Antimicrobial Resistance, Virulence Gene Profiling, and Spa Typing of Staphylococcus aureus Isolated from Retail Chicken Meat in Alabama, USA
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples Collection
2.2. Staphylococcus aureus Isolation and Identification
2.3. Bacterial Strains and Oligonucleotide Primers
2.4. Genomic and Plasmid DNA Extraction
2.5. Antibiotic Susceptibility Assay
2.6. PCR Assay for Enterotoxins and Antibiotic Resistance Genes
2.7. DNA Preparation and PCR Amplification of Spa Gene
2.8. Sequencing and Analysis of the Spa Type t008 Isolate’s Plasmid
3. Results
3.1. Frequency of S. aureus Isolation in Chicken Meat Samples
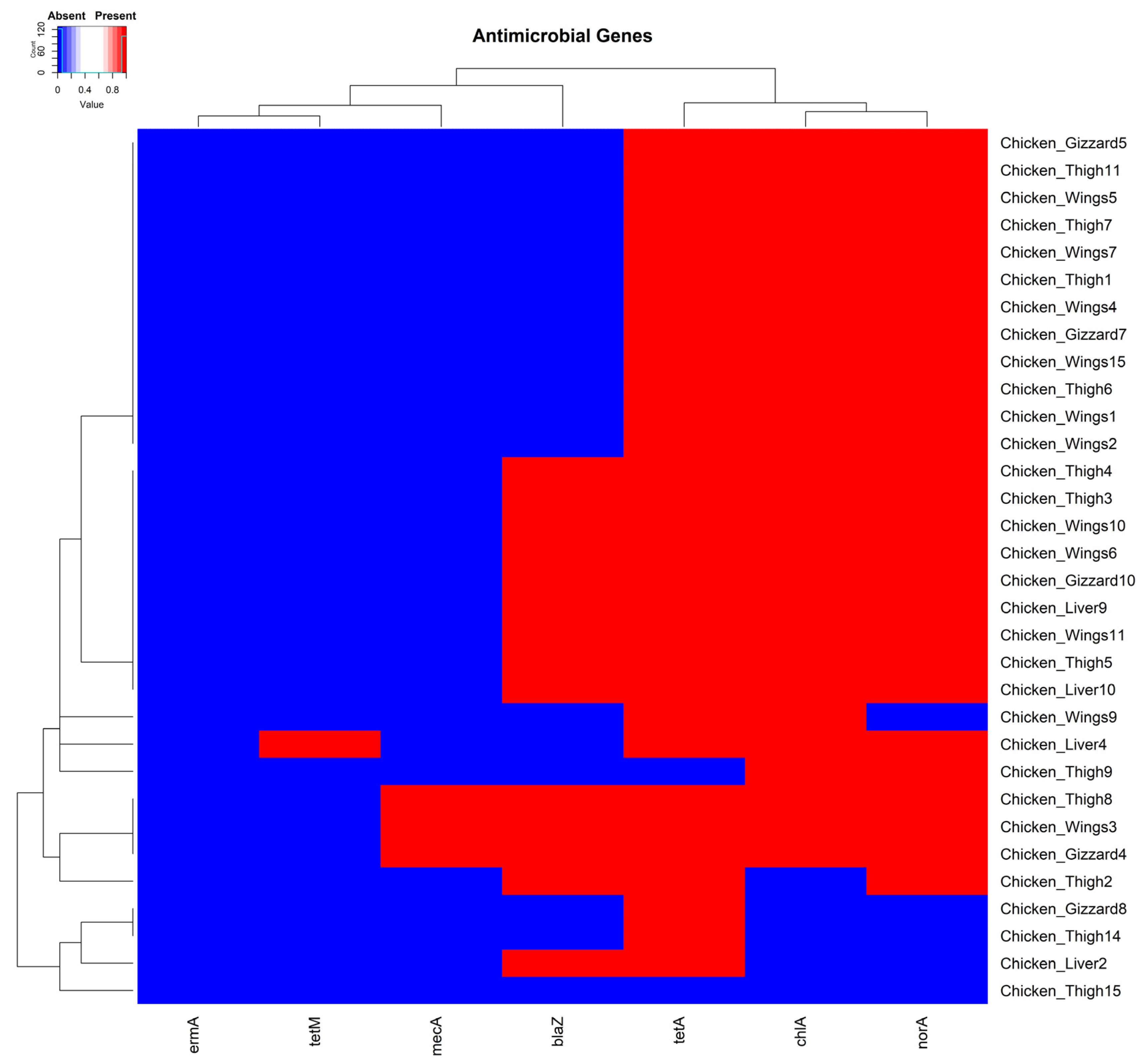
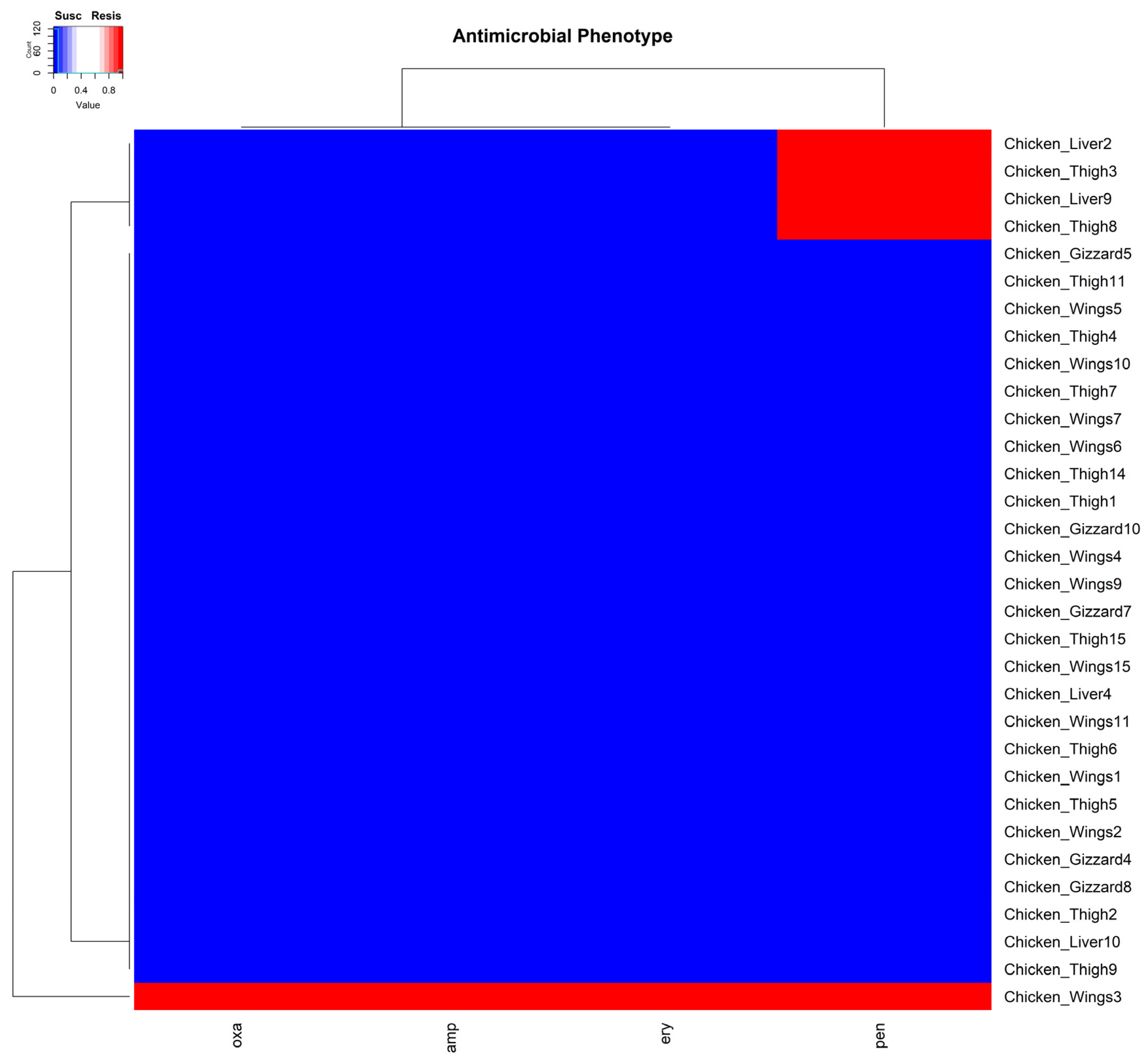
3.2. Minimum Inhibitory Concentration Assay and Antimicrobial Resistance Genes
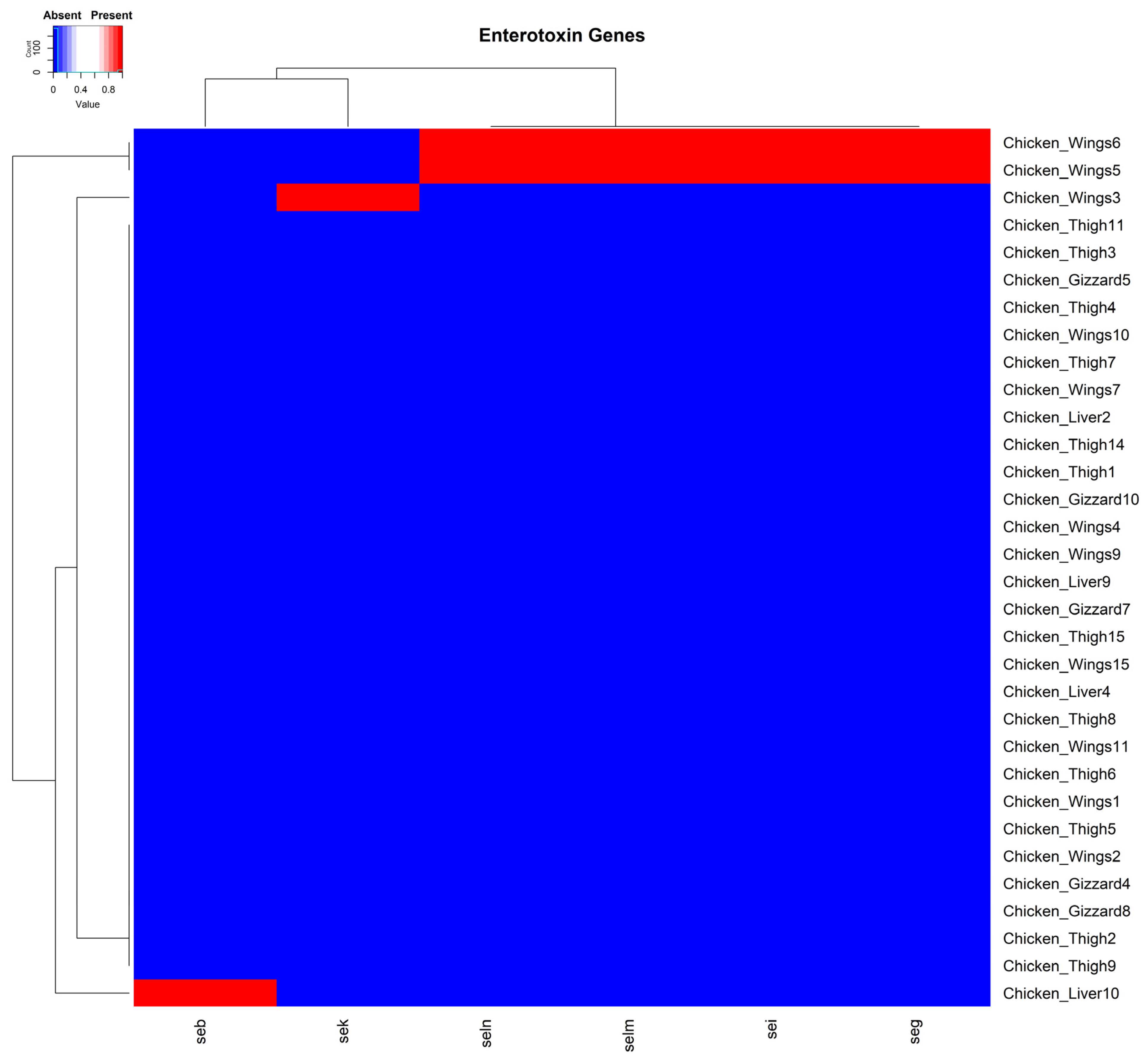
3.3. Distribution of Enterotoxin Genes Within the Plasmids of S. aureus Isolates
3.4. Multidrug Resistance Plasmid Carried Genes Against Three Antibiotic Classes
