Pathogens That Rewrite the Rules: Ascoviruses, Elegant Manipulators of Cell Death Pathways and Architects of the Extracellular Viral Paradigm
Abstract
1. Introduction
2. Discovery, Classification and Evolutionary Origin
3. General Biology of AVs
4. AV Virion Ultrastructure
5. Proteomic Analysis of AV Virions
5.1. Proteins and Peptides Shared by SfAV-1a, HvAV-31 and TnAV-2c Virions
5.2. AV Virions Contain Host-Coded Proteins
Heat Shock Proteins (HSPs), Prophenoloxidases (PPOs), and Epoxide Hydrolase (EH)
6. A Unique Cytopathology Evolved in AVs for Dissemination Efficiency
SfAV-1a VCVs Are Modified Bodies Derived from Regulated Cell Death Pathways
7. AV Cytopathology
7.1. Cellular Invasion
7.2. Oxidative Stress Suppression
7.3. Nucleic Acid Metabolism
7.4. Delaying Immune and Cell Death Pathways—Antiapoptotic Proteins
7.5. Cellular Cleavage and Formation of VCVs
7.6. Proapoptotic Proteins of AVs
7.7. Lipid Metabolism, Ceramide-Sphingosine-1-Phosphate Rheostat, and VCV Formation
7.8. Role of Ascovirus Lipid Enzymes in Programmed Cell Death Pathways
8. Lysosome–Mitochondria Feedback Loop and Intrinsic Apoptotic Induction
8.1. Viral Exploitation of Mitophagy to Balance Apoptosis and Replication
8.2. Ceramide-Driven Feedback and Viral Reprogramming of Apoptosis
9. Factors Influencing the Persistence of AVs in Nature
10. Biocontrol Potential of Ascoviruses: Opportunities and Challenges
11. Future Studies
12. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Federici, B.A.; Bideshi, D.K.; Tan, Y.; Spears, T.; Bigot, Y. Ascoviruses: Superb Manipulators of Apoptosis for Viral Replication and Transmission. Curr. Top. Microbiol. Immunol. 2009, 328, 171–196. [Google Scholar] [CrossRef]
- Bideshi, D.K.; Bigot, Y.; Federici, B.A.; Spears, T. Ascoviruses. In Insect Virology; Asgari, S., Johnson, K.N., Eds.; Caister Academic Press: Norfolk, UK, 2010; pp. 3–34. ISBN 9781904455714. [Google Scholar]
- Federici, B.; Bigot, Y.; Granados, R.R.; Hamm, J.J.; Miller, L.K.; Newton, I.; Stasiak, K.; Vlak, J.M. Family Ascoviridae. In Virus Taxonomy: Eighth Report of the International Committee on Taxonomy of Viruses; Fauquet, C.M., Mayo, M.A., Maniloff, J., Desselberger, U., Ball, L.A., Eds.; Academic Press: San Diego, CA, USA, 2005; pp. 269–274. [Google Scholar]
- Asgari, S.; Bideshi, D.K.; Bigot, Y.; Federici, B.A.; Cheng, X.-W.; ICTV Report Consortium. ICTV Virus Taxonomy Profile: Ascoviridae. J. Gen. Virol. 2017, 98, 4–5. [Google Scholar] [CrossRef] [PubMed]
- Kost, T.A.; Kemp, C.W. Fundamentals of Baculovirus Expression and Applications. Adv. Exp. Med. Biol. 2016, 896, 187–197. [Google Scholar] [CrossRef]
- Beas-Catena, A.; Sánchez-Mirón, A.; García-Camacho, F.; Contreras-Gómez, A.; Molina-Grima, E. Baculovirus Biopesticides: An Overview. J. Anim. Plant Sci. 2014, 24, 362–373. [Google Scholar]
- Cossentine, J.E. The Parasitoid Factor in the Virulence and Spread of Lepidopteran Baculoviruses. Virol. Sin. 2009, 24, 305–314. [Google Scholar] [CrossRef]
- Hong, Q.; Liu, J.; Wei, Y.; Wei, X. Application of Baculovirus Expression Vector System (BEVS) in Vaccine Development. Vaccines 2023, 11, 1218. [Google Scholar] [CrossRef]
- Maori, E.; Lavi, S.; Mozes-Koch, R.; Gantman, Y.; Peretz, Y.; Edelbaum, O.; Tanne, E.; Sela, I. Isolation and Characterization of Israeli Acute Paralysis Virus, a Dicistrovirus Affecting Honeybees in Israel: Evidence for Diversity Due to Intra- and Inter-Species Recombination. J. Gen. Virol. 2007, 88, 3428–3438. [Google Scholar] [CrossRef]
- Bonning, B.C.; Miller, W.A. Dicistroviruses. Annu. Rev. Entomol. 2010, 55, 129–150. [Google Scholar] [CrossRef]
- Valles, S.M.; Chen, Y.; Firth, A.E.; Guérin, D.M.A.; Hashimoto, Y.; Herrero, S.; de Miranda, J.R.; Ryabov, E.; ICTV Report Consortium. ICTV Virus Taxonomy Profile: Dicistroviridae. J. Gen. Virol. 2017, 98, 355–356. [Google Scholar] [CrossRef] [PubMed]
- Bideshi, D.K.; Tan, Y.; Bigot, Y.; Federici, B.A. A Viral Caspase Contributes to Modified Apoptosis for Virus Transmission. Genes Dev. 2005, 19, 1416–1421. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Li, J.-R.; Xiao, H.-Y.; Cao, S.-K.; Chen, B.; Li, N.; Chen, G.; Huang, G.-H. Apoptosis or Antiapoptosis? Interrupted Regulated Cell Death of Host Cells by Ascovirus Infection in Vitro. MBio 2023, 14, e0311922. [Google Scholar] [CrossRef]
- Verburg, S.G.; Lelievre, R.M.; Westerveld, M.J.; Inkol, J.M.; Sun, Y.L.; Workenhe, S.T. Viral-Mediated Activation and Inhibition of Programmed Cell Death. PLoS Pathog. 2022, 18, e1010718. [Google Scholar] [CrossRef]
- Federici, B.A. Enveloped Double-Stranded DNA Insect Virus with Novel Structure and Cytopathology. Proc. Natl. Acad. Sci. USA 1983, 80, 7664–7668. [Google Scholar] [CrossRef]
- Zaghloul, H.A.H.; Arensburger, P.; Federici, B.A. Host Cytoskeleton Gene Expression Is Correlated with the Formation of Ascovirus Reproductive Viral Vesicles. Viruses 2022, 14, 1444. [Google Scholar] [CrossRef]
- Zaghloul, H.A.H.; Hice, R.H.; Arensburger, P.; Bideshi, D.K.; Federici, B.A. Extended in Vivo Transcriptomes of Two Ascoviruses with Different Tissue Tropisms Reveal Alternative Mechanisms for Enhancing Virus Reproduction in Hemolymph. Sci. Rep. 2021, 11, 16402. [Google Scholar] [CrossRef] [PubMed]
- Zaghloul, H.A.H.; Hice, R.; Arensburger, P.; Federici, B.A. Transcriptome Analysis of the Spodoptera frugiperda Ascovirus in Vivo Provides Insights into How Its Apoptosis Inhibitors and Caspase Promote Increased Synthesis of Viral Vesicles and Virion Progeny. J. Virol. 2017, 91, e00874-17. [Google Scholar] [CrossRef] [PubMed]
- Zaghloul, H.A.H.; Hice, R.; Bideshi, D.K.; Arensburger, P.; Federici, B.A. Mitochondrial and Innate Immunity Transcriptomes from Spodoptera frugiperda Larvae Infected with the Spodoptera frugiperda Ascovirus. J. Virol. 2020, 94, e01985-19. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.-H.; Wang, Y.-S.; Wang, X.; Garretson, T.A.; Dai, L.-Y.; Zhang, C.-X.; Cheng, X.-W. Genomic Sequence of Heliothis virescens Ascovirus 3g Isolated from Spodoptera exigua. J. Virol. 2012, 86, 12467–12468. [Google Scholar] [CrossRef]
- Bigot, Y.; Rabouille, A.; Sizaret, P.Y.; Hamelin, M.H.; Periquet, G. Particle and Genomic Characteristics of a New Member of the Ascoviridae: Diadromus Pulchellus Ascovirus. J. Gen. Virol. 1997, 78 Pt 5, 1139–1147. [Google Scholar] [CrossRef]
- Chen, G.; Liu, H.; Mo, B.-C.; Hu, J.; Liu, S.-Q.; Bustos-Segura, C.; Xue, J.; Wang, X. Growth and Development of Helicoverpa armigera (Lepidoptera: Noctuidae) Larvae Infected by Heliothis virescens Ascovirus 3i (HvAV-3i). Front. Physiol. 2020, 11, 93. [Google Scholar] [CrossRef]
- Liu, S.; Sappington, T.W.; Coates, B.S.; Bonning, B.C. Sequences Encoding a Novel Toursvirus Identified from Southern and Northern Corn Rootworms (Coleoptera: Chrysomelidae). Viruses 2022, 14, 397. [Google Scholar] [CrossRef]
- Bigot, Y.; Stasiak, K.; Rouleux-Bonnin, F.; Federici, B.A. Characterization of Repetitive DNA Regions and Methylated DNA in Ascovirus Genomes. J. Gen. Virol. 2000, 81, 3073–3082. [Google Scholar] [CrossRef]
- Stasiak, K.; Renault, S.; Demattei, M.-V.; Bigot, Y.; Federici, B.A. Evidence for the Evolution of Ascoviruses from Iridoviruses. J. Gen. Virol. 2003, 84, 2999–3009. [Google Scholar] [CrossRef] [PubMed]
- Bigot, Y.; Renault, S.; Nicolas, J.; Moundras, C.; Demattei, M.-V.; Samain, S.; Bideshi, D.K.; Federici, B.A. Symbiotic Virus at the Evolutionary Intersection of Three Types of Large DNA Viruses; Iridoviruses, Ascoviruses, and Ichnoviruses. PLoS ONE 2009, 4, e6397. [Google Scholar] [CrossRef]
- Koonin, E.V.; Yutin, N. Evolution of the Large Nucleocytoplasmic DNA Viruses of Eukaryotes and Convergent Origins of Viral Gigantism. Adv. Virus Res. 2019, 103, 167–202. [Google Scholar] [CrossRef]
- Mönttinen, H.A.M.; Bicep, C.; Williams, T.A.; Hirt, R.P. The Genomes of Nucleocytoplasmic Large DNA Viruses: Viral Evolution Writ Large. Microb. Genom. 2021, 7, e000649. [Google Scholar] [CrossRef] [PubMed]
- Bideshi, D.K.; Demattei, M.-V.; Rouleux-Bonnin, F.; Stasiak, K.; Tan, Y.; Bigot, S.; Bigot, Y.; Federici, B.A. Genomic Sequence of Spodoptera frugiperda Ascovirus 1a, an Enveloped, Double-Stranded DNA Insect Virus That Manipulates Apoptosis for Viral Reproduction. J. Virol. 2006, 80, 11791–11805. [Google Scholar] [CrossRef]
- Colson, P.; De Lamballerie, X.; Yutin, N.; Asgari, S.; Bigot, Y.; Bideshi, D.K.; Cheng, X.-W.; Federici, B.A.; Van Etten, J.L.; Koonin, E.V.; et al. “Megavirales”, a Proposed New Order for Eukaryotic Nucleocytoplasmic Large DNA Viruses. Arch. Virol. 2013, 158, 2517–2521. [Google Scholar] [CrossRef]
- Piégu, B.; Asgari, S.; Bideshi, D.; Federici, B.A.; Bigot, Y. Evolutionary Relationships of Iridoviruses and Divergence of Ascoviruses from Invertebrate Iridoviruses in the Superfamily Megavirales. Mol. Phylogenet. Evol. 2015, 84, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Bigot, Y.; Rabouille, A.; Doury, G.; Sizaret, P.Y.; Delbost, F.; Hamelin, M.H.; Periquet, G. Biological and Molecular Features of the Relationships between Diadromus pulchellus Ascovirus, a Parasitoid Hymenopteran Wasp (Diadromus pulchellus) and Its Lepidopteran Host, Acrolepiopsis assectella. J. Gen. Virol. 1997, 78 Pt 5, 1149–1163. [Google Scholar] [CrossRef]
- Kuhn, J.H.; Koonin, E.V. Viriforms—A New Category of Classifiable Virus-Derived Genetic Elements. Biomolecules 2023, 13, 289. [Google Scholar] [CrossRef] [PubMed]
