Clinical Characteristics of Adenovirus Pneumonia in Children
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Specimen Testing
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
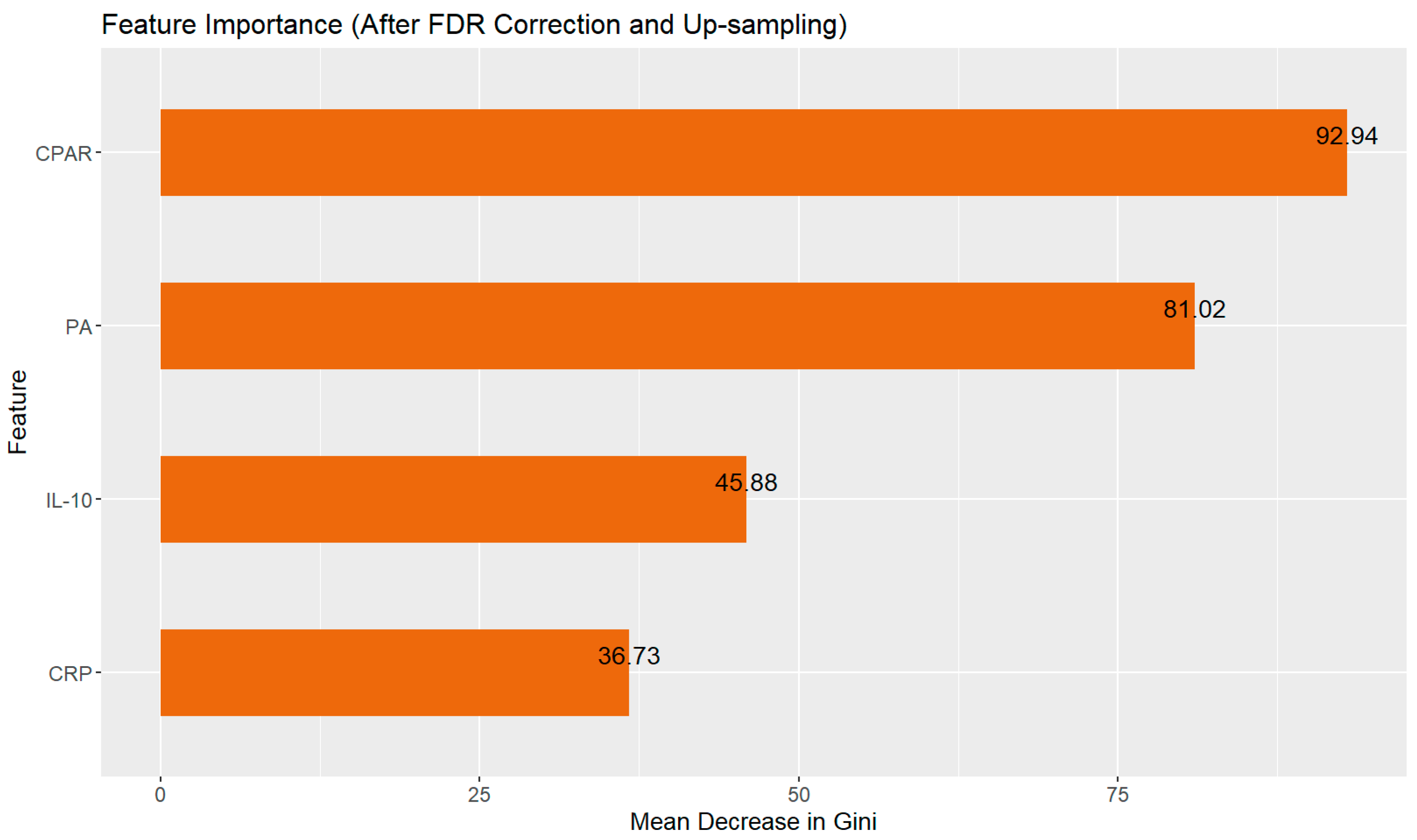
3.2. Variables of Importance
3.3. Random Forest Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SAP | Severe Adenoviral Pneumonia |
| NSAP | Non-Severe Adenoviral Pneumonia |
| PLT | Platelet |
| PA | Prealbumin |
| CRP | C-Reactive Protein |
| IFN-γ | Interferon-γ |
| ROC | Receiver Operating Characteristic |
| AUC | Area Under the Receiver Operating Characteristic Curve |
| CBC | Complete Blood Count |
| PCT | Procalcitonin |
| NEV | Neutrophil Absolute Count |
| LYM | Lymphocyte Count |
| TNF | Tumor Necrosis Factor |
| IL | Interleukins |
Appendix A
| Assessment Items | Mild | Severe |
|---|---|---|
| General condition | Good | Poor |
| Consciousness disorder | Absent | Present |
| Hypoxemia | Absent | Cyanosis; Rapid respiration, RR ≥ 70 breaths/min (infants), RR ≥ 50 breaths/min (>1 year old); Accessory respiration (groaning, nasal flaring, three—depression sign); Intermittent apnea; Oxygen saturation < 92% |
| Fever | Does not meet severe criteria | Hyperpyrexia, persistent high fever > 5 days |
| Dehydration sign/refusal to eat | Absent | Present |
| Chest X-ray or chest CT | Does not meet severe criteria | ≥2/3 of one—side lung infiltration, multi—lobe lung infiltration, pleural effusion, pneumothorax, atelectasis, lung necrosis, lung abscess |
| Extrapulmonary complications | Absent | Present |
| Criteria | All of the above mild conditions are present | Any one of the above severe conditions is present |
Appendix B
| Parameter | Reagent | Manufacturer | Equipment |
|---|---|---|---|
| LYM | Mindray M-6FD DYE; Mindray M-6FN DYE | Mindray, Shenzhen, China | Mindray BC5300 analyzer |
| PLT | |||
| NEV | |||
| CRP | High Sensitivity C-reaction Protein (HS-CRP) Kit | ||
