Bacterial Proteomics and Antibiotic Resistance Identification: Is Single-Cell Analysis a Worthwhile Pursuit?
Abstract
1. Introduction
2. Antibiotic Resistance and Its Identification
3. Bacterial Proteomics and Antibiotic Resistance Identification
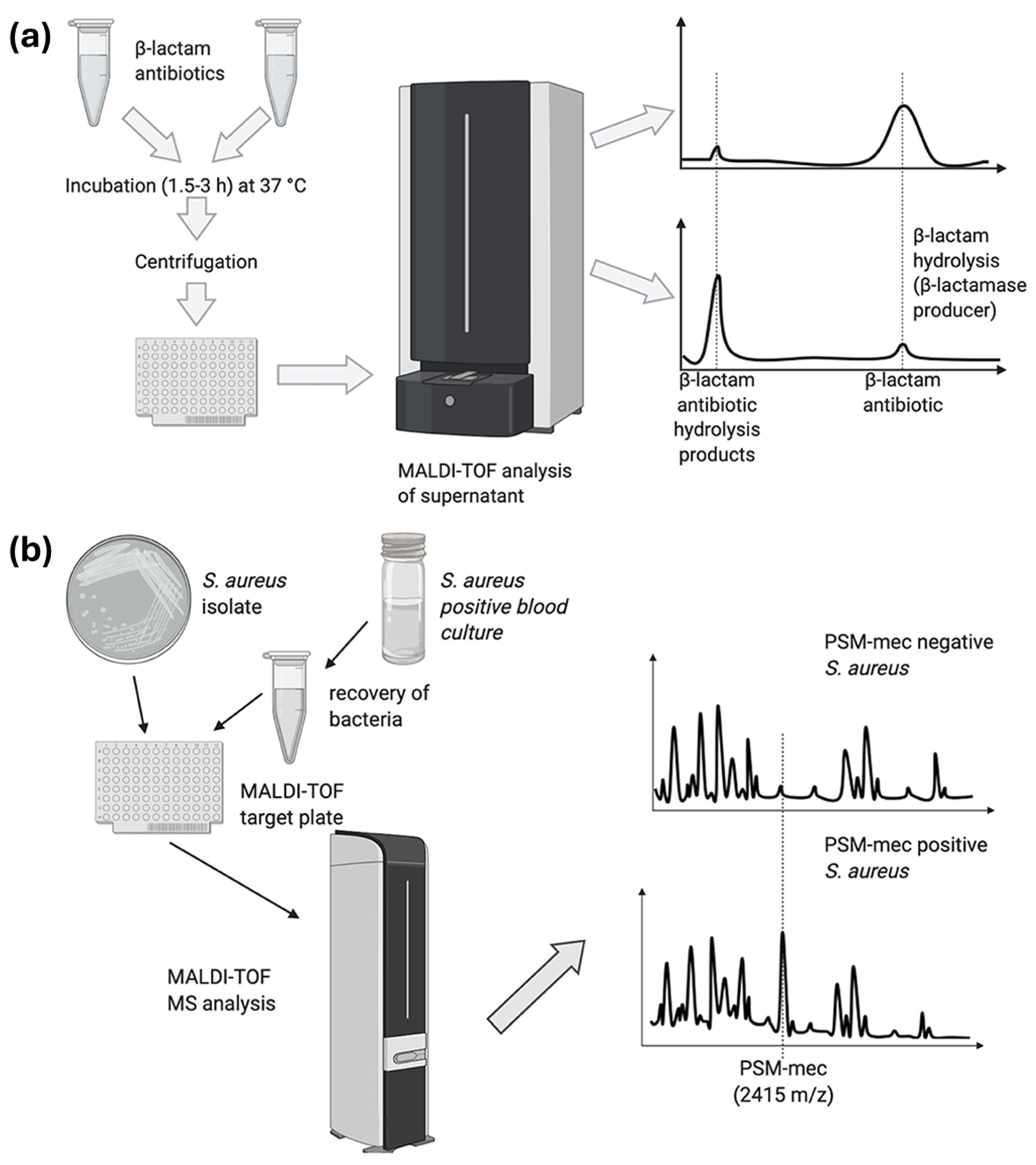
3.1. MALDI-TOF-MS-Based Top-Down Proteomics for Antibiotic Resistance Identification
3.2. Non-MALDI-TOF-MS-Based Top-Down Proteomics for Antibiotic Resistance Identification
3.3. Discovery Bottom-Up Proteomics for Antibiotic Resistance Identification
4. Bacterial Single-Cell Proteomics and Antibiotic Resistance Identification
5. Core Challenges of Bacterial Single-Cell Proteomics
5.1. Diversity of Bacterial Populations
5.2. Structure of Bacterial Cell Exterior
5.3. Bacterial Cell Size and Proteome Amount
5.4. Nature and Composition of Bacterial Proteome
5.5. Identification of Resistance-Inducing Proteins
5.6. Data Analysis
6. Emerging Technologies for Bacterial Single-Cell Proteomics
7. Beyond Bacterial Cellular Proteomes for Antibiotic Resistance Identification
7.1. Bacterial Secretory and Membrane Proteome for Antibiotic Resistance Identification
7.2. Biofilm Proteome for Antibiotic Resistance Identification
8. Future Outlook
9. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Analyzing Protein Structure and Function. In Molecular Biology of the Cell, 4th ed.; Garland Science: New York, NY, USA, 2002. [Google Scholar]
- Garrels, J.I. Proteome. In Encyclopaedia of Genetics; Brenner, S., Miller, J.H., Eds.; Academic Press: London, UK, 2001; pp. 1575–1578. [Google Scholar]
- Beynon, R.J. The dynamics of the proteome: Strategies for measuring protein turnover on a proteome-wide scale. Brief. Funct. Genom. Proteom. 2005, 3, 382–390. [Google Scholar] [CrossRef]
- Graves, P.R.; Haystead, T.A.J. Molecular Biologist’s Guide to Proteomics. Microbiol. Mol. Biol. Rev. 2002, 66, 39–63. [Google Scholar] [CrossRef]
- Ayon, N.J.; Sharma, A.D.; Gutheil, W.G. LC-MS/MS-Based Separation and Quantification of Marfey’s Reagent Derivatized Proteinogenic Amino Acid dl-Stereoisomers. J. Am. Soc. Mass Spectrom. 2019, 30, 448–458. [Google Scholar] [CrossRef]
- Ayon, N.J. Features, roles and chiral analyses of proteinogenic amino acids. AIMS Mol. Sci. 2020, 7, 229–268. [Google Scholar] [CrossRef]
- Ayon, N.J.; Vemula, H.; Gutheil, W.G. Analytical method development for the determination of D-and L-amino acids by high performance liquid chromatography tandem mass spectrometry. In Proceedings of the Pharmaceutics Graduate Student Research Meeting (PGSRM) 2016, Kansas City, MO, USA, 17 June 2016. [Google Scholar]
- Ayon, N.J. Preanalytical Strategies for Native Mass Spectrometry Analysis of Protein Modifications, Complexes, and Higher-Order Structures. Preprints 2025. [Google Scholar] [CrossRef]
- Shuken, S.R. An Introduction to Mass Spectrometry-Based Proteomics. J. Proteome Res. 2023, 22, 2151–2171. [Google Scholar] [CrossRef]
- Bekker-Jensen, D.B.; Martínez-Val, A.; Steigerwald, S.; Rüther, P.; Fort, K.L.; Arrey, T.N.; Harder, A.; Makarov, A.; Olsen, J.V. A Compact Quadrupole-Orbitrap Mass Spectrometer with FAIMS Interface Improves Proteome Coverage in Short LC Gradients. Mol. Cell. Proteom. 2020, 19, 716–729. [Google Scholar] [CrossRef]
- O’Connor, C.; Adams, J.U. Tissues Are Organized Communities of Different Cell Types. In Essentials of Cell Biology; NPG Education: Cambrige, UK, 2010. [Google Scholar]
- Yuan, G.C.; Cai, L.; Elowitz, M.; Enver, T.; Fan, G.; Guo, G.; Irizarry, R.; Kharchenko, P.; Kim, J.; Orkin, S.; et al. Challenges and emerging directions in single-cell analysis. Genome Biol. 2017, 18, 84. [Google Scholar] [CrossRef] [PubMed]
- Diz, A.P.; Truebano, M.; Skibinski, D.O.F. The consequences of sample pooling in proteomics: An empirical study. Electrophoresis 2009, 30, 2967–2975. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Budnik, B. A review of the current state of single-cell proteomics and future perspective. Anal. Bioanal. Chem. 2023, 415, 6889–6899. [Google Scholar] [CrossRef] [PubMed]
- Budnik, B.; Levy, E.; Harmange, G.; Slavov, N. SCoPE-MS: Mass spectrometry of single mammalian cells quantifies proteome heterogeneity during cell differentiation. Genome Biol. 2018, 19, 161. [Google Scholar] [CrossRef]
- Su, P.; Hollas, M.A.R.; Butun, F.A.; Kanchustambham, V.L.; Rubakhin, S.; Ramani, N.; Greer, J.B.; Early, B.P.; Fellers, R.T.; Caldwell, M.A.; et al. Single Cell Analysis of Proteoforms. J. Proteome Res. 2024, 23, 1883–1893. [Google Scholar] [CrossRef]
- Su, P.; Hollas, M.A.R.; Pla, I.; Rubakhin, S.; Butun, F.A.; Greer, J.B.; Early, B.P.; Fellers, R.T.; Caldwell, M.A.; Sweedler, J.V.; et al. Proteoform profiling of endogenous single cells from rat hippocampus at scale. Nat. Biotechnol. 2025. [Google Scholar] [CrossRef] [PubMed]
- Eberwine, J.; Sul, J.-Y.; Bartfai, T.; Kim, J. The promise of single-cell sequencing. Nat. Methods 2014, 11, 25–27. [Google Scholar] [CrossRef] [PubMed]
- Aldridge, S.; Teichmann, S.A. Single cell transcriptomics comes of age. Nat. Commun. 2020, 11, 4307. [Google Scholar] [CrossRef]
- Mansuri, M.S.; Williams, K.; Nairn, A.C. Uncovering biology by single-cell proteomics. Commun. Biol. 2023, 6, 381. [Google Scholar] [CrossRef]
- Kelly, R.T. Single-cell Proteomics: Progress and Prospects. Mol. Cell. Proteom. MCP 2020, 19, 1739–1748. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Avila, X.; de Oliveira, R.M.; Huang, S.; Wang, C.; Kelly, R.T. Trends in Mass Spectrometry-Based Single-Cell Proteomics. Anal. Chem. 2025, 97, 5893–5907. [Google Scholar] [CrossRef]
- Petrosius, V.; Aragon-Fernandez, P.; Arrey, T.N.; Woessmann, J.; Üresin, N.; de Boer, B.; Su, J.; Furtwängler, B.; Stewart, H.; Denisov, E.; et al. Quantitative Label-Free Single-Cell Proteomics on the Orbitrap Astral MS. Mol. Cell. Proteom. 2025, 24, 100982. [Google Scholar] [CrossRef] [PubMed]
- Grenga, L.; Pible, O.; Armengaud, J. Pathogen proteotyping: A rapidly developing application of mass spectrometry to address clinical concerns. Clin. Mass Spectrom. 2019, 14, 9–17. [Google Scholar] [CrossRef]
- Sauer, S.; Kliem, M. Mass spectrometry tools for the classification and identification of bacteria. Nat. Rev. Microbiol. 2010, 8, 74–82. [Google Scholar] [CrossRef]
- Abele, M.; Soleymaniniya, A.; Bayer, F.P.; Lomp, N.; Doll, E.; Meng, C.; Neuhaus, K.; Scherer, S.; Wenning, M.; Wantia, N.; et al. Proteomic Diversity in Bacteria: Insights and Implications for Bacterial Identification. Mol. Cell. Proteom. 2025, 24, 100917. [Google Scholar] [CrossRef]
- Jubaer, N. Qualification of Human Liver Microsomes for Antibacterial Activity Screening of Drug Metabolites. Appl. Microbiol. 2023, 3, 104–118. [Google Scholar] [CrossRef]
- Chabas, M.; Gaillard, J.-C.; Alpha-Bazin, B.; Armengaud, J. Flash MS/MS proteotyping allows identifying microbial isolates in 36 s of mass spectrometry signal. Proteomics 2024, 24, 2300372. [Google Scholar] [CrossRef]
- Gant, M.S.; Chamot-Rooke, J. Present and future perspectives on mass spectrometry for clinical microbiology. Microbes Infect. 2024, 26, 105296. [Google Scholar] [CrossRef] [PubMed]
- Demirev, P.; Sandrin, T.R. (Eds.) Applications of Mass Spectrometry in Microbiology: From Strain Characterization to Rapid Screening for Antibiotic Resistance; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Vemula, H.; Ayon, N.J.; Gutheil, W.G. Cytoplasmic peptidoglycan intermediate levels in Staphylococcus aureus. Biochimie 2016, 121, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Vemula, H.; Ayon, N.J.; Burton, A.; Gutheil, W.G. Antibiotic Effects on Methicillin-Resistant Staphylococcus aureus Cytoplasmic Peptidoglycan Intermediate Levels and Evidence for Potential Metabolite Level Regulatory Loops. Antimicrob. Agents Chemother. 2017, 61, e02253-16. [Google Scholar] [CrossRef]