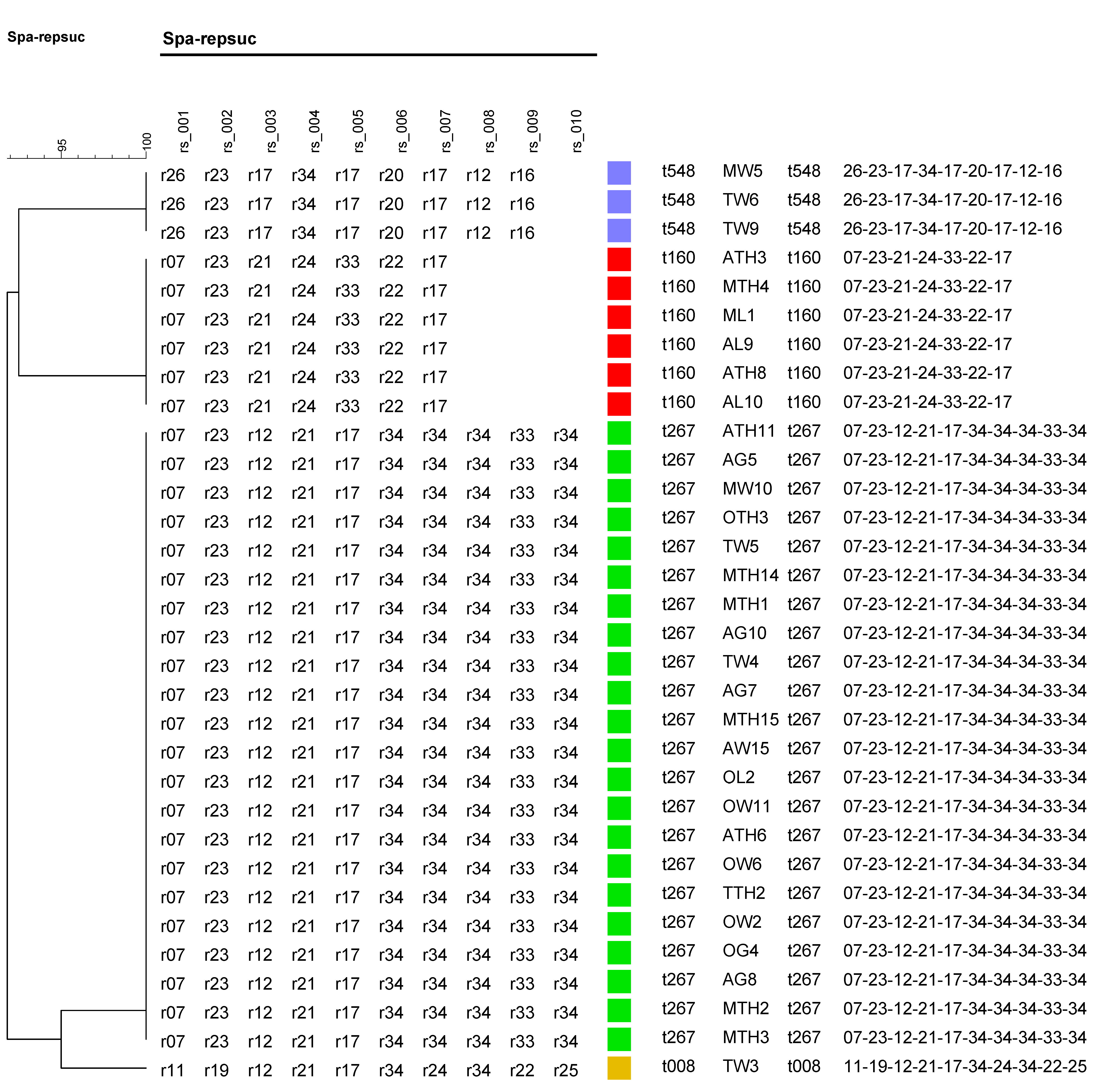
3.5. Molecular Typing of Spa Gene
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Putman, B.; Thoma, G.; Burek, J.; Matlock, M. A Retrospective Analysis of the United States Poultry Industry: 1965 Compared with 2010. Agric. Syst. 2017, 157, 107–117. [Google Scholar] [CrossRef]
- USDA-Economic Research Service Poultry Sector at a Glance. Available online: https://www.ers.usda.gov/topics/animal-products/poultry-eggs/sector-at-a-glance/ (accessed on 18 July 2024).