| Th1/Th2 cytokines | Cell Factor Combined Detection Kit (Immunofluorescence Method) | Jiangxi Cellgene Bio-Tech, Nanchang, China | FACScalibur® flow cytometer |
| PA | Prealbumin (PA) Assay Kit (Immunoturbidimetric Method) | Mindray, Shenzhen, China | Beckman Coulter AU5800 analyzer |
| PCT | Pylon PCT | ET Healthcare, Suzhou, USA |
Appendix C
| Laboratory Data (Reference Range) | NSAP (n = 374) | SAP (n = 54) | p-Value | Adjusted p-Value * | ||
|---|---|---|---|---|---|---|
| Mean ± SD | Median (IQR) | Mean ± SD | Median (IQR) | |||
| LYM (0.70–4.9 × 109/L) | 3.55 ± 2.49 | 3 (1.95) | 3.31 ± 2.14 | 2.75 (2.13) | 0.4158 | 0.532 |
| NEV (1.50–7.80 × 109/L) | 5.85 ± 4.26 | 4.44 (5.78) | 6.22 ± 4.86 | 5.13 (4.83) | 0.6049 | 0.645 |
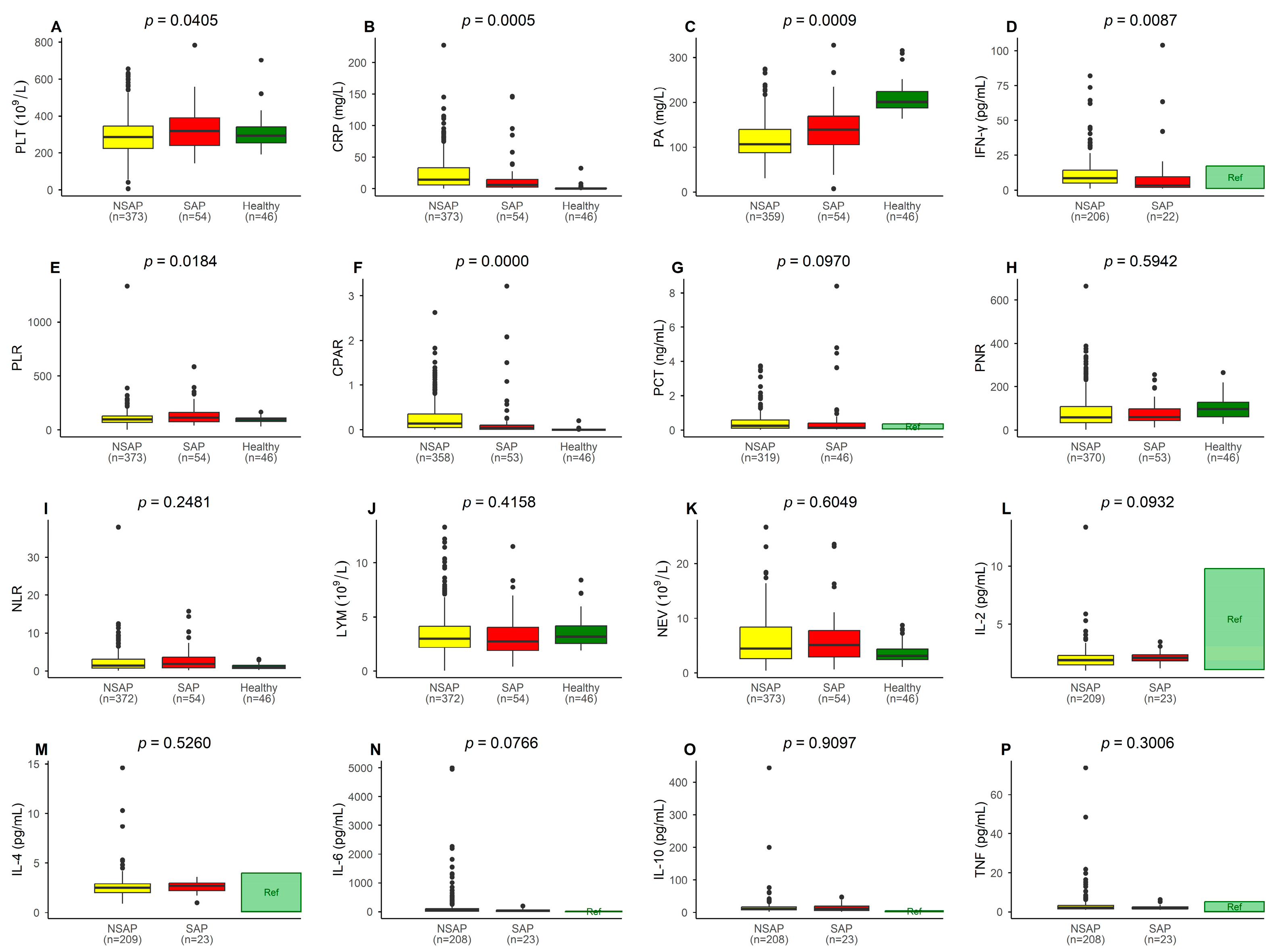
| PLT (100–400 × 109/L) | 295.77 ± 103.28 | 287 (121) | 328.50 ± 115.01 | 318.5 (148.25) | 0.0405 | 0.108 |
| CRP (0.00–8.00 mg/L) | 24.49 ± 27.96 | 14.26 (27.63) | 17.84 ± 31.81 | 6.08 (12.23) | 0.0005 | 0.004 |
| PA (150.0–300.0 mg/L) | 115.68 ± 41.05 | 106.7 (51.85) | 138.46 ± 57.87 | 138.85 (63.35) | 0.0009 | 0.005 |
| PCT (0.07–0.350 ng/mL) | 0.48 ± 0.72 | 0.25 (0.47) | 0.82 ± 1.74 | 0.15 (0.44) | 0.0970 | 0.256 |
| PNR | 87.65 ± 84.42 | 58.14 (75.78) | 84.19 ± 71.66 | 59.79 (57.51) | 0.5942 | 0.601 |
| NLR | 2.33 ± 2.84 | 1.42 (2.28) | 2.94 ± 3.27 | 1.90 (2.75) | 0.2481 | 0.390 |
| PLR | 105.43 ± 81.31 | 95.39 (59.31) | 138.56 ± 100.08 | 112.16 (87.62) | 0.0184 | 0.068 |
| CPAR | 0.26 ± 0.34 | 0.14 (0.30) | 0.47 ± 1.84 | 0.04 (0.12) | 0.0000 | 0.002 |
| IL-2 (1.1–9.8 pg/mL) | 2.02 ± 1.07 | 1.90 (0.80) | 2.15 ± 0.58 | 2.10 (0.50) | 0.0932 | 0.186 |
| IL-4 (0.1–4.0 pg/mL) | 2.64 ± 1.31 | 2.50 (0.90) | 2.56 ± 0.63 | 2.70 (0.80) | 0.5260 | 0.601 |
| IL-6 (1.7–16.6 pg/mL) | 219.65 ± 663.02 | 51.10 (86.30) | 49.62 ± 48.76 | 37.40 (48.40) | 0.0766 | 0.164 |
| IL-10 (2.6–4.9 pg/mL) | 40.55 ± 346.36 | 11.00 (0.40) | 14.98 ± 11.96 | 11.10 (13.70) | 0.9097 | 0.880 |