- Bigot, Y.; Samain, S.; Augé-Gouillou, C.; Federici, B.A. Molecular Evidence for the Evolution of Ichnoviruses from Ascoviruses by Symbiogenesis. BMC Evol. Biol. 2008, 8, 253. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.-Q.; Shi, M.; Huang, J.-H.; Chen, X.-X. Parasitoid Polydnaviruses and Immune Interaction with Secondary Hosts. Dev. Comp. Immunol. 2018, 83, 124–129. [Google Scholar] [CrossRef]
- Sharanowski, B.J.; Ridenbaugh, R.D.; Piekarski, P.K.; Broad, G.R.; Burke, G.R.; Deans, A.R.; Lemmon, A.R.; Moriarty Lemmon, E.C.; Diehl, G.J.; Whitfield, J.B.; et al. Phylogenomics of Ichneumonidae (Hymenoptera) and Implications for Evolution of Mode of Parasitism and Viral Endogenization. Mol. Phylogenet. Evol. 2021, 156, 107023. [Google Scholar] [CrossRef]
- Renault, S.; Petit, A.; Bénédet, F.; Bigot, S.; Bigot, Y. Effects of the Diadromus pulchellus Ascovirus, DpAV-4, on the Hemocytic Encapsulation Response and Capsule Melanization of the Leek-Moth Pupa, Acrolepiopsis assectella. J. Insect Physiol. 2002, 48, 297–302. [Google Scholar] [CrossRef]
- Nakhleh, J.; El Moussawi, L.; Osta, M.A. The Melanization Response in Insect Immunity. In Advances in Insect Physiology; Elsevier: Amsterdam, The Netherlands, 2017; pp. 83–109. ISBN 9780128117750. [Google Scholar]
- Muhammad, A.; Sun, C.; Shao, Y. The Humoral Immune Response of the Lepidopteran Model Insect, Silkworm Bombyx mori L., to Microbial Pathogens. Curr. Res. Insect Sci. 2024, 6, 100097. [Google Scholar] [CrossRef] [PubMed]
- Shelby, K.S.; Webb, B.A. Polydnavirus-Mediated Suppression of Insect Immunity. J. Insect Physiol. 1999, 45, 507–514. [Google Scholar] [CrossRef]
- Schmidt, O.; Theopold, U.; Strand, M. Innate Immunity and Its Evasion and Suppression by Hymenopteran Endoparasitoids. Bioessays 2001, 23, 344–351. [Google Scholar] [CrossRef]
- Zhang, G.; Lu, Z.-Q.; Jiang, H.; Asgari, S. Negative Regulation of Prophenoloxidase (proPO) Activation by a Clip-Domain Serine Proteinase Homolog (SPH) from Endoparasitoid Venom. Insect Biochem. Mol. Biol. 2004, 34, 477–483. [Google Scholar] [CrossRef]
- Gao, S.; Zhou, G.; Sun, T.; Liu, J.; Kong, W.; Wu, H.; Gao, J.; Lu, C.; Gao, B. Apolygus lucorum-Induced Resistance in Vitis vinifera L. Elicits Changes at the Phenotypic, Physiological, and Biochemical Levels. Sci. Hortic. 2022, 298, 110985. [Google Scholar] [CrossRef]
- Kaya, S.; Uçkan, F.; Er, A. Immunosuppressive Influence of Parasitoid Wasp Pimpla turionellae Calyx Fluid on Host Galleria mellonella Cell-Mediated Immune Response and Hemocyte Viability. Bull. Entomol. Res. 2021, 112, 361–369. [Google Scholar] [CrossRef]
- Tan, Y.; Bideshi, D.K.; Johnson, J.J.; Bigot, Y.; Federici, B.A. Proteomic Analysis of the Spodoptera frugiperda Ascovirus 1a Virion Reveals 21 Proteins. J. Gen. Virol. 2009, 90, 359–365. [Google Scholar] [CrossRef]
- Strand, M.R.; Burke, G.R. Polydnaviruses: Evolution and Function. Curr. Issues Mol. Biol. 2020, 34, 163–182. [Google Scholar] [CrossRef]
- Burke, G.R.; Hines, H.M.; Sharanowski, B.J. The Presence of Ancient Core Genes Reveals Endogenization from Diverse Viral Ancestors in Parasitoid Wasps. Genome Biol. Evol. 2021, 13, evab105. [Google Scholar] [CrossRef]
- Carner, G.R.; Hudson, J.S. Histopathology of Virus-like Particles in Heliothis spp. J. Invertebr. Pathol. 1983, 41, 238–249. [Google Scholar] [CrossRef]
- Federici, B.A. A New Type of Insect Pathogen in Larvae of the Clover Cutworm, Scotogramma trifolii. J. Invertebr. Pathol. 1982, 40, 41–54. [Google Scholar] [CrossRef]
- Browning, H.W.; Federici, B.A.; Oatman, E.R. Occurrence of a Disease Caused by a Rickettsia-like Organism in a Larval Population of the Cabbage Looper, Trichoplusia ni, in Southern California. Environ. Entomol. 1982, 11, 550–554. [Google Scholar] [CrossRef]
- Govindarajan, R.; Federici, B.A. Ascovirus Infectivity and Effects of Infection on the Growth and Development of Noctuid Larvae. J. Invertebr. Pathol. 1990, 56, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Hamm, J.J.; Styer, E.L.; Federici, B.A. Comparison of Field-Collected Ascovirus Isolates by DNA Hybridization, Host Range, and Histopathology. J. Invertebr. Pathol. 1998, 72, 138–146. [Google Scholar] [CrossRef]
- Cheng, X.-W.; Carner, G.R.; Arif, B.M. A New Ascovirus from Spodoptera exigua and Its Relatedness to the Isolate from Spodoptera frugiperda. J. Gen. Virol. 2000, 81, 3083–3092. [Google Scholar] [CrossRef]
- Federici, B.A.; Govindarajan, R. Comparative Histopathology of Three Ascovirus Isolates in Larval Noctuids. J. Invertebr. Pathol. 1990, 56, 300–311. [Google Scholar] [CrossRef]
- Li, S.-J.; Wang, X.; Zhou, Z.-S.; Zhu, J.; Hu, J.; Zhao, Y.-P.; Zhou, G.-W.; Huang, G.-H. A Comparison of Growth and Development of Three Major Agricultural Insect Pests Infected with Heliothis virescens Ascovirus 3h (HvAV-3h). PLoS ONE 2013, 8, e85704. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Wang, X.; Zhang, Y.; Zheng, Y.; Zhou, S.; Huang, G.-H. Characterization and Growing Development of Spodoptera exigua (Lepidoptera: Noctuidae) Larvae Infected by Heliothis virescens Ascovirus 3h (HvAV-3h). J. Econ. Entomol. 2016, 109, 2020–2026. [Google Scholar] [CrossRef]
- Lezama-Gutiérrez, R.; Hamm, J.J.; Molina-Ochoa, J.; López-Edwards, M.; Pescador-Rubio, A.; González-Ramirez, M.; Styer, E.L. Occurrence of Entomopathogens of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the Mexican States of Michoacán, Colima, Jalisco and Tamaulipas. Fla. Entomol. 2001, 84, 23. [Google Scholar] [CrossRef]
- Huang, G.-H.; Garretson, T.A.; Cheng, X.-H.; Holztrager, M.S.; Li, S.-J.; Wang, X.; Cheng, X.-W. Phylogenetic Position and Replication Kinetics of Heliothis virescens Ascovirus 3h (HvAV-3h) Isolated from Spodoptera exigua. PLoS ONE 2012, 7, e40225. [Google Scholar] [CrossRef]
- Arai, E.; Ishii, K.; Ishii, H.; Sagawa, S.; Makiyama, N.; Mizutani, T.; Omatsu, T.; Katayama, Y.; Kunimi, Y.; Inoue, M.N.; et al. An Ascovirus Isolated from Spodoptera litura (Noctuidae: Lepidoptera) Transmitted by the Generalist Endoparasitoid Meteorus pulchricornis (Braconidae: Hymenoptera). J. Gen. Virol. 2018, 99, 574–584. [Google Scholar] [CrossRef] [PubMed]
- Newton, I.R. The Biology and Characterisation of the Ascoviruses (Ascoviridae: Ascovirus) of Helicoverpa armigera Hubner and Helicoverpa punctigera Wallengren (Lepidoptera: Noctuidae) in Australia. Ph.D. Thesis, University of Queensland, Brisbane, Australia, 2004. [Google Scholar] [CrossRef]
- Smede, M.; Furlong, M.J.; Asgari, S. Effects of Heliothis virescens Ascovirus (HvAV-3e) on a Novel Host, Crocidolomia pavonana (Lepidoptera: Crambidae). J. Invertebr. Pathol. 2008, 99, 281–285. [Google Scholar] [CrossRef]
- Furlong, M.J.; Asgari, S. Effects of an Ascovirus (HvAV-3e) on Diamondback Moth, Plutella xylostella, and Evidence for Virus Transmission by a Larval Parasitoid. J. Invertebr. Pathol. 2010, 103, 89–95. [Google Scholar] [CrossRef]
- Fauquet, C.M.; Mayo, M.A.; Maniloff, J.; Desselberger, U.; Ball, L.A. Poxviridae. In Virus Taxonomy: Eighth Report of the International Committee on the Taxonomy of Viruses; Fauquet, C.M., Mayo, M.A., Maniloff, J., Desselberger, U., Ball, L.A., Eds.; Elsevier Academic Press: San Diego, CA, USA, 2005; pp. 117–133. [Google Scholar]
- Chinchar, V.G.; Essbauer, S.; He, J.G.; Hyatt; Miyazaki, T.; Seligy, V.; Williams, T. Family Iridoviridae. In Virus Taxonomy: Eighth Report of the International Committee on Taxonomy of Viruses; Fauquet, C.M., Mayo, M., Maniloff, J., Desselberger, U., Ball, L.A., Eds.; Academic Press: San Diego, CA, USA, 2005; pp. 145–162. [Google Scholar]
- Asgari, S. A Caspase-like Gene from Heliothis virescens Ascovirus (HvAV-3e) Is Not Involved in Apoptosis but Is Essential for Virus Replication. Virus Res. 2007, 128, 99–105. [Google Scholar] [CrossRef]
- Theilmann, D.A.; Blissard, G.W.; Boning, B.; Jehle, J.A.; O’Reilly, D.R.; Rohrmann, G.F.; Thiem, S.; Vlak, J.M. Baculoviridae. In Virus Taxonomy; Elsevier: London, UK, 2005; p. II. ISBN 9780122499517. [Google Scholar]
- Cui, L.; Cheng, X.; Li, L.; Li, J. Identification of Trichoplusia ni Ascovirus 2c Virion Structural Proteins. J. Gen. Virol. 2007, 88, 2194–2197. [Google Scholar] [CrossRef]
- Chen, Z.-S.; Cheng, X.-W.; Wang, X.; Hou, D.-H.; Huang, G.-H. Proteomic Analysis of the Heliothis virescens Ascovirus 3i (HvAV-3i) Virion. J. Gen. Virol. 2019, 100, 301–307. [Google Scholar] [CrossRef]
- Ince, I.A.; Boeren, S.A.; van Oers, M.M.; Vervoort, J.J.M.; Vlak, J.M. Proteomic Analysis of Chilo Iridescent Virus. Virology 2010, 405, 253–258. [Google Scholar] [CrossRef][Green Version]
- Cheng, X.-W.; Wan, X.-F.; Xue, J.; Moore, R.C. Ascovirus and Its Evolution. Virol. Sin. 2007, 22, 137–147. [Google Scholar] [CrossRef]
- Harrison, R.L.; Herniou, E.A.; Jehle, J.A.; Theilmann, D.A.; Burand, J.P.; Becnel, J.J.; Krell, P.J.; van Oers, M.M.; Mowery, J.D.; Bauchan, G.R.; et al. ICTV Virus Taxonomy Profile: Baculoviridae. J. Gen. Virol. 2018, 99, 1185–1186. [Google Scholar] [CrossRef]
- King, L.A.; Wilkinson, N.; Miller, D.P.; Marlow, S.A. Entomopoxviruses. In The Insect Viruses; Springer: Boston, MA, USA, 1998; pp. 1–29. ISBN 9781461374374. Available online: https://www.amazon.com/Insect-Viruses-Lois-K-Miller/dp/1461374375 (accessed on 4 August 2025).