- Lord, J.D. The Growth and Localization of the United States Broiler Chicken Industry. Southeast. Geogr. 1971, 11, 29–42. [Google Scholar] [CrossRef]
- Maples, J.G.; Thompson, J.M.; Anderson, J.D.; Anderson, D.P. Estimating COVID-19 Impacts on the Broiler Industry. Appl. Econ. Perspect. Policy 2021, 43, 315–328. [Google Scholar] [CrossRef]
- Kuck, G.; Schnitkey, G. An Overview of Meat Consumption in the United States; Farmdoc Daily: Urbana, IL, USA, 2021. [Google Scholar]
- Mudenda, S.; Malama, S.; Munyeme, M.; Hang’ombe, B.M.; Mainda, G.; Kapona, O.; Mukosha, M.; Yamba, K.; Bumbangi, F.N.; Mfune, R.L.; et al. Awareness of Antimicrobial Resistance and Associated Factors among Layer Poultry Farmers in Zambia: Implications for Surveillance and Antimicrobial Stewardship Programs. Antibiotics 2022, 11, 383. [Google Scholar] [CrossRef]
- Cox, L.M. Antibiotics Shape Microbiota and Weight Gain across the Animal Kingdom. Anim. Front. 2016, 6, 8–14. [Google Scholar] [CrossRef]
- Diaz-Sanchez, S.; Moscoso, S.; Solís De Los Santos, F.; Andino, A.; Hanning, I. Antibiotic Use in Poultry: A Driving Force for Organic Poultry Production. Food Prot. Trends 2015, 35, 440–447. [Google Scholar]
- Kumar, A.; Patyal, A.; Panda, A.K. Sub-Therapeutic Use of Antibiotics in Animal Feed and Their Potential Impact on Environmental and Human Health: A Comprehensive Review Bacteriological Quality of Meat and Meat Products View Project Food Borne Pathogen in Ready to Eat Foods of Animal Origi. J. Anim. Feed Sci. Technol. 2018, 6, 25. [Google Scholar] [CrossRef]
- Wallinga, D.; Smit, L.A.M.; Davis, M.F.; Casey, J.A.; Nachman, K.E. A Review of the Effectiveness of Current US Policies on Antimicrobial Use in Meat and Poultry Production. Curr. Environ. Heal. Rep. 2022, 9, 339–354. [Google Scholar] [CrossRef]
- Hao, H.; Cheng, G.; Iqbal, Z.; Ai, X.; Hussain, H.I.; Huang, L.; Dai, M.; Wang, Y.; Liu, Z.; Yuan, Z. Benefits and Risks of Antimicrobial Use in Food-Producing Animals. Front. Microbiol. 2014, 5, 288. [Google Scholar] [CrossRef]
- Chinemerem Nwobodo, D.; Ugwu, M.C.; Oliseloke Anie, C.; Al-Ouqaili, M.T.S.; Chinedu Ikem, J.; Victor Chigozie, U.; Saki, M. Antibiotic Resistance: The Challenges and Some Emerging Strategies for Tackling a Global Menace. J. Clin. Lab. Anal. 2022, 36, e24655. [Google Scholar] [CrossRef] [PubMed]
- Chambers, H.F.; DeLeo, F.R. Waves of Resistance: Staphylococcus aureus in the Antibiotic Era. Nat. Rev. Microbiol. 2009, 7, 629–641. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Duan, N.; Gu, H.; Hao, L.; Ye, H.; Gong, W.; Wang, Z. A Review of the Methods for Detection of Staphylococcus aureus Enterotoxins. Toxins 2016, 8, 176. [Google Scholar] [CrossRef]
- Parvin, M.S.; Ali, M.Y.; Talukder, S.; Nahar, A.; Chowdhury, E.H.; Rahman, M.T.; Islam, M.T. Prevalence and Multidrug Resistance Pattern of Methicillin Resistant S. Aureus Isolated from Frozen Chicken Meat in Bangladesh. Microorganisms 2021, 9, 636. [Google Scholar] [CrossRef]
- Buzón-Durán, L.; Capita, R.; Alonso-Calleja, C. Microbial Loads and Antibiotic Resistance Patterns of Staphylococcus aureus in Different Types of Raw Poultry-Based Meat Preparations. Poult. Sci. 2017, 96, 4046–4052. [Google Scholar] [CrossRef] [PubMed]
- Tsehayneh, B.; Yayeh, T.; Agmas, B. Evaluation of Bacterial Load and Antibiotic Resistance Pattern of Staphylococcus aureus from Ready-to-Eat Raw Beef in Bahir Dar City, Ethiopia. Int. J. Microbiol. 2021, 2021, 5560596. [Google Scholar] [CrossRef] [PubMed]
- Zeaki, N.; Johler, S.; Skandamis, P.N.; Schelin, J. The Role of Regulatory Mechanisms and Environmental Parameters in Staphylococcal Food Poisoning and Resulting Challenges to Risk Assessment. Front. Microbiol. 2019, 10, 1307. [Google Scholar] [CrossRef] [PubMed]
- Peterson, E.; Kaur, P. Antibiotic Resistance Mechanisms in Bacteria: Relationships Between Resistance Determinants of Antibiotic Producers, Environmental Bacteria, and Clinical Pathogens. Front. Microbiol. 2018, 9, 2928. [Google Scholar] [CrossRef] [PubMed]
- Argudín, M.Á.; Mendoza, M.C.; Rodicio, M.R. Food Poisoning and Staphylococcus aureus Enterotoxins. Toxins 2010, 2, 1751–1773. [Google Scholar] [CrossRef] [PubMed]