| TNF (0.1–5.2 pg/mL) | 5.13 ± 22.94 | 2.20 (1.80) | 2.26 ± 1.29 | 1.80 (1.00) | 0.3006 | 0.415 |
| IFN-γ (1.6–17.3 pg/mL) | 35.87 ± 222.17 | 8.50 (9.70) | 20.28 ± 38.85 | 3.60 (12.95) | 0.0087 | 0.068 |
References
- Lynch, J.P., 3rd; Kajon, A.E. Adenovirus: Epidemiology, Global Spread of Novel Types, and Approach to Treatment. Semin. Respir. Crit. Care Med. 2021, 42, 800–821. [Google Scholar] [CrossRef]
- Peng, J.; Zou, W.W.; Wang, X.L.; Zhang, Z.G.; Huo, R.; Yang, L. Viral-mediated gene therapy in pediatric neurological disorders. World J. Pediatr. 2024, 20, 533–555. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Zeng, Y.; Zhong, Z.; Yang, L.; Li, H.; Zhang, H.M.; Xia, H.; Jiang, M.Y. Clinical characteristics and severity prediction score of Adenovirus pneumonia in immunocompetent adult. PLoS ONE 2023, 18, e0281590. [Google Scholar] [CrossRef]
- Xu, N.; Chen, P.; Wang, Y. Evaluation of Risk Factors for Exacerbations in Children with Adenoviral Pneumonia. Biomed. Res. Int. 2020, 31, 4878635. [Google Scholar] [CrossRef]
- Radke, J.R.; Cook, J.L. Human adenovirus lung disease: Outbreaks, models of immune-response-driven acute lung injury and pandemic potential. Curr. Opin. Infect. Dis. 2023, 36, 164–170. [Google Scholar] [CrossRef]
- Ling, Y.; Yang, D.; Yang, S. Clinical characteristics, early blood biochemical indicators, and prognostic status of children with bronchopneumonia. Medicine 2023, 102, e36162. [Google Scholar] [CrossRef]
- Zheng, N.; Zhu, D.; Han, Y. Procalcitonin and C-reactive protein perform better than the neutrophil/lymphocyte count ratio in evaluating hospital acquired pneumonia. BMC Pulm. Med. 2020, 20, 166–176. [Google Scholar] [CrossRef]