- Dunford, J.C.; Somma, L.A.; Serrano, D.; Rutledge, C.R.; Capinera, J.L.; Smagghe, G.; Shaaya, E.; Riley, D.G.; Capinera, J.L.; Dreistadt, S.H.; et al. Entomopoxvirus. In Encyclopedia of Entomology; Springer: Dordrecht, The Netherlands, 2008; pp. 1345–1347. ISBN 9781402062421. [Google Scholar]
- Thézé, J.; Takatsuka, J.; Li, Z.; Gallais, J.; Doucet, D.; Arif, B.; Nakai, M.; Herniou, E.A. New Insights into the Evolution of Entomopoxvirinae from the Complete Genome Sequences of Four Entomopoxviruses Infecting Adoxophyes honmai, Choristoneura biennis, Choristoneura rosaceana, and Mythimna separata. J. Virol. 2013, 87, 7992–8003. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Rodriguez, G.; Di Nunzio, F. The Viral Capsid: A Master Key to Access the Host Nucleus. Viruses 2021, 13, 1178. [Google Scholar] [CrossRef] [PubMed]
- Sherman, M.B.; Smith, H.Q.; Smith, T.J. The Dynamic Life of Virus Capsids. Viruses 2020, 12, 618. [Google Scholar] [CrossRef]
- Bideshi, D.K.; Spears, T.; Zaghloul, H.A.H.; Tan, Y.; Bigot, Y.; Federici, B.A. Ascovirus P64 Homologs: A Novel Family of Large Cationic Proteins That Condense Viral Genomic DNA for Encapsidation. Biology 2018, 7, 44. [Google Scholar] [CrossRef]
- Tan, Y.; Spears, T.; Bideshi, D.K.; Johnson, J.J.; Hice, R.; Bigot, Y.; Federici, B.A. P64, a Novel Major Virion DNA-Binding Protein Potentially Involved in Condensing the Spodoptera frugiperda Ascovirus 1a Genome. J. Virol. 2009, 83, 2708–2714. [Google Scholar] [CrossRef]
- Carpenter, D.A.; Ip, W. Neurofilament Triplet Protein Interactions: Evidence for the Preferred Formation of NF-L-Containing Dimers and a Putative Function for the End Domains. J. Cell Sci. 1996, 109 Pt 10, 2493–2498. [Google Scholar] [CrossRef]
- Herrmann, H.; Aebi, U. Neurofilament Triplet Proteins. In Guidebook to the Cytoskeletal and Motor Proteins; Oxford University Press: Oxford, UK, 1999; pp. 313–316. ISBN 9780198599579. Available online: https://doi.org/10.1093/oso/9780198599579.003.00101 (accessed on 4 August 2025).
- Iyer, L.M.; Koonin, E.V.; Leipe, D.D.; Aravind, L. Origin and Evolution of the Archaeo-Eukaryotic Primase Superfamily and Related Palm-Domain Proteins: Structural Insights and New Members. Nucleic Acids Res. 2005, 33, 3875–3896. [Google Scholar] [CrossRef]
- Bulach, D.M.; Kumar, C.A.; Zaia, A.; Liang, B.; Tribe, D.E. Group II Nucleopolyhedrovirus Subgroups Revealed by Phylogenetic Analysis of Polyhedrin and DNA Polymerase Gene Sequences. J. Invertebr. Pathol. 1999, 73, 59–73. [Google Scholar] [CrossRef]
- Campos, E.I.; Reinberg, D. Histones: Annotating Chromatin. Annu. Rev. Genet. 2009, 43, 559–599. [Google Scholar] [CrossRef]
- La Bella, F.; Vesco, C. Late Modifications of Simian Virus 40 Chromatin during the Lytic Cycle Occur in an Immature Form of Virion. J. Virol. 1980, 33, 1138–1150. [Google Scholar] [CrossRef]
- Imai, K.; Kamio, N.; Cueno, M.E.; Saito, Y.; Inoue, H.; Saito, I.; Ochiai, K. Role of the Histone H3 Lysine 9 Methyltransferase Suv39 h1 in Maintaining Epstein-Barr Virus Latency in B95-8 Cells. FEBS J. 2014, 281, 2148–2158. [Google Scholar] [CrossRef]
- Talbert, P.B.; Armache, K.-J.; Henikoff, S. Viral Histones: Pickpocket’s Prize or Primordial Progenitor? Epigenetics Chromatin 2022, 15, 21. [Google Scholar] [CrossRef] [PubMed]
- Jacob, T.; Van den Broeke, C.; Favoreel, H.W. Viral Serine/threonine Protein Kinases. J. Virol. 2011, 85, 1158–1173. [Google Scholar] [CrossRef] [PubMed]
- Kamenski, T.; Heilmeier, S.; Meinhart, A.; Cramer, P. Structure and Mechanism of RNA Polymerase II CTD Phosphatases. Mol. Cell 2004, 15, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yu, H.; He, L.; Li, N.; Huang, G.-H. 3H-117, a Structural Protein of Heliothis virescens Ascovirus 3h (HvAV-3h). Virus Genes 2019, 55, 688–695. [Google Scholar] [CrossRef]
- Clem, R.J. Viral IAPs, Then and Now. Semin. Cell Dev. Biol. 2015, 39, 72–79. [Google Scholar] [CrossRef]
- Szöllősi, D.; Stockner, T. Sodium Binding Stabilizes the Outward-Open State of SERT by Limiting Bundle Domain Motions. Cells 2022, 11, 255. [Google Scholar] [CrossRef]
- Flaus, A.; Martin, D.M.A.; Barton, G.J.; Owen-Hughes, T. Identification of Multiple Distinct Snf2 Subfamilies with Conserved Structural Motifs. Nucleic Acids Res. 2006, 34, 2887–2905. [Google Scholar] [CrossRef]
- Byrd, A.K.; Raney, K.D. Superfamily 2 Helicases. Front. Biosci. (Landmark Ed.) 2012, 17, 2070–2088. [Google Scholar] [CrossRef]
- Udenwobele, D.I.; Su, R.-C.; Good, S.V.; Ball, T.B.; Varma Shrivastav, S.; Shrivastav, A. Myristoylation: An Important Protein Modification in the Immune Response. Front. Immunol. 2017, 8, 751. [Google Scholar] [CrossRef]
- Martin, D.D.O.; Beauchamp, E.; Berthiaume, L.G. Post-Translational Myristoylation: Fat Matters in Cellular Life and Death. Biochimie 2011, 93, 18–31. [Google Scholar] [CrossRef]
- Wang, B.; Dai, T.; Sun, W.; Wei, Y.; Ren, J.; Zhang, L.; Zhang, M.; Zhou, F. Protein N-Myristoylation: Functions and Mechanisms in Control of Innate Immunity. Cell. Mol. Immunol. 2021, 18, 878–888. [Google Scholar] [CrossRef] [PubMed]
- Hakim, M.; Mandelbaum, A.; Fass, D. Structure of a Baculovirus Sulfhydryl Oxidase, a Highly Divergent Member of the Erv Flavoenzyme Family. J. Virol. 2011, 85, 9406–9413. [Google Scholar] [CrossRef]
- Li, S.; Shen, G.; Li, W. Intramembrane Thiol Oxidoreductases: Evolutionary Convergence and Structural Controversy. Biochemistry 2018, 57, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Faccio, G.; Nivala, O.; Kruus, K.; Buchert, J.; Saloheimo, M. Sulfhydryl Oxidases: Sources, Properties, Production and Applications. Appl. Microbiol. Biotechnol. 2011, 91, 957–966. [Google Scholar] [CrossRef]
- Koval, T.; Dohnálek, J. Characteristics and Application of S1-P1 Nucleases in Biotechnology and Medicine. Biotechnol. Adv. 2018, 36, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Baker, S.C. An “Old” Protein with a New Story: Coronavirus Endoribonuclease Is Important for Evading Host Antiviral Defenses. Virology 2018, 517, 157–163. [Google Scholar] [CrossRef]
- Wang, Z.; Geraghty, R.J. Viral Nucleases. Viruses 2023, 15, 740. [Google Scholar] [CrossRef] [PubMed]
- Rao, L.; Gennerich, A. Structure and Function of Dynein’s Non-Catalytic Subunits. Cells 2024, 13, 330. [Google Scholar] [CrossRef] [PubMed]
- Dodding, M.P.; Way, M. Coupling Viruses to Dynein and Kinesin-1: Coupling Viruses to Dynein and Kinesin-1. EMBO J. 2011, 30, 3527–3539. [Google Scholar] [CrossRef] [PubMed]
- Osseman, Q.; Gallucci, L.; Au, S.; Cazenave, C.; Berdance, E.; Blondot, M.-L.; Cassany, A.; Bégu, D.; Ragues, J.; Aknin, C.; et al. The Chaperone Dynein LL1 Mediates Cytoplasmic Transport of Empty and Mature Hepatitis B Virus Capsids. J. Hepatol. 2018, 68, 441–448. [Google Scholar] [CrossRef]
- Ravindran, M.S.; Spriggs, C.C.; Verhey, K.J.; Tsai, B. Dynein Engages and Disassembles Cytosol-Localized Simian Virus 40 to Promote Infection. J. Virol. 2018, 92, e00353-18. [Google Scholar] [CrossRef] [PubMed]
- Tati, S.M.; Alisaraie, L. Recruitment of Dynein and Kinesin to Viral Particles. FASEB J. 2022, 36, e22311. [Google Scholar] [CrossRef]
- Wilson, D.W. Motor Skills: Recruitment of Kinesins, Myosins and Dynein during Assembly and Egress of Alphaherpesviruses. Viruses 2021, 13, 1622. [Google Scholar] [CrossRef] [PubMed]
- Baxevanis, A.D.; Landsman, D. The HMG-1 Box Protein Family: Classification and Functional Relationships. Nucleic Acids Res. 1995, 23, 1604–1613. [Google Scholar] [CrossRef]
- Zhao, S.-P.; Lu, D.; Yu, T.-F.; Ji, Y.-J.; Zheng, W.-J.; Zhang, S.-X.; Chai, S.-C.; Chen, Z.-Y.; Cui, X.-Y. Genome-Wide Analysis of the YABBY Family in Soybean and Functional Identification of GmYABBY10 Involvement in High Salt and Drought Stresses. Plant Physiol. Biochem. 2017, 119, 132–146. [Google Scholar] [CrossRef]
- Hussain, M.; Javed, M.M.; Sami, A.; Shafiq, M.; Ali, Q.; Mazhar, H.S.-U.-D.; Tabassum, J.; Javed, M.A.; Haider, M.Z.; Hussain, M.; et al. Genome-Wide Analysis of Plant Specific YABBY Transcription Factor Gene Family in Carrot (Dacus Carota) and Its Comparison with Arabidopsis. BMC Genom. Data 2024, 25, 26. [Google Scholar] [CrossRef] [PubMed]