- Blumenscheit, C.; Pfeifer, Y.; Werner, G.; John, C.; Schneider, A.; Lasch, P.; Doellinger, J. Unbiased Antimicrobial Resistance Detection from Clinical Bacterial Isolates Using Proteomics. Anal. Chem. 2021, 93, 14599–14608. [Google Scholar] [CrossRef]
- Alves, G.; Ogurtsov, A.; Karlsson, R.; Jaén-Luchoro, D.; Piñeiro-Iglesias, B.; Salvà-Serra, F.; Andersson, B.; Moore, E.R.B.; Yu, Y.-K. Identification of Antibiotic Resistance Proteins via MiCId’s Augmented Workflow. A Mass Spectrometry-Based Proteomics Approach. J. Am. Soc. Mass Spectrom. 2022, 33, 917–931. [Google Scholar] [CrossRef]
- Mancuso, G.; Midiri, A.; Gerace, E.; Biondo, C. Bacterial Antibiotic Resistance: The Most Critical Pathogens. Pathogens 2021, 10, 1310. [Google Scholar] [CrossRef] [PubMed]
- Munita Jose, M.; Arias Cesar, A. Mechanisms of Antibiotic Resistance. Microbiol. Spectr. 2016, 4, 464–473. [Google Scholar] [CrossRef]
- Ahmed, S.K.; Hussein, S.; Qurbani, K.; Ibrahim, R.H.; Fareeq, A.; Mahmood, K.A.; Mohamed, M.G. Antimicrobial resistance: Impacts, challenges, and future prospects. J. Med. Surg. Public Health 2024, 2, 100081. [Google Scholar] [CrossRef]
- Habboush, Y.; Guzman, N. Antibiotic Resistance. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Parras-Moltó, M.; Lund, D.; Ebmeyer, S.; Larsson, D.G.J.; Johnning, A.; Kristiansson, E. The transfer of antibiotic resistance genes between evolutionarily distant bacteria. mSphere 2025, 10, e00114–e00125. [Google Scholar] [CrossRef] [PubMed]
- Ventola, C.L. The antibiotic resistance crisis: Part 1: Causes and threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Ayon, N.J. High-Throughput Screening of Natural Product and Synthetic Molecule Libraries for Antibacterial Drug Discovery. Metabolites 2023, 13, 625. [Google Scholar] [CrossRef]
- Dadgostar, P. Antimicrobial Resistance: Implications and Costs. Infect. Drug Resist. 2019, 12, 3903–3910. [Google Scholar] [CrossRef]
- Anderson, M.; Clift, C.; Schulze, K.; Sagan, A.; Nahrgang, S.; Ait Ouakrim, D.; Mossialos, E. European Observatory Policy Briefs. In Averting the AMR Crisis: What Are the Avenues for Policy Action for Countries in Europe? European Observatory on Health Systems and Policies: Copenhagen, Denmark, 2019. [Google Scholar]
- Chokshi, A.; Sifri, Z.; Cennimo, D.; Horng, H. Global Contributors to Antibiotic Resistance. J. Glob. Infect. Dis. 2019, 11, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Gajic, I.; Kabic, J.; Kekic, D.; Jovicevic, M.; Milenkovic, M.; Mitic Culafic, D.; Trudic, A.; Ranin, L.; Opavski, N. Antimicrobial Susceptibility Testing: A Comprehensive Review of Currently Used Methods. Antibiotics 2022, 11, 427. [Google Scholar] [CrossRef] [PubMed]
- Naylor, N.R.; Atun, R.; Zhu, N.; Kulasabanathan, K.; Silva, S.; Chatterjee, A.; Knight, G.M.; Robotham, J.V. Estimating the burden of antimicrobial resistance: A systematic literature review. Antimicrob. Resist. Infect. Control 2018, 7, 58. [Google Scholar] [CrossRef]
- Urban-Chmiel, R.; Marek, A.; Stępień-Pyśniak, D.; Wieczorek, K.; Dec, M.; Nowaczek, A.; Osek, J. Antibiotic Resistance in Bacteria—A Review. Antibiotics 2022, 11, 1079. [Google Scholar] [CrossRef]
- Kaprou, G.D.; Bergšpica, I.; Alexa, E.A.; Alvarez-Ordóñez, A.; Prieto, M. Rapid Methods for Antimicrobial Resistance Diagnostics. Antibiotics 2021, 10, 209. [Google Scholar] [CrossRef] [PubMed]
- Yamin, D.; Uskoković, V.; Wakil, A.M.; Goni, M.D.; Shamsuddin, S.H.; Mustafa, F.H.; Alfouzan, W.A.; Alissa, M.; Alshengeti, A.; Almaghrabi, R.H.; et al. Current and Future Technologies for the Detection of Antibiotic-Resistant Bacteria. Diagnostics 2023, 13, 3246. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, J.H.; Ferraro, M.J. Antimicrobial susceptibility testing: A review of general principles and contemporary practices. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2009, 49, 1749–1755. [Google Scholar] [CrossRef]
- Banerjee, R.; Patel, R. Molecular diagnostics for genotypic detection of antibiotic resistance: Current landscape and future directions. JAC-Antimicrob. Resist. 2023, 5, dlad018. [Google Scholar] [CrossRef]
- Faksri, K.; Kaewprasert, O.; Ong, R.T.-H.; Suriyaphol, P.; Prammananan, T.; Teo, Y.-Y.; Srilohasin, P.; Chaiprasert, A. Comparisons of whole-genome sequencing and phenotypic drug susceptibility testing for Mycobacterium tuberculosis causing MDR-TB and XDR-TB in Thailand. Int. J. Antimicrob. Agents 2019, 54, 109–116. [Google Scholar] [CrossRef]
- Rossen, J.W.A.; Friedrich, A.W.; Moran-Gilad, J. Practical issues in implementing whole-genome-sequencing in routine diagnostic microbiology. Clin. Microbiol. Infect. 2018, 24, 355–360. [Google Scholar] [CrossRef]
- Florio, W.; Baldeschi, L.; Rizzato, C.; Tavanti, A.; Ghelardi, E.; Lupetti, A. Detection of Antibiotic-Resistance by MALDI-TOF Mass Spectrometry: An Expanding Area. Front. Cell. Infect. Microbiol. 2020, 10, 572909. [Google Scholar] [CrossRef]
- Charretier, Y.; Schrenzel, J. Mass spectrometry methods for predicting antibiotic resistance. Proteom. Clin. Appl. 2016, 10, 964–981. [Google Scholar] [CrossRef]
- Panda, A.; Kurapati, S.; Samantaray, J.C.; Srinivasan, A.; Khalil, S. MALDI-TOF mass spectrometry proteomic based identification of clinical bacterial isolates. Indian J. Med. Res. 2014, 140, 770–777. [Google Scholar]
- Welker, M.; van Belkum, A. One System for All: Is Mass Spectrometry a Future Alternative for Conventional Antibiotic Susceptibility Testing? Front. Microbiol. 2019, 10, 2711. [Google Scholar] [CrossRef] [PubMed]
- Alcock, B.P.; Raphenya, A.R.; Lau, T.T.Y.; Tsang, K.K.; Bouchard, M.; Edalatmand, A.; Huynh, W.; Nguyen, A.-L.V.; Cheng, A.A.; Liu, S.; et al. CARD 2020: Antibiotic resistome surveillance with the comprehensive antibiotic resistance database. Nucleic Acids Res. 2019, 48, D517–D525. [Google Scholar] [CrossRef]
- Pérez-Llarena, F.J.; Bou, G. Proteomics as a Tool for Studying Bacterial Virulence and Antimicrobial Resistance. Front. Microbiol. 2016, 7, 410. [Google Scholar] [CrossRef]
- Chang, C.-J.; Lin, J.-H.; Chang, K.-C.; Lai, M.-J.; Rohini, R.; Hu, A. Diagnosis of β-Lactam Resistance in Acinetobacter baumannii Using Shotgun Proteomics and LC-Nano-Electrospray Ionization Ion Trap Mass Spectrometry. Anal. Chem. 2013, 85, 2802–2808. [Google Scholar] [CrossRef] [PubMed]
- Trip, H.; Mende, K.; Majchrzykiewicz-Koehorst, J.A.; Sedee, N.J.A.; Hulst, A.G.; Jansen, H.-J.; Murray, C.K.; Paauw, A. Simultaneous Identification of Multiple β-Lactamases in Acinetobacter baumannii in Relation to Carbapenem and Ceftazidime Resistance, Using Liquid Chromatography-Tandem Mass Spectrometry. J. Clin. Microbiol. 2015, 53, 1927–1930. [Google Scholar] [CrossRef]
- Fleurbaaij, F.; Goessens, W.; van Leeuwen, H.C.; Kraakman, M.E.M.; Bernards, S.T.; Hensbergen, P.J.; Kuijper, E.J. Direct detection of extended-spectrum beta-lactamases (CTX-M) from blood cultures by LC-MS/MS bottom-up proteomics. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 1621–1628. [Google Scholar] [CrossRef]
- Jaén-Luchoro, D.; Busquets, A.; Karlsson, R.; Salvà-Serra, F.; Åhrén, C.; Karami, N.; Moore, E.R.B. Genomic and Proteomic Characterization of the Extended-Spectrum β-Lactamase (ESBL)-Producing Escherichia coli Strain CCUG 73778: A Virulent, Nosocomial Outbreak Strain. Microorganisms 2020, 8, 893. [Google Scholar] [CrossRef]