- Zhang, H.F.; Li, L.Q.; Ge, Y.L.; Zhang, J.B.; Fu, A.S.; Liu, C.H.; Shao, D.F.; Bai, J.; Zhu, X.Y. Serum Prealbumin Improves the Sensitivity of Pneumonia Severity Index in Predicting 30-day Mortality of CAP Patients. Clin. Lab. 2020, 66, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Gorelik, O.; Izhakian, S.; Barchel, D.; Almoznino-Sarafian, D.; Tzur, I.; Swarka, M.; Beberashvili, I.; Feldman, L.; Cohen, N.; Shteinshnaider, M. Prognostic significance of platelet count changes during hospitalization for community-acquired pneumonia. Platelets 2017, 28, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Soares, A.C.; Souza, D.G.; Pinho, V.; Vieira, A.T.; Nicoli, J.R.; Cunha, F.Q.; Mantovani, A.; Reis, L.F.; Dias, A.A.; Teixeira, M.M. Dual function of the long pentraxin PTX3 in resistance against pulmonary infection with Klebsiella pneumoniae in transgenic mice. Microbes Infect. 2006, 8, 1321–1329. [Google Scholar] [CrossRef]
- Zhang, Z.; Dou, H.; Tu, P.; Shi, D.; Wei, R.; Wan, R.; Jia, C.; Ning, L.; Wang, D.; Li, J.; et al. Serum cytokine profiling reveals different immune response patterns during general and severe Mycoplasma pneumoniae pneumonia. Front. Immunol. 2022, 13, 1088725. [Google Scholar] [CrossRef] [PubMed]
- Xiang, W.Q.; Li, L.; Wang, B.H.; Ali, A.F.; Li, W. Profiles and predictive value of cytokines in children with human metapneumovirus pneumonia. Virol. J. 2022, 19, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Abbas, S.; Jalil, Z.; Javed, A.R.; Batool, I.; Khan, M.Z.; Noorwali, A.; Gadekallu, T.R.; Akbar, A. BCD-WERT: A novel approach for breast cancer detection using whale optimization based efficient features and extremely randomized tree algorithm. PeerJ Comput. Sci. 2021, 7, e390. [Google Scholar] [CrossRef] [PubMed]
- Fagherazzi, G.; Aguayo, G.A.; Zhang, L.; Hanaire, H.; Picard, S.; Sablone, L.; Vergès, B.; Hamamouche, N.; Detournay, B.; Joubert, M.; et al. Heterogeneity of glycaemic phenotypes in type 1 diabetes. Diabetologia 2024, 67, 1567–1581. [Google Scholar] [CrossRef]
