Description of a Virulent Systemic Feline Calicivirus Infection in a Kitten with Footpads Oedema and Fatal Pneumonia
Abstract
1. Introduction
2. Materials and Methods
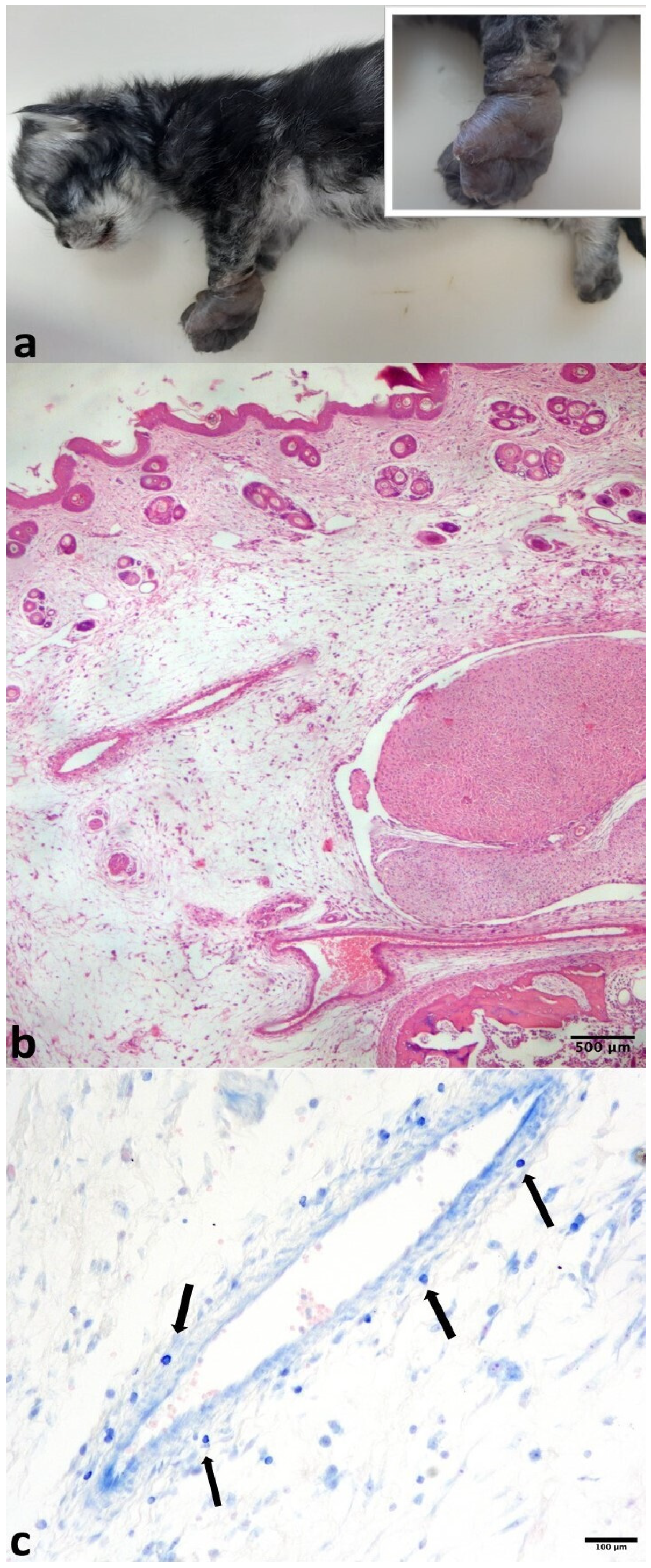
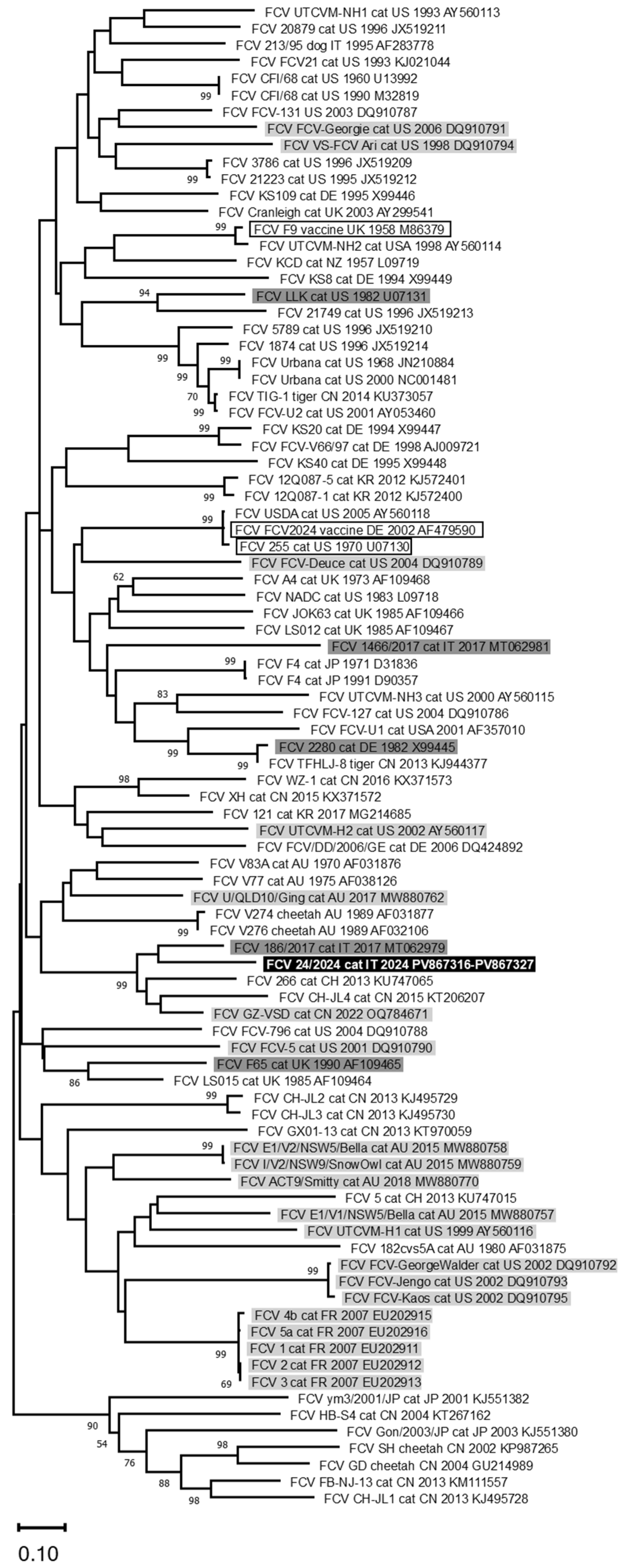
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Radford, A.D.; Coyne, K.P.; Dawson, S.; Porter, C.J.; Gaskell, R.M. Feline Calicivirus. Vet. Res. 2007, 38, 319–335. [Google Scholar] [CrossRef]
- Bannasch, M.J.; Foley, J.E. Epidemiologic evaluation of multiple respiratory pathogens in cats in animal shelters. J. Feline Med. Surg. 2005, 7, 109–119. [Google Scholar] [CrossRef]
- Coyne, K.P.; Jones, B.R.D.; Kipar, A.; Chantrey, J.; Porter, C.J.; Barber, P.J.; Dawson, S.; Gaskell, R.M.; Radford, A.D. Lethal outbreak of disease associated with feline calicivirus infection in cats. Vet. Rec. 2006, 158, 544–550. [Google Scholar] [CrossRef]
- Hofmann-Lehmann, R.; Hosie, M.J.; Hartmann, K.; Egberink, H.; Truyen, U.; Tasker, S.; Belák, S.; Boucraut-Baralon, C.; Frymus, T.; Lloret, A.; et al. Calicivirus infection in cats. Viruses 2022, 14, 937. [Google Scholar] [CrossRef]
- Clay, S.; Maherchandani, S.; Malik, Y.S.; Goyal, S.M. Survival on uncommon fomites of feline calicivirus, a surrogate of noroviruses. Am. J. Infect. Control 2006, 34, 41–43. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Lee, D.; Hong, Y.J.; Hwang, C.Y.; Hyun, J.E. Outbreaks of nosocomial feline calicivirus-associated virulent systemic disease in Korea. J. Vet. Sci. 2024, 25, e51. [Google Scholar] [CrossRef]
- Spiri, A.M. An Update on Feline Calicivirus. Schweiz. Arch. Tierheilkd. 2022, 164, 225–241. [Google Scholar] [CrossRef] [PubMed]
- Wardley, R.C. Feline calicivirus carrier state a study of the host/virus relationship. Arch. Virol. 1976, 52, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Coyne, K.P.; Dawson, S.; Radford, A.D.; Cripps, P.J.; Porter, C.J.; McCraken, C.M.; Gaskell, R.M. Long term analysis of feline calicivirus prevalence and viral shedding patterns in naturally infected colonies of domestic cats. Vet. Microbiol. 2006, 118, 12–25. [Google Scholar] [CrossRef]
- Fried, W.A.; Soltero-Rivera, M.; Ramesh, A.; Lommer, M.J.; Arzi, B.; DeRisi, J.L.; Horst, J.A. Use of unbiased metagenomic and transcriptomic analyses to investigate the association between feline calicivirus and feline chronic gingivostomatitis in domestic cats. Am. J. Vet. Res. 2021, 82, 381. [Google Scholar] [CrossRef]