- Kadariya, J.; Smith, T.C.; Thapaliya, D. Staphylococcus aureus and Staphylococcal Food-Borne Disease: An Ongoing Challenge in Public Health. BioMed Res. Int. 2014, 2014, 827965. [Google Scholar] [CrossRef] [PubMed]
- Otto, M. Staphylococcus aureus Toxins. Curr. Opin. Microbiol. 2014, 17, 32–37. [Google Scholar] [CrossRef]
- Chajęcka-Wierzchowska, W.; Gajewska, J.; Wiśniewski, P.; Zadernowska, A. Enterotoxigenic Potential of Coagulase-Negative Staphylococci from Ready-to-Eat Food. Pathogens 2020, 9, 734. [Google Scholar] [CrossRef] [PubMed]
- Hennekinne, J.A.; De Buyser, M.L.; Dragacci, S. Staphylococcus aureus and Its Food Poisoning Toxins: Characterization and Outbreak Investigation. FEMS Microbiol. Rev. 2012, 36, 815–836. [Google Scholar] [CrossRef]
- Pinchuk, I.V.; Beswick, E.J.; Reyes, V.E. Staphylococcal Enterotoxins. Toxins 2010, 2, 2177. [Google Scholar] [CrossRef] [PubMed]
- Fraser, J.D.; Proft, T. The Bacterial Superantigen and Superantigen-like Proteins. Immunol. Rev. 2008, 225, 226–243. [Google Scholar] [CrossRef] [PubMed]
- Fournier, P.E.; Dubourg, G.; Raoult, D. Clinical Detection and Characterization of Bacterial Pathogens in the Genomics Era. Genome Med. 2014, 6, 1–15. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, F.P.; Suaya, J.A.; Ray, G.T.; Baxter, R.; Brown, M.L.; Mera, R.M.; Close, N.M.; Thomas, E.; Amrine-Madsen, H. Spa Typing and Multilocus Sequence Typing Show Comparable Performance in a Macroepidemiologic Study of Staphylococcus aureus in the United States. Microb. Drug Resist. 2016, 22, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Bosch, T.; Verkade, E.; van Luit, M.; Landman, F.; Kluytmans, J.; Schouls, L.M. Transmission and Persistence of Livestock-Associated Methicillin-Resistant Staphylococcus aureus among Veterinarians and Their Household Members. Appl. Environ. Microbiol. 2015, 81, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Darban-Sarokhalil, D.; Khoramrooz, S.S.; Marashifard, M.; Malek Hosseini, S.A.A.; Parhizgari, N.; Yazdanpanah, M.; Gharibpour, F.; Mirzaii, M.; Sharifi, B.; Haeili, M. Molecular Characterization of Staphylococcus aureus Isolates from Southwest of Iran Using Spa and SCCmec Typing Methods. Microb. Pathog. 2016, 98, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Rouger, A.; Tresse, O.; Zagorec, M. Bacterial Contaminants of Poultry Meat: Sources, Species, and Dynamics. Microorganisms 2017, 5, 50. [Google Scholar] [CrossRef]
- Rohde, A.; Hammerl, J.A.; Appel, B.; Dieckmann, R.; Al Dahouk, S. Sampling and Homogenization Strategies Significantly Influence the Detection of Foodborne Pathogens in Meat. BioMed Res. Int. 2015, 2015, 145437. [Google Scholar] [CrossRef] [PubMed]
- Kateete, D.P.; Kimani, C.N.; Katabazi, F.A.; Okeng, A.; Okee, M.S.; Nanteza, A.; Joloba, M.L.; Najjuka, F.C. Identification of Staphylococcus aureus: DNase and Mannitol Salt Agar Improve the Efficiency of the Tube Coagulase Test. Ann. Clin. Microbiol. Antimicrob. 2010, 9, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bergdoll, M.S. The Nature of Bacterial Toxins. Clin. Toxicol. 1972, 5, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.D.; Velu, D.; Bhuvana, M.; Krithiga, N.; Banerjee, A.; Shome, R.; Rahman, H.; Ghosh, S.K.; Shome, B.R. Staphylococcus aureus Spa Type T267, Clonal Ancestor of Bovine Subclinical Mastitis in India. J. Appl. Microbiol. 2013, 114, 1604–1615. [Google Scholar] [CrossRef] [PubMed]
- Roetzer, A.; Gruener, C.S.; Haller, G.; Beyerly, J.; Model, N.; Eibl, M.M. Enterotoxin Gene Cluster-Encoded SEI and SElN from Staphylococcus aureus Isolates Are Crucial for the Induction of Human Blood Cell Proliferation and Pathogenicity in Rabbits. Toxins 2016, 8, 314. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.; Falcão, C.; Viveiros, M.; MacHado, D.; Martins, M.; Melo-Cristino, J.; Amaral, L.; Couto, I. Exploring the Contribution of Efflux on the Resistance to Fluoroquinolones in Clinical Isolates of Staphylococcus aureus. BMC Microbiol. 2011, 11, 1–12. [Google Scholar] [CrossRef]
- Couto, I.; Costa, S.S.; Viveiros, M.; Martins, M.; Amaral, L. Efflux-Mediated Response of Staphylococcus aureus Exposed to Ethidium Bromide. J. Antimicrob. Chemother. 2008, 62, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, M.R.; Bajaksouzian, S.; Appelbaum, P.C. Telithromycin Post-Antibiotic and Post-Antibiotic Sub-MIC Effects for 10 Gram-Positive Cocci. J. Antimicrob. Chemother. 2003, 52, 809–812. [Google Scholar] [CrossRef]