- Issa, N.T.; Stathias, V.; Schürer, S.; Dakshanamurthy, S. Machine and deep learning approaches for cancer drug repurposing. Semin. Cancer Biol. 2021, 68, 132–142. [Google Scholar] [CrossRef]
- Gao, T.; Nong, Z.; Luo, Y.; Mo, M.; Chen, Z.; Yang, Z.; Pan, L. Machine learning-based prediction of in-hospital mortality for critically ill patients with sepsis-associated acute kidney injury. Ren. Fail. 2024, 46, 2316267. [Google Scholar] [CrossRef]
- Lee, E.E.; Torous, J.; De Choudhury, M.; Depp, C.A.; Graham, S.A.; Kim, H.C.; Paulus, M.P.; Krystal, J.H.; Jeste, D.V. Artificial Intelligence for Mental Health Care: Clinical Applications, Barriers, Facilitators, and Artificial Wisdom. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2021, 6, 856–864. [Google Scholar] [CrossRef]
- National Health Commission of the People’s Republic of China; State Administration of Traditional Chinese Medicine. Guideline for diagnosis and treatment of adenovirus pneumonia in children (2019 version). Chin. J. Clin. Infect. Dis. 2019, 12, 161–166. [Google Scholar] [CrossRef]
- Li, X.; Zhong, X.; Wang, Y.; Zeng, X.; Luo, T.; Liu, Q. Clinical determinants of the severity of COVID-19: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0250602. [Google Scholar] [CrossRef]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Valenzuela-Sánchez, F.; Valenzuela-Méndez, B.; Rodríguez-Gutiérrez, J.F.; Estella, Á. Latest developments in early diagnosis and specific treatment of severe influenza infection. J. Intensive Med. 2023, 4, 160–174. [Google Scholar] [CrossRef]
- Pan, D.; Zheng, J.; Chen, Q.; Zeng, L.E.; Lin, C.; You, Y.; Lin, J. Clinical Characteristics and Genotyping of Pediatric Adenovirus Pneumonia Disease and Coinfection in Southeast China. Genet. Test. Mol. Biomark. 2023, 27, 306–316. [Google Scholar] [CrossRef]