- Balboni, A.; Verin, R.; Buldrini, I.; Zamagni, S.; Morini, M.; Terrusi, A.; Gallina, L.; Urbani, L.; Dondi, F.; Battilani, M. Natural cases of polyarthritis associated with feline calicivirus infection in cats. Vet. Res. Commun. 2022, 46, 613–619. [Google Scholar] [CrossRef]
- Di Martino, B.; Lanave, G.; Di Profio, F.; Melegari, I.; Marsilio, F.; Camero, M.; Catella, C.; Capozza, P.; Bányai, K.; Barrs, V.R.; et al. Identification of feline calicivirus in cats with enteritis. Transbound Emerg. Dis. 2020, 67, 2579–2588. [Google Scholar] [CrossRef]
- Caringella, F.; Elia, G.; Decaro, N.; Martella, V.; Lanave, G.; Varello, K.; Catella, C.; Diakoudi, G.; Carelli, G.; Colaianni, M.L.; et al. Feline calicivirus infection in cats with virulent systemic disease, Italy. Res. Vet. Sci. 2019, 124, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Bordicchia, M.; Fumian, T.M.; Van Brussel, K.; Russo, A.G.; Carrai, M.; Le, S.-J.; Pesavento, P.A.; Holmes, E.C.; Martella, V.; White, P.; et al. Feline calicivirus virulent systemic disease: Clinical epidemiology, analysis of viral isolates and in vitro efficacy of novel antivirals in Australian outbreaks. Virus 2021, 13, 2040. [Google Scholar] [CrossRef]
- Radford, A.D.; Afonso, M.; Sykes, J.E. Feline calicivirus infections. In Greene’s Infectious Diseases of the Dog and Cat, 5th ed.; Sykes, J.E., Ed.; Elsevier: St. Louis, MO, USA, 2023; pp. 443–454. [Google Scholar]
- Duclos, A.A.; Guzmàn Ramos, P.J.; Mooney, C.T. Virulent systemic feline calicivirus infection: A case report and first description in Ireland. Ir. Vet. J. 2024, 77, 1. [Google Scholar] [CrossRef]
- Wei, Y.; Zeng, Q.; Gou, H.; Bao, S. Update on feline calicivirus: Viral evolution, pathogenesis, epidemiology, prevention and control. Front. Microbiol. 2024, 15, 1388420. [Google Scholar] [CrossRef]
- Pedersen, N.C.; Elliott, J.B.; Glasgow, A.; Poland, A.; Keel, K. An isolated epizootic of hemorrhagic-like fever in cats caused by a novel and highly virulent strain of feline calicivirus. Vet. Microbiol. 2000, 73, 281–300. [Google Scholar] [CrossRef] [PubMed]
- Hurley, K.F.; Sykes, J.E. Update on feline calicivirus: New trends. The Veterinary clinics of North America. Small Anim. Pract. 2003, 33, 759–772. [Google Scholar] [CrossRef] [PubMed]
- Battilani, M.; Vaccari, F.; Carelle, M.S.; Morandi, F.; Benazzi, C.; Kipar, A.; Dondi, F.; Scagliarini, A. Virulent feline calicivirus disease in a shelter in Italy: A case description. Res. Vet. Sci. 2013, 95, 283–290. [Google Scholar] [CrossRef]
