Hidden Circulation and Socio-Sanitary Vulnerability: Rotavirus A and Human Adenovirus Prevalence in Symptomatic and Asymptomatic Children in Central Brazil Post-COVID-19
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. General Characteristics of the Study Population
3.2. Symptoms Association to RVA and HAdV Positivity
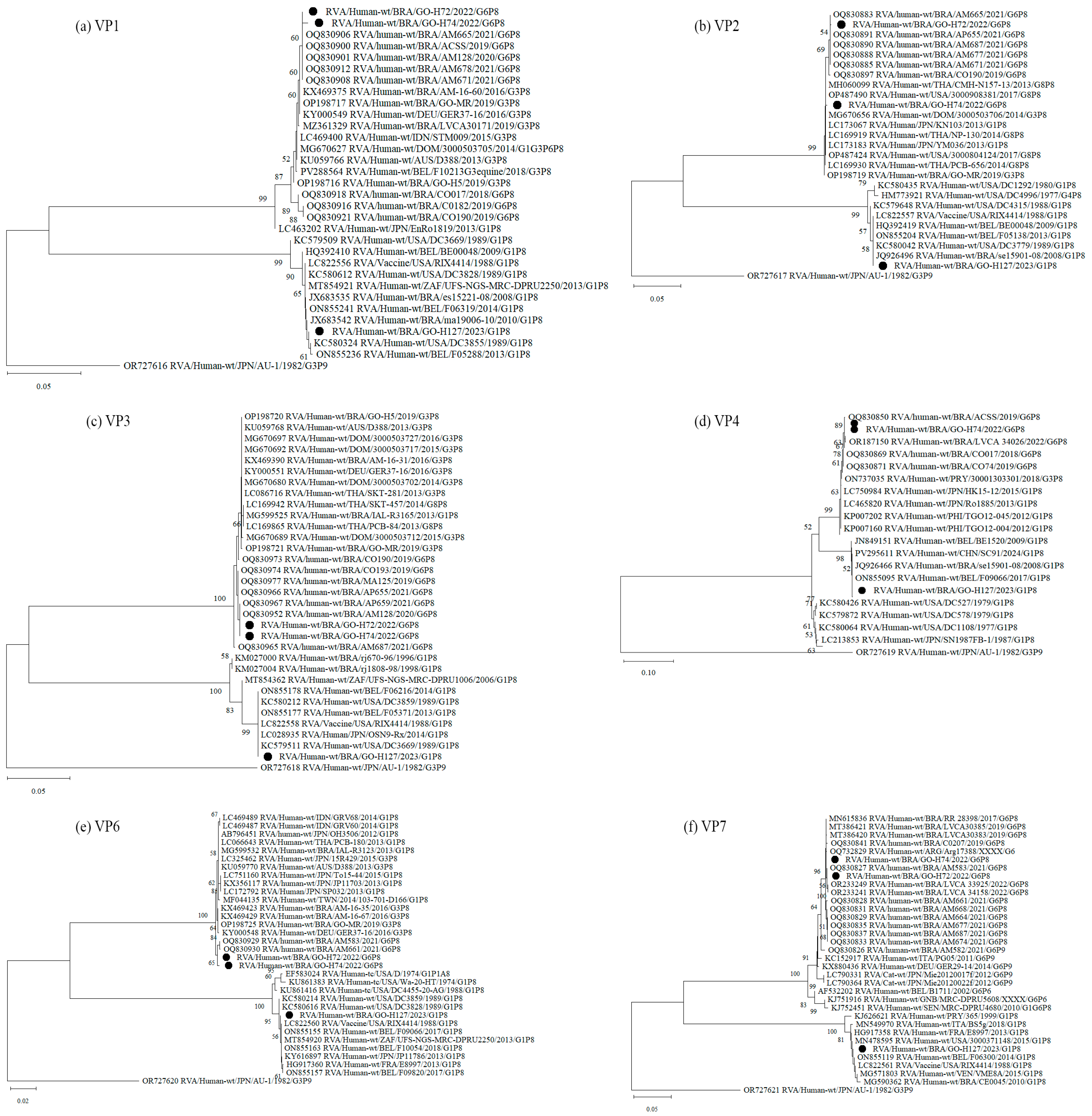
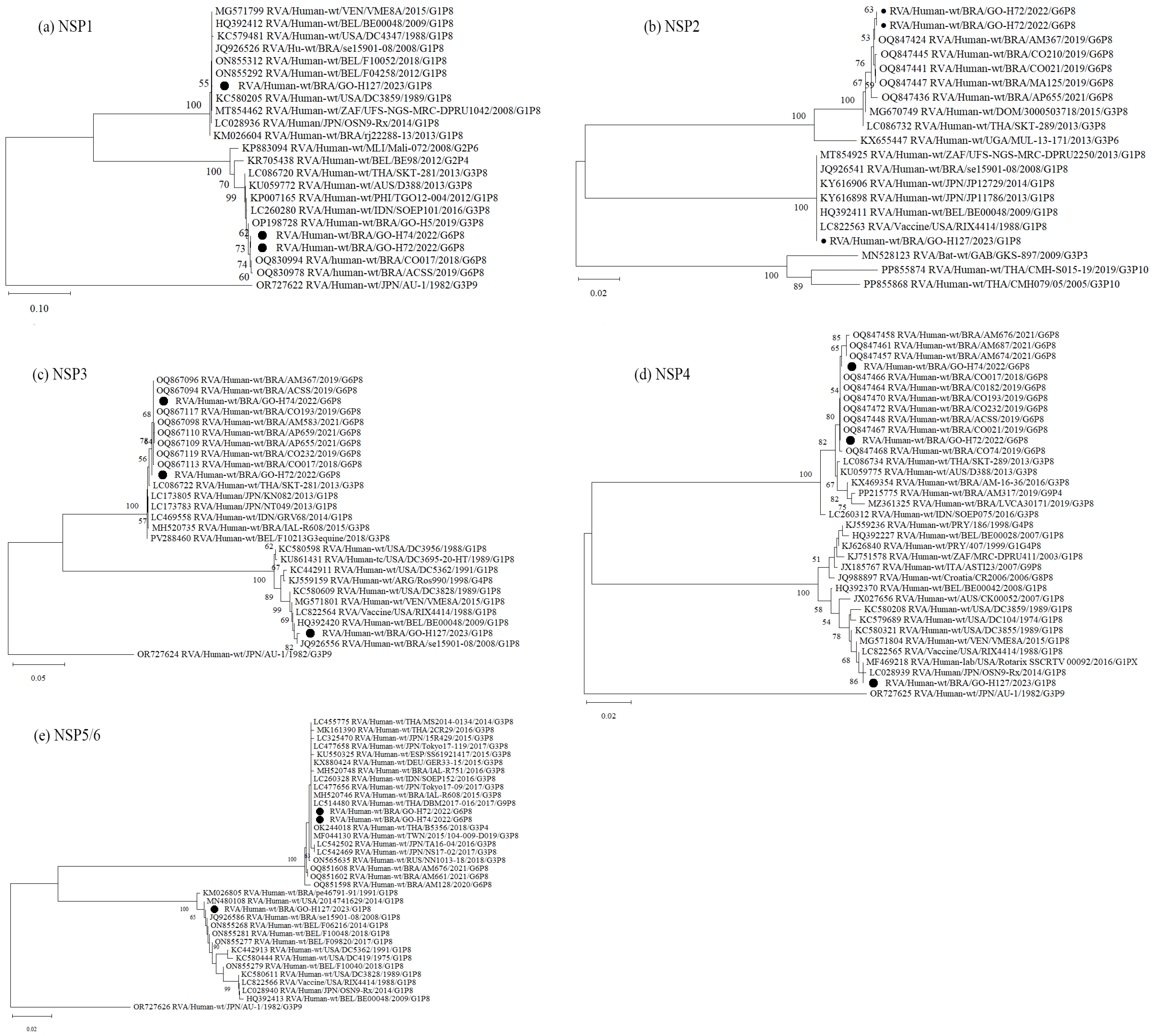
3.3. Nucleotide Sequencing and Phylogenetic Analysis of RVA-Positive Samples
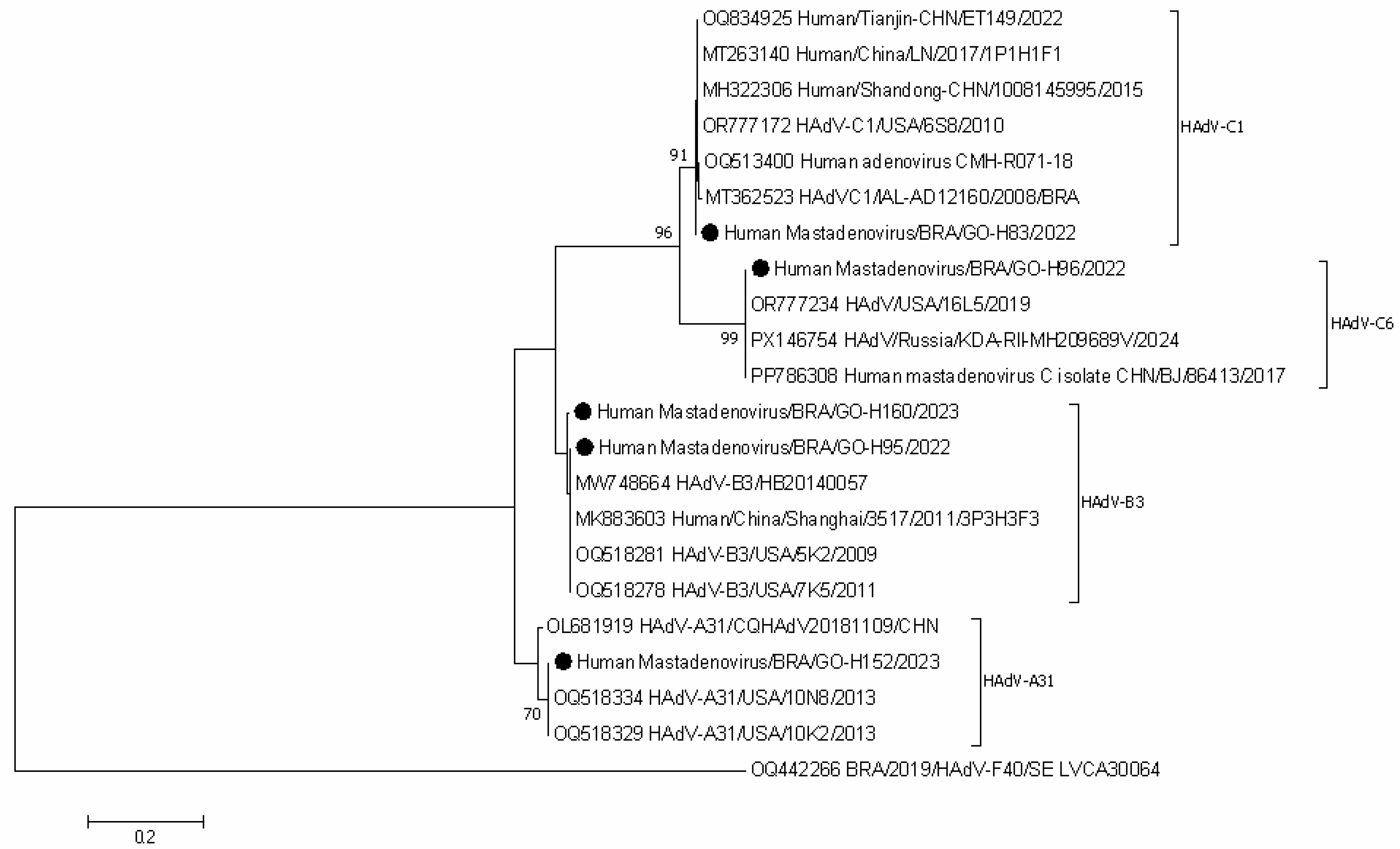
3.4. Nucleotide Sequencing and Phylogenetic Analysis of HAdV-Positive Samples
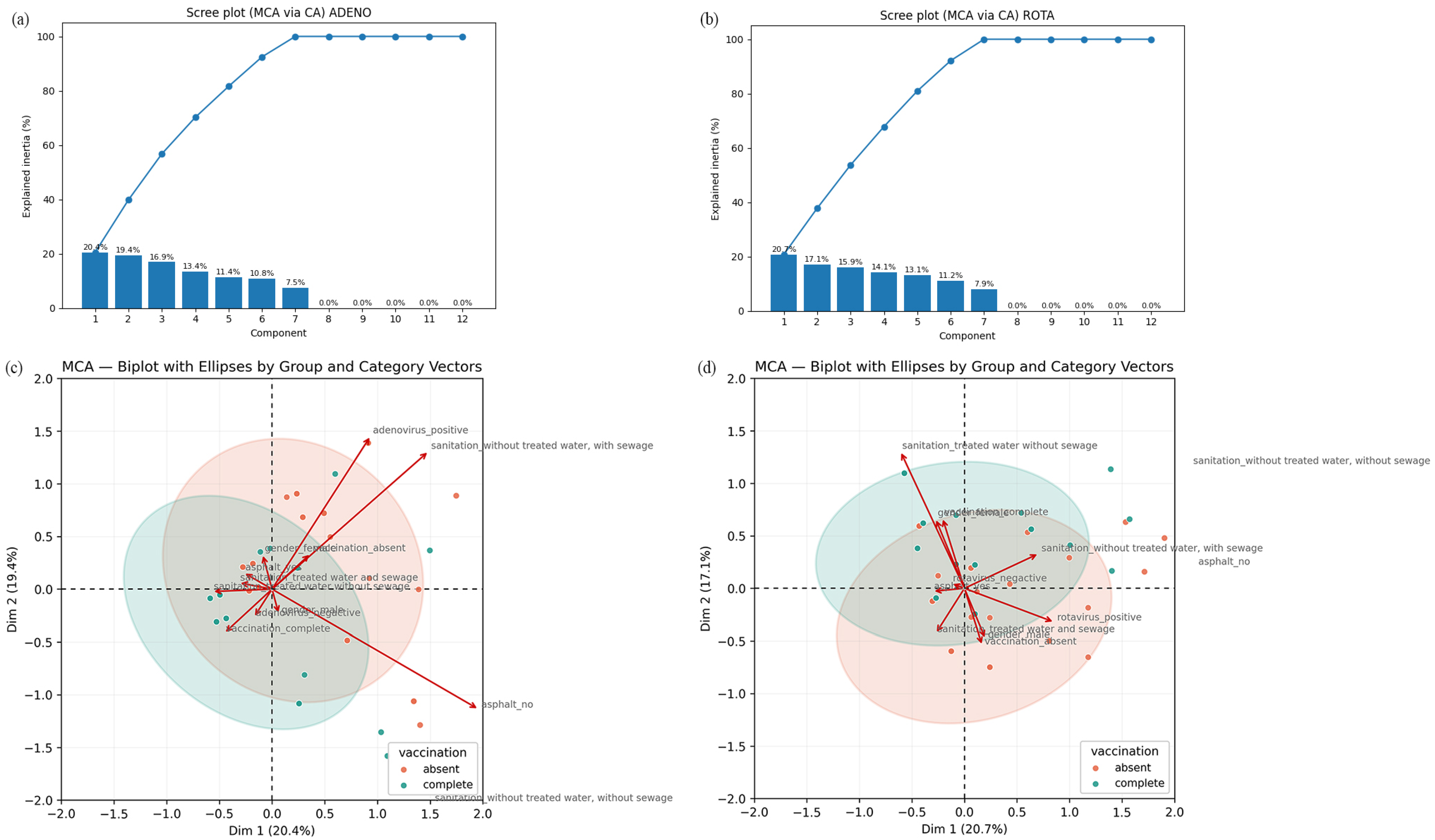
3.5. Multiple Correspondence Analysis (MCA) for RVA and HAdV Positivity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Gastroenterology Organisation. Acute Diarrhea in Adults and Children: A Global Perspective; World Gastroenterology Organisation: Milwaukee, WI, USA, 2012. [Google Scholar]
- Brasil, M.d.S. Foodborne and Waterborne Diseases Surveillance: Training Manual; Ministério da Saúde: Brasília, DF, Brazil, 2021.
- Brasil Ministério da Saúde. Guia de Vigilância em Saúde; Ministério da Saúde: Brasília, DF, Brazil, 2024.
- Lee, B.; Damon, C.F.; Platts-Mills, J.A. Pediatric acute gastroenteritis associated with adenovirus 40/41 in low-income and middle-income countries. Curr. Opin. Infect. Dis. 2020, 33, 398–403. [Google Scholar] [CrossRef]
