Construction of a Risk Assessment Model for Short-Term Mortality in Patients with Invasive Fungal Diseases Post-Cardiac Surgery Based on Multivariate Analysis
Abstract
1. Introduction
2. Materials and Methods
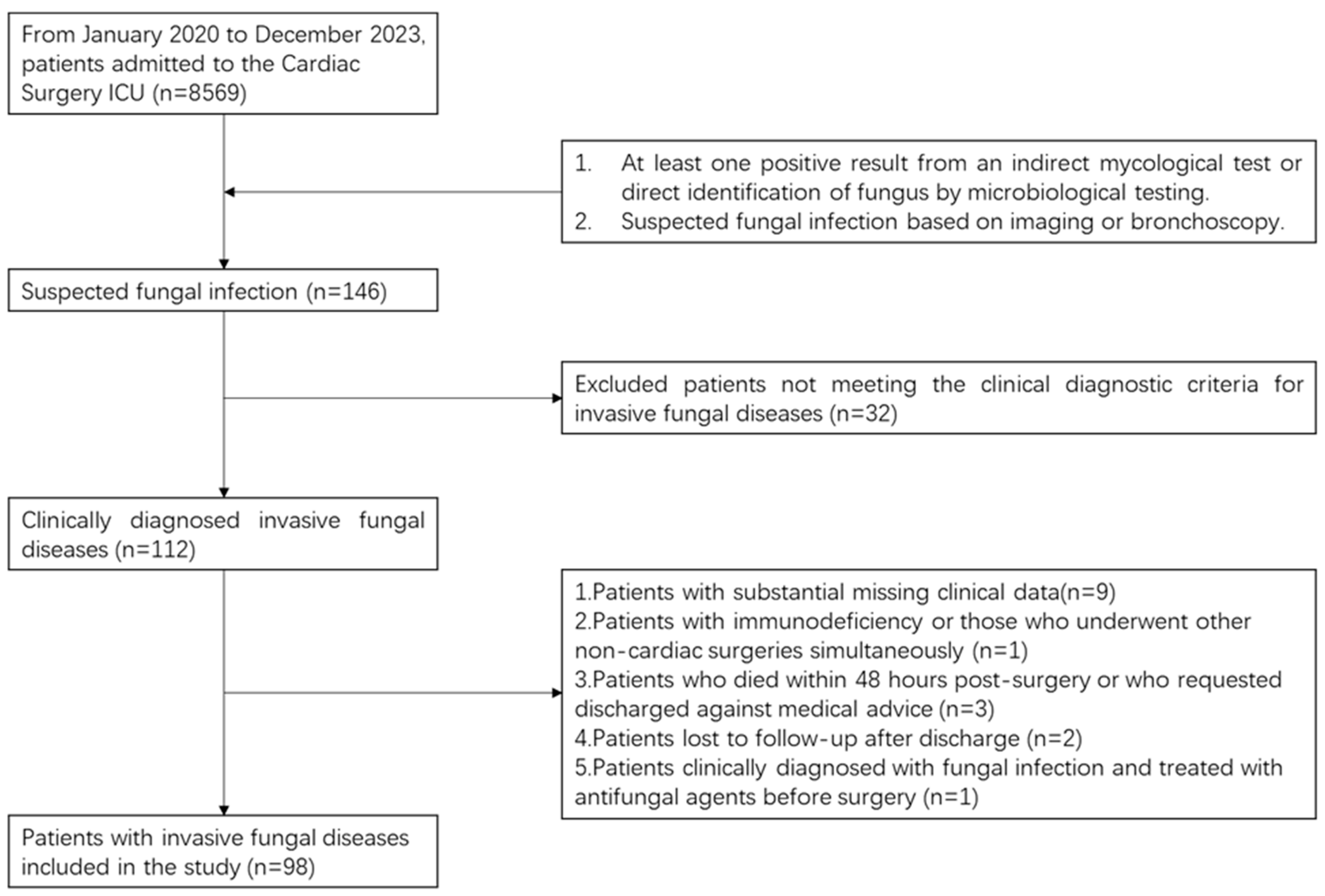
2.1. Study Population
2.2. Diagnostic Criteria
2.2.1. Probable Invasive Fungal Disease
2.2.2. Confirmed Candidemia
2.3. Inclusion and Exclusion Criteria
2.3.1. Inclusion Criteria
2.3.2. Exclusion Criteria
2.4. Clinical Data Collection
2.5. Statistical Analysis
2.6. Ethical Statement
3. Results
3.1. Baseline Characteristics
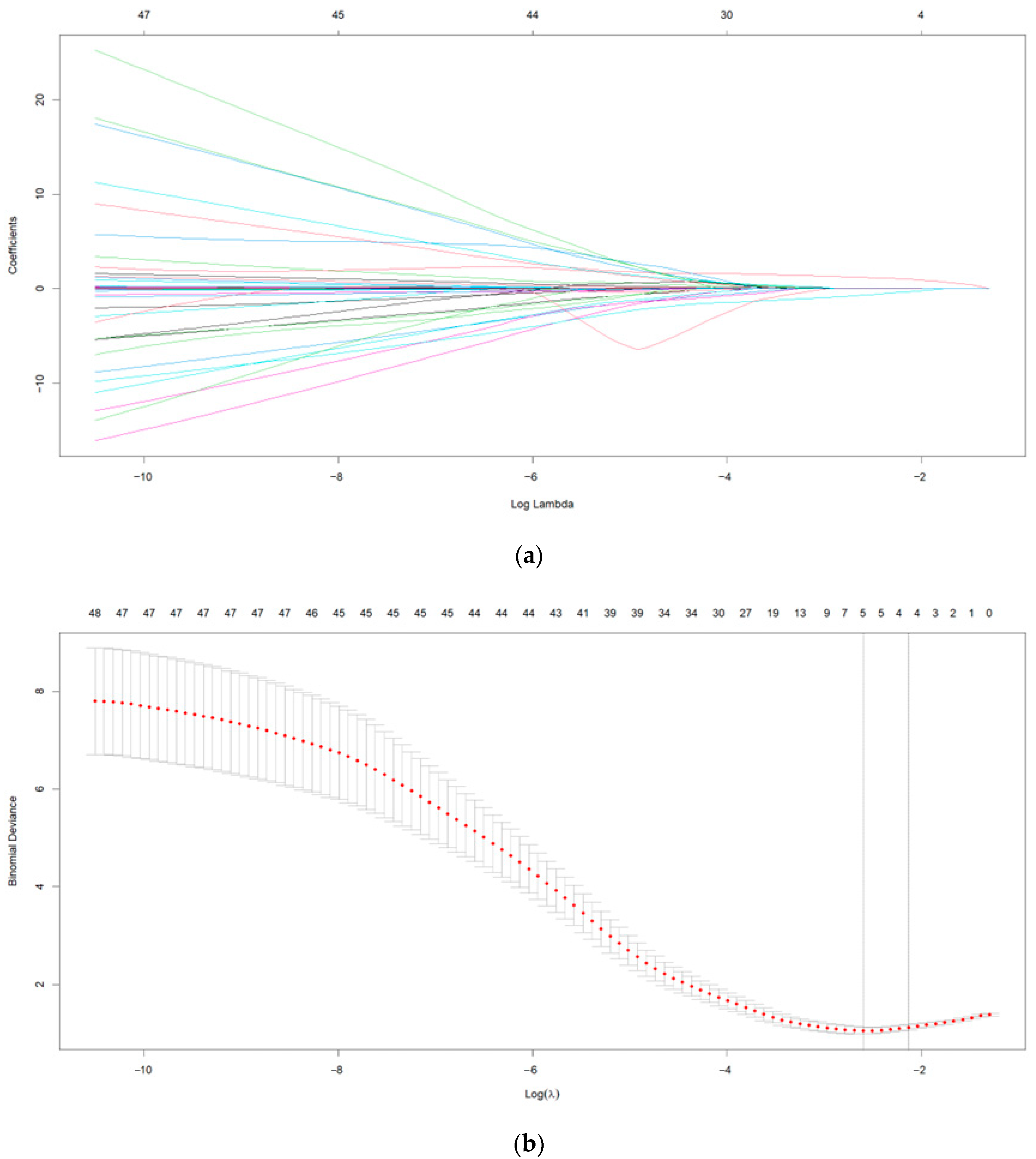
3.2. Results of LASSO-Logistic Regression Analysis
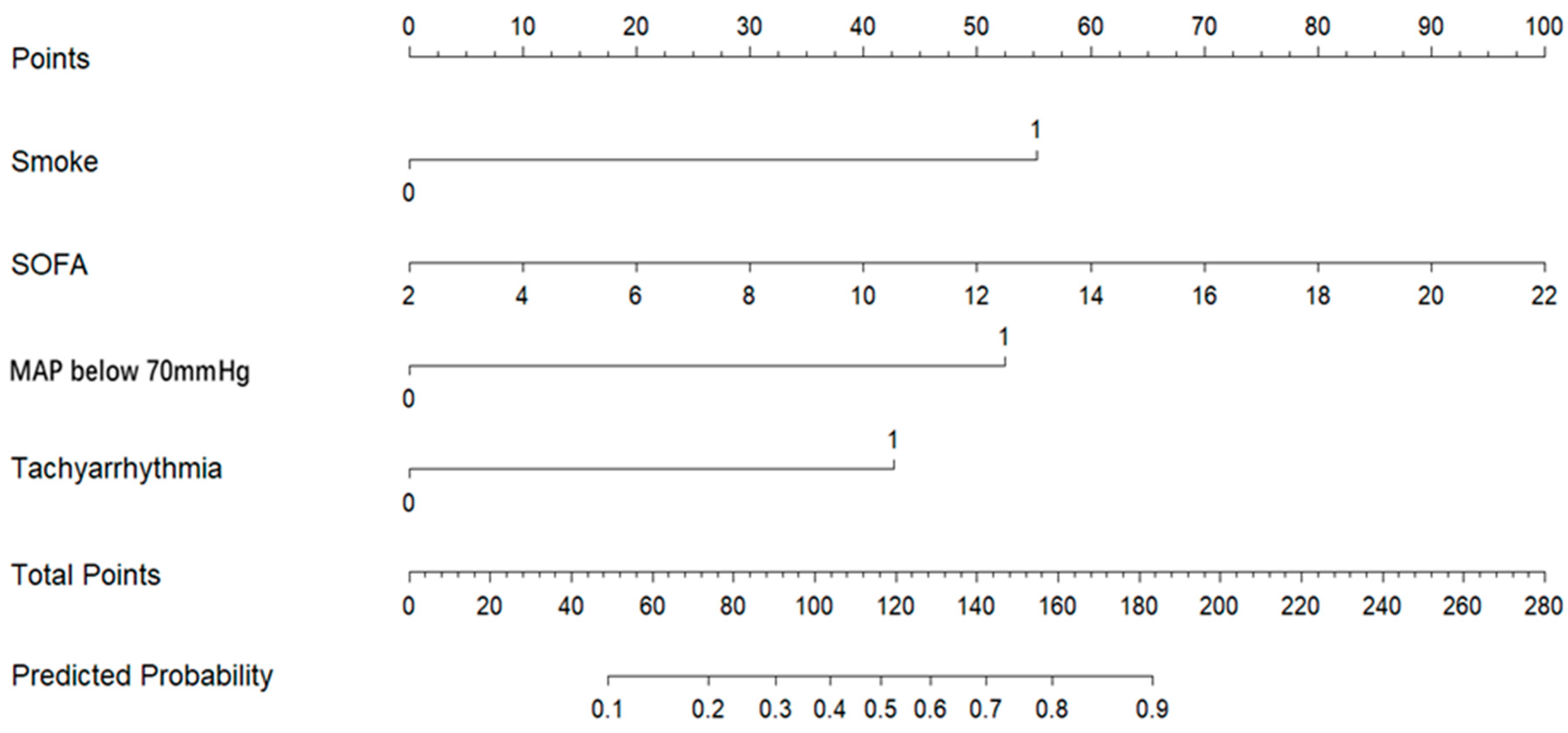
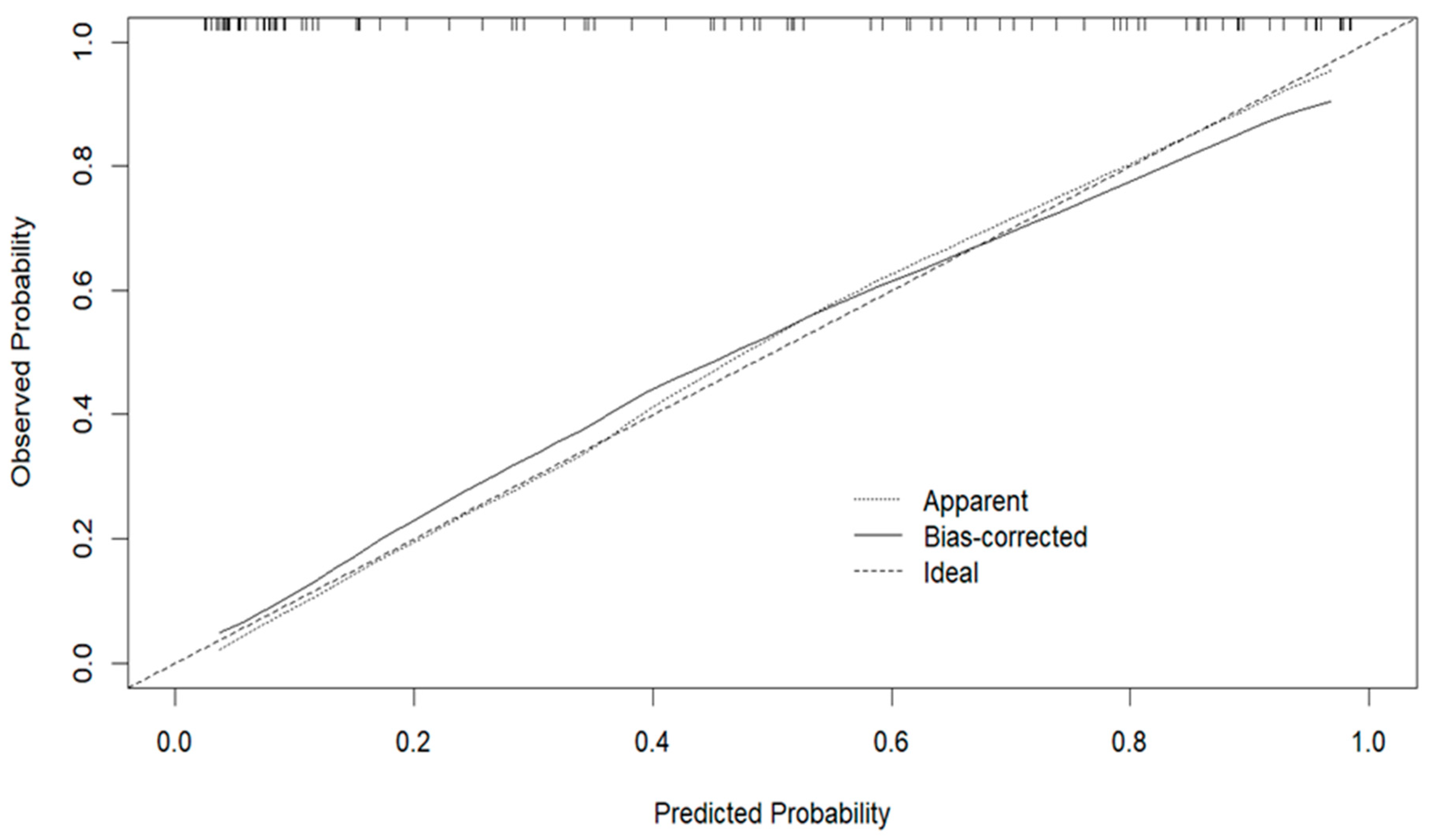
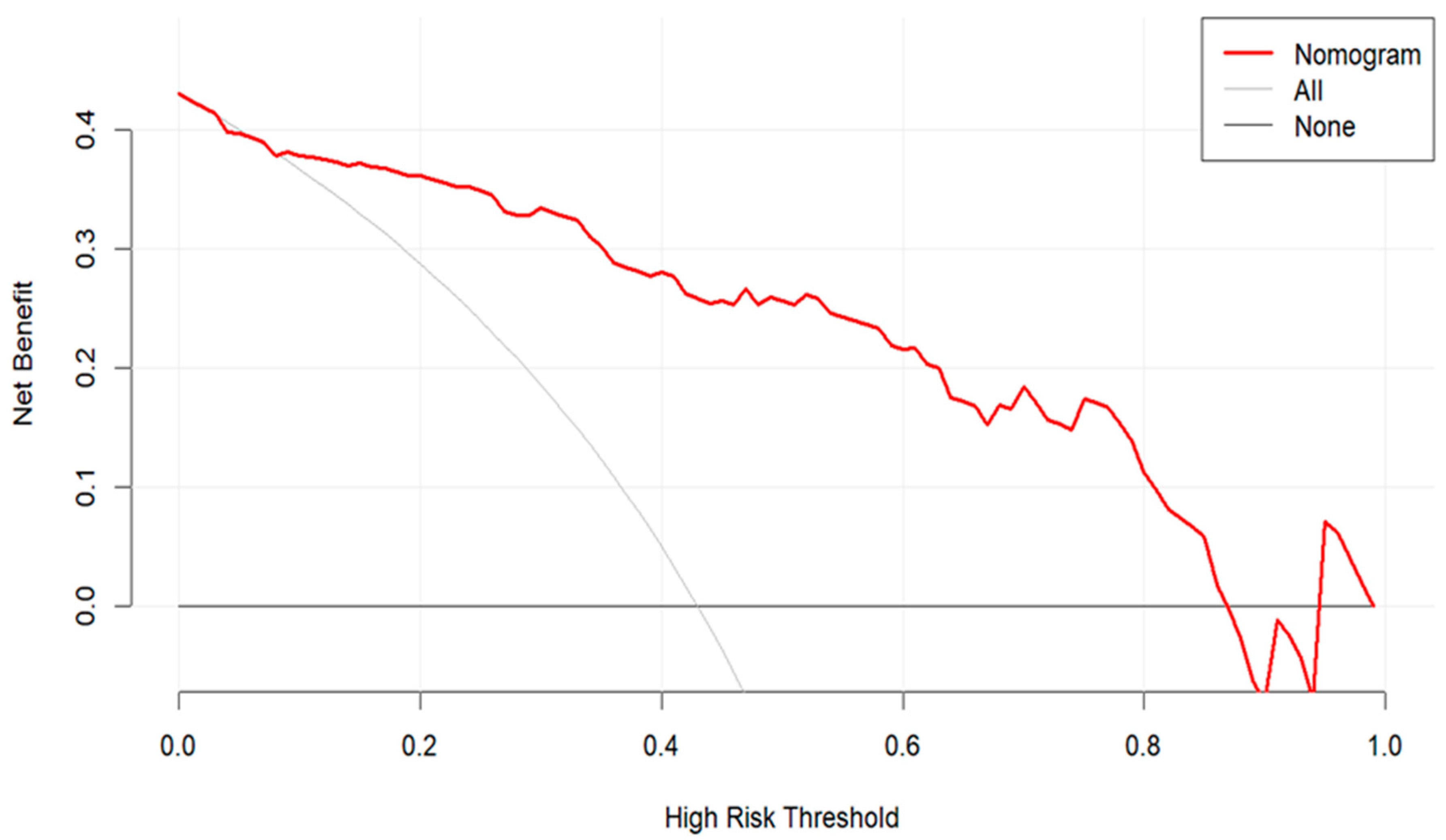
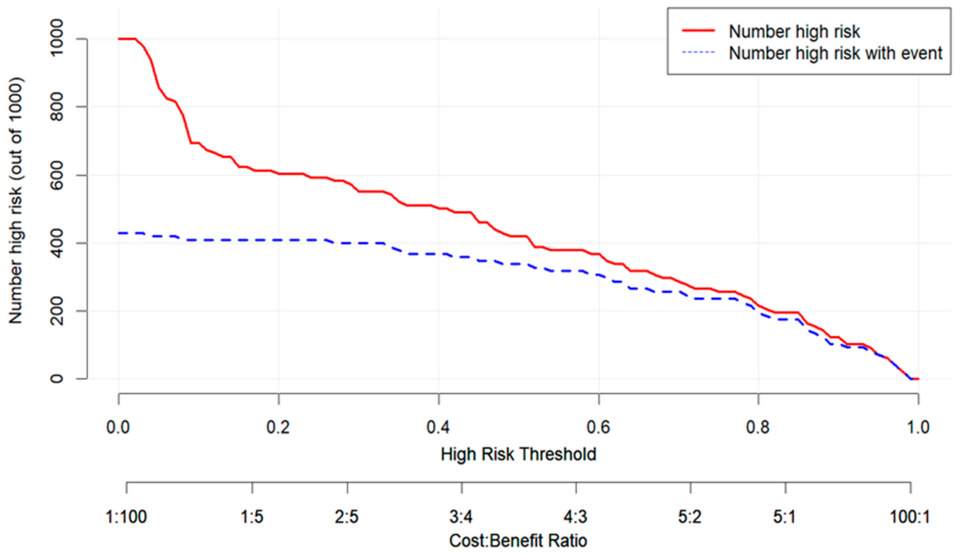
3.3. Development and Validation of the Predictive Model
4. Discussion
5. Limitations and Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Perez-Granda, M.J.; Barrio, J.M.; Cuerpo, G.; Valerio, M.; Munoz, P.; Hortal, J.; Pinto, A.G.; Bouza, E.; On behalf of the Cardiovascular Infection Study Group. Infectious complications following major heart surgery from the day of the surgery to hospital discharge. BMC Infect. Dis. 2024, 24, 73. [Google Scholar] [CrossRef]
- Zukowska, A.; Zukowski, M. Surgical Site Infection in Cardiac Surgery. J. Clin. Med. 2022, 11, 6991. [Google Scholar] [CrossRef]