- Foudraine, D.E.; Dekker, L.J.M.; Strepis, N.; Bexkens, M.L.; Klaassen, C.H.W.; Luider, T.M.; Goessens, W.H.F. Accurate Detection of the Four Most Prevalent Carbapenemases in E. coli and K. pneumoniae by High-Resolution Mass Spectrometry. Front. Microbiol. 2019, 10, 2760. [Google Scholar] [CrossRef]
- Charretier, Y.; Dauwalder, O.; Franceschi, C.; Degout-Charmette, E.; Zambardi, G.; Cecchini, T.; Bardet, C.; Lacoux, X.; Dufour, P.; Veron, L.; et al. Rapid Bacterial Identification, Resistance, Virulence and Type Profiling Using Selected Reaction Monitoring Mass Spectrometry. Sci. Rep. 2015, 5, 13944. [Google Scholar] [CrossRef] [PubMed]
- Elbehiry, A.; Aldubaib, M.; Abalkhail, A.; Marzouk, E.; A, A.L.; Moussa, I.; Ibrahem, M.; Albazie, H.; Alqarni, A.; Anagreyyah, S.; et al. How MALDI-TOF Mass Spectrometry Technology Contributes to Microbial Infection Control in Healthcare Settings. Vaccines 2022, 10, 1881. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, S.K.; Cavey, G.S.; Pearson, J.D. Single Ribosomal Protein Mutations in Antibiotic-Resistant Bacteria Analyzed by Mass Spectrometry. Antimicrob. Agents Chemother. 2001, 45, 3046–3055. [Google Scholar] [CrossRef]
- Sulaiman, J.E.; Lam, H. Proteomics in antibiotic resistance and tolerance research: Mapping the resistome and the tolerome of bacterial pathogens. Proteomics 2022, 22, 2100409. [Google Scholar] [CrossRef] [PubMed]
- Lopatkin, A.J.; Bening, S.C.; Manson, A.L.; Stokes, J.M.; Kohanski, M.A.; Badran, A.H.; Earl, A.M.; Cheney, N.J.; Yang, J.H.; Collins, J.J. Clinically relevant mutations in core metabolic genes confer antibiotic resistance. Science 2021, 371, eaba0862. [Google Scholar] [CrossRef] [PubMed]
- Kohanski, M.A.; Dwyer, D.J.; Collins, J.J. How antibiotics kill bacteria: From targets to networks. Nat. Rev. Microbiol. 2010, 8, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Rex, D.A.B.; Schuster, D.; Neely, B.A.; Rosano, G.L.; Volkmar, N.; Momenzadeh, A.; Peters-Clarke, T.M.; Egbert, S.B.; Kreimer, S.; et al. Comprehensive Overview of Bottom-Up Proteomics Using Mass Spectrometry. ACS Meas. Sci. Au 2024, 4, 338–417. [Google Scholar] [CrossRef]
- Miller, R.M.; Smith, L.M. Overview and considerations in bottom-up proteomics. Analyst 2023, 148, 475–486. [Google Scholar] [CrossRef]
- Forgrave, L.M.; Wang, M.; Yang, D.; DeMarco, M.L. Proteoforms and their expanding role in laboratory medicine. Pract. Lab. Med. 2022, 28, e00260. [Google Scholar] [CrossRef]
- Liu, X.; Hengel, S.; Wu, S.; Tolić, N.; Pasa-Tolić, L.; Pevzner, P.A. Identification of Ultramodified Proteins Using Top-Down Tandem Mass Spectra. J. Proteome Res. 2013, 12, 5830–5838. [Google Scholar] [CrossRef]
- Savaryn, J.P.; Catherman, A.D.; Thomas, P.M.; Abecassis, M.M.; Kelleher, N.L. The emergence of top-down proteomics in clinical research. Genome Med. 2013, 5, 53. [Google Scholar] [CrossRef]
- Anhalt, J.P.; Fenselau, C. Identification of bacteria using mass spectrometry. Anal. Chem. 1975, 47, 219–225. [Google Scholar] [CrossRef]
- Dieckmann, R.; Helmuth, R.; Erhard, M.; Malorny, B. Rapid classification and identification of salmonellae at the species and subspecies levels by whole-cell matrix-assisted laser desorption ionization-time of flight mass spectrometry. Appl. Environ. Microbiol. 2008, 74, 7767–7778. [Google Scholar] [CrossRef]
- Fagerquist, C.K.; Dodd, C.E. Top-down proteomic identification of plasmid and host proteins produced by pathogenic Escherichia coli using MALDI-TOF-TOF tandem mass spectrometry. PLoS ONE 2021, 16, e0260650. [Google Scholar] [CrossRef] [PubMed]
- Meier-Credo, J.; Preiss, L.; Wüllenweber, I.; Resemann, A.; Nordmann, C.; Zabret, J.; Suckau, D.; Michel, H.; Nowaczyk, M.M.; Meier, T.; et al. Top-Down Identification and Sequence Analysis of Small Membrane Proteins Using MALDI-MS/MS. J. Am. Soc. Mass Spectrom. 2022, 33, 1293–1302. [Google Scholar] [CrossRef]
- Hrabák, J.; Chudáčková, E.; Walková, R. Matrix-Assisted Laser Desorption Ionization–Time of Flight (MALDI-TOF) Mass Spectrometry for Detection of Antibiotic Resistance Mechanisms: From Research to Routine Diagnosis. Clin. Microbiol. Rev. 2013, 26, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Kostrzewa, M.; Sparbier, K.; Maier, T.; Schubert, S. MALDI-TOF MS: An upcoming tool for rapid detection of antibiotic resistance in microorganisms. Proteom. Clin. Appl. 2013, 7, 767–778. [Google Scholar] [CrossRef]
- Vrioni, G.; Tsiamis, C.; Oikonomidis, G.; Theodoridou, K.; Kapsimali, V.; Tsakris, A. MALDI-TOF mass spectrometry technology for detecting biomarkers of antimicrobial resistance: Current achievements and future perspectives. Ann. Transl. Med. 2018, 6, 240. [Google Scholar] [CrossRef]
- Viboud, G.; Asaro, H.; Huang, M.B. Use of matrix-assisted laser desorption ionization time of flight (MALDI-TOF) to detect antibiotic resistance in bacteria: A scoping review. Am. J. Clin. Pathol. 2023, 161, 317–328. [Google Scholar] [CrossRef]
- Maus, A.; Bisha, B.; Fagerquist, C.; Basile, F. Detection and identification of a protein biomarker in antibiotic-resistant Escherichia coli using intact protein LC offline MALDI-MS and MS/MS. J. Appl. Microbiol. 2020, 128, 697–709. [Google Scholar] [CrossRef]
- Kocsis, T.; Győrffy, A.; Pomázi, A. Application of MALDI-TOF MS and FT-IR spectroscopy in identification and antibiotic resistance profiling of lactic acid bacteria. Appl. Microbiol. Biotechnol. 2025, 109, 165. [Google Scholar] [CrossRef]
- Santiago, L.S.; Guerrero-López, A.; Sevilla-Salcedo, C.; Rodríguez-Temporal, D.; Rodríguez-Sánchez, B.; Gómez-Verdejo, V. Machine Learning applied to MALDI-TOF data in a clinical setting: A systematic review. bioRxiv 2025. [Google Scholar] [CrossRef]
- Visonà, G.; Duroux, D.; Miranda, L.; Sükei, E.; Li, Y.; Borgwardt, K.; Oliver, C. Multimodal learning in clinical proteomics: Enhancing antimicrobial resistance prediction models with chemical information. Bioinformatics 2023, 39, btad717. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.-A.; Peleg, A.Y.; Song, J.; Antony, B.; Webb, G.I.; Wisniewski, J.A.; Blakeway, L.V.; Badoordeen, G.Z.; Theegala, R.; Zisis, H.; et al. Predicting Pseudomonas aeruginosa drug resistance using artificial intelligence and clinical MALDI-TOF mass spectra. mSystems 2024, 9, e00789-00724. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.; Chen, Q.; Zhang, J. Repurposing MALDI-TOF MS for effective antibiotic resistance screening in Staphylococcus epidermidis using machine learning. Sci. Rep. 2024, 14, 24139. [Google Scholar] [CrossRef]
- Victor Lin, H.-T.; Yang, T.-W.; Lu, W.-J.; Chiang, H.-J.; Hsu, P.-H. Machine learning-enhanced MALDI-TOF MS for real-time detection of antibiotic-resistant E. coli in food processing. LWT 2025, 224, 117860. [Google Scholar] [CrossRef]
- Trimpin, S.; Inutan, E.D.; Herath, T.N.; McEwen, C.N. Laserspray ionization, a new atmospheric pressure MALDI method for producing highly charged gas-phase ions of peptides and proteins directly from solid solutions. Mol. Cell. Proteom. MCP 2010, 9, 362–367. [Google Scholar] [CrossRef]
- Levy, E.; Slavov, N. Single cell protein analysis for systems biology. Essays Biochem. 2018, 62, 595–605. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Z.; Pan, J.; Ma, X.; Zhang, W.; Ouyang, Z. Single-Cell Mass Spectrometry Analysis of Metabolites Facilitated by Cell Electro-Migration and Electroporation. Anal. Chem. 2020, 92, 10138–10144. [Google Scholar] [CrossRef]
- Li, Y.; Bouza, M.; Wu, C.; Guo, H.; Huang, D.; Doron, G.; Temenoff, J.S.; Stecenko, A.A.; Wang, Z.L.; Fernández, F.M. Sub-nanoliter metabolomics via mass spectrometry to characterize volume-limited samples. Nat. Commun. 2020, 11, 5625. [Google Scholar] [CrossRef] [PubMed]