- Krupa, P.; Bystron, J.; Bania, J.; Podkowik, M.; Empel, J.; Mroczkowska, A. Genotypes and Oxacillin Resistance of Staphylococcus aureus from Chicken and Chicken Meat in Poland. Poult. Sci. 2014, 93, 3179–3186. [Google Scholar] [CrossRef]
- Arfatahery, N.; Davoodabadi, A.; Abedimohtasab, T. Characterization of Toxin Genes and Antimicrobial Susceptibility of Staphylococcus aureus Isolates in Fishery Products in Iran. Sci. Rep. 2016, 6, 1–76. [Google Scholar] [CrossRef]
- Tang, J.; Zhang, R.; Chen, J.; Zhao, Y.; Tang, C.; Yue, H.; Li, J.; Wang, Q.; Shi, H. Incidence and Characterization of Staphylococcus aureus Strains Isolated from Food Markets. Ann. Microbiol. 2015, 65, 279–286. [Google Scholar] [CrossRef]
- Lee, J.H. Methicillin (Oxacillin)-Resistant Staphylococcus aureus Strains Isolated from Major Food Animals and Their Potential Transmission to Humans. Appl. Environ. Microbiol. 2003, 69, 6489–6494. [Google Scholar] [CrossRef] [PubMed]
- Martineau, F.; Picard, F.J.; Grenier, L.; Roy, P.H.; Ouellette, M.; Bergeron, M.G. Multiplex PCR Assays for the Detection of Clinically Relevant Antibiotic Resistance Genes in Staphylococci Isolated from Patients Infected after Cardiac Surgery. J. Antimicrob. Chemother. 2000, 46, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Warnes, G.; Bolker, B.; Bonebakker, L.; Gentleman, R.; Huber, W.; Liaw, A.; Lumley, T.; Maechler, M.; Magnusson, A.; Moeller, S.; et al. gplots: Various R Programming Tools for Plotting Data. R Package Version 3.1.3.1. 2024, pp. 1–23. Available online: https://cran.r-project.org/web/packages/gplots/index.html (accessed on 10 November 2024).
- R Core Team. R Core Team 2023 R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Microsoft Corporation. Microsoft Excel. 2018. Available online: https://office.microsoft.com/excel (accessed on 1 November 2024).
- Chiang, Y.C.; Chang, L.T.; Lin, C.W.; Yang, C.Y.; Tsen, H.Y. PCR Primers for the Detection of Staphylococcal Enterotoxins K, L, and M and Survey of Staphylococcal Enterotoxin Types in Staphylococcus aureus Isolates from Food Poisoning Cases in Taiwan. J. Food Prot. 2006, 69, 1072–1079. [Google Scholar] [CrossRef] [PubMed]
- Aminov, R.I.; Garrigues-Jeanjean, N.; Mackie, R.I. Molecular Ecology of Tetracycline Resistance: Development and Validation of Primers for Detection of Tetracycline Resistance Genes Encoding Ribosomal Protection Proteins. Appl. Environ. Microbiol. 2001, 67, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Harmsen, D.; Claus, H.; Witte, W.; Rothgänger, J.; Claus, H.; Turnwald, D.; Vogel, U. Typing of Methicillin-Resistant Staphylococcus aureus in a University Hospital Setting by Using Novel Software for Spa Repeat Determination and Database Management. J. Clin. Microbiol. 2003, 41, 5442–5448. [Google Scholar] [CrossRef] [PubMed]
- Strommenger, B.; Braulke, C.; Heuck, D.; Schmidt, C.; Pasemann, B.; Nübel, U.; Witte, W. Spa Typing of Staphylococcus aureus as a Frontline Tool in Epidemiological Typing. J. Clin. Microbiol. 2008, 46, 574–581. [Google Scholar] [CrossRef]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data; The Babraham Institute: Cambridge, UK, 2010; Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 30 September 2024).
- Wick, R.R.; Judd, L.M.; Gorrie, C.L.; Holt, K.E. Completing Bacterial Genome Assemblies with Multiplex MinION Sequencing. Microb. Genom. 2017, 3, e000132. [Google Scholar] [CrossRef]
- Kolmogorov, M.; Yuan, J.; Lin, Y.; Pevzner, P.A. Assembly of Long, Error-Prone Reads Using Repeat Graphs. Nat. Biotechnol. 2019, 37, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Feldgarden, M.; Brover, V.; Haft, D.; Prasad, A.; Slotta, D.; Tolstoy, I. Validating the AMRFinder Tool and Resistance Gene Database by Using Antimicrobial Resistance Genotype-Phenotype Correlations in a Collection of Isolates. Antimicrob. Agents Chemother. 2019, 63, e00483. [Google Scholar] [CrossRef]
- Dhungel, S.; Rijal, K.R.; Yadav, B.; Dhungel, B.; Adhikari, N.; Shrestha, U.T.; Adhikari, B.; Banjara, M.R.; Ghimire, P. Methicillin-Resistant Staphylococcus aureus (MRSA): Prevalence, Antimicrobial Susceptibility Pattern, and Detection of Mec A Gene among Cardiac Patients from a Tertiary Care Heart Center in Kathmandu, Nepal. Infect. Dis. Res. Treat. 2021, 14, 117863372110373. [Google Scholar] [CrossRef]