- Vicente-Martínez, L.; Vicente-Guillen, R.; Calabuig, E.; Escribá, F.; Pajares, A.; Argente, P. Experience of a fungal infection after cardiac surgery. Rev. Española Anestesiol. Reanim. 2019, 66, 307–314. [Google Scholar] [CrossRef]
- Giacobbe, D.R.; Salsano, A.; Del Puente, F.; Miette, A.; Vena, A.; Corcione, S.; Bartoletti, M.; Mularoni, A.; Maraolo, A.E.; Peghin, M.; et al. Risk Factors for Candidemia After Open Heart Surgery: Results from a Multicenter Case—Control Study. Open Forum Infect. Dis. 2020, 7, ofaa233. [Google Scholar] [CrossRef]
- Bassetti, M.; Azoulay, E.; Kullberg, B.J.; Ruhnke, M.; Shoham, S.; Vazquez, J.; Giacobbe, D.R.; Calandra, T. EORTC/MSGERC Definitions of Invasive Fungal Diseases: Summary of Activities of the Intensive Care Unit Working Group. Clin. Infect. Dis. 2021, 72 (Suppl. 2), S121–S127. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Giacobbe, D.R.; Agvald-Ohman, C.; Akova, M.; Alastruey-Izquierdo, A.; Arikan-Akdagli, S.; Azoulay, E.; Blot, S.; Cornely, O.A.; Cuenca-Estrella, M.; et al. Invasive Fungal Diseases in Adult Patients in Intensive Care Unit (FUNDICU): 2024 consensus definitions from ESGCIP, EFISG, ESICM, ECMM, MSGERC, ISAC, and ISHAM. Intensive Care Med. 2024, 50, 502–515. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, J.P.; Chen, S.C.; Kauffman, C.A.; Steinbach, W.J.; Baddley, J.W.; Verweij, P.E.; Clancy, C.J.; Wingard, J.R.; Lockhart, S.R.; Groll, A.H.; et al. Revision and Update of the Consensus Definitions of Invasive Fungal Disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin. Infect. Dis. 2020, 71, 1367–1376. [Google Scholar] [CrossRef] [PubMed]
- Tajdini, M.; Behnoush, A.H.; Pashang, M.; Jameie, M.; Khalaji, A.; Sadeghian, S.; Vasheghani-Farahani, A.; Poorhosseini, H.; Masoudkabir, F.; Hosseini, K.; et al. Heart surgery over two decades: What we have learned about results and changing risks. BMC Cardiovasc. Disord. 2024, 24, 195. [Google Scholar] [CrossRef]
- Roeschl, T.; Hinrichs, N.; Hommel, M.; Pfahringer, B.; Balzer, F.; Falk, V.; O’Brien, B.; Ott, S.C.; Potapov, E.; Schoenrath, F.; et al. Systematic Assessment of Shock Severity in Postoperative Cardiac Surgery Patients. JACC 2023, 82, 1691–1706. [Google Scholar] [CrossRef]
- Wang, J.; Wang, T.; Zhang, H.; Zhang, Q.; Liu, G.; Yan, S.; Wang, Q.; Teng, Y.; Wang, J.; Hu, Q.; et al. Effect of mild hypothermia vs normothermia cardiopulmonary bypass on postoperative bleeding in patients undergoing coronary artery bypass grafting: Protocol of a multi-center, randomized, controlled trial. BMC Surg. 2024, 24, 323. [Google Scholar] [CrossRef]
- Salmanton-Garcia, J.; Cornely, O.A.; Stemler, J.; Barac, A.; Steinmann, J.; Sivakova, A.; Akalin, E.H.; Arikan-Akdagli, S.; Loughlin, L.; Toscano, C.; et al. Attributable mortality of candidemia—Results from the ECMM Candida III multinational European Observational Cohort Study. J. Infect. 