- Karampatsas, K.; Osborne, L.; Seah, M.L.; Tong, C.Y.W.; Prendergast, A.J. Clinical characteristics and complications of rotavirus gastroenteritis in children in east London: A retrospective case-control study. PLoS ONE 2018, 13, e0194009. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, P.; Borte, M.; Zimmer, K.P.; Huppertz, H.I. Complications in hospitalized children with acute gastroenteritis caused by rotavirus: A retrospective analysis. Eur. J. Pediatr. 2012, 171, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.; Duan, Q.; Zhang, W. Vaccines against gastroenteritis, current progress and challenges. Gut Microbes 2020, 11, 1486–1517. [Google Scholar] [CrossRef] [PubMed]
- Ndwandwe, D.; Runeyi, S.; Mathebula, L.; Wiysonge, C. Rotavirus vaccine clinical trials: A cross-sectional analysis of clinical trials registries. Trials 2022, 23, 945. [Google Scholar] [CrossRef]
- GBD 2016 Diarrhoeal Disease Collaborators. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect. Dis. 2018, 18, 1211–1228. [Google Scholar] [CrossRef]
- Guarino, A.; Aguilar, J.; Berkley, J.; Broekaert, I.; Vazquez-Frias, R.; Holtz, L.; Lo Vecchio, A.; Meskini, T.; Moore, S.; Rivera Medina, J.F.; et al. Acute Gastroenteritis in Children of the World: What Needs to Be Done? J. Pediatr. Gastroenterol. Nutr. 2020, 70, 694–701. [Google Scholar] [CrossRef]
- Clark, A.; van Zandvoort, K.; Flasche, S.; Sanderson, C.; Bines, J.; Tate, J.; Parashar, U.; Jit, M. Efficacy of live oral rotavirus vaccines by duration of follow-up: A meta-regression of randomised controlled trials. Lancet Infect. Dis. 2019, 19, 717–727. [Google Scholar] [CrossRef]
