Parasitology 1993, 107 (Suppl. S1), S107–S123. [Google Scholar] [CrossRef] [PubMed]
- McGarvey, S.; Aligui, G.; Graham, K.; Peters, P.; Olds, G.; Olveda, R. Schistosomiasis japonica and childhood nutritional status in northeastern Leyte, the Philippines: A randomized trial of praziquantel versus placebo. Am. J. Trop. Med. Hyg. 1996, 54, 498–502. [Google Scholar] [CrossRef]
- Munisi, D.Z.; Buza, J.; Mpolya, E.A.; Angelo, T.; Kinung’hi, S.M. The Efficacy of Single-Dose versus Double-Dose Praziquantel Treatments on Schistosoma mansoni Infections: Its Implication on Undernutrition and Anaemia among Primary Schoolchildren in Two On-Shore Communities, Northwestern Tanzania. BioMed Res. Int. 2017, 2017, 7035025. [Google Scholar] [CrossRef] [PubMed]
- Latham, M.C.; Stephenson, L.S.; Kurz, K.M.; Kinoti, S.N. Metrifonate or praziquantel treatment improves physical fitness and appetite of Kenyan schoolboys with Schistosoma haematobium and hookworm infections. Am. J. Trop. Med. Hyg. 1990, 43, 170–179. [Google Scholar] [CrossRef]
- Stephenson, L.S.; Latham, M.C.; Kurz, K.M.; Kinoti, S.N. Single dose metrifonate or praziquantel treatment in Kenyan children. II. Effects on growth in relation to Schistosoma haematobium and hookworm egg counts. Am. J. Trop. Med. Hyg. 1989, 41, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Degarege, A.; Erko, B. Association between intestinal helminth infections and underweight among school children in Tikur Wuha Elementary School, Northwestern Ethiopia. J. Infect. Public Health 2013, 6, 125–133. [Google Scholar] [CrossRef]
- Yimam, Y.; Degarege, A.; Erko, B. Effect of anthelminthic treatment on helminth infection and related anaemia among school-age children in northwestern Ethiopia. BMC Infect. Dis. 2016, 16, 613. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, H.M.; Acosta, L.P.; McGarvey, S.T.; Jarilla, B.; Jiz, M.; Pablo, A.; Su, L.; Manalo, D.L.; Olveda, R.M.; Kurtis, J.D.; et al. Nutritional status improves after treatment of schistosoma japonicum-infected children and adolescents. J. Nutr. 2006, 136, 138–188. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Martorell, R.; Khan, L.K.; Schroeder, D.G. Reversibility of stunting: Epidemiological findings in children from developing countries. Eur. J. Clin. Nutr. 1994, 48, S45–S57. [Google Scholar]
- Walker, S.P.; Grantham-McGregor, S.M.; Himes, J.H.; Powell, C.A.; Chang, S.M. Early Childhood Supplementation Does Not Benefit the Long-Term Growth of Stunted Children in Jamaica. Community Int. Nutr. 1996, 126, 3017–3024. [Google Scholar] [CrossRef] [PubMed]
- Fok, L.; Brett-Major, D.M.; Erko, B.; Linville, J.; Dai, H.D.; Negash, Y.; Animut, A.; Degarege, A. Efficacy of Praziquantel in Treating Schistosoma haematobium Infection Among Ethiopian Children. Biomedicines 2024, 12, 2463. [Google Scholar] [CrossRef]
- Chala, B.; Torben, W. An Epidemiological Trend of Urogenital Schistosomiasis in Ethiopia. Front. Public Health 2018, 6, 60. [Google Scholar] [CrossRef]
- Degarege, A.; Mekonnen, Z.; Levecke, B.; Legesse, M.; Negash, Y.; Vercruysse, J.; Erko, B. Prevalence of Schistosoma haematobium Infection among School-Age Children in Afar Area, Northeastern Ethiopia. PLoS ONE 2015, 10, 8. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Degarege, A.; Animut, A.; Negash, Y.; Erko, B. Performance of Urine Reagent Strips in Detecting the Presence and Estimating the Prevalence and Intensity of Schistosoma haematobium Infection. Microorganisms 2022, 10, 2062. [Google Scholar] [CrossRef]