2024, 89, 106229. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Giacobbe, D.R.; Vena, A.; Trucchi, C.; Ansaldi, F.; Antonelli, M.; Adamkova, V.; Alicino, C.; Almyroudi, M.P.; Atchade, E.; et al. Incidence and outcome of invasive candidiasis in intensive care units (ICUs) in Europe: Results of the EUCANDICU project. Crit. Care 2019, 23, 219. [Google Scholar] [CrossRef]
- Montagna, M.T.; Caggiano, G.; Lovero, G.; De Giglio, O.; Coretti, C.; Cuna, T.; Iatta, R.; Giglio, M.; Dalfino, L.; Bruno, F.; et al. Epidemiology of invasive fungal infections in the intensive care unit: Results of a multicenter Italian survey (AURORA Project). Infection 2013, 41, 645–653. [Google Scholar] [CrossRef]
- Pourbaix, A.; Lafont Rapnouil, B.; Guery, R.; Lanternier, F.; Lortholary, O.; Cohen, J.F. Smoking as a Risk Factor of Invasive Fungal Disease: Systematic Review and Meta-Analysis. Clin. Infect. Dis. 2020, 71, 1106–1119. [Google Scholar] [CrossRef]
- Nishimoto, Y.; Ohbe, H.; Matsui, H.; Nakajima, M.; Sasabuchi, Y.; Goto, T.; Morita, K.; Fushimi, K.; Sato, Y.; Yasunaga, H. Predictive ability of the sequential organ failure assessment score for in-hospital mortality in patients with cardiac critical illnesses: A nationwide observational study. Eur. Heart J. Acute Cardiovasc. Care 2022, 11, 312–321. [Google Scholar] [CrossRef]