- Yandle, Z.; Coughlan, S.; Dean, J.; Hare, D.; De Gascun, C.F. Indirect impact of rotavirus vaccination on viral causes of acute gastroenteritis in the elderly. J. Clin. Virol. 2021, 137, 104780. [Google Scholar] [CrossRef]
- Moszak, M.; Szulinska, M.; Bogdanski, P. You Are What You Eat-The Relationship between Diet, Microbiota, and Metabolic Disorders-A Review. Nutrients 2020, 12, 1096. [Google Scholar] [CrossRef]
- UNICEF. Diarrhoea Remains a Leading Killer of Young Children, Despite the Availability of a Simple Treatment. 2024. Available online: https://data.unicef.org/topic/child-health/diarrhoeal-disease/ (accessed on 30 October 2025).
- World Health Organization. Diarrhoeal Disease. 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/diarrhoeal-disease (accessed on 12 September 2025).
- Banyai, K.; Estes, M.K.; Martella, V.; Parashar, U.D. Viral gastroenteritis. Lancet 2018, 392, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Posovszky, C.; Buderus, S.; Classen, M.; Lawrenz, B.; Keller, K.M.; Koletzko, S. Acute Infectious Gastroenteritis in Infancy and Childhood. Dtsch. Ärzteblatt Int. 2020, 117, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Brasil. Ministério da Saúde. DATASUS. Informações de Saúde (TABNET): Mortalidade e Morbidade por Doenças Diarreicas Agudas, Brasil, 2000–2024. 2024. Available online: https://datasus.saude.gov.br/ (accessed on 30 October 2025).