- Broyles, S.S. Vaccinia Virus Transcription. J. Gen. Virol. 2003, 84, 2293–2303. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.H. Viral Polymerases. Adv. Exp. Med. Biol. 2012, 726, 267–304. [Google Scholar] [CrossRef]
- Ramaswamy, K.; Rashid, M.; Ramasamy, S.; Jayavelu, T.; Venkataraman, S. Revisiting Viral RNA-Dependent RNA Polymerases: Insights from Recent Structural Studies. Viruses 2022, 14, 2200. [Google Scholar] [CrossRef]
- Maxwell, K.L.; Frappier, L. Viral Proteomics. Microbiol. Mol. Biol. Rev. 2007, 71, 398–411. [Google Scholar] [CrossRef]
- Shaw, M.L.; Stone, K.L.; Colangelo, C.M.; Gulcicek, E.E.; Palese, P. Cellular Proteins in Influenza Virus Particles. PLoS Pathog. 2008, 4, e1000085. [Google Scholar] [CrossRef]
- Loret, S.; Guay, G.; Lippé, R. Comprehensive Characterization of Extracellular Herpes Simplex Virus Type 1 Virions. J. Virol. 2008, 82, 8605–8618. [Google Scholar] [CrossRef]
- Lippé, R. Deciphering Novel Host-Herpesvirus Interactions by Virion Proteomics. Front. Microbiol. 2012, 3, 181. [Google Scholar] [CrossRef]
- Stegen, C.; Yakova, Y.; Henaff, D.; Nadjar, J.; Duron, J.; Lippé, R. Analysis of Virion-Incorporated Host Proteins Required for Herpes Simplex Virus Type 1 Infection through a RNA Interference Screen. PLoS ONE 2013, 8, e53276. [Google Scholar] [CrossRef]
- Gale, T.V.; Horton, T.M.; Hoffmann, A.R.; Branco, L.M.; Garry, R.F. Host Proteins Identified in Extracellular Viral Particles as Targets for Broad-Spectrum Antiviral Inhibitors. J. Proteome Res. 2019, 18, 7–17. [Google Scholar] [CrossRef] [PubMed]
- El Bilali, N.; Khadivjam, B.; Bonneil, E.; Thibault, P.; Lippé, R. Proteomics of Herpes Simplex Virus Type 1 Nuclear Capsids. J. Virol. 2021, 95, e01842-19. [Google Scholar] [CrossRef]
- Khadivjam, B.; Bonneil, É.; Thibault, P.; Lippé, R. RNA Helicase DDX3X Modulates Herpes Simplex Virus 1 Nuclear Egress. Commun. Biol. 2023, 6, 134. [Google Scholar] [CrossRef]
- Murigneux, E.; Softic, L.; Aubé, C.; Grandi, C.; Judith, D.; Bruce, J.; Le Gall, M.; Guillonneau, F.; Schmitt, A.; Parissi, V.; et al. Proteomic Analysis of SARS-CoV-2 Particles Unveils a Key Role of G3BP Proteins in Viral Assembly. Nat. Commun. 2024, 15, 640. [Google Scholar] [CrossRef]
- Wang, R.; Deng, F.; Hou, D.; Zhao, Y.; Guo, L.; Wang, H.; Hu, Z. Proteomics of the Autographa californica Nucleopolyhedrovirus Budded Virions. J. Virol. 2010, 84, 7233–7242. [Google Scholar] [CrossRef]
- Yu, H.; Xiao, H.-Y.; Li, N.; Yang, C.-J.; Huang, G.-H. An Ascovirus Utilizes Different Types of Host Larval Regulated Cell Death Mechanisms to Produce and Release Vesicles. J. Virol. 2022, 97, e0156622. [Google Scholar] [CrossRef]
- Yu, H.; Chen, H.; Li, N.; Yang, C.-J.; Xiao, H.-Y.; Chen, G.; Huang, G.-H. Flexible Changes to the Heliothis virescens Ascovirus 3h (HvAV-3h) Virion Components Affect Pathogenicity against Different Host Larvae Species. Microbiol. Spectr. 2023, 11, e0248823. [Google Scholar] [CrossRef]
- Camini, F.C.; da Silva Caetano, C.C.; Almeida, L.T.; de Brito Magalhães, C.L. Implications of Oxidative Stress on Viral Pathogenesis. Arch. Virol. 2017, 162, 907–917. [Google Scholar] [CrossRef]
- Santoro, M.G.; Amici, C.; Rossi, A. Role of Heat Shock Proteins in Viral Infection. In Heat Shock Proteins; Springer: Dordrecht, The Netherlands, 2009; pp. 51–84. ISBN 9789048129751. [Google Scholar]
- Zaghloul, H.A.H.; Xiao, Z.; Tang, J.; Xiao, T.; Gao, J.; Hu, J.; Huang, G.-H. An Aegerolysin-like Protein from Heliothis virescens Ascovirus 3h (HvAV-3h) Shows Immune Suppression and Antibacterial Activity. J. Gen. Virol. 2025, 106, 002107. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhao, Y.; Wang, D.; Chen, Z. Mode of Action of Heat Shock Protein (HSP) Inhibitors against Viruses through Host HSP and Virus Interactions. Genes 2023, 14, 792. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Abdullah, S.W.; Li, P.; Wu, J.E.; Pei, C.; Mu, S.; Wang, Y.; Sun, S.; Guo, H. Heat Shock Protein 60 Is Involved in Viral Replication Complex Formation and Facilitates Foot and Mouth Virus Replication by Stabilizing Viral Nonstructural Proteins 3A and 2C. MBio 2022, 13, e01434-22. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Abdullah, S.W.; Sun, S.; Guo, H. Heat Shock Protein 60 Manipulates Foot-and-Mouth Disease Virus Replication by Regulating Mitophagy. Cell. Mol. Life Sci. 2025, 82, 141. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yu, W. Heat Shock Proteins and Viral Infection. Front. Immunol. 2022, 13, 947789. [Google Scholar] [CrossRef]
- Lubkowska, A.; Pluta, W.; Strońska, A.; Lalko, A. Role of Heat Shock Proteins (HSP70 and HSP90) in Viral Infection. Int. J. Mol. Sci. 2021, 22, 9366. [Google Scholar] [CrossRef]
- Vidyasagar, A.; Wilson, N.A.; Djamali, A. Heat Shock Protein 27 (HSP27): Biomarker of Disease and Therapeutic Target. Fibrogenesis Tissue Repair 2012, 5, 7. [Google Scholar] [CrossRef]
- Lu, A.; Zhang, Q.; Zhang, J.; Yang, B.; Wu, K.; Xie, W.; Luan, Y.-X.; Ling, E. Insect Prophenoloxidase: The View beyond Immunity. Front. Physiol. 2014, 5, 252. [Google Scholar] [CrossRef]
- Marieshwari, B.N.; Bhuvaragavan, S.; Sruthi, K.; Mullainadhan, P.; Janarthanan, S. Insect Phenoloxidase and Its Diverse Roles: Melanogenesis and beyond. J. Comp. Physiol. B 2023, 193, 1–23. [Google Scholar] [CrossRef]
- Zdybicka-Barabas, A.; Stączek, S.; Kunat-Budzyńska, M.; Cytryńska, M. Innate Immunity in Insects: The Lights and Shadows of Phenoloxidase System Activation. Int. J. Mol. Sci. 2025, 26, 1320. [Google Scholar] [CrossRef] [PubMed]
- Morisseau, C.; Hammock, B.D. Epoxide Hydrolases: Mechanisms, Inhibitor Designs, and Biological Roles. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 311–333. [Google Scholar] [CrossRef]
- Morisseau, C. Role of Epoxide Hydrolases in Lipid Metabolism. Biochimie 2013, 95, 91–95. [Google Scholar] [CrossRef]
- Morisseau, C.; Hammock, B.D. Impact of Soluble Epoxide Hydrolase and Epoxyeicosanoids on Human Health. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 37–58. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Morisseau, C.; Yang, J.; Mamatha, D.M.; Hammock, B.D. Epoxide Hydrolase Activities and Epoxy Fatty Acids in the Mosquito Culex Quinquefasciatus. Insect Biochem. Mol. Biol. 2015, 59, 41–49. [Google Scholar] [CrossRef]
- Ripon, M.A.R.; Bhowmik, D.R.; Amin, M.T.; Hossain, M.S. Role of Arachidonic Cascade in COVID-19 Infection: A Review. Prostaglandins Other Lipid Mediat. 2021, 154, 106539. [Google Scholar] [CrossRef]
- Morisseau, C. The Role of Hydrolases in Biology and Xenobiotics Metabolism. Int. J. Mol. Sci. 2022, 23, 4870. [Google Scholar] [CrossRef]
- Wojtasek, H.; Prestwich, G.D. An Insect Juvenile Hormone-Specific Epoxide Hydrolase Is Related to Vertebrate Microsomal Epoxide Hydrolases. Biochem. Biophys. Res. Commun. 1996, 220, 323–329. [Google Scholar] [CrossRef] [PubMed]
- de Kort, C.A.D.; Granger, N.A. Regulation of JH Titers: The Relevance of Degradative Enzymes and Binding Proteins. Arch. Insect Biochem. Physiol. 1996, 33, 1–26. [Google Scholar] [CrossRef]
- Seino, A.; Ogura, T.; Tsubota, T.; Shimomura, M.; Nakakura, T.; Tan, A.; Mita, K.; Shinoda, T.; Nakagawa, Y.; Shiotsuki, T. Characterization of Juvenile Hormone Epoxide Hydrolase and Related Genes in the Larval Development of the Silkworm Bombyx Mori. Biosci. Biotechnol. Biochem. 2010, 74, 1421–1429. [Google Scholar] [CrossRef]
- Bigot, Y. Ascovirus. In The Springer Index of Viruses; Springer: New York, NY, USA, 2011; pp. 73–78. ISBN 9780387959184. [Google Scholar]
- Danthi, P. Enter the Kill Zone: Initiation of Death Signaling during Virus Entry. Virology 2011, 411, 316–324. [Google Scholar] [CrossRef] [PubMed]
- He, B. Viruses, Endoplasmic Reticulum Stress, and Interferon Responses. Cell Death Differ. 2006, 13, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Prelli Bozzo, C.; Kmiec, D.; Kirchhoff, F. When Good Turns Bad: How Viruses Exploit Innate Immunity Factors. Curr. Opin. Virol. 2022, 52, 60–67. [Google Scholar] [CrossRef]