- Liu, W.; Qiu, S.; Zhang, L.; Wu, H.; Tian, X.; Li, X.; Xu, D.; Dai, J.; Gu, S.; Liu, Q.; et al. Analysis of severe human adenovirus infection outbreak in Guangdong Province, southern China in 2019. Virol. Sin. 2022, 37, 331–340. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, H.; Tian, S.; Deng, J. Adenovirus viremia may predict adenovirus pneumonia severity in immunocompetent children. BMC Infect. Dis. 2021, 21, 213–219. [Google Scholar] [CrossRef]
- Furuya, H.; Kawachi, S.; Shigematsu, M.; Suzuki, K.; Watanabe, T. Clinical factors associated with severity in hospitalized children infected with avian influenza (H5N1). Environ. Health Prev. Med. 2011, 16, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Du, Z.; Zhu, Y.; Li, W.; Miao, H.; Li, Z. Evaluation of organ function in patients with severe COVID-19 infections. Med. Clin. 2020, 155, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wang, Q.M.; Li, D.X. An Analysis of Predictive Factors for Severe Neonatal Infection and the Construction of a Prediction Model. Infect. Drug Resist. 2023, 16, 3561–3574. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, R.C.; Campbell, A.P.; Taylor, C.A.; Chai, S.J.; Kawasaki, B.; Meek, J.; Anderson, E.J.; Weigel, A.; Monroe, M.L.; Reeg, L.; et al. Risk Factors for Severe COVID-19 in Children. Pediatrics 2022, 149, e2021053418. [Google Scholar] [CrossRef]
- Zou, S.; Liu, J.; Yang, Z.; Xiao, D.; Cao, D. SAA and CRP are potential indicators in distinction and severity assessment for children with influenza. Int. J. Infect. Dis. 2021, 108, 357–362. [Google Scholar] [CrossRef]
- Bashir, A.; Khan, R.; Thompson, S.; Caceres, M. A retrospective observational study of biomarker levels and severity assessment in pediatric community-acquired pneumonia. Medicine 2022, 101, e30010. [Google Scholar] [CrossRef]
- Candeias, S.M.; Gaipl, U.S. The Immune System in Cancer Prevention, Development and Therapy. Anticancer Agents Med. Chem. 2016, 16, 101–107. [Google Scholar] [CrossRef]