- Pagani, L.; Kivisild, T.; Tarekegn, A.; Ekong, R.; Plaster, C.; Gallego Romero, I.; Ayub, Q.; Mehdi, S.Q.; Thomas, M.G.; Luiselli, D.; et al. Ethiopian Genetic Diversity Reveals Linguistic Stratification and Complex Influences on the Ethiopian Gene Pool. Am. J. Hum. Genet. 2012, 91, 83–96. [Google Scholar] [CrossRef]
- Osakunor, D.N.; Mduluza, T.; Osei-Hyiaman, D.; Burgess, K.; Woolhouse, M.E.; Mutapi, F. Schistosoma haematobium infection is associated with alterations in energy and purine-related metabolism in preschool-aged children. PLoS Negl. Trop. Dis. 2020, 14, e0008866. [Google Scholar] [CrossRef]
- Raj, E.; Calvo-Urbano, B.; Heffernan, C.; Halder, J.; Webster, J.P. Systematic review to evaluate a potential association between helminth infection and physical stunting in children. Parasites Vectors 2022, 15, 135. [Google Scholar] [CrossRef]
- Webb, E.L.; Edielu, A.; Wu, H.W.; Kabatereine, N.B.; Tukahebwa, E.M.; Mubangizi, A.; Adriko, M.; Elliott, A.M.; Hope, W.W.; Mawa, P.A.; et al. The praziquantel in preschoolers (PIP) trial: Study protocol for a phase II PK/PD-driven randomised controlled trial of praziquantel in children under 4 years of age. Trials 2021, 22, 601. [Google Scholar] [CrossRef] [PubMed]
- British Nutrition Foundation; Buttriss, J.L. Nutrition and Development: Short- and Long-Term Consequences for Health; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2013. [Google Scholar]
- Dettori, J.R. Loss to follow-up. Evid. Based Spine Care J. 2011, 2, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Howe, C.J.; Cole, S.R.; Lau, B.; Napravnik, S.; Eron, J.J., Jr. Selection bias due to loss to follow up in cohort studies. Epidemiology 2016, 27, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Birhanu, M.Y.; Bekele, G.M.; Yirdaw, G.; Demissie, B.S.; Getahun, G.K.; Jemberie, S.S. Incidence and predictors of loss to follow-up among Ethiopian children on antiretroviral therapy: A systematic review and meta-analysis. BMC Public Health 2024, 24, 169. [Google Scholar] [CrossRef]
- Obasohan, P.E.; Walters, S.J.; Jacques, R.; Khatab, K. Risk Factors Associated with Malnutrition among Children Under-Five Years in Sub-Saharan African Countries: A Scoping Review. Int. J. Environ. Res. Public Health 2020, 17, 8782. [Google Scholar] [CrossRef] [PubMed]
- Beiersmann, C.; Bermejo Lorenzo, J.; Bountogo, M.; Tiendrébeogo, J.; Gabrysch, S.; Yé, M.; Jahn, A.; Müller, O. Malnutrition determinants in young children from Burkina Faso. J. Trop. Pediatr. 2013, 59, 372–379. [Google Scholar] [CrossRef]
- Alcoba, G.; Kerac, M.; Breysse, S.; Salpeteur, C.; Galetto-Lacour, A.; Briend, A.; Gervaix, A. Do children with uncomplicated severe acute malnutrition need antibiotics? A systematic review and meta-analysis. PLoS ONE 2013, 8, e53184. [Google Scholar] [CrossRef]
- Sié, A.; Ouattara, M.; Bountogo, M.; Dah, C.; Ouedraogo, T.; Boudo, V.; Lebas, E.; Hu, H.; Arnold, B.F.; O’Brien, K.S.; et al. Single-dose azithromycin for infant growth in Burkina Faso: Prespecified secondary anthropometric outcomes from a randomized controlled trial. PLoS Med. 2024, 21, e1004345. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).