- Schoe, A.; Bakhshi-Raiez, F.; de Keizer, N.; van Dissel, J.T.; de Jonge, E. Mortality prediction by SOFA score in ICU-patients after cardiac surgery; comparison with traditional prognostic-models. BMC Anesthesiol. 2020, 20, 65. [Google Scholar] [CrossRef]
- Follath, F.; Yilmaz, M.B.; Delgado, J.F.; Parissis, J.T.; Porcher, R.; Gayat, E.; Burrows, N.; McLean, A.; Vilas-Boas, F.; Mebazaa, A. Clinical presentation, management and outcomes in the Acute Heart Failure Global Survey of Standard Treatment (ALARM-HF). Intensive Care Med. 2011, 37, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Li, W.; Wang, Y.; Chen, X.; He, D.; Liu, M.; Yuan, J.; Xiao, C.; Li, Q.; Chen, L.; et al. Investigating the Association Between Mean Arterial Pressure on 28-Day Mortality Risk in Patients with Sepsis: Retrospective Cohort Study Based on the MIMIC-IV Database. Interact. J. Med. Res. 2025, 14, e63291. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-López, J.M.; Iglesias-González, J.L.; Lozano-Sánchez, F.S.; Palomero-Rodríguez, M.; Sánchez-Conde, P. Inflammatory Response, Immunosuppression and Arginase Activity after Cardiac Surgery Using Cardiopulmonary Bypass. J. Clin. Med. 2022, 11, 4187. [Google Scholar] [CrossRef]