- Cheng, L.; Rui, Y.; Wang, Y.; Chen, S.; Su, J.; Yu, X.-F. A Glimpse into Viral Warfare: Decoding the Intriguing Role of Highly Pathogenic Coronavirus Proteins in Apoptosis Regulation. J. Biomed. Sci. 2024, 31, 70. [Google Scholar] [CrossRef]
- Mardi, N.; Haiaty, S.; Rahbarghazi, R.; Mobarak, H.; Milani, M.; Zarebkohan, A.; Nouri, M. Exosomal Transmission of Viruses, a Two-Edged Biological Sword. Cell Commun. Signal. 2023, 21, 19. [Google Scholar] [CrossRef]
- Gheitasi, H.; Sabbaghian, M.; Shekarchi, A.A.; Mirmazhary, A.A.; Poortahmasebi, V. Exosome-Mediated Regulation of Inflammatory Pathway during Respiratory Viral Disease. Virol. J. 2024, 21, 30. [Google Scholar] [CrossRef]
- Malherbe. Viral Cytopathology, 1st ed.; CRC Press: Boca Raton, FL, USA, 1980. [Google Scholar] [CrossRef]
- Caruso, J.L.; Childs, J.M.; Howell, D.N. Surgical Pathology and Diagnostic Cytology of Viral Infections. In Lennette’s Laboratory Diagnosis of Viral Infections; Jerome, K.R., Ed.; Informa Healthcare USA, Inc.: New York, NY, USA, 2010; pp. 151–172. ISBN 9781420084955. [Google Scholar]
- Suchman, E.; Blair, C. Cytopathic Effects of Viruses Protocols. In Proceedings of the ASM Conference for Undergraduate Educators, Buffalo, NY, USA, 18–20 May 2007. [Google Scholar]
- Bailey and Scott’s Diagnostic Microbiology; Tille, P.M., Ed.; Elsevier, Inc.: Amsterdam, The Netherlands, 2017; pp. 881–915. ISBN 9780323354820. [Google Scholar]
- López-Ferber, M.; Caballero, P.; Williams, T. Baculovirus Genetic Diversity and Population Structure. Viruses 2025, 17, 142. [Google Scholar] [CrossRef] [PubMed]
- Charman, M.; Weitzman, M.D. Replication Compartments of DNA Viruses in the Nucleus: Location, Location, Location. Viruses 2020, 12, 151. [Google Scholar] [CrossRef]
- Greseth, M.D.; Traktman, P. The Life Cycle of the Vaccinia Virus Genome. Annu. Rev. Virol. 2022, 9, 239–259. [Google Scholar] [CrossRef]
- Schmid, M.; Speiseder, T.; Dobner, T.; Gonzalez, R.A. DNA Virus Replication Compartments. J. Virol. 2014, 88, 1404–1420. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Cao, H.; Wang, Y.; Li, J.; Dai, J.; Li, L.-F.; Qiu, H.-J.; Li, S. Viral Replication Organelles: The Highly Complex and Programmed Replication Machinery. Front. Microbiol. 2024, 15, 1450060. [Google Scholar] [CrossRef] [PubMed]
- Besse, S.; Puvion-Dutilleul, F. Anchorage of Adenoviral RNAs to Clusters of Interchromatin Granules. Gene Expr. 1995, 5, 79–92. [Google Scholar]
- Lettin, L.; Erbay, B.; Blair, G.E. Viruses and Cajal Bodies: A Critical Cellular Target in Virus Infection? Viruses 2023, 15, 2311. [Google Scholar] [CrossRef]
- Ryabchenko, B.; Šroller, V.; Horníková, L.; Lovtsov, A.; Forstová, J.; Huérfano, S. The Interactions between PML Nuclear Bodies and Small and Medium Size DNA Viruses. Virol. J. 2023, 20, 82. [Google Scholar] [CrossRef]
- Seok, J.Y.; An, J.; Ha, S.Y.; Chung, D.H.; Lee, S.; Kim, H. Morphologic Analysis of Cytomegalovirus Infected Cells in Bronchial Washing Cytology: Comparison of Liquid-Based Preparation and Conventional Smear. J. Pathol. Transl. Med. 2016, 50, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Ketelut-Carneiro, N.; Fitzgerald, K.A. Apoptosis, Pyroptosis, and Necroptosis-Oh My! The Many Ways a Cell Can Die. J. Mol. Biol. 2022, 434, 167378. [Google Scholar] [CrossRef] [PubMed]
- Lemke, G. How Macrophages Deal with Death. Nat. Rev. Immunol. 2019, 19, 539–549. [Google Scholar] [CrossRef]
- Xing, J.; Wang, K.; Xu, Y.-C.; Pei, Z.-J.; Yu, Q.-X.; Liu, X.-Y.; Dong, Y.-L.; Li, S.-F.; Chen, Y.; Zhao, Y.-J.; et al. Efferocytosis: Unveiling Its Potential in Autoimmune Disease and Treatment Strategies. Autoimmun. Rev. 2024, 23, 103578. [Google Scholar] [CrossRef]
- Nagata, S. Apoptosis and Clearance of Apoptotic Cells. Annu. Rev. Immunol. 2018, 36, 489–517. [Google Scholar] [CrossRef] [PubMed]
- Trzeciak, A.; Wang, Y.-T.; Perry, J.S.A. First We Eat, Then We Do Everything Else: The Dynamic Metabolic Regulation of Efferocytosis. Cell Metab. 2021, 33, 2126–2141. [Google Scholar] [CrossRef] [PubMed]
- Nössing, C.; Ryan, K.M. 50 Years on and Still Very Much Alive: “Apoptosis: A Basic Biological Phenomenon with Wide-Ranging Implications in Tissue Kinetics”. Br. J. Cancer 2023, 128, 426–431. [Google Scholar] [CrossRef]
- Ilchev Takov, D.; Ivanov Tchorbanov, A.; Kirilova Pilarska, D.; Vladislavov Ostoich, P. Lepidopterans as Model Organisms in Studies of Insect Immunity: A Review. Pol. J. Entomol. 2020, 89, 207–225. [Google Scholar] [CrossRef]
- Eleftherianos, I.; Heryanto, C.; Bassal, T.; Zhang, W.; Tettamanti, G.; Mohamed, A. Haemocyte-Mediated Immunity in Insects: Cells, Processes and Associated Components in the Fight against Pathogens and Parasites. Immunology 2021, 164, 401–432. [Google Scholar] [CrossRef]
- Lavine, M.D.; Strand, M.R. Insect Hemocytes and Their Role in Immunity. Insect Biochem. Mol. Biol. 2002, 32, 1295–1309. [Google Scholar] [CrossRef]
- Mahanta, D.K.; Bhoi, T.K.; Komal, J.; Samal, I.; Nikhil, R.M.; Paschapur, A.U.; Singh, G.; Kumar, P.V.D.; Desai, H.R.; Ahmad, M.A.; et al. Insect-Pathogen Crosstalk and the Cellular-Molecular Mechanisms of Insect Immunity: Uncovering the Underlying Signaling Pathways and Immune Regulatory Function of Non-Coding RNAs. Front. Immunol. 2023, 14, 1169152. [Google Scholar] [CrossRef]
- Triveni, N.; Vootla, S.K. Molecular Signatures of Host–pathogen Interactions in Virus-Infected Lepidopterans. In Learning Materials in Biosciences; Springer International Publishing: Cham, Switzerland, 2023; pp. 93–116. ISBN 9783031267758. [Google Scholar]
- Zhao, L.; Niu, J.; Feng, D.; Wang, X.; Zhang, R. Immune Functions of Pattern Recognition Receptors in Lepidoptera. Front. Immunol. 2023, 14, 1203061. [Google Scholar] [CrossRef]
- Jiang, H.; Vilcinskas, A.; Kanost, M.R. Immunity in Lepidopteran Insects. Adv. Exp. Med. Biol. 2010, 708, 181–204. [Google Scholar] [CrossRef] [PubMed]
- Karaji, N.; Sattentau, Q.J. Efferocytosis of Pathogen-Infected Cells. Front. Immunol. 2017, 8, 1863. [Google Scholar] [CrossRef]
- Mohammad-Rafiei, F.; Moadab, F.; Mahmoudi, A.; Navashenaq, J.G.; Gheibihayat, S.M. Efferocytosis: A Double-Edged Sword in Microbial Immunity. Arch. Microbiol. 2023, 205, 370. [Google Scholar] [CrossRef]
- Droesbeke, B.; Balmelle, N.; Nauwynck, H.J.; Favoreel, H.; Tignon, M. African swine fever virus infection of porcine peripheral blood monocyte-derived macrophages induces the formation of tunneling nanotube-connected large vesicle-like cell segments: A potential mechanism for intercellular ASFV trafficking. Vet. Res. 2025, 56, 148. [Google Scholar] [CrossRef]
- Salina, A.C.G.; Dos-Santos, D.; Rodrigues, T.S.; Fortes-Rocha, M.; Freitas-Filho, E.G.; Alzamora-Terrel, D.L.; Castro, I.M.S.; Fraga da Silva, T.F.C.; de Lima, M.H.F.; Nascimento, D.C.; et al. Efferocytosis of SARS-CoV-2-Infected Dying Cells Impairs Macrophage Anti-Inflammatory Functions and Clearance of Apoptotic Cells. Elife 2022, 11, e74443. [Google Scholar] [CrossRef]
- Gao, P.; Zhou, L.; Wu, J.; Weng, W.; Wang, H.; Ye, M.; Qu, Y.; Hao, Y.; Zhang, Y.; Ge, X.; et al. Riding Apoptotic Bodies for Cell-Cell Transmission by African Swine Fever Virus. Proc. Natl. Acad. Sci. USA 2023, 120, e2309506120. [Google Scholar] [CrossRef]
- Zaghloul, H.A.H.; Hice, R.; Arensburger, P.; Federici, B.A. Early in Vivo Transcriptome of Trichoplusia ni Ascovirus Core Genes. J. Gen. Virol. 2022, 103, 001737. [Google Scholar] [CrossRef]
- Sobhy, H. A Comparative Review of Viral Entry and Attachment during Large and Giant dsDNA Virus Infections. Arch. Virol. 2017, 162, 3567–3585. [Google Scholar] [CrossRef] [PubMed]
- Meier, O.; Greber, U.F. Adenovirus Endocytosis. J. Gene Med. 2004, 6 (Suppl. 1), S152–S163. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.P.; Koyuncu, O.O.; Enquist, L.W. Subversion of the Actin Cytoskeleton during Viral Infection. Nat. Rev. Microbiol. 2011, 9, 427–439. [Google Scholar] [CrossRef]
- Laliberte, J.P.; Weisberg, A.S.; Moss, B. The Membrane Fusion Step of Vaccinia Virus Entry Is Cooperatively Mediated by Multiple Viral Proteins and Host Cell Components. PLoS Pathog. 2011, 7, e1002446. [Google Scholar] [CrossRef]
- Hussain, M.; Garrad, S.; Asgari, S. The Role of Actin Filaments in Ascovirus Replication and Pathology. Arch. Virol. 2009, 154, 1737–1743. [Google Scholar] [CrossRef]