- Schirmer, M.; Wink, K.; Ohla, S.; Belder, D.; Schmid, A.; Dusny, C. Conversion Efficiencies of a Few Living Microbial Cells Detected at a High Throughput by Droplet-Based ESI-MS. Anal. Chem. 2020, 92, 10700–10708. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Chang, K.-C.; Payne, E.M.; Modavi, C.; Liu, L.; Palmer, C.M.; Tao, N.; Alper, H.S.; Kennedy, R.T.; Cornett, D.S.; et al. Mapping enzyme catalysis with metabolic biosensing. Nat. Commun. 2021, 12, 6803. [Google Scholar] [CrossRef]
- Smith, L.M.; Kelleher, N.L.; Linial, M.; Goodlett, D.; Langridge-Smith, P.; Ah Goo, Y.; Safford, G.; Bonilla, L.; Kruppa, G.; Zubarev, R.; et al. Proteoform: A single term describing protein complexity. Nat. Methods 2013, 10, 186–187. [Google Scholar] [CrossRef]
- Gault, J.; Malosse, C.; Machata, S.; Millien, C.; Podglajen, I.; Ploy, M.-C.; Costello, C.E.; Duménil, G.; Chamot-Rooke, J. Complete posttranslational modification mapping of pathogenic Neisseria meningitidis pilins requires top-down mass spectrometry. Proteomics 2014, 14, 1141–1151. [Google Scholar] [CrossRef]
- Melo, R.M.; de Souza, J.M.F.; Williams, T.C.R.; Fontes, W.; de Sousa, M.V.; Ricart, C.A.O.; do Vale, L.H.F. Revealing Corynebacterium glutamicum proteoforms through top-down proteomics. Sci. Rep. 2023, 13, 2602. [Google Scholar] [CrossRef]
- Genth, J.; Schäfer, K.; Cassidy, L.; Graspeuntner, S.; Rupp, J.; Tholey, A. Identification of proteoforms of short open reading frame-encoded peptides in Blautia producta under different cultivation conditions. Genom. Proteom. 2023, 11, e02528-02523. [Google Scholar] [CrossRef]
- Ansong, C.; Wu, S.; Meng, D.; Liu, X.; Brewer, H.M.; Deatherage Kaiser, B.L.; Nakayasu, E.S.; Cort, J.R.; Pevzner, P.; Smith, R.D.; et al. Top-down proteomics reveals a unique protein S-thiolation switch in Salmonella Typhimurium in response to infection-like conditions. Proc. Natl. Acad. Sci. USA 2013, 110, 10153–10158. [Google Scholar] [CrossRef] [PubMed]
- Kelleher, N.L.; Thomas, P.M.; Ntai, I.; Compton, P.D.; LeDuc, R.D. Deep and quantitative top-down proteomics in clinical and translational research. Expert Rev. Proteom. 2014, 11, 649–651. [Google Scholar] [CrossRef] [PubMed]
- Gault, J.; Vorontsov, E.; Dupré, M.; Calvaresi, V.; Duchateau, M.; Lima, D.B.; Malosse, C.; Chamot-Rooke, J. Top-Down Proteomics in the Study of Microbial Pathogenicity. In MALDI-TOF and Tandem MS for Clinical Microbiology; Wiley: Hoboken, NJ, USA, 2017; pp. 493–504. [Google Scholar]
- McFarland, M.A.; Andrzejewski, D.; Musser, S.M.; Callahan, J.H. Platform for Identification of Salmonella Serovar Differentiating Bacterial Proteins by Top-Down Mass Spectrometry: S. Typhimurium vs. S. Heidelberg. Anal. Chem. 2014, 86, 6879–6886. [Google Scholar] [CrossRef] [PubMed]
- Walukiewicz, H.E.; Farris, Y.; Burnet, M.C.; Feid, S.C.; You, Y.; Kim, H.; Bank, T.; Christensen, D.; Payne, S.H.; Wolfe, A.J.; et al. Regulation of bacterial stringent response by an evolutionarily conserved ribosomal protein L11 methylation. mBio 2024, 15, e0177324. [Google Scholar] [CrossRef]
- Nadler, W.M.; Waidelich, D.; Kerner, A.; Hanke, S.; Berg, R.; Trumpp, A.; Rösli, C. MALDI versus ESI: The Impact of the Ion Source on Peptide Identification. J. Proteome Res. 2017, 16, 1207–1215. [Google Scholar] [CrossRef]
- Dupré, M.; Duchateau, M.; Malosse, C.; Borges-Lima, D.; Calvaresi, V.; Podglajen, I.; Clermont, D.; Rey, M.; Chamot-Rooke, J. Optimization of a Top-Down Proteomics Platform for Closely Related Pathogenic Bacterial Discrimination. J. Proteome Res. 2021, 20, 202–211. [Google Scholar] [CrossRef]
- Neil, J.R.; Verma, A.; Kronewitter, S.R.; McGee, W.M.; Mullen, C.; Viirtola, M.; Kotovuori, A.; Friedrich, H.; Finell, J.; Rannisto, J.; et al. Rapid MRSA detection via tandem mass spectrometry of the intact 80 kDa PBP2a resistance protein. Sci. Rep. 2021, 11, 18309. [Google Scholar] [CrossRef]
- Lenski, R.E.; Travisano, M. Dynamics of adaptation and diversification: A 10,000-generation experiment with bacterial populations. Proc. Natl. Acad. Sci. USA 1994, 91, 6808–6814. [Google Scholar] [CrossRef] [PubMed]
- Williams, T.L.; Monday, S.R.; Edelson-Mammel, S.; Buchanan, R.; Musser, S.M. A top-down proteomics approach for differentiating thermal resistant strains of Enterobacter sakazakii. Proteomics 2005, 5, 4161–4169. [Google Scholar] [CrossRef]
- Williams, T.L.; Musser, S.M.; Nordstrom, J.L.; DePaola, A.; Monday, S.R. Identification of a protein biomarker unique to the pandemic O3:K6 clone of Vibrio parahaemolyticus. J. Clin. Microbiol. 2004, 42, 1657–1665. [Google Scholar] [CrossRef]
- Kondori, N.; Kurtovic, A.; Piñeiro-Iglesias, B.; Salvà-Serra, F.; Jaén-Luchoro, D.; Andersson, B.; Alves, G.; Ogurtsov, A.; Thorsell, A.; Fuchs, J.; et al. Mass Spectrometry Proteotyping-Based Detection and Identification of Staphylococcus aureus, Escherichia coli, and Candida albicans in Blood. Front. Cell. Infect. Microbiol. 2021, 11, 634215. [Google Scholar] [CrossRef]
- Kulak, N.A.; Pichler, G.; Paron, I.; Nagaraj, N.; Mann, M. Minimal, encapsulated proteomic-sample processing applied to copy-number estimation in eukaryotic cells. Nat. Methods 2014, 11, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, J.; Fu, Q.; Guo, S.; Ta, L.; Sun, P. Proteomic Analyses Uncover the Mechanisms Underlying Antibiotic Resistance Differences among Three Acinetobacter baumannii Isolates. J. Mol. Microbiol. Biotechnol. 2016, 26, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Boulund, F.; Karlsson, R.; Gonzales-Siles, L.; Johnning, A.; Karami, N.; Al-Bayati, O.; Åhrén, C.; Moore, E.R.B.; Kristiansson, E. Typing and Characterization of Bacteria Using Bottom-up Tandem Mass Spectrometry Proteomics. Mol. Cell. Proteom. MCP 2017, 16, 1052–1063. [Google Scholar] [CrossRef]
- Foudraine, D.E.; Strepis, N.; Stingl, C.; ten Kate, M.T.; Verbon, A.; Klaassen, C.H.W.; Goessens, W.H.F.; Luider, T.M.; Dekker, L.J.M. Exploring antimicrobial resistance to beta-lactams, aminoglycosides and fluoroquinolones in E. coli and K. pneumoniae using proteogenomics. Sci. Rep. 2021, 11, 12472. [Google Scholar] [CrossRef]
- Piras, C.; De Fazio, R.; Di Francesco, A.; Oppedisano, F.; Spina, A.A.; Cunsolo, V.; Roncada, P.; Cramer, R.; Britti, D. Detection of Antimicrobial Proteins/Peptides and Bacterial Proteins Involved in Antimicrobial Resistance in Raw Cow’s Milk from Different Breeds. Antibiotics 2024, 13, 838. [Google Scholar] [CrossRef]
- Mehrotra, T.; Konar, D.; Pragasam, A.K.; Kumar, S.; Jana, P.; Babele, P.; Paul, D.; Purohit, A.; Tanwar, S.; Bakshi, S.; et al. Antimicrobial resistance heterogeneity among multidrug-resistant Gram-negative pathogens: Phenotypic, genotypic, and proteomic analysis. Proc. Natl. Acad. Sci. USA 2023, 120, e2305465120. [Google Scholar] [CrossRef]
- Végvári, Á.; Zhang, X.; Zubarev, R.A. Toward Single Bacterium Proteomics. J. Am. Soc. Mass Spectrom. 2023, 34, 2098–2106. [Google Scholar] [CrossRef]
- Soufi, B.; Krug, K.; Harst, A.; Macek, B. Characterization of the E. coli proteome and its modifications during growth and ethanol stress. Front. Microbiol. 2015, 6, 103. [Google Scholar] [CrossRef]
- Wiens, J.J. How many species are there on Earth? Progress and problems. PLoS Biol. 2023, 21, e3002388. [Google Scholar] [CrossRef] [PubMed]
- Dykhuizen, D. Species Numbers in Bacteria. Proc. Calif. Acad. Sci. 2005, 56, 62–71. [Google Scholar] [PubMed]
- Khan, Y.S.; Farhana, A. Histology, Cell. Available online: https://www.ncbi.nlm.nih.gov/books/NBK554382/ (accessed on 27 September 2025).