- Brasil. Ministério da Saúde. SIVEP-DDA—Sistema de Informação de Agravos de Notificação—Doença Diarreica Aguda; Ministério da Saúde: Brasília, DF, Brazil, 2006.
- Lestari, F.B.; Vongpunsawad, S.; Wanlapakorn, N.; Poovorawan, Y. Rotavirus infection in children in Southeast Asia 2008–2018: Disease burden, genotype distribution, seasonality, and vaccination. J. Biomed. Sci. 2020, 27, 66. [Google Scholar] [CrossRef] [PubMed]
- Sadiq, A.; Bostan, N.; Yinda, K.C.; Naseem, S.; Sattar, S. Rotavirus: Genetics, pathogenesis and vaccine advances. Rev. Med. Virol. 2018, 28, e2003. [Google Scholar] [CrossRef]
- World Health Organization; UNICEF; Gavi. Increases in Vaccine-Preventable Disease Outbreaks Threaten Years of Progress, warn WHO, UNICEF, Gavi. 24 April 2025. Available online: https://www.who.int/news/item/24-04-2025-increases-in-vaccine-preventable-disease-outbreaks-threaten-years-of-progress--warn-who--unicef--gavi?utm_source=chatgpt.com (accessed on 4 November 2025).
- Bigouette, J.P.; Callaghan, A.W.; Donadel, M.; Porter, A.M.; Rosencrans, L.; Lickness, J.S.; Blough, S.; Li, X.; Perry, R.T.; Williams, A.J.; et al. Effects of COVID-19 on Vaccine-Preventable Disease Surveillance Systems in the World Health Organization African Region, 2020. Emerg. Infect. Dis. 2022, 28, S203–S207. [Google Scholar] [CrossRef]
- Zeng, S.Q.; Halkosalo, A.; Salminen, M.; Szakal, E.D.; Puustinen, L.; Vesikari, T. One-step quantitative RT-PCR for the detection of rotavirus in acute gastroenteritis. J. Virol. Methods 2008, 153, 238–240. [Google Scholar] [CrossRef]
- Hernroth, B.E.; Conden-Hansson, A.C.; Rehnstam-Holm, A.S.; Girones, R.; Allard, A.K. Environmental factors influencing human viral pathogens and their potential indicator organisms in the blue mussel, Mytilus edulis: The first Scandinavian report. Appl. Environ. Microbiol. 2002, 68, 4523–4533. [Google Scholar] [CrossRef]
- da Silva, M.F.M.; Rose, T.L.; Gomez, M.M.; Carvalho-Costa, F.A.; Fialho, A.M.; de Assis, R.M.S.; de Andrade, J.; Volotao, E.M.; Leite, J.P.G. G1P[8] species A rotavirus over 27 years—Pre- and post-vaccination eras—In Brazil: Full genomic constellation analysis and no evidence for selection pressure by Rotarix® vaccine. Infect. Genet. Evol. 2015, 30, 206–218. [Google Scholar] [CrossRef]
- Souza, E.V.; de Souza, Y.; Medeiros, R.S.; de Azevedo, L.S.; de Queiroz, T.G.A.; Sanz-Duro, R.L.; Marinho, R.; Komninakis, S.V.; Timenetsky, M.; Luchs, A. Diversity of enteric and non-enteric human adenovirus strains in Brazil, 2006–2011. Arch. Virol. 2021, 166, 897–903. [Google Scholar] [CrossRef]
- Hall, T. BioEdit: A User-Friendly Biological Sequence Alignment Editor and Analysis Program for Windows 95/98/NT. Nucl. Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Sadiq, A.; Khan, J. Rotavirus in developing countries: Molecular diversity, epidemiological insights, and strategies for effective vaccination. Front. Microbiol. 2023, 14, 1297269. [Google Scholar] [CrossRef] [PubMed]
- De Jesus, M.C.S.; Santos, V.S.; Storti-Melo, L.M.; De Souza, C.D.F.; Barreto, I.D.C.; Paes, M.V.C.; Lima, P.A.S.; Bohland, A.K.; Berezin, E.N.; Machado, R.L.D.; et al. Impact of a twelve-year rotavirus vaccine program on acute diarrhea mortality and hospitalization in Brazil: 2006–2018. Expert Rev. Vaccines 2020, 19, 585–593. [Google Scholar] [CrossRef]
- do Carmo, G.M.; Yen, C.; Cortes, J.; Siqueira, A.A.; de Oliveira, W.K.; Cortez-Escalante, J.J.; Lopman, B.; Flannery, B.; de Oliveira, L.H.; Carmo, E.H.; et al. Decline in diarrhea mortality and admissions after routine childhood rotavirus immunization in Brazil: A time-series analysis. PLoS Med. 2011, 8, e1001024. [Google Scholar] [CrossRef] [PubMed]
- Carvalho-Costa, F.A.; de Assis, R.M.S.; Fialho, A.M.; Araujo, I.T.; Silva, M.F.; Gomez, M.M.; Andrade, J.S.; Rose, T.L.; Fumian, T.M.; Volotao, E.M.; et al. The evolving epidemiology of rotavirus A infection in Brazil a decade after the introduction of universal vaccination with Rotarix®. BMC Pediatr. 2019, 19, 42. [Google Scholar] [CrossRef]
- Reis, T.A.; Assis, A.S.; do Valle, D.A.; Barletta, V.H.; de Carvalho, I.P.; Rose, T.L.; Portes, S.A.; Leite, J.P.; da Rosa e Silva, M.L. The role of human adenoviruses type 41 in acute diarrheal disease in Minas Gerais after rotavirus vaccination. Braz. J. Microbiol. 2016, 47, 243–250. [Google Scholar] [CrossRef]
- do Nascimento, L.G.; Fialho, A.M.; de Andrade, J.; de Assis, R.M.S.; Fumian, T.M. Human enteric adenovirus F40/41 as a major cause of acute gastroenteritis in children in Brazil, 2018 to 2020. Sci. Rep. 2022, 12, 11220. [Google Scholar] [CrossRef]
- Mello, M.S.; Malta, F.C.; Fialho, A.M.; Burlandy, F.M.; Fumian, T.M. Molecular Epidemiology of Human Adenovirus from Acute Gastroenteritis Cases in Brazil After the COVID-19 Pandemic Period, 2021–2023. Viruses 2025, 17, 577. [Google Scholar] [CrossRef]
- Primo, D.; Pacheco, G.T.; Timenetsky, M.; Luchs, A. Surveillance and molecular characterization of human adenovirus in patients with acute gastroenteritis in the era of rotavirus vaccine, Brazil, 2012–2017. J. Clin. Virol. 2018, 109, 35–40. [Google Scholar] [CrossRef]
- Dian, Z.; Sun, Y.; Zhang, G.; Xu, Y.; Fan, X.; Yang, X.; Pan, Q.; Peppelenbosch, M.; Miao, Z. Rotavirus-related systemic diseases: Clinical manifestation, evidence and pathogenesis. Crit. Rev. Microbiol. 2021, 47, 580–595. [Google Scholar] [CrossRef]
- Gomez-Rial, J.; Rivero-Calle, I.; Salas, A.; Martinon-Torres, F. Rotavirus and autoimmunity. J. Infect. 2020, 81, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, M.B.; de Assis, R.M.S.; Andrade, J.; Fialho, A.M.; Fumian, T.M. Rotavirus A during the COVID-19 Pandemic in Brazil, 2020–2022: Emergence of G6P[8] Genotype. Viruses 2023, 15, 1619. [Google Scholar] [CrossRef]
- Raboni, S.M.; Damasio, G.A.; Ferreira, C.E.; Pereira, L.A.; Nogueira, M.B.; Vidal, L.R.; Cruz, C.R.; Almeida, S.M. Acute gastroenteritis and enteric viruses in hospitalised children in southern Brazil: Aetiology, seasonality and clinical outcomes. Mem. Inst. Oswaldo Cruz 2014, 109, 428–435. [Google Scholar] [CrossRef] [PubMed]
- DATASUS, Ministério da Saúde. Tabnet: Imunizações—Cobertura. 2025. Available online: https://datasus.saude.gov.br/informacoes-de-saude-tabnet/ (accessed on 20 November 2025).