- Buendia, I.; Michalska, P.; Navarro, E.; Gameiro, I.; Egea, J.; León, R. Nrf2-ARE Pathway: An Emerging Target against Oxidative Stress and Neuroinflammation in Neurodegenerative Diseases. Pharmacol. Ther. 2016, 157, 84–104. [Google Scholar] [CrossRef]
- Jin, R.; Xiao, Z.; Nakai, M.; Huang, G.-H. Insight into the Regulation of the Nrf2 Pathway in Response to Ascovirus Infection in Spodoptera exigua. Pest Manag. Sci. 2023, 79, 1123–1130. [Google Scholar] [CrossRef]
- Leipe, D.D.; Aravind, L.; Koonin, E.V. Did DNA Replication Evolve Twice Independently? Nucleic Acids Res. 1999, 27, 3389–3401. [Google Scholar] [CrossRef]
- Kazlauskas, D.; Krupovic, M.; Guglielmini, J.; Forterre, P.; Venclovas, Č. Diversity and Evolution of B-Family DNA Polymerases. Nucleic Acids Res. 2020, 48, 10142–10156. [Google Scholar] [CrossRef]
- Iyer, L.M.; Balaji, S.; Koonin, E.V.; Aravind, L. Evolutionary Genomics of Nucleo-Cytoplasmic Large DNA Viruses. Virus Res. 2006, 117, 156–184. [Google Scholar] [CrossRef]
- O’Reilly, D.R.; Crawford, A.M.; Miller, L.K. Viral Proliferating Cell Nuclear Antigen. Nature 1989, 337, 606. [Google Scholar] [CrossRef] [PubMed]
- Mayanagi, K.; Kiyonari, S.; Nishida, H.; Saito, M.; Kohda, D.; Ishino, Y.; Shirai, T.; Morikawa, K. Architecture of the DNA Polymerase B-Proliferating Cell Nuclear Antigen (PCNA)-DNA Ternary Complex. Proc. Natl. Acad. Sci. USA 2011, 108, 1845–1849. [Google Scholar] [CrossRef]
- Gorbalenya, A.E.; Koonin, E.V.; Wolf, Y.I. A New Superfamily of Putative NTP-Binding Domains Encoded by Genomes of Small DNA and RNA Viruses. FEBS Lett. 1990, 262, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Evans, E.; Klemperer, N.; Ghosh, R.; Traktman, P. The Vaccinia Virus D5 Protein, Which Is Required for DNA Replication, Is a Nucleic Acid-Independent Nucleoside Triphosphatase. J. Virol. 1995, 69, 5353–5361. [Google Scholar] [CrossRef]
- Hutin, S.; Ling, W.L.; Round, A.; Effantin, G.; Reich, S.; Iseni, F.; Tarbouriech, N.; Schoehn, G.; Burmeister, W.P. Domain Organization of Vaccinia Virus Helicase-Primase D5. J. Virol. 2016, 90, 4604–4613. [Google Scholar] [CrossRef]
- Hutin, S.; Ling, W.L.; Tarbouriech, N.; Schoehn, G.; Grimm, C.; Fischer, U.; Burmeister, W.P. The Vaccinia Virus DNA Helicase Structure from Combined Single-Particle Cryo-Electron Microscopy and AlphaFold2 Prediction. Viruses 2022, 14, 2206. [Google Scholar] [CrossRef]
- Efstathiou, S.; Kemp, S.; Darby, G.; Minson, A.C. The Role of Herpes Simplex Virus Type 1 Thymidine Kinase in Pathogenesis. J. Gen. Virol. 1989, 70 Pt 4, 869–879. [Google Scholar] [CrossRef] [PubMed]
- Gentry, G.A. Viral Thymidine Kinases and Their Relatives. Pharmacol. Ther. 1992, 54, 319–355. [Google Scholar] [CrossRef] [PubMed]
- Pommier, Y.; Sun, Y.; Huang, S.-Y.N.; Nitiss, J.L. Roles of Eukaryotic Topoisomerases in Transcription, Replication and Genomic Stability. Nat. Rev. Mol. Cell Biol. 2016, 17, 703–721. [Google Scholar] [CrossRef]
- Sriskanda, V.; Shuman, S. Specificity and Fidelity of Strand Joining by Chlorella Virus DNA Ligase. Nucleic Acids Res. 1998, 26, 3536–3541. [Google Scholar] [CrossRef]
- Ahmad, S.I.; Kirk, S.H.; Eisenstark, A. Thymine Metabolism and Thymineless Death in Prokaryotes and Eukaryotes. Annu. Rev. Microbiol. 1998, 52, 591–625. [Google Scholar] [CrossRef]
- Cromie, G.A.; Connelly, J.C.; Leach, D.R. Recombination at Double-Strand Breaks and DNA Ends: Conserved Mechanisms from Phage to Humans. Mol. Cell 2001, 8, 1163–1174. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Roizman, B. State and Role of SRC Family Kinases in Replication of Herpes Simplex Virus 1. J. Virol. 2006, 80, 3349–3359. [Google Scholar] [CrossRef][Green Version]
- Münter, S.; Way, M.; Frischknecht, F. Signaling during Pathogen Infection. Sci. STKE 2006, 2006, re5. [Google Scholar] [CrossRef]
- Okano, K.; Vanarsdall, A.L.; Mikhailov, V.S.; Rohrmann, G.F. Conserved Molecular Systems of the Baculoviridae. Virology 2006, 344, 77–87. [Google Scholar] [CrossRef]
- Smith, G.R. Homologous Recombination near and far from DNA Breaks: Alternative Roles and Contrasting Views. Annu. Rev. Genet. 2001, 35, 243–274. [Google Scholar] [CrossRef]
- Traktman, P.; Boyle, K. Methods for Analysis of Poxvirus DNA Replication. Methods Mol. Biol. 2004, 269, 169–186. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Gong, H.; Sheng, N.; Zhou, X.; Gulari, E.; Gao, X.; Church, G. Accurate Multiplex Gene Synthesis from Programmable DNA Microchips. Nature 2004, 432, 1050–1054. [Google Scholar] [CrossRef]
- da Fonseca, F.G.; Weisberg, A.S.; Caeiro, M.F.; Moss, B. Vaccinia Virus Mutants with Alanine Substitutions in the Conserved G5R Gene Fail to Initiate Morphogenesis at the Nonpermissive Temperature. J. Virol. 2004, 78, 10238–10248. [Google Scholar] [CrossRef]
- Da Silva, M.; Shen, L.; Tcherepanov, V.; Watson, C.; Upton, C. Predicted Function of the Vaccinia Virus G5R Protein. Bioinformatics 2006, 22, 2846–2850. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Meister, R.J.; Oldenhof, H.; Bowman, J.L.; Gasser, C.S. Multiple Protein Regions Contribute to Differential Activities of YABBY Proteins in Reproductive Development. Plant Physiol. 2005, 137, 651–662. [Google Scholar] [CrossRef]
- Bianchi, M.E.; Agresti, A. HMG Proteins: Dynamic Players in Gene Regulation and Differentiation. Curr. Opin. Genet. Dev. 2005, 15, 496–506. [Google Scholar] [CrossRef]
- Han, K.; Lai, M.; Zhao, T.; Yang, X.; An, X.; Chen, Z. Plant YABBY Transcription Factors: A Review of Gene Expression, Biological Functions, and Prospects. Crit. Rev. Biotechnol. 2025, 45, 214–235. [Google Scholar] [CrossRef]
- Lin, H.-W.; Chang, Y.-Y.; Wong, M.-L.; Lin, J.-W.; Chang, T.-J. Functional Analysis of Virion Host Shutoff Protein of Pseudorabies Virus. Virology 2004, 324, 412–418. [Google Scholar] [CrossRef]
- Smiley, J.R. Herpes Simplex Virus Virion Host Shutoff Protein: Immune Evasion Mediated by a Viral RNase? J. Virol. 2004, 78, 1063–1068. [Google Scholar] [CrossRef]
- Tombácz, D.; Tóth, J.S.; Boldogkoi, Z. Deletion of the Virion Host Shut: Off Gene of Pseudorabies Virus Results in Selective Upregulation of the Expression of Early Viral Genes in the Late Stage of Infection. Genomics 2011, 98, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Aravind, L.; Koonin, E.V. A Natural Classification of Ribonucleases. Methods Enzymol. 2001, 341, 3–28. [Google Scholar] [CrossRef] [PubMed]
- Drider, D.; Condon, C. The Continuing Story of Endoribonuclease III. J. Mol. Microbiol. Biotechnol. 2004, 8, 195–200. [Google Scholar] [CrossRef]
- Nicholson, A.W. Ribonuclease III Mechanisms of Double-Stranded RNA Cleavage: Ribonuclease III Mechanisms of Double-Stranded RNA Cleavage. Wiley Interdiscip. Rev. RNA 2014, 5, 31–48. [Google Scholar] [CrossRef]
- Lejars, M.; Kobayashi, A.; Hajnsdorf, E. RNase III, Ribosome Biogenesis and beyond. Microorganisms 2021, 9, 2608. [Google Scholar] [CrossRef]
- Su, X.; Jin, Y.; Shen, Y.; Kim, I.-M.; Weintraub, N.L.; Tang, Y. RNAase III-Type Enzyme Dicer Regulates Mitochondrial Fatty Acid Oxidative Metabolism in Cardiac Mesenchymal Stem Cells. Int. J. Mol. Sci. 2019, 20, 5554. [Google Scholar] [CrossRef] [PubMed]
- Cuellar, W.J.; Kreuze, J.F.; Rajamäki, M.-L.; Cruzado, K.R.; Untiveros, M.; Valkonen, J.P.T. Elimination of Antiviral Defense by Viral RNase III. Proc. Natl. Acad. Sci. USA 2009, 106, 10354–10358. [Google Scholar] [CrossRef]
- Aguado, L.C.; tenOever, B.R. RNase III Nucleases and the Evolution of Antiviral Systems. Bioessays 2018, 40, 1700173. [Google Scholar] [CrossRef]
- Zhang, Y.; Calin-Jageman, I.; Gurnon, J.R.; Choi, T.-J.; Adams, B.; Nicholson, A.W.; Van Etten, J.L. Characterization of a Chlorella Virus PBCV-1 Encoded Ribonuclease III. Virology 2003, 317, 73–83. [Google Scholar] [CrossRef]
- Zenke, K.; Kim, K.H. Functional Characterization of the RNase III Gene of Rock Bream Iridovirus. Arch. Virol. 2008, 153, 1651–1656. [Google Scholar] [CrossRef]