- Qu, F.; Yang, W.; He, N.; Qu, S.; Zhou, X.; Ma, H.; Jiang, X. Effect of Postoperative Atrial Fibrillation After Cardiac Surgery: A Meta-Analysis. Clin. Cardiol. 2024, 47, e70053. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Du, A.; An, Y. The Impact of New-Onset Atrial Fibrillation on Short- and Long-Term Mortality of Critically Ill Patients with Candidemia. Res. Sq. 2021. preprint. [Google Scholar] [CrossRef]

| Variable | Overall | Survivors | Non-Survivors | p-Value |
|---|---|---|---|---|
| (a) | ||||
| (N = 98) | (N = 56) | (N = 42) | ||
| Age (years, mean ± SD) | 63.1 ± 11.1 | 63.9 ± 9.4 | 62.1 ± 13.0 | 0.51 |
| Male, n (%) | 53 (54.1%) | 29 (51.8%) | 24 (57.1%) | 0.62 |
| BMI (kg/m2, mean ± SD) | 24.26 ± 4.31 | 24.40 ± 4.18 | 24.06 ± 4.53 | 0.7 |
| Smoking, n (%) | 48 (49.0%) | 14 (25.0%) | 34 (81.0%) | <0.001 |
| Diabetes mellitus, n (%) | 25 (25.5%) | 13 (23.2%) | 12 (28.6%) | 0.56 |
| Hypertension, n (%) | 62 (63.3%) | 33 (58.9%) | 29 (69.0%) | 0.32 |
| LVEF [IQR] | 0.58 (0.45,0.63) | 0.59 (0.48,0.63) | 0.55 (0.41,0.62) | 0.65 |
| NYHA class III–IV, n (%) | 93 (94.90%) | 54 (96.43%) | 39 (92.86%) | 0.65 |
| Pulmonary hypertension, median [IQR] | 29.5 [24.0–45.8] | 28.5 [22.8–42.0] | 30.0 [27.0–46.8] | 0.42 |
| (b) | ||||
| Type of surgery, n (%) | 0.95 | |||
| – Isolated CABG | 30 (30.6%) | 16 (28.6%) | 14 (33.3%) | |
| – Valve surgery (repair or replacement) | 20 (20.4%) | 11 (19.6%) | 9 (21.4%) | |
| – Combined Valve + CABG surgery | 16 (16.3%) | 9 (16.1%) | 7 (16.7%) | |
| – Thoracic aortic surgery | 24 (24.5%) | 16 (28.6%) | 8 (19.0%) | |
| – Congenital heart surgery | 2 (2.0%) | 1 (1.8%) | 1 (2.4%) | |