- Monné Rodriguez, J.M.; Soare, T.; Malbon, A.; Blundell, R.; Papoula-Pereira, R.; Leeming, G.; Köhler, K.; Kipar, A. Alveolar macrophages are the main target cells in feline calicivirus-associated pneumonia. Vet. J. 2014, 201, 156–165. [Google Scholar] [CrossRef]
- Helps, C.; Lait, P.; Tasker, S.; Harbour, D. Melting curve analysis of feline calicivirus isolates detected by real-time reverse transcription PCR. J. Virol. Methods 2002, 106, 241–244. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Gallina, L.; Facile, V.; Roda, N.; Sabetti, M.C.; Terrusi, A.; Urbani, L.; Magliocca, M.; Vasylyeva, K.; Dondi, F.; Balboni, A.; et al. Molecular investigation and genetic characterization of feline leukemia virus (FeLV) in cats referred to a veterinary teaching hospital in Northern Italy. Vet. Res. Commun. 2024, 48, 2683–2689. [Google Scholar] [CrossRef]
- Radford, A.D.; Dawson, S.; Kerins, A.M.; Sommerville, L.M.; Ryvar, R.; Gaskell, R.M. Molecular analysis of isolates of feline calicivirus from a population of cats in a rescue shelter. Vet. Record 2001, 149, 477–481. [Google Scholar] [CrossRef]
- Lanave, G.; Buonavoglia, A.; Pellegrini, F.; Di Martino, B.; Di Profio, F.; Diakoudi, G.; Catella, C.; Omar, A.H.; Vasinioti, V.I.; Cardone, R.; et al. An outbreak of limping syndrome associated with feline calicivirus. Animals 2023, 13, 1778. [Google Scholar] [CrossRef]
- Palombieri, A.; Sarchese, V.; Giordano, M.V.; Fruci, P.; Crisi, P.E.; Aste, G.; Bongiovanni, L.; Rinaldi, V.; Sposato, A.; Camero, M.; et al. Detection and Characterization of Feline Calicivirus Associated with Paw and Mouth Disease. Animals 2022, 13, 65. [Google Scholar] [CrossRef]
- Helps, C.R.; Lait, P.; Damhuis, A.; Björnehammar, U.; Bolta, D.; Brovida, C.; Chabanne, L.; Egberink, H.; Ferrand, G.; Fontbonne, A.; et al. Factors associated with upper respiratory tract disease caused by feline herpesvirus, feline calicivirus, Chlamydophila felis and Bordetella bronchiseptica in cats: Experience from 218 European catteries. Vet. Record 2005, 156, 669–673. [Google Scholar] [CrossRef] [PubMed]
- Hurley, K.E.; Pesavento, P.A.; Pedersen, N.C.; Poland, A.M.; Wilson, E.; Foley, J.E. An outbreak of virulent systemic feline calicivirus disease. J. Am. Vet. Med. Assoc. 2004, 224, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Foley, J.; Hurley, K.; Pesavento, P.A.; Poland, A.; Pedersen, N.C. Virulent systemic feline calicivirus infection: Local cytokine modulation and contribution of viral mutants. J. Feline Med. Surg. 2006, 8, 55–61. [Google Scholar] [CrossRef]