- Kreuze, J.F.; Savenkov, E.I.; Cuellar, W.; Li, X.; Valkonen, J.P.T. Viral Class 1 RNase III Involved in Suppression of RNA Silencing. J. Virol. 2005, 79, 7227–7238. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Taft, R.J.; Asgari, S. An Insect Virus-Encoded microRNA Regulates Viral Replication. J. Virol. 2008, 82, 9164–9170. [Google Scholar] [CrossRef]
- Hussain, M.; Abraham, A.M.; Asgari, S. An Ascovirus-Encoded RNase III Autoregulates Its Expression and Suppresses RNA Interference-Mediated Gene Silencing. J. Virol. 2010, 84, 3624–3630. [Google Scholar] [CrossRef]
- Marques, J.T.; Imler, J.-L. The Diversity of Insect Antiviral Immunity: Insights from Viruses. Curr. Opin. Microbiol. 2016, 32, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Upton, J.W.; Chan, F.K.-M. Staying Alive: Cell Death in Antiviral Immunity. Mol. Cell 2014, 54, 273–280. [Google Scholar] [CrossRef]
- Best, S.M. Viral Subversion of Apoptotic Enzymes: Escape from Death Row. Annu. Rev. Microbiol. 2008, 62, 171–192. [Google Scholar] [CrossRef]
- Ryerson, M.R.; Richards, M.M.; Kvansakul, M.; Hawkins, C.J.; Shisler, J.L. Vaccinia Virus Encodes a Novel Inhibitor of Apoptosis That Associates with the Apoptosome. J. Virol. 2017, 91, e01385-17. [Google Scholar] [CrossRef] [PubMed]
- Schimmer, A.D. Inhibitor of Apoptosis Proteins: Translating Basic Knowledge into Clinical Practice. Cancer Res. 2004, 64, 7183–7190. [Google Scholar] [CrossRef] [PubMed]
- Dubrez-Daloz, L.; Dupoux, A.; Cartier, J. IAPs: More than Just Inhibitors of Apoptosis Proteins. Cell Cycle 2008, 7, 1036–1046. [Google Scholar] [CrossRef]
- Sahoo, G.; Samal, D.; Khandayataray, P.; Murthy, M.K. A Review on Caspases: Key Regulators of Biological Activities and Apoptosis. Mol. Neurobiol. 2023, 60, 5805–5837. [Google Scholar] [CrossRef]
- Crook, N.E.; Clem, R.J.; Miller, L.K. An Apoptosis-Inhibiting Baculovirus Gene with a Zinc Finger-like Motif. J. Virol. 1993, 67, 2168–2174. [Google Scholar] [CrossRef]
- Birnbaum, M.J.; Clem, R.J.; Miller, L.K. An Apoptosis-Inhibiting Gene from a Nuclear Polyhedrosis Virus Encoding a Polypeptide with Cys/His Sequence Motifs. J. Virol. 1994, 68, 2521–2528. [Google Scholar] [CrossRef] [PubMed]
- Clem, R.J. Baculoviruses and Apoptosis: The Good, the Bad, and the Ugly. Cell Death Differ. 2001, 8, 137–143. [Google Scholar] [CrossRef]
- Liu, Y.-Y.; Xian, W.-F.; Xue, J.; Wei, Y.-L.; Cheng, X.-W.; Wang, X. Complete Genome Sequence of a Renamed Isolate, Trichoplusia ni Ascovirus 6b, from the United States. Genome Announc. 2018, 6, e00148-18. [Google Scholar] [CrossRef]
- Silke, J.; Vince, J. IAPs and Cell Death. Curr. Top. Microbiol. Immunol. 2017, 403, 95–117. [Google Scholar] [CrossRef]
- Bertheloot, D.; Latz, E.; Franklin, B.S. Necroptosis, Pyroptosis and Apoptosis: An Intricate Game of Cell Death. Cell. Mol. Immunol. 2021, 18, 1106–1121. [Google Scholar] [CrossRef]
- Yang, B.; Qi, Z.; Armas Diaz, Y.; Cassotta, M.; Grosso, G.; Cianciosi, D.; Zhang, D.; Zou, X.; Quiles, J.L.; Battino, M.; et al. Pyroptosis: A Novel Therapeutic Target for Bioactive Compounds in Human Disease Treatment? A Narrative Review. Nutrients 2025, 17, 461. [Google Scholar] [CrossRef]
- Gyrd-Hansen, M.; Darding, M.; Miasari, M.; Santoro, M.M.; Zender, L.; Xue, W.; Tenev, T.; da Fonseca, P.C.A.; Zvelebil, M.; Bujnicki, J.M.; et al. IAPs Contain an Evolutionarily Conserved Ubiquitin-Binding Domain That Regulates NF-kappaB as Well as Cell Survival and Oncogenesis. Nat. Cell Biol. 2008, 10, 1309–1317. [Google Scholar] [CrossRef]
- Rumble, J.M.; Duckett, C.S. Diverse Functions within the IAP Family. J. Cell Sci. 2008, 121, 3505–3507. [Google Scholar] [CrossRef]
- Cheung, C.H.A.; Chang, Y.-C.; Lin, T.-Y.; Cheng, S.M.; Leung, E. Anti-Apoptotic Proteins in the Autophagic World: An Update on Functions of XIAP, Survivin, and BRUCE. J. Biomed. Sci. 2020, 27, 31. [Google Scholar] [CrossRef] [PubMed]
- Dumétier, B.; Zadoroznyj, A.; Dubrez, L. IAP-Mediated Protein Ubiquitination in Regulating Cell Signaling. Cells 2020, 9, 1118. [Google Scholar] [CrossRef]
- Coste, F.; Kemp, C.; Bobezeau, V.; Hetru, C.; Kellenberger, C.; Imler, J.-L.; Roussel, A. Crystal Structure of Diedel, a Marker of the Immune Response of Drosophila Melanogaster. PLoS ONE 2012, 7, e33416. [Google Scholar] [CrossRef]
- Fahrbach, S.E.; Nambu, J.R.; Schwartz, L.M. Programmed Cell Death in Insects. In Insect Molecular Biology and Biochemistry; Gilbert, L.I., Ed.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 419–449. ISBN 9780123847478. [Google Scholar]
- Romero, A.; Novoa, B.; Figueras, A. The Complexity of Apoptotic Cell Death in Mollusks: An Update. Fish Shellfish Immunol. 2015, 46, 79–87. [Google Scholar] [CrossRef]
- Yuan, J.; Ofengeim, D. A Guide to Cell Death Pathways. Nat. Rev. Mol. Cell Biol. 2024, 25, 379–395. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, L.B.; Shaham, S. Apoptotic and Nonapoptotic Cell Death in Caenorhabditis elegans Development. Annu. Rev. Genet. 2024, 58, 113–134. [Google Scholar] [CrossRef]
- Cooper, D.M.; Granville, D.J.; Lowenberger, C. The Insect Caspases. Apoptosis 2009, 14, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Ghavami, S.; Hashemi, M.; Ande, S.R.; Yeganeh, B.; Xiao, W.; Eshraghi, M.; Bus, C.J.; Kadkhoda, K.; Wiechec, E.; Halayko, A.J.; et al. Apoptosis and Cancer: Mutations within Caspase Genes. J. Med. Genet. 2009, 46, 497–510. [Google Scholar] [CrossRef] [PubMed]
- Parrish, A.B.; Freel, C.D.; Kornbluth, S. Cellular Mechanisms Controlling Caspase Activation and Function. Cold Spring Harb. Perspect. Biol. 2013, 5, a008672. [Google Scholar] [CrossRef]
- Mustafa, M.; Ahmad, R.; Tantry, I.Q.; Ahmad, W.; Siddiqui, S.; Alam, M.; Abbas, K.; Moinuddin; Hassan, M.I.; Habib, S.; et al. Apoptosis: A Comprehensive Overview of Signaling Pathways, Morphological Changes, and Physiological Significance and Therapeutic Implications. Cells 2024, 13, 1838. [Google Scholar] [CrossRef] [PubMed]
- Liston, P.; Fong, W.G.; Korneluk, R.G. The Inhibitors of Apoptosis: There Is More to Life than Bcl2. Oncogene 2003, 22, 8568–8580. [Google Scholar] [CrossRef]
- Huber, K.L.; Serrano, B.P.; Hardy, J.A. Caspase-9 CARD: Core Domain Interactions Require a Properly Formed Active Site. Biochem. J. 2018, 475, 1177–1196. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Zhuang, X.-Z.; Li, J.; Zhou, X. Targeting the Inhibitors of Apoptosis Proteins (IAPs) to Combat Drug Resistance in Cancers. Front. Pharmacol. 2025, 16, 1562167. [Google Scholar] [CrossRef]
- Wang, P.; Shi, T.; Ma, D. Cloning of a Novel Human Caspase-9 Splice Variant Containing Only the CARD Domain. Life Sci. 2006, 79, 934–940. [Google Scholar] [CrossRef]
- Chaïbi, C.; Cotte-Laffitte, J.; Sandré, C.; Esclatine, A.; Servin, A.L.; Quéro, A.-M.; Géniteau-Legendre, M. Rotavirus Induces Apoptosis in Fully Differentiated Human Intestinal Caco-2 Cells. Virology 2005, 332, 480–490. [Google Scholar] [CrossRef]
- Bagchi, P.; Dutta, D.; Chattopadhyay, S.; Mukherjee, A.; Halder, U.C.; Sarkar, S.; Kobayashi, N.; Komoto, S.; Taniguchi, K.; Chawla-Sarkar, M. Rotavirus Nonstructural Protein 1 Suppresses Virus-Induced Cellular Apoptosis to Facilitate Viral Growth by Activating the Cell Survival Pathways during Early Stages of Infection. J. Virol. 2010, 84, 6834–6845. [Google Scholar] [CrossRef]
- Connolly, P.F.; Fearnhead, H.O. Viral Hijacking of Host Caspases: An Emerging Category of Pathogen-Host Interactions. Cell Death Differ. 2017, 24, 1401–1410. [Google Scholar] [CrossRef]
- Dai, X.; Hakizimana, O.; Zhang, X.; Kaushik, A.C.; Zhang, J. Orchestrated Efforts on Host Network Hijacking: Processes Governing Virus Replication. Virulence 2020, 11, 183–198. [Google Scholar] [CrossRef]
- Conus, S.; Simon, H.-U. Cathepsins: Key Modulators of Cell Death and Inflammatory Responses. Biochem. Pharmacol. 2008, 76, 1374–1382. [Google Scholar] [CrossRef]
- Jakob, N.J.; Müller, K.; Bahr, U.; Darai, G. Analysis of the First Complete DNA Sequence of an Invertebrate Iridovirus: Coding Strategy of the Genome of Chilo Iridescent Virus. Virology 2001, 286, 182–196. [Google Scholar] [CrossRef]
- Slack, J.M.; Kuzio, J.; Faulkner, P. Characterization of v-Cath, a Cathepsin L-like Proteinase Expressed by the Baculovirus Autographa californica Multiple Nuclear Polyhedrosis Virus. J. Gen. Virol. 1995, 76 Pt 5, 1091–1098. [Google Scholar] [CrossRef]