- Oliveira, I.S.; Cardoso, L.S.; Ferreira, I.G.; Alexandre-Silva, G.M.; Jacob, B.; Cerni, F.A.; Monteiro, W.M.; Zottich, U.; Pucca, M.B. Anti-vaccination movements in the world and in Brazil. Rev. Soc. Bras. Med. Trop. 2022, 55, e05922021. [Google Scholar] [CrossRef] [PubMed]
- Vasconcellos-Silva, P.R.; Castiel, L.D.; Griep, R.H. The media-driven risk society, the anti-vaccination movement and risk of autismo. Cienc. Saude Coletiva 2015, 20, 607–616. [Google Scholar] [CrossRef]
- Lo Vecchio, A.; Conelli, M.L.; Guarino, A. Infections and Chronic Diarrhea in Children. Pediatr. Infect. Dis. J. 2021, 40, e255–e258. [Google Scholar] [CrossRef]
- Joshi, M.S.; Lole, K.S.; Barve, U.S.; Salve, D.S.; Ganorkar, N.N.; Chavan, N.A.; Shinde, M.S.; Gopalkrishna, V. Investigation of a large waterborne acute gastroenteritis outbreak caused by group B rotavirus in Maharashtra state, India. J. Med. Virol. 2019, 91, 1877–1881. [Google Scholar] [CrossRef]
- Troeger, C.; Khalil, I.A.; Rao, P.C.; Cao, S.; Blacker, B.F.; Ahmed, T.; Armah, G.; Bines, J.E.; Brewer, T.G.; Colombara, D.V.; et al. Rotavirus Vaccination and the Global Burden of Rotavirus Diarrhea Among Children Younger Than 5 Years. JAMA Pediatr. 2018, 172, 958–965. [Google Scholar] [CrossRef]
- Burke, R.M.; Tate, J.E.; Parashar, U.D. Global Experience With Rotavirus Vaccines. J. Infect. Dis. 2021, 224 (Suppl. S7), S792–S800. [Google Scholar] [CrossRef]
- IBGE. Censo Demográfico 2022—Características dos Domicílios: Resultados do Universo; IBGE: Rio de Janeiro, Brazil, 2024.
- Waggie, Z.; Hawkridge, A.; Hussey, G.D. Review of rotavirus studies in Africa: 1976–2006. J. Infect. Dis. 2010, 202 (Suppl. S1), S23–S33. [Google Scholar] [CrossRef]
- Modaress, S.; Rahbarimanesh, A.A.; Edalat, R.; Sohrabi, A.; Modarres, S.; Gomari, H.; Motamedirad, M.; Sayari, A.A. Human rotavirus genotypes detection among hospitalized children, a study in Tehran, Iran. Arch. Iran. Med. 2011, 14, 39–45. [Google Scholar] [PubMed]
- Leite, J.P.; Carvalho-Costa, F.A.; Linhares, A.C. Group A rotavirus genotypes and the ongoing Brazilian experience: A review. Mem. Inst. Oswaldo Cruz 2008, 103, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Carvalho-Costa, F.A.; Volotao Ede, M.; de Assis, R.M.; Fialho, A.M.; de Andrade Jda, S.; Rocha, L.N.; Tort, L.F.; da Silva, M.F.; Gomez, M.M.; de Souza, P.M.; et al. Laboratory-based rotavirus surveillance during the introduction of a vaccination program, Brazil, 2005–2009. Pediatr. Infect. Dis. J. 2011, 30, S35–S41. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, M.B.; Fialho, A.M.; Maranhao, A.G.; Malta, F.C.; Andrade, J.; Assis, R.M.S.; Mouta, S.; Miagostovich, M.P.; Leite, J.P.G.; Machado Fumian, T. Rotavirus A in Brazil: Molecular Epidemiology and Surveillance during 2018–2019. Pathogens 2020, 9, 515. [Google Scholar] [CrossRef]
- Banyai, K.; Laszlo, B.; Duque, J.; Steele, A.D.; Nelson, E.A.; Gentsch, J.R.; Parashar, U.D. Systematic review of regional and temporal trends in global rotavirus strain diversity in the pre rotavirus vaccine era: Insights for understanding the impact of rotavirus vaccination programs. Vaccine 2012, 30 (Suppl. S1), A122–A130. [Google Scholar] [CrossRef]
- Santos, F.S.; Sousa Junior, E.C.; Guerra, S.F.S.; Lobo, P.S.; Penha Junior, E.T.; Lima, A.B.F.; Vinente, C.B.G.; Chagas, E.H.N.; Justino, M.C.A.; Linhares, A.C.; et al. G1P[8] Rotavirus in children with severe diarrhea in the post-vaccine introduction era in Brazil: Evidence of reassortments and structural modifications of the antigenic VP7 and VP4 regions. Infect. Genet. Evol. 2019, 69, 255–266. [Google Scholar] [CrossRef]
- Gutierrez, M.B.; de Figueiredo, M.R.; Fialho, A.M.; Cantelli, C.P.; Miagostovich, M.P.; Fumian, T.M. Nosocomial acute gastroenteritis outbreak caused by an equine-like G3P[8] DS-1-like rotavirus and GII.4 Sydney[P16] norovirus at a pediatric hospital in Rio de Janeiro, Brazil, 2019. Hum. Vaccines Immunother. 2021, 17, 4654–4660. [Google Scholar] [CrossRef]
- Oliveira Matos, A.; Araujo, M.; Paulino, J.; Franco, F.C.; Luchs, A.; Sales-Campos, H.; Fiaccadori, F.; Souza, M.; Silva-Sales, M. Mutations in the main antigenic sites of VP7 and VP8* from G3P[8] rotavirus a strains circulating in Brazil may impact immune evasion to rotavirus vaccination. Braz. J. Microbiol. 2025, 56, 319–330. [Google Scholar] [CrossRef]
- Li, Y.; Wang, S.; Liang, F.; Teng, S.; Wang, F. Prevalence and genetic diversity of rotavirus among children under 5 years of age in China: A meta-analysis. Front. Immunol. 2024, 15, 1364429. [Google Scholar] [CrossRef]