- Hanson, B.M.; Dressler, A.E.; Harper, A.L.; Scheibel, R.P.; Wardyn, S.E.; Roberts, L.K.; Kroeger, J.S.; Smith, T.C. Prevalence of Staphylococcus aureus and Methicillin-Resistant Staphylococcus aureus (MRSA) on Retail Meat in Iowa. J. Infect. Public Health 2011, 4, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Waters, A.E.; Contente-Cuomo, T.; Buchhagen, J.; Liu, C.M.; Watson, L.; Pearce, K.; Foster, J.T.; Bowers, J.; Driebe, E.M.; Engelthaler, D.M.; et al. Multidrug-Resistant Staphylococcus aureus in US Meat and Poultry. Clin. Infect. Dis. 2011, 52, 1227–1230. [Google Scholar] [CrossRef]
- El-Ghany, W.A.A. Staphylococcus aureus in Poultry, with Special Emphasis on Methicillin-Resistant Strain Infection: A Comprehensive Review from One Health Perspective. Int. J. One Heal. 2021, 7, 257–267. [Google Scholar] [CrossRef]
- Velasco, V.; Quezada-Aguiluz, M.; Bello-Toledo, H.; Velasco, V.; Quezada-Aguiluz, M.; Bello-Toledo, H. Staphylococcus aureus in the Meat Supply Chain: Detection Methods, Antimicrobial Resistance, and Virulence Factors. In Staphylococcus and Streptococcus; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Omoe, K.; Hu, D.L.; Ono, H.K.; Shimizu, S.; Takahashi-Omoe, H.; Nakane, A.; Uchiyama, T.; Shinagawa, K.; Imanishi, K. Emetic Potentials of Newly Identified Staphylococcal Enterotoxin-like Toxins. Infect. Immun. 2013, 81, 3627–3631. [Google Scholar] [CrossRef] [PubMed]
- Hennekinne, J.A.; Ostyn, A.; Guillier, F.; Herbin, S.; Prufer, A.L.; Dragacci, S. How Should Staphylococcal Food Poisoning Outbreaks Be Characterized? Toxins 2010, 2, 2106. [Google Scholar] [CrossRef]
- Deekshit, V.K.; Srikumar, S. ‘To Be, or Not to Be’—The Dilemma of ‘Silent’ Antimicrobial Resistance Genes in Bacteria. J. Appl. Microbiol. 2022, 133, 2902–2914. [Google Scholar] [CrossRef] [PubMed]
- Duran, N.; Ozer, B.; Duran, G.G.; Onlen, Y.; Demir, C. Antibiotic Resistance Genes & Susceptibility Patterns in Staphylococci. Indian J. Med. Res. 2012, 135, 389. [Google Scholar] [PubMed]
- Malachowa, N.; Deleo, F.R. Mobile Genetic Elements of Staphylococcus aureus. Cell. Mol. Life Sci. C. 2010, 67, 3057–3071. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Hayashi, T.; Takami, H.; Ohnishi, M.; Murata, T.; Nakayama, K.; Asakawa, K.; Ohara, M.; Komatsuzawa, H.; Sugai, M. Complete Nucleotide Sequence of a Staphylococcus aureus Exfoliative Toxin B Plasmid and Identification of a Novel ADP-Ribosyltransferase, EDIN-C. Infect. Immun. 2001, 69, 7760. [Google Scholar] [CrossRef]
- Jensen, S.O.; Lyon, B.R. Genetics of Antimicrobial Resistance in Staphylococcus aureus. Future Microbiol. 2009, 4, 565–582. [Google Scholar] [CrossRef]
- McCarthy, A.J.; Lindsay, J.A. The Distribution of Plasmids That Carry Virulence and Resistance Genes in Staphylococcus aureus Is Lineage Associated. BMC Microbiol. 2012, 12, 104. [Google Scholar] [CrossRef] [PubMed]
- Hudson, L.O.; Murphy, C.R.; Spratt, B.G.; Enright, M.C.; Elkins, K.; Nguyen, C.; Terpstra, L.; Gombosev, A.; Kim, D.; Hannah, P.; et al. Diversity of Methicillin-Resistant Staphylococcus aureus (MRSA) Strains Isolated from Inpatients of 30 Hospitals in Orange County, California. PLoS ONE 2013, 8, e62117. [Google Scholar] [CrossRef] [PubMed]
- Van Loo, I.H.M.; Diederen, B.M.W.; Savelkoul, P.H.M.; Woudenberg, J.H.C.; Roosendaal, R.; Van Belkum, A.; Lemmens-Den Toom, N.; Verhulst, C.; Van Keulen, P.H.J.; Kluytmans, J.A.J.W. Methicillin-Resistant Staphylococcus aureus in Meat Products, the Netherlands. Emerg. Infect. Dis. 2007, 13, 1753. [Google Scholar] [CrossRef] [PubMed]
- Pu, S.; Han, F.; Ge, B. Isolation and Characterization of Methicillin-Resistant Staphylococcus aureus Strains from Louisiana Retail Meats. Appl. Environ. Microbiol. 2009, 75, 265–267. [Google Scholar] [CrossRef] [PubMed]
- Nulens, E.; Stobberingh, E.E.; Van Desse, H.; Sebastian, S.; Van Tiel, F.H.; Beisser, P.S.; Deurenberg, R.H. Molecular Characterization of Staphylococcus aureus Bloodstream Isolates Collected in a Dutch University Hospital between 1999 and 2006. J. Clin. Microbiol. 2008, 46, 2438–2441. [Google Scholar] [CrossRef] [PubMed]