| – Others | 6 (6.1%) | 3 (5.4%) | 3 (7.1%) | |
| Operation time (min, Mean ± SD) | 400.05 ± 133.19 | 401.43 ± 122.45 | 398.21 ± 147.80 | 0.91 |
| Cardiopulmonary bypass time (min, mean ± SD) | 202.00 (163.50,261.50) | 199.50 (164.75,268.50) | 202.00 (154.00,233.50) | 0.57 |
| Blood transfusion (yes, n%) | 94 (95.92%) | 52 (92.86%) | 42 (100.00%) | 0.13 |
| (c) | ||||
| SOFA score, median [IQR] | 11.0 [8.0–15.0] | 9.5 [7.0–12.0] | 14.5 [11.0–17.0] | <0.001 |
| APACHE II, median [IQR] | 24.0 [18.0–29.0] | 20.0 [16.0–24.8] | 28.5 [25.0–38.0] | <0.001 |
| Use of ECMO or IABP, n (%) | 18(18.4%) | 10(17.9%) | 8(19.0%) | 0.88 |
| MAP below 70 mmHg, n (%) | 29 (29.6%) | 7 (12.5%) | 22 (52.4%) | <0.001 |
| Tachyarrhythmia, n (%) | 31 (31.6%) | 11 (19.6%) | 20 (47.6%) | 0.004 |
| AKI, n (%) | 69 (70.4%) | 32 (57.1%) | 37 (88.1%) | 0.002 |
| RRT required, n (%) | 38 (38.8%) | 15 (26.8%) | 23 (54.8%) | 0.007 |
| Hepatic dysfunction, n (%) | 69 (70.4%) | 35 (62.5%) | 34 (81.0%) | 0.06 |
| Invasive mechanical ventilation > 48 h, n (%) | 84 (85.7%) | 43 (76.8%) | 41 (97.6%) | 0.006 |
| Combination antibiotic therapy (≥2 agents), n (%) | 76 (77.6%) | 41 (73.2%) | 35 (83.3%) | 0.24 |
| ICU stay > 7 days, n (%) | 87 (88.8%) | 47 (83.9%) | 40 (95.2%) | 0.08 |
| Variable | β | SE | Wald Z | p-Value | Odds Ratio (95% CI) |
|---|---|---|---|---|---|
| Intercept | −4.1299 | 1.0222 | −4.04 | <0.0001 | - |