- Willi, B.; Spiri, A.M.; Meli, M.L.; Samman, A.; Hoffmann, K.; Sydler, T.; Cattori, V.; Graf, F.; Diserens, K.A.; Padrutt, I.; et al. Molecular characterization and virus neutralization patterns of severe, non-epizootics forms of feline calicivirus infections resembling virulent systemic disease in cats in Switzerland and in Liechtenstein. Vet. Microbiol. 2016, 182, 202–212. [Google Scholar] [CrossRef]
- Pesavento, P.A.; Maclachlan, N.J.; Dillard-Telm, L.; Grant, C.K.; Hurley, K.F. Pathologic, immunohistochemical, and electron microscopic findings in naturally occurring virulent systemic feline calicivirus infection in cats. Vet. Pathol. 2004, 41, 257–263. [Google Scholar] [CrossRef]
- Cai, Y.; Fukushi, H.; Koyasu, S.; Kuroda, E.; Yamaguchi, T.; Hirai, K. An etiological investigation of domestic cats with conjunctivitis and upper respiratory tract disease in Japan. J. Vet. Med. Sci. 2002, 64, 215–219. [Google Scholar] [CrossRef]
- Gerriets, W.; Joy, N.; Huebner-Guthardt, J.; Eule, J.C. Feline calicivirus: A neglected cause of feline ocular surface infecions? Vet. Ophthalmol. 2012, 15, 172–179. [Google Scholar] [CrossRef]
- Ossiboff, R.J.; Sheh, A.; Shotton, J.; Pesavento, P.A.; Parker, J.S.L. Feline caliciviruses (FCVs) isolated from cats with virulent systemic disease possess in vitro phenotypes distinct from those of other FCV isolates. J. Gen. Virol. 2007, 88, 506–517. [Google Scholar] [CrossRef]
- Pesavento, P.A.; Chang, K.O.; Parker, J.S.L. Molecular Virology of Feline Calicivirus. Vet. Clin. N. Am. Small Anim. Pract. 2008, 38, 775–786. [Google Scholar] [CrossRef]
- Reynolds, B.S.; Poulet, H.; Pingret, J.L.; Jas, D.; Brunet, S.; Lemeter, C.; Etievant, M.; Boucraut-Baralon, C. A nosocomial outbreak of feline calicivirus associated virulent systemic disease in France. J. Feline Med. Surg. 2009, 11, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Coyne, K.P.; Gaskell, R.M.; Dawson, S.; Porter, C.J.; Radford, A.D. Evolutionary mechanisms of persistence and diversification of a calicivirus within endemically infected natural host populations. J. Virol. 2007, 81, 1961–1971. [Google Scholar] [CrossRef]
- Radford, A.D.; Dawson, S.; Ryvar, R.; Coyne, K.; Johnson, D.R.; Cox, M.B.; Acke, E.F.J.; Addie, D.D.; Gaskell, R.M. High genetic diversity of the immunodominant region of the feline calicivirus capsid gene in endemically infected cat colonies. Virus Genes 2003, 27, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Slaviero, M.; Ehlers, L.P.; Argenta, F.F.; Savi, C.; Lopes, B.C.; Pavarini, S.P.; Driemeier, D.; Sonne, L. Causes and lesions of fatal pneumonia in domestic cats. J. Comp. Pathol. 2021, 189, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Sánchez-Vizcaíno, F.; McGahie, D.; Lesbros, C.; Almeras, T.; Howarth, D.; O’Hara, V.; Dawson, S.; Radford, A.D. European molecular epidemiology and strain diversity of feline calicivirus. Vet. Record 2016, 178, 114–115. [Google Scholar] [CrossRef]