- Redda, Y.T.; Adamu, H.; Bergholm, J.; Lindahl, J.F.; Blomstrom, A.L.; Berg, M.; Sisay Tessema, T. Rotavirus A genotype diversity and antigenic profile in Central Ethiopia: Implications for rotarix® vaccine efficacy. Front. Microbiol. 2025, 16, 1656797. [Google Scholar] [CrossRef]
- Gonzalez, G.; Carr, M.J.; Byrne, H.; Colgan, A.; Hare, D.; Sawa, H.; De Gascun, C.F.; Matthijnssens, J.; Yandle, Z. Complex evolutionary dynamics including reassortment drive genome diversity in human rotavirus species A circulating in Ireland. Infect. Genet. Evol. 2025, 135, 105848. [Google Scholar] [CrossRef]
- Saikia, K.; Ahmed, R.; Das, B.; Paul, S.; Ray, S.K.; Chandra Deka, R.; Borah, P.P.; Saharia, N.; Namsa, N.D. Impact of Rotavac Vaccine on Hospital-Based Disease Prevalence and Strain Diversity in India: A Systematic Review and Meta-Analysis. Rev. Med. Virol. 2025, 35, e70066. [Google Scholar] [CrossRef]
- Sangsiriwut, K.; Thitanuwat, B.; Saita, T.; Prasertsopon, J.; Lerdsamran, H.; Puthavathana, P.; Noisumdaeng, P. Molecular Surveillance and Genotypic Distribution of Rotavirus A, Norovirus GI and GII in Bangkok Wastewater Treatment Plants During COVID-19 Phase in 2023, Thailand. Food Environ. Virol. 2025, 17, 53. [Google Scholar] [CrossRef]
- Banyai, K.; Gentsch, J.R.; Griffin, D.D.; Holmes, J.L.; Glass, R.I.; Szucs, G. Genetic variability among serotype G6 human rotaviruses: Identification of a novel lineage isolated in Hungary. J. Med. Virol. 2003, 71, 124–134. [Google Scholar] [CrossRef] [PubMed]
- De Grazia, S.; Martella, V.; Rotolo, V.; Bonura, F.; Matthijnssens, J.; Banyai, K.; Ciarlet, M.; Giammanco, G.M. Molecular characterization of genotype G6 human rotavirus strains detected in Italy from 1986 to 2009. Infect. Genet. Evol. 2011, 11, 1449–1455. [Google Scholar] [CrossRef] [PubMed]
- Gerna, G.; Sarasini, A.; Parea, M.; Arista, S.; Miranda, P.; Brussow, H.; Hoshino, Y.; Flores, J. Isolation and characterization of two distinct human rotavirus strains with G6 specificity. J. Clin. Microbiol. 1992, 30, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Ianiro, G.; Delogu, R.; Camilloni, B.; Lorini, C.; Ruggeri, F.M.; Fiore, L. Detection of unusual G6 rotavirus strains in Italian children with diarrhoea during the 2011 surveillance season. J. Med. Virol. 2013, 85, 1860–1869. [Google Scholar] [CrossRef]
- Ndze, V.N.; Esona, M.D.; Achidi, E.A.; Gonsu, K.H.; Doro, R.; Marton, S.; Farkas, S.; Ngeng, M.B.; Ngu, A.F.; Obama-Abena, M.T.; et al. Full genome characterization of human Rotavirus A strains isolated in Cameroon, 2010–2011: Diverse combinations of the G and P genes and lack of reassortment of the backbone genes. Infect. Genet. Evol. 2014, 28, 537–560. [Google Scholar] [CrossRef]
- Nordgren, J.; Nitiema, L.W.; Sharma, S.; Ouermi, D.; Traore, A.S.; Simpore, J.; Svensson, L. Emergence of unusual G6P[6] rotaviruses in children, Burkina Faso, 2009–2010. Emerg. Infect. Dis. 2012, 18, 589–597. [Google Scholar] [CrossRef]
- Simsek, C.; Bloemen, M.; Jansen, D.; Descheemaeker, P.; Reynders, M.; Van Ranst, M.; Matthijnssens, J. Rotavirus vaccine-derived cases in Belgium: Evidence for reversion of attenuating mutations and alternative causes of gastroenteritis. Vaccine 2022, 40, 5114–5125. [Google Scholar] [CrossRef]
- Degiuseppe, J.I.; Martelli, A.; Barrios Mathieur, C.; Stupka, J.A.; Argentinean Viral Gastroenteritis Surveillance, N. Genetic diversity of rotavirus A in Argentina during 2019–2022: Detection of G6 strains and insights regarding its dissemination. Arch. Virol. 2023, 168, 251. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, Y.; Kusuhara, H.; Takai-Todaka, R.; Haga, K.; Katayama, K.; Tsugawa, T. Human transmission and outbreaks of feline-like G6 rotavirus revealed with whole-genome analysis of G6P[9] feline rotavirus. J. Med. Virol. 2024, 96, e29565. [Google Scholar] [CrossRef] [PubMed]
- Silva-Sales, M.; Leal, E.; Milagres, F.A.P.; Brustulin, R.; Morais, V.D.S.; Marcatti, R.; Araujo, E.L.L.; Witkin, S.S.; Deng, X.; Sabino, E.C.; et al. Genomic constellation of human Rotavirus A strains identified in Northern Brazil: A 6-year follow-up (2010–2016). Rev. Inst. Med. Trop. Sao Paulo 2020, 62, e98. [Google Scholar] [CrossRef] [PubMed]
- Moura, P.O.; Roberto, A.F.; Hein, N.; Baldacci, E.; Vieira, S.E.; Ejzenberg, B.; Perrini, P.; Stewien, K.E.; Durigon, E.L.; Mehnert, D.U.; et al. Molecular epidemiology of human adenovirus isolated from children hospitalized with acute respiratory infection in Sao Paulo, Brazil. J. Med. Virol. 2007, 79, 174–181. [Google Scholar] [CrossRef]