- Bartlett, A.; Padfield, D.; Lear, L.; Bendall, R.; Vos, M. A comprehensive list of bacterial pathogens infecting humans. Microbiology 2022, 168, 001269. [Google Scholar] [CrossRef] [PubMed]
- Vemula, H.; Ayon, N. Profiling of bacterial cell wall peptidoglycan pathway inhibitors using LC-MS/MS-based quantification of cytoplasmic pathway intermediates. In Abstracts of Papers of the American Chemical Society; American Chemical Society: Washington, DC, USA, 2016. [Google Scholar]
- Baran, A.; Kwiatkowska, A.; Potocki, L. Antibiotics and Bacterial Resistance—A Short Story of an Endless Arms Race. Int. J. Mol. Sci. 2023, 24, 5777. [Google Scholar] [CrossRef]
- Carr, G.B.; Murgel, C.A.F. The Use of the Operating Microscope in Endodontics. Dent. Clin. N. Am. 2010, 54, 191–214. [Google Scholar] [CrossRef]
- Savage, J.C.; Picard, K.; González-Ibáñez, F.; Tremblay, M. A Brief History of Microglial Ultrastructure: Distinctive Features, Phenotypes, and Functions Discovered Over the Past 60 Years by Electron Microscopy. Front. Immunol. 2018, 9, 803. [Google Scholar] [CrossRef]
- Bekker-Jensen, D.B.; Kelstrup, C.D.; Batth, T.S.; Larsen, S.C.; Haldrup, C.; Bramsen, J.B.; Sørensen, K.D.; Høyer, S.; Ørntoft, T.F.; Andersen, C.L.; et al. An Optimized Shotgun Strategy for the Rapid Generation of Comprehensive Human Proteomes. Cell Syst. 2017, 4, 587–599.e584. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Kroenke, C.D.; Song, J.; Piwnica-Worms, D.; Ackerman, J.J.; Neil, J.J. Intracellular water-specific MR of microbead-adherent cells: The HeLa cell intracellular water exchange lifetime. NMR Biomed. 2008, 21, 159–164. [Google Scholar] [CrossRef]
- James, M.B.; Giorgio, T.D. Nuclear-associated plasmid, but not cell-associated plasmid, is correlated with transgene expression in cultured mammalian cells. Mol. Ther. J. Am. Soc. Gene Ther. 2000, 1, 339–346. [Google Scholar] [CrossRef]
- Schulz, H.N.; Jørgensen, B.B. Big Bacteria. Annu. Rev. Microbiol. 2001, 55, 105–137. [Google Scholar] [CrossRef]
- Jiang, R.D.; Shen, H.; Piao, Y.J. The morphometrical analysis on the ultrastructure of A549 cells. Rom. J. Morphol. Embryol. Rev. Roum. Morphol. Et Embryol. 2010, 51, 663–667. [Google Scholar]
- Phillips, R.; Kondev, J.; Theriot, J.; Garcia, H. Physical Biology of the Cell, 2nd ed.; Garland Science: New York, NY, USA, 2012. [Google Scholar]
- De Ley, J.; Park, I.W. Dissimilarity Between Human and Bacterial Deoxyribonucleic Acids. Nature 1966, 211, 1002. [Google Scholar] [CrossRef]
- Neurohr, G.E.; Amon, A. Relevance and Regulation of Cell Density. Trends Cell Biol. 2020, 30, 213–225. [Google Scholar] [CrossRef]
- Mairet, F.; Gouzé, J.-L.; de Jong, H. Optimal proteome allocation and the temperature dependence of microbial growth laws. npj Syst. Biol. Appl. 2021, 7, 14. [Google Scholar] [CrossRef]
- Kratz, J.C.; Banerjee, S. Dynamic proteome trade-offs regulate bacterial cell size and growth in fluctuating nutrient environments. Commun. Biol. 2023, 6, 486. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Balakrishnan, R.; Braniff, N.; Mori, M.; Manzanarez, G.; Zhang, Z.; Hwa, T. Cellular perception of growth rate and the mechanistic origin of bacterial growth law. Proc. Natl. Acad. Sci. USA 2022, 119, e2201585119. [Google Scholar] [CrossRef] [PubMed]
- Fortuin, S.; Nel, A.J.M.; Blackburn, J.M.; Soares, N.C. Comparison between the proteome of Escherichia coli single colony and during liquid culture. J. Proteom. 2020, 228, 103929. [Google Scholar] [CrossRef]
- Tu, C.; Rudnick, P.A.; Martinez, M.Y.; Cheek, K.L.; Stein, S.E.; Slebos, R.J.; Liebler, D.C. Depletion of abundant plasma proteins and limitations of plasma proteomics. J. Proteome Res. 2010, 9, 4982–4991. [Google Scholar] [CrossRef] [PubMed]
- Midha, M.K.; Kusebauch, U.; Shteynberg, D.; Kapil, C.; Bader, S.L.; Reddy, P.J.; Campbell, D.S.; Baliga, N.S.; Moritz, R.L. A comprehensive spectral assay library to quantify the Escherichia coli proteome by DIA/SWATH-MS. Sci. Data 2020, 7, 389. [Google Scholar] [CrossRef] [PubMed]
- Sun, S. Emerging antibiotic resistance by various novel proteins/enzymes. Eur. J. Clin. Microbiol. Infect. Dis. 2025, 44, 1551–1566. [Google Scholar] [CrossRef] [PubMed]
- Gaurav, A.; Bakht, P.; Saini, M.; Pandey, S.; Pathania, R. Role of bacterial efflux pumps in antibiotic resistance, virulence, and strategies to discover novel efflux pump inhibitors. Microbiology 2023, 169, 001333. [Google Scholar] [CrossRef]
- Nishino, K.; Yamasaki, S.; Nakashima, R.; Zwama, M.; Hayashi-Nishino, M. Function and Inhibitory Mechanisms of Multidrug Efflux Pumps. Front. Microbiol. 2021, 12, 737288. [Google Scholar] [CrossRef]
- Schulz, G.E. The Structures of General Porins. In Bacterial and Eukaryotic Porins; Benz, R., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2004. [Google Scholar]
- Vergalli, J.; Bodrenko, I.V.; Masi, M.; Moynié, L.; Acosta-Gutiérrez, S.; Naismith, J.H.; Davin-Regli, A.; Ceccarelli, M.; van den Berg, B.; Winterhalter, M.; et al. Porins and small-molecule translocation across the outer membrane of Gram-negative bacteria. Nat. Rev. Microbiol. 2020, 18, 164–176. [Google Scholar] [CrossRef]
- Pöhl, S.; Giacomelli, G.; Meyer, F.M.; Kleeberg, V.; Cohen, E.J.; Biboy, J.; Rosum, J.; Glatter, T.; Vollmer, W.; van Teeseling, M.C.F.; et al. An outer membrane porin-lipoprotein complex modulates elongasome movement to establish cell curvature in Rhodospirillum rubrum. Nat. Commun. 2024, 15, 7616. [Google Scholar] [CrossRef]
- Yu, M.S.C.; Chiang, D.M.; Reithmair, M.; Meidert, A.; Brandes, F.; Schelling, G.; Ludwig, C.; Meng, C.; Kirchner, B.; Zenner, C.; et al. The proteome of bacterial membrane vesicles in Escherichia coli-a time course comparison study in two different media. Front. Microbiol. 2024, 15, 1361270. [Google Scholar] [CrossRef] [PubMed]
- McGarvey, K.M.; Queitsch, K.; Fields, S. Wide variation in antibiotic resistance proteins identified by functional metagenomic screening of a soil DNA library. Appl. Environ. Microbiol. 2012, 78, 1708–1714. [Google Scholar] [CrossRef]
- González, L.J.; Bahr, G.; González, M.M.; Bonomo, R.A.; Vila, A.J. In-cell kinetic stability is an essential trait in metallo-β-lactamase evolution. Nat. Chem. Biol. 2023, 19, 1116–1126. [Google Scholar] [CrossRef]
- Zhang, F.; Cheng, W. The Mechanism of Bacterial Resistance and Potential Bacteriostatic Strategies. Antibiotics 2022, 11, 1215. [Google Scholar] [CrossRef]
- Lähnemann, D.; Köster, J.; Szczurek, E.; McCarthy, D.J.; Hicks, S.C.; Robinson, M.D.; Vallejos, C.A.; Campbell, K.R.; Beerenwinkel, N.; Mahfouz, A.; et al. Eleven grand challenges in single-cell data science. Genome Biol. 2020, 21, 31. [Google Scholar] [CrossRef]
- Kassem, S.; van der Pan, K.; de Jager, A.L.; Naber, B.A.E.; de Laat, I.F.; Louis, A.; van Dongen, J.J.M.; Teodosio, C.; Díez, P. Proteomics for Low Cell Numbers: How to Optimize the Sample Preparation Workflow for Mass Spectrometry Analysis. J. Proteome Res. 2021, 20, 4217–4230. [Google Scholar] [CrossRef]
- Harris, L.; Fondrie, W.E.; Oh, S.; Noble, W.S. Evaluating Proteomics Imputation Methods with Improved Criteria. J. Proteome Res. 2023, 22, 3427–3438. [Google Scholar] [CrossRef] [PubMed]
- Franks, A.; Airoldi, E.; Slavov, N. Post-transcriptional regulation across human tissues. PLoS Comput. Biol. 2017, 13, e1005535. [Google Scholar] [CrossRef] [PubMed]
- Gatto, L.; Aebersold, R.; Cox, J.; Demichev, V.; Derks, J.; Emmott, E.; Franks, A.M.; Ivanov, A.R.; Kelly, R.T.; Khoury, L.; et al. Initial recommendations for performing, benchmarking and reporting single-cell proteomics experiments. Nat. Methods 2023, 20, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Lian, X.; Zhang, Y.; Zhou, Y.; Sun, X.; Huang, S.; Dai, H.; Han, L.; Zhu, F. SingPro: A knowledge base providing single-cell proteomic data. Nucleic Acids Res. 2024, 52, D552–D561. [Google Scholar] [CrossRef]