- Schorr-Evans, E.M.; Poland, A.; Johnson, W.E.; Pedersen, N.C. An epizootic of highly virulent feline calicivirus disease in a hospital setting in New England. J. Feline Med. Surg. 2003, 5, 217–226. [Google Scholar] [CrossRef] [PubMed]

| Sample | Target RNA Quantity * | Ct |
|---|---|---|
| Lung | 9 × 105 | 18 |
| Right forelimb | 3 × 102 | 31 |
| Left forelimb | 5 × 103 | 26 |
| Right hind limb | 2 × 102 | 31 |
| Left hind limb | 2 × 102 | 31 |
| Spleen | 9 × 102 | 29 |
| Liver | 5 × 102 | 30 |
| Upper respiratory system | 3 × 102 | 30 |
| Heart | 1 × 103 | 28 |
| Kidney | 5 × 101 | 33 |
| Brain | 6 × 100 | 36 |
| Bone marrow | negative | - |
| Oropharyngeal swab | 3 × 104 | 24 |
| Conjunctival swab | negative | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magliocca, M.; Mandrioli, L.; Battilani, M.; Bacci, B.; Ballotta, G.; Anjomanibenisi, M.; Urbani, L.; Martella, L.; Facile, V.; Scarpellini, R.; et al. Description of a Virulent Systemic Feline Calicivirus Infection in a Kitten with Footpads Oedema and Fatal Pneumonia. Pathogens 2025, 14, 1183. https://doi.org/10.3390/pathogens14111183
Magliocca M, Mandrioli L, Battilani M, Bacci B, Ballotta G, Anjomanibenisi M, Urbani L, Martella L, Facile V, Scarpellini R, et al. Description of a Virulent Systemic Feline Calicivirus Infection in a Kitten with Footpads Oedema and Fatal Pneumonia. Pathogens. 2025; 14(11):1183. https://doi.org/10.3390/pathogens14111183
Chicago/Turabian StyleMagliocca, Martina, Luciana Mandrioli, Mara Battilani, Barbara Bacci, Giulia Ballotta, Maral Anjomanibenisi, Lorenza Urbani, Liliana Martella, Veronica Facile, Raffaele Scarpellini, and et al. 2025. "Description of a Virulent Systemic Feline Calicivirus Infection in a Kitten with Footpads Oedema and Fatal Pneumonia" Pathogens 14, no. 11: 1183. https://doi.org/10.3390/pathogens14111183
APA StyleMagliocca, M., Mandrioli, L., Battilani, M., Bacci, B., Ballotta, G., Anjomanibenisi, M., Urbani, L., Martella, L., Facile, V., Scarpellini, R., Ascenzi, I., Gallina, L., & Balboni, A. (2025). Description of a Virulent Systemic Feline Calicivirus Infection in a Kitten with Footpads Oedema and Fatal Pneumonia. Pathogens, 14(11), 1183. https://doi.org/10.3390/pathogens14111183