- Portal, T.M.; Reymao, T.K.A.; Quindere Neto, G.A.; Fiuza, M.; Teixeira, D.M.; Lima, I.C.G.; Sousa Junior, E.C.; Bandeira, R.D.S.; De Deus, D.R.; Justino, M.C.A.; et al. Detection and genotyping of enteric viruses in hospitalized children with acute gastroenteritis in Belem, Brazil: Occurrence of adenovirus viremia by species F, types 40/41. J. Med. Virol. 2019, 91, 378–384. [Google Scholar] [CrossRef]
- Marsh, K.; Tayler, R.; Pollock, L.; Roy, K.; Lakha, F.; Ho, A.; Henderson, D.; Divala, T.; Currie, S.; Yirrell, D.; et al. Investigation into cases of hepatitis of unknown aetiology among young children, Scotland, 1 January 2022 to 12 April 2022. Eurosurveillance 2022, 27, 2200318. [Google Scholar] [CrossRef]
- Rabaan, A.A.; Bakhrebah, M.A.; Nassar, M.S.; Natto, Z.S.; Al Mutair, A.; Alhumaid, S.; Aljeldah, M.; Garout, M.; Alfouzan, W.A.; Alshahrani, F.S.; et al. Suspected Adenovirus Causing an Emerging HEPATITIS among Children below 10 Years: A Review. Pathogens 2022, 11, 712. [Google Scholar] [CrossRef]
- Sallam, M.; Mahafzah, A.; Sahin, G.O.; On Behalf Of Escmid Study Group For Viral, H.-E. Hepatitis of Unknown Origin and Etiology (Acute Non HepA-E Hepatitis) among Children in 2021/2022: Review of the Current Findings. Healthcare 2022, 10, 973. [Google Scholar] [CrossRef]
- Benko, M.; Aoki, K.; Arnberg, N.; Davison, A.J.; Echavarria, M.; Hess, M.; Jones, M.S.; Kajan, G.L.; Kajon, A.E.; Mittal, S.K.; et al. ICTV Virus Taxonomy Profile: Adenoviridae 2022. J. Gen. Virol. 2022, 103, 001721. [Google Scholar] [CrossRef]
- Radke, J.R.; Cook, J.L. Human adenovirus lung disease: Outbreaks, models of immune-response-driven acute lung injury and pandemic potential. Curr. Opin. Infect. Dis. 2023, 36, 164–170. [Google Scholar] [CrossRef]
- Ruivo, A.P.; da Cruz Bauermann, M.; Gregianini, T.S.; Dos Santos, F.M.; Godinho, F.; Baethgen, L.F.; Machado, T.R.M.; Martins, L.G.; Mondini, R.P.; Port, C.N.; et al. Surveillance of respiratory viruses in severe acute respiratory infections in Southern Brazil, 2023–2024. BMC Infect. Dis. 2025, 25, 1163. [Google Scholar] [CrossRef] [PubMed]
- Kachooei, A.; Ataei-Pirkooh, A.; Mir-Hosseinian, M.; Behnezhad, F.; Eftekhari, M.; Hoseini-Fakhr, S.S.; Jalilvand, S.; Latifi, T.; Khouy, R.A.; Shoja, Z. Viral co-infections in pediatric acute gastroenteritis: Epidemiology of rotavirus, norovirus, adenovirus, and astrovirus in Tehran, Iran (2021–2022). BMC Infect. Dis. 2025, 25, 1319. [Google Scholar] [CrossRef] [PubMed]
- Tahmasebi, R.; Costa, A.C.D.; Tardy, K.; Tinker, R.J.; Milagres, F.A.P.; Brustulin, R.; Teles, M.; Chagas, R.T.D.; Soares, C.; Watanabe, A.S.A.; et al. Genomic Analyses of Potential Novel Recombinant Human Adenovirus C in Brazil. Viruses 2020, 12, 508. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.S.; Huang, Z.M.; Weng, Y.W.; Chen, F.Q.; Zhang, Y.L.; Lin, W.D.; Yu, T.T. Prevalence and Genotypes of Rotavirus A and Human Adenovirus among Hospitalized Children with Acute Gastroenteritis in Fujian, China, 2009–2017. Biomed. Environ. Sci. 2019, 32, 210–214. [Google Scholar] [CrossRef]
- Tang, X.; Hu, Y.; Zhong, X.; Xu, H. Molecular Epidemiology of Human Adenovirus, Astrovirus, and Sapovirus Among Outpatient Children With Acute Diarrhea in Chongqing, China, 2017–2019. Front. Pediatr. 2022, 10, 826600. [Google Scholar] [CrossRef]
- Kumthip, K.; Khamrin, P.; Ushijima, H.; Maneekarn, N. Enteric and non-enteric adenoviruses associated with acute gastroenteritis in pediatric patients in Thailand, 2011 to 2017. PLoS ONE 2019, 14, e0220263. [Google Scholar] [CrossRef]
- Gelaw, A.; Pietsch, C.; Liebert, U.G. Genetic diversity of human adenovirus and human astrovirus in children with acute gastroenteritis in Northwest Ethiopia. Arch. Virol. 2019, 164, 2985–2993. [Google Scholar] [CrossRef]
- Qiu, F.Z.; Shen, X.X.; Li, G.X.; Zhao, L.; Chen, C.; Duan, S.X.; Guo, J.Y.; Zhao, M.C.; Yan, T.F.; Qi, J.J.; et al. Adenovirus associated with acute diarrhea: A case-control study. BMC Infect. Dis. 2018, 18, 450. [Google Scholar] [CrossRef]
- Gotting, J.; Baier, C.; Panagiota, V.; Maecker-Kolhoff, B.; Dhingra, A.; Heim, A. High genetic stability of co-circulating human adenovirus type 31 lineages over 59 years. Virus Evol. 2022, 8, veac067. [Google Scholar] [CrossRef]
- Fattouh, R.; Stapleton, P.J.; Eshaghi, A.; Thomas, A.D.; Science, M.E.; Schechter, T.; Streitenberger, L.; Hubacek, P.; Yau, Y.C.W.; Brown, M.; et al. A Prolonged Outbreak of Human Adenovirus A31 (HAdV-A31) Infection on a Pediatric Hematopoietic Stem Cell Transplantation Ward with Whole Genome Sequencing Evidence of International Linkages. J. Clin. Microbiol. 2022, 60, e0066522. [Google Scholar] [CrossRef]
- Lu, L.; Zhong, H.; Xu, M.; Su, L.; Cao, L.; Jia, R.; Xu, J. Molecular and epidemiological characterization of human adenovirus and classic human astrovirus in children with acute diarrhea in Shanghai, 2017–2018. BMC Infect. Dis. 2021, 21, 713. [Google Scholar] [CrossRef]