- Ctortecka, C.; Stejskal, K.; Krššáková, G.; Mendjan, S.; Mechtler, K. Quantitative Accuracy and Precision in Multiplexed Single-Cell Proteomics. Anal. Chem. 2022, 94, 2434–2443. [Google Scholar] [CrossRef]
- Ma, A.; McDermaid, A.; Xu, J.; Chang, Y.; Ma, Q. Integrative Methods and Practical Challenges for Single-Cell Multi-omics. Trends Biotechnol 2020, 38, 1007–1022. [Google Scholar] [CrossRef] [PubMed]
- Grapov, D.; Fahrmann, J.; Wanichthanarak, K.; Khoomrung, S. Rise of Deep Learning for Genomic, Proteomic, and Metabolomic Data Integration in Precision Medicine. OMICS A J. Integr. Biol. 2018, 22, 630–636. [Google Scholar] [CrossRef]
- Perakakis, N.; Yazdani, A.; Karniadakis, G.E.; Mantzoros, C. Omics, big data and machine learning as tools to propel understanding of biological mechanisms and to discover novel diagnostics and therapeutics. Metabolism 2018, 87, A1–A9. [Google Scholar] [CrossRef]
- Li, Y.; Yang, X.; Zhao, W. Emerging Microtechnologies and Automated Systems for Rapid Bacterial Identification and Antibiotic Susceptibility Testing. SLAS Technol. 2017, 22, 585–608. [Google Scholar] [CrossRef] [PubMed]
- Ayon, N.J.; Gutheil, W.G. Dimensionally Enhanced Antibacterial Library Screening. ACS Chem. Biol. 2019, 14, 2887–2894. [Google Scholar] [CrossRef] [PubMed]
- Ayon, N.J. Metabolomics and Chemical Library Screening for Antibacterial Drug Discovery. Ph.D. Thesis, University of Missouri, Kansas City, MO, USA, 2020. [Google Scholar]
- Ayon, N.J.; Gutheil, W.G. Enhanced Chemical Diversity through Library In Situ Pre-Metabolism: Using Biological Chemistry for Novel Lead Discovery. In Abstracts of Papers of the American Chemical Society; American Chemical Society: Washington, DC, USA, 2016. [Google Scholar]
- Gargvanshi, S.; Heravi, G.; Ayon Navid, J.; Gutheil William, G. Screening the NCI diversity set V for anti-MRSA activity: Cefoxitin synergy and LC-MS/MS confirmation of folate/thymidine biosynthesis inhibition. Microbiol. Spectr. 2023, 11, e00541-23. [Google Scholar] [CrossRef]
- Tajik, M.; Baharfar, M.; Donald, W.A. Single-cell mass spectrometry. Trends Biotechnol. 2022, 40, 1374–1392. [Google Scholar] [CrossRef]
- Ye, Z.; Sabatier, P.; van der Hoeven, L.; Lechner, M.Y.; Phlairaharn, T.; Guzman, U.H.; Liu, Z.; Huang, H.; Huang, M.; Li, X.; et al. Enhanced sensitivity and scalability with a Chip-Tip workflow enables deep single-cell proteomics. Nat. Methods 2025, 22, 499–509. [Google Scholar] [CrossRef]
- Yang, Z.; Jin, K.; Chen, Y.; Liu, Q.; Chen, H.; Hu, S.; Wang, Y.; Pan, Z.; Feng, F.; Shi, M.; et al. AM-DMF-SCP: Integrated Single-Cell Proteomics Analysis on an Active Matrix Digital Microfluidic Chip. JACS Au 2024, 4, 1811–1823. [Google Scholar] [CrossRef]
- Dib, Y.; Carret, L.; Thion, C. High Throughput Generation of Bacterial Isolate Libraries Using Direct Liquid Cultivation with cellenONE; Cellenion: Lyon, France, 2021. [Google Scholar]
- Mauger, S.; Monard, C.; Thion, C.; Vandenkoornhuyse, P. Contribution of single-cell omics to microbial ecology. Trends Ecol. Evol. 2022, 37, 67–78. [Google Scholar] [CrossRef]
- Riba, J.; Gleichmann, T.; Zimmermann, S.; Zengerle, R.; Koltay, P. Label-free isolation and deposition of single bacterial cells from heterogeneous samples for clonal culturing. Sci. Rep. 2016, 6, 32837. [Google Scholar] [CrossRef]
- Burmeister, A.; Grünberger, A. Microfluidic cultivation and analysis tools for interaction studies of microbial co-cultures. Curr. Opin. Biotechnol. 2020, 62, 106–115. [Google Scholar] [CrossRef]
- Geersens, É.; Vuilleumier, S.; Ryckelynck, M. Growth-Associated Droplet Shrinkage for Bacterial Quantification, Growth Monitoring, and Separation by Ultrahigh-Throughput Microfluidics. ACS Omega 2022, 7, 12039–12047. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, K.; Falke, F.; Frick, O.; Dusny, C.; Schmid, A. An Inert Continuous Microreactor for the Isolation and Analysis of a Single Microbial Cell. Micromachines 2015, 6, 1836–1855. [Google Scholar] [CrossRef]
- Chaudhary, D.K.; Khulan, A.; Kim, J. Development of a novel cultivation technique for uncultured soil bacteria. Sci. Rep. 2019, 9, 6666. [Google Scholar] [CrossRef] [PubMed]
- Sedgwick, H.; Caron, F.; Monaghan, P.B.; Kolch, W.; Cooper, J.M. Lab-on-a-chip technologies for proteomic analysis from isolated cells. J. R. Soc. Interface 2008, 5 (Suppl. 2), S123–S130. [Google Scholar] [CrossRef]
- Piendl, S.K.; Schönfelder, T.; Polack, M.; Weigelt, L.; van der Zwaag, T.; Teutenberg, T.; Beckert, E.; Belder, D. Integration of segmented microflow chemistry and online HPLC/MS analysis on a microfluidic chip system enabling enantioselective analyses at the nanoliter scale. Lab A Chip 2021, 21, 2614–2624. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, C.-F.; Tsao, C.-W.; Chang, C.-C.; Chu, C.-C.; DeVoe, D.L. Polymer Microchips Integrating Solid-Phase Extraction and High-Performance Liquid Chromatography Using Reversed-Phase Polymethacrylate Monoliths. Anal. Chem. 2009, 81, 2545–2554. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Murray, C.I.; Karpov, O.A.; Van Eyk, J.E. Automated proteomic sample preparation: The key component for high throughput and quantitative mass spectrometry analysis. Mass Spectrom. Rev. 2023, 42, e21750. [Google Scholar] [CrossRef]
- Reder, A.; Hentschker, C.; Steil, L.; Gesell Salazar, M.; Hammer, E.; Dhople, V.M.; Sura, T.; Lissner, U.; Wolfgramm, H.; Dittmar, D.; et al. MassSpecPreppy—An end-to-end solution for automated protein concentration determination and flexible sample digestion for proteomics applications. Proteomics 2024, 24, 2300294. [Google Scholar] [CrossRef]
- Chen, Y.; Guenther, J.M.; Gin, J.W.; Chan, L.J.G.; Costello, Z.; Ogorzalek, T.L.; Tran, H.M.; Blake-Hedges, J.M.; Keasling, J.D.; Adams, P.D.; et al. Automated “Cells-To-Peptides” Sample Preparation Workflow for High-Throughput, Quantitative Proteomic Assays of Microbes. J. Proteome Res. 2019, 18, 3752–3761. [Google Scholar] [CrossRef]
- Zecha, J.; Satpathy, S.; Kanashova, T.; Avanessian, S.C.; Kane, M.H.; Clauser, K.R.; Mertins, P.; Carr, S.A.; Kuster, B. TMT Labeling for the Masses: A Robust and Cost-efficient, In-solution Labeling Approach. Mol. Cell. Proteom. MCP 2019, 18, 1468–1478. [Google Scholar] [CrossRef]
- Peng, J.; Chan, C.; Zhang, S.; Sklavounos, A.A.; Olson, M.E.; Scott, E.Y.; Hu, Y.; Rajesh, V.; Li, B.B.; Chamberlain, M.D.; et al. All-in-One digital microfluidics pipeline for proteomic sample preparation and analysis. Chem. Sci. 2023, 14, 2887–2900. [Google Scholar] [CrossRef]
- Momenzadeh, A.; Meyer, J.G. Single-cell proteomics using mass spectrometry. Cell Genom. 2025, 5, 100973. [Google Scholar] [CrossRef] [PubMed]
- Höcker, O.; Montealegre, C.; Neusüß, C. Characterization of a nanoflow sheath liquid interface and comparison to a sheath liquid and a sheathless porous-tip interface for CE-ESI-MS in positive and negative ionization. Anal. Bioanal. Chem. 2018, 410, 5265–5275. [Google Scholar] [CrossRef]
- Li, J.; Huang, L.; Guo, Y.; Cupp-Sutton, K.A.; Wu, S. An automated spray-capillary platform for the microsampling and CE-MS analysis of picoliter- and nanoliter-volume samples. Anal. Bioanal. Chem. 2023, 415, 6961–6973. [Google Scholar] [CrossRef]
- Laxminarayan, R. Antibiotic effectiveness: Balancing conservation against innovation. Science 2014, 345, 1299–1301. [Google Scholar] [CrossRef]
- Wilke, M.S.; Lovering, A.L.; Strynadka, N.C.J. β-Lactam antibiotic resistance: A current structural perspective. Curr. Opin. Microbiol. 2005, 8, 525–533. [Google Scholar] [CrossRef]
- Pandey, N.; Cascella, M. Beta-Lactam Antibiotics. Available online: https://www.ncbi.nlm.nih.gov/books/NBK545311/ (accessed on 10 February 2025).