- Sergelidis, D.; Abrahim, A.; Papadopoulos, T.; Soultos, N.; Martziou, E.; Koulourida, V.; Govaris, A.; Pexara, A.; Zdragas, A.; Papa, A. Isolation of Methicillin-resistant Staphylococcus Spp. from Ready-to-eat Fish Products. Lett. Appl. Microbiol. 2014, 59, 500–506. [Google Scholar] [CrossRef]
- Jackson, C.R.; Davis, J.A.; Barrett, J.B. Prevalence and Characterization of Methicillin-Resistant Staphylococcus aureus Isolates from Retail Meat and Humans in Georgia. J. Clin. Microbiol. 2013, 51, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Zhen, X.; Lundborg, C.S.; Zhang, M.; Sun, X.; Li, Y.; Hu, X.; Gu, S.; Gu, Y.; Wei, J.; Dong, H. Clinical and Economic Impact of Methicillin-Resistant Staphylococcus aureus: A Multicentre Study in China. Sci. Rep. 2020, 10, 3900. [Google Scholar] [CrossRef] [PubMed]

| Enterotoxins | Oligonucleotide Sequences (5′-3′) | Expected Size bp | Reference |
|---|---|---|---|
| F and R Primers | |||
| SEA | CCTTTGGAAACGGTTAAAACG | 127 | [41] |
| TCTGAACCTTCCCATCAAAAAC | |||
| SEB | TCGCATCAAACTGACAAACGA | 410 | [48] |
| CACTTTTTCTTTGTCGTAAGATAA | |||
| SEC | AGATGAAGTAGTTGATGTGTATGG | 451 | [48] |
| CACACTTTTAGAATCAACCG | |||
| SED | CCAATAATAGGAGAAAATAAAAG | 278 | [48] |
| ATTGGTATTTTTTTTCGTTC | |||
| SEE | CAGTACCTATAGATAAAGTTAAAACAAGC | 178 | [48] |
| TAACTTACCGTGGACCCTTC | |||
| SEG | GCTATCGACACACTACAACC′ | 538 | [48] |
| CCAAGTGATTGTCTATTGTCG | |||
| SEH | CACATCATATGCGAAAGC | 584 | [48] |
| CGAATGAGTAATCTCTAGG | |||
| SEI | GATACTGGAACAGGACAAGC | 789 | [34] |
| CTTACAGGCAGTCCATCTCC | |||
| SEJ | CTCCCTGACGTTAACACTACTAATAA | 666 | [48] |
| TTGTCTGGATATTGACCTATAACATT | |||
| SEK | CACAGCTACTAACGAATATC | 378 | [48] |
| TGGAATTTCTCAGACTCTAC | |||
| SEL | CATACAGTCTTATCTAACGG | 275 | [48] |
| TTTTCTGCTTTAGTAACACC | |||
| SER | AGATGTGTTTGGAATACCCTAT’ | 123 | [36] |
| CTATCAGCTGTGGAGTGCAT | |||
| SElM | CTTGTCCTGTTCCAGTATC | 329 | [48] |
| ATACGGTGGAGTTACATTAG | |||
| SElN | GATGAAGAGAAAGTTATAGGCGT | 268 | [36] |
| AACTCTGCTCCCACTGAACC | |||
| SELlU | AATGGCTCTAAAATTGATGGTTC | 142 | [36] |
| CCATATTATCCGCTGAAAAATAG | |||
| SElJ | GTTCTGGTGGTAAACCA | 132 | [36] |
| GCGGAA CAACAGTTCTGA |
| Antibiotic Resistance Genes | Oligonucleotide Sequences (5′-3′) | Expected Size bp | References |
|---|---|---|---|
| F and R Primers | |||
| blaZ | ACTTCAACACCTGCTGCTTTC | 173 | [44] |
| TGACCACTTTTATCAGCAACC | |||
| mecA | AAAATCGATGGTAAAGGTTGGC | 533 | [43] |
| AGTTCTGCAGTACCGGATTTGC | |||
| tetA | CTAAAGAGGACCAGAAGACT | 512 | [42] |
| ATAACCCGGTACTAATAACA | |||
| tetM | ACAGAAAGCTTATTATATAAC | 171 | [49] |
| TGGCGTGTCTATGATGTTCAC | |||
| chlA | CCTGCTAACAATAGACCTGA | 768 | [42] |
| CGCTTTAACATTTGCGATAT | |||
| norA | TGTTAAGTCTTGGTCATCTGCA | 761 | [37,38] |
| CCATAAATCCACCAATCCC | |||
| ermA | CTTCGATAGTTTATTAATATTAG | 645 | [39] |
| TTCTAAAAAGCATGTAAAAGAA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faraj, R.; Ramadan, H.; Bentum, K.E.; Alkaraghulli, B.; Woube, Y.; Hassan, Z.; Samuel, T.; Adesiyun, A.; Jackson, C.R.; Abebe, W. Antimicrobial Resistance, Virulence Gene Profiling, and Spa Typing of Staphylococcus aureus Isolated from Retail Chicken Meat in Alabama, USA. Pathogens 2025, 14, 107. https://doi.org/10.3390/pathogens14020107
Faraj R, Ramadan H, Bentum KE, Alkaraghulli B, Woube Y, Hassan Z, Samuel T, Adesiyun A, Jackson CR, Abebe W. Antimicrobial Resistance, Virulence Gene Profiling, and Spa Typing of Staphylococcus aureus Isolated from Retail Chicken Meat in Alabama, USA. Pathogens. 2025; 14(2):107. https://doi.org/10.3390/pathogens14020107
Chicago/Turabian StyleFaraj, Rawah, Hazem Ramadan, Kingsley E. Bentum, Bilal Alkaraghulli, Yilkal Woube, Zakaria Hassan, Temesgen Samuel, Abiodun Adesiyun, Charlene R. Jackson, and Woubit Abebe. 2025. "Antimicrobial Resistance, Virulence Gene Profiling, and Spa Typing of Staphylococcus aureus Isolated from Retail Chicken Meat in Alabama, USA" Pathogens 14, no. 2: 107. https://doi.org/10.3390/pathogens14020107
APA StyleFaraj, R., Ramadan, H., Bentum, K. E., Alkaraghulli, B., Woube, Y., Hassan, Z., Samuel, T., Adesiyun, A., Jackson, C. R., & Abebe, W. (2025). Antimicrobial Resistance, Virulence Gene Profiling, and Spa Typing of Staphylococcus aureus Isolated from Retail Chicken Meat in Alabama, USA. Pathogens, 14(2), 107. https://doi.org/10.3390/pathogens14020107