- Liu, L.; Qian, Y.; Zhang, Y.; Zhao, L.; Jia, L.; Dong, H. Epidemiological aspects of rotavirus and adenovirus in hospitalized children with diarrhea: A 5-year survey in Beijing. BMC Infect. Dis. 2016, 16, 508. [Google Scholar] [CrossRef]
| Characteristics | Overall (n = 114) | HAdV Positive (n = 16) | RVA Positive (n = 13) | Coinfection (n = 2) | p1 (n = 114) |
|---|---|---|---|---|---|
| Age | |||||
| 0–6 months | 56 (49.1%) | 6 (37.5%) | 7 (53.8%) | 1 (50%) | 0.76 |
| 7–12 months | 12 (10.5%) | 2 (12.5%) | 0 (0%) | 0 (0%) | |
| 13–24 months | 20 (17.5%) | 5 (31.25%) | 2 (15.4%) | 0 (0%) | |
| 25–60 months | 26 (22.8%) | 3 (18.75%) | 4 (30.8%) | 1 (50%) | |
| Gender | |||||
| Male | 66 (57.9%) | 7 (43.75%) | 8 (61.54%) | 2 (100%) | 0.41 |
| Female | 48 (42.1%) | 9 (56.25%) | 5 (38.46%) | 0 (0%) | |
| Mesoregions | |||||
| Center | 90 (78.95%) | 13 (81.25%) | 12 (92.3%) | 2 (100%) | 0.792 |
| East | 3 (2.6%) | 1 (6.25%) | 0 (0%) | 0 (0%) | |
| Northwest | 2 (1.75%) | 1 (6.25%) | 0 (0%) | 0 (0%) | |
| North | 0 (0%) | 0 | 0 (0%) | 0 (0%) | |
| South | 13 (11.4%) | 1 (6.25%) | 0 (0%) | 0 (0%) | |
| Other states | 6 (5.3%) | 0 | 1 (7.7%) | 0 (0%) | |
| Family income | |||||
| 0–1 salary | 44 (38.6%) | 9 (56.25%) | 7 (53.85%) | 0 (0%) | 0.142 |
| 1.1–2 salaries | 27 (23.7%) | 4 (25%) | 1 (7.7%) | 0 (0%) | |
| 2.1–3 salaries | 11 (9.6%) | 0 (0%) | 2 (15.4%) | 0 (0%) | |
| 3.1–4 salaries | 2 (1.8%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| >4 salaries | 12 (10.5%) | 2 (12.5%) | 0 (0%) | 0 (0%) | |
| N.I. | 18 (15.8%) | 1 (6.25%) | 3 (23.1%) | 2 (100%) | |
| Vaccination | |||||
| No | 51 (44.73%) | 3 (18.75%) | 6 (46.1%) | 1 (50%) | 0.383 |
| Incomplete | 15 (13.16%) | 2 (12.5%) | 2 (15.4%) | 0 (0%) | |
| Complete | 48 (42.1%) | 11 (68.75%) | 5 (38.5%) | 1 (50%) | |
| Pavement | |||||
| No | 14 (12.3%) | 1 (6.25%) | 2 (15.4%) | 1 (50%) | 0.347 |
| Yes | 100 (87.7%) | 15 (93.75%) | 11 (84.6%) | 1 (50%) | |
| Treated water | |||||
| No | 22 (19.3%) | 5 (31.25%) | 3 (23.1%) | 1 (50%) | 0.321 |
| Yes | 92 (80.7%) | 11 (68.75%) | 10 (76.9%) | 1 (50%) | |
| Sewage system | |||||
| No | 24 (21.1%) | 3 (18.75%) | 3 (23.1%) | 0 (0%) | 0.888 |
| Yes | 90 (78.9%) | 13 (81.25%) | 10 (76.9%) | 2 (100%) | |
| School attendance | |||||
| No | 83 (72.8%) | 12 (75%) | 10 (76.9%) | 0 (0%) | 0.194 |
| Yes | 25 (21.9%) | 4 (25%) | 2 (15.4%) | 2 (100%) | |
| N.I. | 6 (5.3%) | 0 (0%) | 1 (7.7%) | 0 (0%) |
| Characteristics | HAdV Positive (n = 16) | RVA Positive (n = 13) | Co-Infection (n = 2) | p1 |
|---|---|---|---|---|
| Number of symptoms | ||||
| 0 | 4 (25%) | 3 (23.1%) | 1 (50%) | 0.101 |
| 1 | 1 (6.25%) | 4 (30.8%) | 0 (0%) | |
| 2 | 9 (56.25%) | 2 (15.4%) | 0 (0%) | |
| 3 | 2 (12.5%) | 2 (15.4%) | 0 (0%) | |
| 4 | 0 (0%) | 2 (15.4%) | 1 (50%) | |
| Symptoms | ||||
| Diarrhea | 7 | 8 | 1 | 0.634 |
| Vomiting | 5 | 5 | 1 | 0.835 |
| Fever | 6 | 5 | 1 | 0.943 |
| Abdominal pain | 7 | 5 | 1 | 0.933 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paulino, J.G.; Matos, A.d.O.; Passos, Y.G.; Franco, F.; Fiaccadori, F.S.; Sales-Campos, H.; Luchs, A.; Souza, M.; Silva-Sales, M. Hidden Circulation and Socio-Sanitary Vulnerability: Rotavirus A and Human Adenovirus Prevalence in Symptomatic and Asymptomatic Children in Central Brazil Post-COVID-19. Pathogens 2025, 14, 1258. https://doi.org/10.3390/pathogens14121258
Paulino JG, Matos AdO, Passos YG, Franco F, Fiaccadori FS, Sales-Campos H, Luchs A, Souza M, Silva-Sales M. Hidden Circulation and Socio-Sanitary Vulnerability: Rotavirus A and Human Adenovirus Prevalence in Symptomatic and Asymptomatic Children in Central Brazil Post-COVID-19. Pathogens. 2025; 14(12):1258. https://doi.org/10.3390/pathogens14121258
Chicago/Turabian StylePaulino, Jordana Gomes, Amanda de Oliveira Matos, Yasmin Gomes Passos, Fernanda Franco, Fabiola Souza Fiaccadori, Helioswilton Sales-Campos, Adriana Luchs, Menira Souza, and Marcelle Silva-Sales. 2025. "Hidden Circulation and Socio-Sanitary Vulnerability: Rotavirus A and Human Adenovirus Prevalence in Symptomatic and Asymptomatic Children in Central Brazil Post-COVID-19" Pathogens 14, no. 12: 1258. https://doi.org/10.3390/pathogens14121258
APA StylePaulino, J. G., Matos, A. d. O., Passos, Y. G., Franco, F., Fiaccadori, F. S., Sales-Campos, H., Luchs, A., Souza, M., & Silva-Sales, M. (2025). Hidden Circulation and Socio-Sanitary Vulnerability: Rotavirus A and Human Adenovirus Prevalence in Symptomatic and Asymptomatic Children in Central Brazil Post-COVID-19. Pathogens, 14(12), 1258. https://doi.org/10.3390/pathogens14121258