- Maffei, B.; Francetic, O.; Subtil, A. Tracking Proteins Secreted by Bacteria: What’s in the Toolbox? Front. Cell. Infect. Microbiol. 2017, 7, 221. [Google Scholar] [CrossRef]
- Green, E.R.; Mecsas, J. Bacterial Secretion Systems: An Overview. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef]
- Hamilton, H.L.; Dillard, J.P. Natural transformation of Neisseria gonorrhoeae: From DNA donation to homologous recombination. Mol. Microbiol. 2006, 59, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Backert, S.; Meyer, T.F. Type IV secretion systems and their effectors in bacterial pathogenesis. Curr. Opin. Microbiol. 2006, 9, 207–217. [Google Scholar] [CrossRef]
- Ghosh, J.; Caparon, M.G. Specificity of Streptococcus pyogenes NAD+ glycohydrolase in cytolysin-mediated translocation. Mol. Microbiol. 2006, 62, 1203–1214. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K.N.; Liao, R.; Guinn, K.M.; Hickey, M.J.; Smith, S.; Behr, M.A.; Sherman, D.R. Deletion of RD1 from Mycobacterium tuberculosis Mimics Bacille Calmette-Guérin Attenuation. J. Infect. Dis. 2003, 187, 117–123. [Google Scholar] [CrossRef]
- Peterson, E.; Kaur, P. Antibiotic Resistance Mechanisms in Bacteria: Relationships Between Resistance Determinants of Antibiotic Producers, Environmental Bacteria, and Clinical Pathogens. Front. Microbiol. 2018, 9, 2928. [Google Scholar] [CrossRef]
- Wilson, M.M.; Anderson, D.E.; Bernstein, H.D. Analysis of the Outer Membrane Proteome and Secretome of Bacteroides fragilis Reveals a Multiplicity of Secretion Mechanisms. PLoS ONE 2015, 10, e0117732. [Google Scholar] [CrossRef]
- Hood, R.D.; Singh, P.; Hsu, F.; Güvener, T.; Carl, M.A.; Trinidad, R.R.S.; Silverman, J.M.; Ohlson, B.B.; Hicks, K.G.; Plemel, R.L.; et al. A Type VI Secretion System of Pseudomonas aeruginosa Targets a Toxin to Bacteria. Cell Host Microbe 2010, 7, 25–37. [Google Scholar] [CrossRef]
- Mills, E.; Baruch, K.; Aviv, G.; Nitzan, M.; Rosenshine, I. Dynamics of the Type III Secretion System Activity of Enteropathogenic Escherichia coli. mBio 2013, 4, 10-1128. [Google Scholar] [CrossRef]
- Pucelik, B.; Dąbrowski, J.M. Chapter Three—Photodynamic inactivation (PDI) as a promising alternative to current pharmaceuticals for the treatment of resistant microorganisms. In Advances in Inorganic Chemistry; van Eldik, R., Hubbard, C.D., Eds.; Academic Press: Cambridge, MA, USA, 2022; Volume 79, pp. 65–108. [Google Scholar]
- Symmons, M.F.; Bokma, E.; Koronakis, E.; Hughes, C.; Koronakis, V. The assembled structure of a complete tripartite bacterial multidrug efflux pump. Proc. Natl. Acad. Sci. USA 2009, 106, 7173–7178. [Google Scholar] [CrossRef]
- Hajiagha, M.N.; Kafil, H.S. Efflux pumps and microbial biofilm formation. Infect. Genet. Evol. 2023, 112, 105459. [Google Scholar] [CrossRef]
- Du, D.; Wang-Kan, X.; Neuberger, A.; van Veen, H.W.; Pos, K.M.; Piddock, L.J.V.; Luisi, B.F. Multidrug efflux pumps: Structure, function and regulation. Nat. Rev. Microbiol. 2018, 16, 523–539. [Google Scholar] [CrossRef]
- Ebbensgaard, A.E.; Løbner-Olesen, A.; Frimodt-Møller, J. The Role of Efflux Pumps in the Transition from Low-Level to Clinical Antibiotic Resistance. Antibiotics 2020, 9, 855. [Google Scholar] [CrossRef]
- Smith, B.L.; Fernando, S.; King, M.D. Escherichia coli resistance mechanism AcrAB-TolC efflux pump interactions with commonly used antibiotics: A molecular dynamics study. Sci. Rep. 2024, 14, 2742. [Google Scholar] [CrossRef]
- Lorusso, A.B.; Carrara, J.A.; Barroso, C.D.N.; Tuon, F.F.; Faoro, H. Role of Efflux Pumps on Antimicrobial Resistance in Pseudomonas aeruginosa. Int. J. Mol. Sci. 2022, 23, 15779. [Google Scholar] [CrossRef]
- Dulanto Chiang, A.; Dekker, J.P. Efflux pump-mediated resistance to new beta lactam antibiotics in multidrug-resistant gram-negative bacteria. Commun. Med. 2024, 4, 170. [Google Scholar] [CrossRef]
- Zhang, L.; Tian, X.; Sun, L.; Mi, K.; Wang, R.; Gong, F.; Huang, L. Bacterial Efflux Pump Inhibitors Reduce Antibiotic Resistance. Pharmaceutics 2024, 16, 170. [Google Scholar] [CrossRef]
- Zack, K.M.; Sorenson, T.; Joshi, S.G. Types and Mechanisms of Efflux Pump Systems and the Potential of Efflux Pump Inhibitors in the Restoration of Antimicrobial Susceptibility, with a Special Reference to Acinetobacter baumannii. Pathogens 2024, 13, 197. [Google Scholar] [CrossRef]
- Rosas, N.C.; Lithgow, T. Targeting bacterial outer-membrane remodelling to impact antimicrobial drug resistance. Trends Microbiol. 2022, 30, 544–552. [Google Scholar] [CrossRef]
- Cai, Y.; Chen, C.; Zhao, M.; Yu, X.; Lan, K.; Liao, K.; Guo, P.; Zhang, W.; Ma, X.; He, Y.; et al. High Prevalence of Metallo-β-Lactamase-Producing Enterobacter cloacae From Three Tertiary Hospitals in China. Front. Microbiol. 2019, 10, 1610. [Google Scholar] [CrossRef]
- Yuan, P.-B.; Ling, J.-H.; Zhu, J.-H.; Peng, C.; Chen, E.-Z.; Zhong, Y.-X.; Liu, W.-T.; Wang, L.-J.; Yang, L.; Chen, D.-Q. Proteomics profiling of ertapenem challenged major porin deficient carbapenem-resistant Klebsiella pneumoniae. J. Proteom. 2022, 268, 104715. [Google Scholar] [CrossRef]
- Russell Lewis, B.; Lawrence, R.; Hammerschmid, D.; Reading, E. Structural mass spectrometry approaches to understand multidrug efflux systems. Essays Biochem. 2023, 67, 255–267. [Google Scholar] [CrossRef]
- Cowan, S.W.; Schirmer, T.; Rummel, G.; Steiert, M.; Ghosh, R.; Pauptit, R.A.; Jansonius, J.N.; Rosenbusch, J.P. Crystal structures explain functional properties of two E. coli porins. Nature 1992, 358, 727–733. [Google Scholar] [CrossRef]
- Sharif, S.; Yadav, A.K. Bacterial biofilm and its role in antibiotic resistance. Microbe 2025, 7, 100356. [Google Scholar] [CrossRef]
- Richter, A.; Feßler, A.T.; Böttner, A.; Köper, L.M.; Wallmann, J.; Schwarz, S. Reasons for antimicrobial treatment failures and predictive value of in-vitro susceptibility testing in veterinary practice: An overview. Vet. Microbiol. 2020, 245, 108694. [Google Scholar] [CrossRef]
- Fisher, R.A.; Gollan, B.; Helaine, S. Persistent bacterial infections and persister cells. Nat. Rev. Microbiol. 2017, 15, 453–464. [Google Scholar] [CrossRef]
- Jiao, Y.; D’Haeseleer, P.; Dill Brian, D.; Shah, M.; VerBerkmoes Nathan, C.; Hettich Robert, L.; Banfield Jillian, F.; Thelen Michael, P. Identification of Biofilm Matrix-Associated Proteins from an Acid Mine Drainage Microbial Community. Appl. Environ. Microbiol. 2011, 77, 5230–5237. [Google Scholar] [CrossRef] [PubMed]
- Madsen, J.S.; Burmølle, M.; Hansen, L.H.; Sørensen, S.J. The interconnection between biofilm formation and horizontal gene transfer. FEMS Immunol. Med. Microbiol. 2012, 65, 183–195. [Google Scholar] [CrossRef]
- Rahman, M.A.; Amirkhani, A.; Chowdhury, D.; Mempin, M.; Molloy, M.P.; Deva, A.K.; Vickery, K.; Hu, H. Proteome of Staphylococcus aureus Biofilm Changes Significantly with Aging. Int. J. Mol. Sci. 2022, 23, 6415. [Google Scholar] [CrossRef]
- Ali, S.; Stavropoulos, A.; Jenkins, B.; Graves, S.; Ahmadi, A.; Marzbanrad, V.; Che, G.; Cheng, J.; Tan, H.; Wei, X.; et al. Comparative proteomics of biofilm development in Pseudoalteromonas tunicata discovers a distinct family of Ca2+-dependent adhesins. mBio 2025, 16, e01069-25. [Google Scholar] [CrossRef]
- Kuik, C.; de Boer, C.; van Hoogstraten, S.W.G.; Freulings, K.; Honing, M.; Arts, J.J.C.; Cillero-Pastor, B. Proteomic signatures of Staphylococcus aureus biofilm maturation on orthopaedic implants. Biofilm 2025, 9, 100287. [Google Scholar] [CrossRef]
- Di Bonaventura, G.; Picciani, C.; Lupetti, V.; Pompilio, A. Comparative Proteomic Analysis of Protein Patterns of Stenotrophomonas maltophilia in Biofilm and Planktonic Lifestyles. Microorganisms 2023, 11, 442. [Google Scholar] [CrossRef]
- Song, H.; Lou, N.; Liu, J.; Xiang, H.; Shang, D. Label-free quantitative proteomic analysis of the inhibition effect of Lactobacillus rhamnosus GG on Escherichia coli biofilm formation in co-culture. Proteome Sci. 2021, 19, 4. [Google Scholar] [CrossRef]
- Stewart, P.S.; Franklin, M.J. Physiological heterogeneity in biofilms. Nat. Rev. Microbiol. 2008, 6, 199–210. [Google Scholar] [CrossRef]
- Rather, M.A.; Gupta, K.; Mandal, M. Microbial biofilm: Formation, architecture, antibiotic resistance, and control strategies. Braz. J. Microbiol. [Publ. Braz. Soc. Microbiol.] 2021, 52, 1701–1718. [Google Scholar] [CrossRef] [PubMed]
- Grooters, K.E.; Ku, J.C.; Richter, D.M.; Krinock, M.J.; Minor, A.; Li, P.; Kim, A.; Sawyer, R.; Li, Y. Strategies for combating antibiotic resistance in bacterial biofilms. Front. Cell. Infect. Microbiol. 2024, 14, 1352273. [Google Scholar] [CrossRef] [PubMed]
- Usui, M.; Yoshii, Y.; Thiriet-Rupert, S.; Ghigo, J.-M.; Beloin, C. Intermittent antibiotic treatment of bacterial biofilms favors the rapid evolution of resistance. Commun. Biol. 2023, 6, 275. [Google Scholar] [CrossRef]
- Schiebel, J.; Böhm, A.; Nitschke, J.; Burdukiewicz, M.; Weinreich, J.; Ali, A.; Roggenbuck, D.; Rödiger, S.; Schierack, P. Genotypic and Phenotypic Characteristics Associated with Biofilm Formation by Human Clinical Escherichia coli Isolates of Different Pathotypes. Appl. Environ. Microbiol. 2017, 83, e01660-17. [Google Scholar] [CrossRef]