- Kaneko, H.; Matsui, E.; Asano, T.; Kato, Z.; Teramoto, T.; Aoki, M.; Kawamoto, N.; Lian, L.A.; Kasahara, K.; Kondo, N. Suppression of IFN-gamma production in atopic group at the acute phase of RSV infection. Pediatr. Allergy Immunol. 2006, 17, 370–375. [Google Scholar] [CrossRef]
- Liu, X.Y.; Zhang, X.F.; Li, J.; Zhu, F.; Yu, Q.L.; Wei, S.Q.; Wang, M.Z. Value of heparin-binding protein in the diagnosis of severe adenovirus pneumonia in children. Chin. J. Contemp. Pediatr. 2022, 24, 1014–1019. [Google Scholar] [CrossRef]
- Komorowski, M. Clinical management of sepsis can be improved by artificial intelligence: Yes. Intensive Care Med. 2020, 46, 375–377. [Google Scholar] [CrossRef]
- Topol, E.J. High-performance medicine: The convergence of human and artificial intelligence. Nat. Med. 2019, 25, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Wong-Chew, R.M.; García-León, M.L.; Noyola, D.E.; Perez-Gonzalez, L.F.; Gaitan-Meza, J.; Vilaseñor-Sierra, A.; Martinez-Aguilar, G.; Rivera-Nuñez, V.H.; Newton-Sánchez, O.A.; Firo-Reyes, V.; et al. Respiratory viruses detected in Mexican children younger than 5 years old with community-acquired pneumonia: A national multicenter study. Int. J. Infect. Dis. 2017, 62, 32–38. [Google Scholar] [CrossRef]
- Peng, L.; Liu, S.; Xie, T.; Li, Y.; Yang, Z.; Chen, Y.; Deng, L.; Huang, H.; Ding, X.; Chen, M.; et al. Predictive Value of Adenoviral Load for Bronchial Mucus Plugs Formation in Children with Adenovirus Pneumonia. Can. Respir. J. 2022, 8, 9595184. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Zang, N.; Zhang, J.; He, Y.; Huang, H.; Liu, X.; Xu, X.; Ren, L.; Deng, Y.; Wu, J.; et al. Genome and proteomic analysis of risk factors for fatal outcome in children with severe community-acquired pneumonia caused by human adenovirus 7. J. Med. Virol. 2023, 95, e29182. [Google Scholar] [CrossRef]
- Wang, L.; Hu, X.; Huang, Z.; Zhang, Y.; Zhao, X.; Liu, X.; Mao, H.; Hao, H.; Xue, W. Analysis of the typing of adenovirus and its clinical characteristics in children with acute respiratory tract infection. BMC Pediatr. 2023, 23, 25–31. [Google Scholar] [CrossRef]