| Smoke | 1.8100 | 0.5863 | 3.09 | 0.0020 | 6.11 (1.94–19.28) |
| SOFA | 0.1636 | 0.0819 | 2.00 | 0.0457 | 1.18 (1.00–1.38) |
| MAP below 70 mmHg | 1.7174 | 0.6373 | 2.69 | 0.0070 | 5.57 (1.60–19.42) |
| Tachyarrhythmia | 1.3960 | 0.6095 | 2.29 | 0.0220 | 4.04 (1.22–13.34) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, D.; Shen, Q.; Zhai, Q. Construction of a Risk Assessment Model for Short-Term Mortality in Patients with Invasive Fungal Diseases Post-Cardiac Surgery Based on Multivariate Analysis. Pathogens 2025, 14, 1116. https://doi.org/10.3390/pathogens14111116
Wei D, Shen Q, Zhai Q. Construction of a Risk Assessment Model for Short-Term Mortality in Patients with Invasive Fungal Diseases Post-Cardiac Surgery Based on Multivariate Analysis. Pathogens. 2025; 14(11):1116. https://doi.org/10.3390/pathogens14111116
Chicago/Turabian StyleWei, Dong, Qi Shen, and Qian Zhai. 2025. "Construction of a Risk Assessment Model for Short-Term Mortality in Patients with Invasive Fungal Diseases Post-Cardiac Surgery Based on Multivariate Analysis" Pathogens 14, no. 11: 1116. https://doi.org/10.3390/pathogens14111116
APA StyleWei, D., Shen, Q., & Zhai, Q. (2025). Construction of a Risk Assessment Model for Short-Term Mortality in Patients with Invasive Fungal Diseases Post-Cardiac Surgery Based on Multivariate Analysis. Pathogens, 14(11), 1116. https://doi.org/10.3390/pathogens14111116