- Ero, R.; Yan, X.F.; Gao, Y.G. Ribosome Protection Proteins-“New” Players in the Global Arms Race with Antibiotic-Resistant Pathogens. Int. J. Mol. Sci. 2021, 22, 5356. [Google Scholar] [CrossRef]
- Salzer, A.; Wolz, C. Role of (p)ppGpp in antibiotic resistance, tolerance, persistence and survival in Firmicutes. microLife 2023, 4, uqad009. [Google Scholar] [CrossRef]
- Wang, E. RNA amplification for successful gene profiling analysis. J. Transl. Med. 2005, 3, 28. [Google Scholar] [CrossRef]
- Guo, W.; Liu, Y.; Han, Y.; Tang, H.; Fan, X.; Wang, C.; Chen, P.R. Amplifiable protein identification via residue-resolved barcoding and composition code counting. Natl. Sci. Rev. 2024, 11, nwae183. [Google Scholar] [CrossRef]
- Singh, A. Nanopores for sequencing proteins. Nat. Methods 2023, 20, 1870. [Google Scholar] [CrossRef]
- Lu, C.; Bonini, A.; Viel, J.H.; Maglia, G. Toward single-molecule protein sequencing using nanopores. Nat. Biotechnol. 2025, 43, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Kimani, R.; Musundi, S.; Wakaba, P.; Mbogo, D.; Essuman, S.; Kanoi, B.N.; Gitaka, J. Application of nanopore sequencing to identify antimicrobial resistance genes, mobile genetic elements and virulence factors in clinical isolates. bioRxiv 2023. [Google Scholar] [CrossRef]
- Zagajewski, A.; Turner, P.; Feehily, C.; El Sayyed, H.; Andersson, M.; Barrett, L.; Oakley, S.; Stracy, M.; Crook, D.; Nellåker, C.; et al. Deep learning and single-cell phenotyping for rapid antimicrobial susceptibility detection in Escherichia coli. Commun. Biol. 2023, 6, 1164. [Google Scholar] [CrossRef] [PubMed]
- Reszetnik, G.; Hammond, K.; Mahshid, S.; AbdElFatah, T.; Nguyen, D.; Corsini, R.; Caya, C.; Papenburg, J.; Cheng, M.P.; Yansouni, C.P. Next-generation rapid phenotypic antimicrobial susceptibility testing. Nat. Commun. 2024, 15, 9719. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Kim, J.; Kim, J.S.; Kim, W.; Lee, C.S. Label-free single-cell antimicrobial susceptibility testing in droplets with concentration gradient generation. Lab Chip 2024, 24, 5274–5289. [Google Scholar] [CrossRef]
- Li, H.; Torab, P.; Mach, K.E.; Surrette, C.; England, M.R.; Craft, D.W.; Thomas, N.J.; Liao, J.C.; Puleo, C.; Wong, P.K. Adaptable microfluidic system for single-cell pathogen classification and antimicrobial susceptibility testing. Proc. Natl. Acad. Sci. USA 2019, 116, 10270–10279. [Google Scholar] [CrossRef]
- Choi, J.; Yoo, J.; Lee, M.; Kim, E.-G.; Lee, J.S.; Lee, S.; Joo, S.; Song, S.H.; Kim, E.-C.; Lee, J.C.; et al. A rapid antimicrobial susceptibility test based on single-cell morphological analysis. Sci. Transl. Med. 2014, 6, 267ra174. [Google Scholar] [CrossRef]
- Soares, N.C.; Spät, P.; Méndez, J.A.; Nakedi, K.; Aranda, J.; Bou, G. Ser/Thr/Tyr phosphoproteome characterization of Acinetobacter baumannii: Comparison between a reference strain and a highly invasive multidrug-resistant clinical isolate. J. Proteom. 2014, 102, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Janiszewska, D.; Szultka-Młyńska, M.; Pomastowski, P.; Buszewski, B. “Omic” Approaches to Bacteria and Antibiotic Resistance Identification. Int. J. Mol. Sci. 2022, 23, 9601. [Google Scholar] [CrossRef]
- Ghosh, A.; Vang, C.K.; Brenner, E.P.; Ravi, J. Unlocking antimicrobial resistance with multiomics and machine learning. Trends Microbiol. 2025, 33, 1048–1051. [Google Scholar] [CrossRef]
- Soni, V.; Wang, Z.; Singh, V. Editorial: Bacterial metabolomics approach towards antimicrobials and resistance. Front. Microbiol. 2023, 14, 1222594. [Google Scholar] [CrossRef]
- Wang, H.; de Carvalho, L.P.S. Metabolomic profiling reveals bacterial metabolic adaptation strategies and new metabolites. Curr. Opin. Chem. Biol. 2023, 74, 102287. [Google Scholar] [CrossRef]
- Louwen, J.J.R.; Van Der Hooft, J.J. Comprehensive Large-Scale Integrative Analysis of Omics Data to Accelerate Specialized Metabolite Discovery. mSystems 2021, 6, 10-1128. [Google Scholar] [CrossRef]
- Caesar, L.K.; Butun, F.A.; Robey, M.T.; Ayon, N.J.; Gupta, R.; Dainko, D.; Bok, J.W.; Nickles, G.; Stankey, R.J.; Johnson, D.; et al. Correlative metabologenomics of 110 fungi reveals metabolite–gene cluster pairs. Nat. Chem. Biol. 2023, 19, 846–854. [Google Scholar] [CrossRef]
- Ayon, N.J.; Earp, C.E.; Gupta, R.; Butun, F.A.; Clements, A.E.; Lee, A.G.; Dainko, D.; Robey, M.T.; Khin, M.; Mardiana, L.; et al. Bioactivity-driven fungal metabologenomics identifies antiproliferative stemphone analogs and their biosynthetic gene cluster. Metabolomics 2024, 20, 90. [Google Scholar] [CrossRef]
- Schwab, W. Metabolome diversity: Too few genes, too many metabolites? Phytochemistry 2003, 62, 837–849. [Google Scholar] [CrossRef]
- Mohammadi, M.; Bishop, S.L.; Aburashed, R.; Luqman, S.; Groves, R.A.; Bihan, D.G.; Rydzak, T.; Lewis, I.A. Microbial containment device: A platform for comprehensive analysis of microbial metabolism without sample preparation. Front. Microbiol. 2022, 13, 958785. [Google Scholar] [CrossRef]
- Bartmanski, B.J.; Rocha, M.; Zimmermann-Kogadeeva, M. Recent advances in data- and knowledge-driven approaches to explore primary microbial metabolism. Curr. Opin. Chem. Biol. 2023, 75, 102324. [Google Scholar] [CrossRef]
- Schirmer, M.; Dusny, C. Microbial single-cell mass spectrometry: Status, challenges, and prospects. Curr. Opin. Biotechnol. 2023, 83, 102977. [Google Scholar] [CrossRef]
- Papagiannopoulou, C.; Parchen, R.; Rubbens, P.; Waegeman, W. Fast Pathogen Identification Using Single-Cell Matrix-Assisted Laser Desorption/Ionization-Aerosol Time-of-Flight Mass Spectrometry Data and Deep Learning Methods. Anal. Chem. 2020, 92, 7523–7531. [Google Scholar] [CrossRef]
- Josten, M.; Reif, M.; Szekat, C.; Al-Sabti, N.; Roemer, T.; Sparbier, K.; Kostrzewa, M.; Rohde, H.; Sahl, H.-G.; Bierbaum, G. Analysis of the Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrum of Staphylococcus aureus Identifies Mutations That Allow Differentiation of the Main Clonal Lineages. J. Clin. Microbiol. 2013, 51, 1809–1817. [Google Scholar] [CrossRef]
- Pineda, F.J.; Antoine, M.D.; Demirev, P.A.; Feldman, A.B.; Jackman, J.; Longenecker, M.; Lin, J.S. Microorganism Identification by Matrix-Assisted Laser/Desorption Ionization Mass Spectrometry and Model-Derived Ribosomal Protein Biomarkers. Anal. Chem. 2003, 75, 3817–3822. [Google Scholar] [CrossRef]






| Tested Antibiotic/Antibiotic Class | Bacterial Species | Sample Type | Marker Proteins/ Peptides/Genes | Quantification | Additional Test | Ref. |
|---|---|---|---|---|---|---|
| Ceftriaxone Gentamicin Carbapenem | A. baumannii (3 isolates) | Respiratory tract, blood, and urine samples from hospitalized patients |
| iTRAQ (isobaric tags for relative and absolute quantification) | Gene ontology functional enrichment analysis | [114] |
| Cefotaxime | E. coli, P. aeruginosa, S. aureus, S. pneumoniae, M. catarrhalis, H. influenzae | Respiratory tract and urine samples of an infected 2-year-old boy |
| Not performed | In silico analysis | [115] |
| ||||||
| Beta-lactams Aminoglycosides Fluroquinolones | E. coli (78 clinical isolates) K. pneumoniae (109 clinical isolates) | Stored clinical isolates | b1a. Carbapenemases (NDM, OXA-48, KPC, VIM) b1b. Beta-lactamases CTX-M, TEM, OXA-1 | Label-free quantification | Susceptibility testing, WGS | [116] |
| b2. 16S-RMTases b (armA, rmtB, rmtC, rmtF, RmtB) b3. QnrA, QnrB, AAC(6′)-Ib-cr, oqxA, oqxB | ||||||
| Beta-lactams Tetracycline | Tested against different bacterial phyla (see reference for complete list) | Raw cow milk | bTET, LRA-19, IND-16, OXA-658, MPHN, TET (52), NIMC, BRO-2, CFRC | Label-free quantification | LAP-MALDI, metaproteomics | [117] |
| Beta-lactams Streptomycin Macrolide Aminoglycosides Sulfonamide Polymyxin | E. coli, K. pneumoniae, A. baumannii, P. aeruginosa, S. enterica | Blood, urine, stool, respiratory tract, and tissue samples | c16S rRNA methyltransferase, O-phosphotransferase, N-acetyltransferase, Macrolide phosphotransferase, Nucleotidyltransferase, Beta-lactamase, PBP1b, MFS, RND efflux pumps, Sulfonamide resistant sul, Bifunctional polymyxin resistance protein (arnA) | Label-free quantification | Genomic analysis | [118] |
| Key Challenge | Key Consideration | Ref. |
|---|---|---|
|
| [155,156] |
|
| [157] |
|
| [157] |
|
| [157,158] |
|
| [159] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayon, N.J. Bacterial Proteomics and Antibiotic Resistance Identification: Is Single-Cell Analysis a Worthwhile Pursuit? Pathogens 2025, 14, 1127. https://doi.org/10.3390/pathogens14111127
Ayon NJ. Bacterial Proteomics and Antibiotic Resistance Identification: Is Single-Cell Analysis a Worthwhile Pursuit? Pathogens. 2025; 14(11):1127. https://doi.org/10.3390/pathogens14111127
Chicago/Turabian StyleAyon, Navid J. 2025. "Bacterial Proteomics and Antibiotic Resistance Identification: Is Single-Cell Analysis a Worthwhile Pursuit?" Pathogens 14, no. 11: 1127. https://doi.org/10.3390/pathogens14111127
APA StyleAyon, N. J. (2025). Bacterial Proteomics and Antibiotic Resistance Identification: Is Single-Cell Analysis a Worthwhile Pursuit? Pathogens, 14(11), 1127. https://doi.org/10.3390/pathogens14111127