| Parameter | Classification | NSAP (n = 374) 1 | SAP (n = 54) 1 | p | |
|---|---|---|---|---|---|
| Gender | Male | 221 (59.1) | 38 (70.4) | 2.0626 | 0.1509 |
| Female | 153 (40.9) | 16 (29.6) | |||
| Age 2 | >6 M and ≤3 Y | 33 (8.8) | 12 (22.2) | 9.2758 | 0.0258 * |
| >3 Y and ≤6 Y | 171 (45.7) | 20 (37.0) | |||
| >6 Y and ≤9 Y | 131 (35.0) | 16 (29.6) | |||
| >9 Y | 39 (10.4) | 6 (11.1) | |||
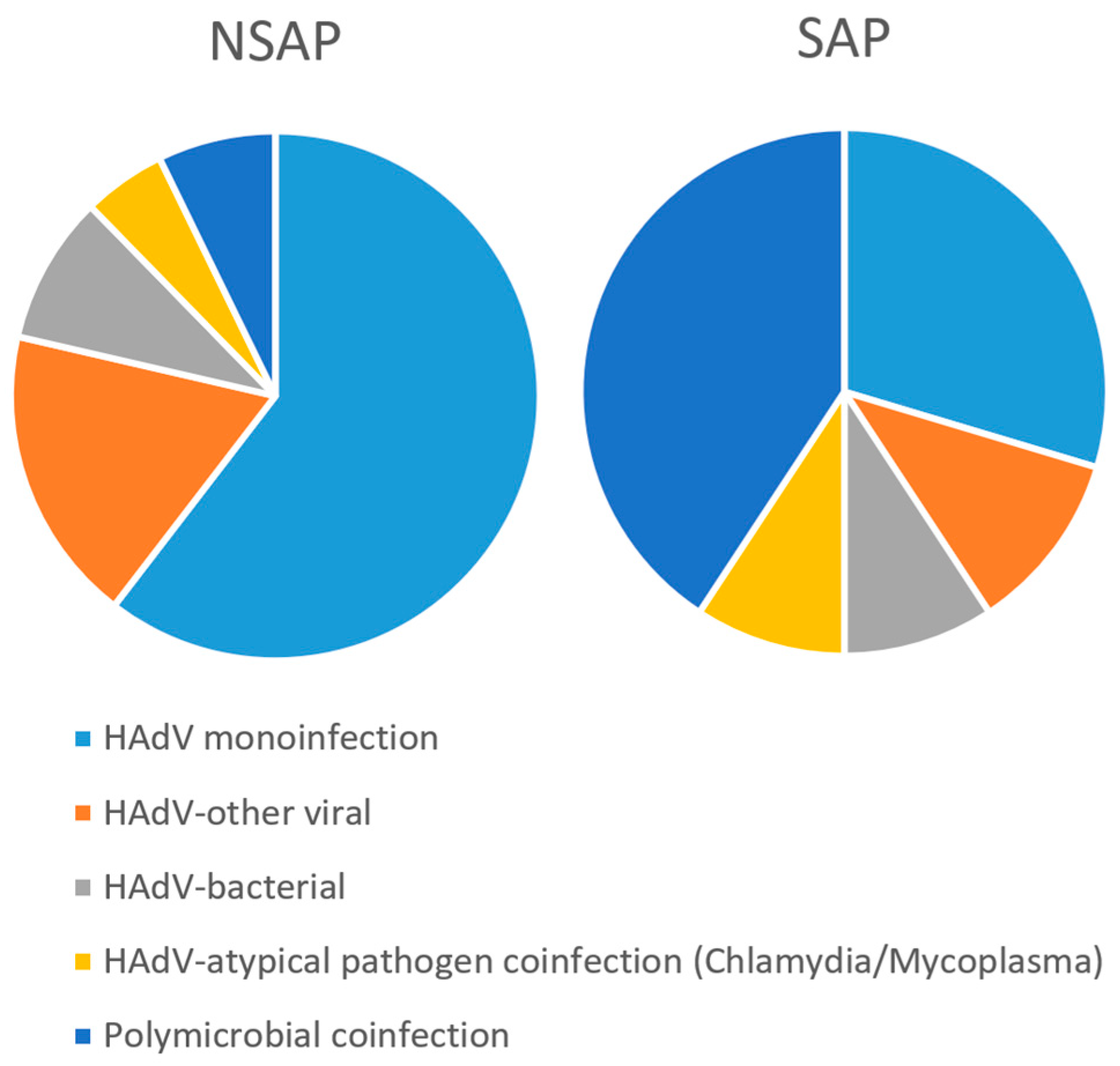
| Polymicrobial coinfection 3 | HAdV monoinfection | 226 (64.4) | 16 (28.1) | 57.0658 | 1.20 × 10−11 |
| HAdV-other viral | 68 (18.2) | 6 (11.1) | |||
| HAdV-bacterial | 34 (9.1) | 5 (9.3) | |||
| HAdV-atypical pathogen coinfection (Chlamydia/Mycoplasma) | 19 (5.1) | 5 (9.3) | |||
| Polymicrobial coinfection | 27 (7.2) | 22 (40.7) |
| Metric | Value |
|---|---|
| Accuracy | 0.845 |
| Precision | 0.915 |
| Sensitivity | 0.915 |
| F1-Score | 0.915 |
| Specificity | 0.167 |
| AUC | 0.699 |
| Balanced Accuracy | 0.541 |
| MCC | 0.081 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, H.; Chen, W.; Lai, Q.; Chen, Y.; Guo, Y.; Chen, J.; Li, W. Clinical Characteristics of Adenovirus Pneumonia in Children. Pathogens 2025, 14, 1110. https://doi.org/10.3390/pathogens14111110
Xu H, Chen W, Lai Q, Chen Y, Guo Y, Chen J, Li W. Clinical Characteristics of Adenovirus Pneumonia in Children. Pathogens. 2025; 14(11):1110. https://doi.org/10.3390/pathogens14111110
Chicago/Turabian StyleXu, Huifen, Wei Chen, Qinrui Lai, Yingying Chen, Yajun Guo, Jing Chen, and Wei Li. 2025. "Clinical Characteristics of Adenovirus Pneumonia in Children" Pathogens 14, no. 11: 1110. https://doi.org/10.3390/pathogens14111110
APA StyleXu, H., Chen, W., Lai, Q., Chen, Y., Guo, Y., Chen, J., & Li, W. (2025). Clinical Characteristics of Adenovirus Pneumonia in Children. Pathogens, 14(11), 1110. https://doi.org/10.3390/pathogens14111110