- Hawtin, R.E.; Zarkowska, T.; Arnold, K.; Thomas, C.J.; Gooday, G.W.; King, L.A.; Kuzio, J.A.; Possee, R.D. Liquefaction of Autographa californica Nucleopolyhedrovirus-Infected Insects Is Dependent on the Integrity of Virus-Encoded Chitinase and Cathepsin Genes. Virology 1997, 238, 243–253. [Google Scholar] [CrossRef]
- Foghsgaard, L.; Wissing, D.; Mauch, D.; Lademann, U.; Bastholm, L.; Boes, M.; Elling, F.; Leist, M.; Jäättelä, M. Cathepsin B Acts as a Dominant Execution Protease in Tumor Cell Apoptosis Induced by Tumor Necrosis Factor. J. Cell Biol. 2001, 153, 999–1010. [Google Scholar] [CrossRef]
- Chwieralski, C.E.; Welte, T.; Bühling, F. Cathepsin-Regulated Apoptosis. Apoptosis 2006, 11, 143–149. [Google Scholar] [CrossRef]
- Droga-Mazovec, G.; Bojic, L.; Petelin, A.; Ivanova, S.; Romih, R.; Repnik, U.; Salvesen, G.S.; Stoka, V.; Turk, V.; Turk, B. Cysteine Cathepsins Trigger Caspase-Dependent Cell Death through Cleavage of Bid and Antiapoptotic Bcl-2 Homologues. J. Biol. Chem. 2008, 283, 19140–19150. [Google Scholar] [CrossRef]
- Repnik, U.; Hafner Česen, M.; Turk, B. Lysosomal Membrane Permeabilization in Cell Death: Concepts and Challenges. Mitochondrion 2014, 19 Pt A, 49–57. [Google Scholar] [CrossRef]
- Xie, Z.; Zhao, M.; Yan, C.; Kong, W.; Lan, F.; Narengaowa; Zhao, S.; Yang, Q.; Bai, Z.; Qing, H.; et al. Cathepsin B in Programmed Cell Death Machinery: Mechanisms of Execution and Regulatory Pathways. Cell Death Dis. 2023, 14, 255. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Ou-Yang, Y.-Y.; Yang, C.-J.; Li, N.; Nakai, M.; Huang, G.-H. 3H-31, A Non-Structural Protein of Heliothis virescens Ascovirus 3h, Inhibits the Host Larval Cathepsin and Chitinase Activities. Virol. Sin. 2021, 36, 1036–1051. [Google Scholar] [CrossRef]
- Lin, Z.; Long, F.; Kang, R.; Klionsky, D.J.; Yang, M.; Tang, D. The Lipid Basis of Cell Death and Autophagy. Autophagy 2024, 20, 469–488. [Google Scholar] [CrossRef] [PubMed]
- Mazzon, M.; Mercer, J. Lipid Interactions during Virus Entry and Infection: Lipids and Viruses. Cell. Microbiol. 2014, 16, 1493–1502. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, E.L.; Lagunoff, M. Viral Activation of Cellular Metabolism. Virology 2015, 479–480, 609–618. [Google Scholar] [CrossRef]
- Newton, J.; Lima, S.; Maceyka, M.; Spiegel, S. Revisiting the Sphingolipid Rheostat: Evolving Concepts in Cancer Therapy. Exp. Cell Res. 2015, 333, 195–200. [Google Scholar] [CrossRef]
- Ueda, N. A Rheostat of Ceramide and Sphingosine-1-Phosphate as a Determinant of Oxidative Stress-Mediated Kidney Injury. Int. J. Mol. Sci. 2022, 23, 4010. [Google Scholar] [CrossRef]
- Soudani, N.; Hage-Sleiman, R.; Karam, W.; Dbaibo, G.; Zaraket, H. Ceramide Suppresses Influenza A Virus Replication in Vitro. J. Virol. 2019, 93, e00053-19. [Google Scholar] [CrossRef] [PubMed]
- Avota, E.; Bodem, J.; Chithelen, J.; Mandasari, P.; Beyersdorf, N.; Schneider-Schaulies, J. The Manifold Roles of Sphingolipids in Viral Infections. Front. Physiol. 2021, 12, 715527. [Google Scholar] [CrossRef]
- Schneider-Schaulies, S.; Schumacher, F.; Wigger, D.; Schöl, M.; Waghmare, T.; Schlegel, J.; Seibel, J.; Kleuser, B. Sphingolipids: Effectors and Achilles Heals in Viral Infections? Cells 2021, 10, 2175. [Google Scholar] [CrossRef]
- Thomas, S.; Samuel, S.V.; Hoch, A.; Syphurs, C.; Diray-Arce, J. The Implication of Sphingolipids in Viral Infections. Int. J. Mol. Sci. 2023, 24, 17303. [Google Scholar] [CrossRef]
- Dai, J.; Feng, Y.; Liao, Y.; Tan, L.; Sun, Y.; Song, C.; Qiu, X.; Ding, C. Virus Infection and Sphingolipid Metabolism. Antivir. Res. 2024, 228, 105942. [Google Scholar] [CrossRef]
- Li, R.; Xiao, Y.; Li, K.; Tian, L. Transcription and Post-translational Regulation of Autophagy in Insects. Front. Physiol. 2022, 13, 825202. [Google Scholar] [CrossRef]
- Shimano, H.; Sato, R. SREBP-Regulated Lipid Metabolism: Convergent Physiology-Divergent Pathophysiology. Nat. Rev. Endocrinol. 2017, 13, 710–730. [Google Scholar] [CrossRef]
- DeBose-Boyd, R.A.; Ye, J. SREBPs in Lipid Metabolism, Insulin Signaling, and beyond. Trends Biochem. Sci. 2018, 43, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Lange, P.T.; Lagunoff, M.; Tarakanova, V.L. Chewing the Fat: The Conserved Ability of DNA Viruses to Hijack Cellular Lipid Metabolism. Viruses 2019, 11, 119. [Google Scholar] [CrossRef]
- Vaidyanathan, S.; Salmi, T.M.; Sathiqu, R.M.; McConville, M.J.; Cox, A.G.; Brown, K.K. YAP Regulates an SGK1/mTORC1/SREBP-Dependent Lipogenic Program to Support Proliferation and Tissue Growth. Dev. Cell 2022, 57, 719–731.e8. [Google Scholar] [CrossRef]
- Bishop, W.R.; Bell, R.M. Assembly of Phospholipids into Cellular Membranes: Biosynthesis, Transmembrane Movement and Intracellular Translocation. Annu. Rev. Cell Biol. 1988, 4, 579–610. [Google Scholar] [CrossRef] [PubMed]
- Martin-Acebes, M.A.; Vazquez-Calvo, A.; Caridi, F.; Saiz, J.-C.; Sobrino, F. Lipid Involvement in Viral Infections: Present and Future Perspectives for the Design of Antiviral Strategies. In Lipid Metabolism; Baez, R.V., Ed.; InTechOpen: London, UK, 2013; ISBN 9789535109440. [Google Scholar]
- Wilkerson, J.L.; Tatum, S.M.; Holland, W.L.; Summers, S.A. Ceramides Are Fuel Gauges on the Drive to Cardiometabolic Disease. Physiol. Rev. 2024, 104, 1061–1119. [Google Scholar] [CrossRef]
- Chitkara, S.; Gozali, N.; Kuzmin, A.; Pliss, A.; Prasad, P.; Sancak, Y.; Atilla-Gokcumen, G.E. ER-localized Ceramide Accumulation due to Disrupted CERT Transport Contributes to Replicative Senescence. bioRxiv 2025. bioRxiv:2025.05.16.654528. [Google Scholar] [CrossRef]
- Thakkar, H.; Vincent, V.; Chaurasia, B. Ceramide Signaling in Immunity: A Molecular Perspective. Lipids Health Dis. 2025, 24, 225. [Google Scholar] [CrossRef]
- Bill, C.A.; Vines, C.M. Phospholipase C. Adv. Exp. Med. Biol. 2020, 1131, 215–242. [Google Scholar] [CrossRef]
- Korbecki, J.; Bosiacki, M.; Pilarczyk, M.; Gąssowska-Dobrowolska, M.; Jarmużek, P.; Szućko-Kociuba, I.; Kulik-Sajewicz, J.; Chlubek, D.; Baranowska-Bosiacka, I. Phospholipid Acyltransferases: Characterization and Involvement of the Enzymes in Metabolic and Cancer Diseases. Cancers 2024, 16, 2115. [Google Scholar] [CrossRef]
- Smede, M.; Hussain, M.; Asgari, S. A Lipase-like Gene from Heliothis virescens Ascovirus (HvAV-3e) Is Essential for Virus Replication and Cell Cleavage. Virus Genes 2009, 39, 409–417. [Google Scholar] [CrossRef]
- Baek, S.H.; Kwak, J.Y.; Lee, S.H.; Lee, T.; Ryu, S.H.; Uhlinger, D.J.; Lambeth, J.D. Lipase Activities of p37, the Major Envelope Protein of Vaccinia Virus. J. Biol. Chem. 1997, 272, 32042–32049. [Google Scholar] [CrossRef] [PubMed]
- Blasco, R.; Moss, B. Extracellular Vaccinia Virus Formation and Cell-to-Cell Virus Transmission Are Prevented by Deletion of the Gene Encoding the 37,000-Dalton Outer Envelope Protein. J. Virol. 1991, 65, 5910–5920. [Google Scholar] [CrossRef]
- Kamil, J.P.; Tischer, B.K.; Trapp, S.; Nair, V.K.; Osterrieder, N.; Kung, H.-J. vLIP, a Viral Lipase Homologue, Is a Virulence Factor of Marek’s Disease Virus. J. Virol. 2005, 79, 6984–6996. [Google Scholar] [CrossRef]
- Haslam, T.M.; Kunst, L. Extending the Story of Very-Long-Chain Fatty Acid Elongation. Plant Sci. 2013, 210, 93–107. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Sun, Q.; Huang, L.; Li, J. Integrated Transcriptome and Metabolome Analyses Uncover Cholesterol-Responsive Gene Networks. Int. J. Mol. Sci. 2025, 26, 7108. [Google Scholar] [CrossRef]
- Oh, C.S.; Toke, D.A.; Mandala, S.; Martin, C.E. ELO2 and ELO3, Homologues of the Saccharomyces Cerevisiae ELO1 Gene, Function in Fatty Acid Elongation and Are Required for Sphingolipid Formation. J. Biol. Chem. 1997, 272, 17376–17384. [Google Scholar] [CrossRef]
- Gaigg, B.; Timischl, B.; Corbino, L.; Schneiter, R. Synthesis of Sphingolipids with Very Long Chain Fatty Acids but Not Ergosterol Is Required for Routing of Newly Synthesized Plasma Membrane ATPase to the Cell Surface of Yeast. J. Biol. Chem. 2005, 280, 22515–22522. [Google Scholar] [CrossRef]
- Shiota, T.; Li, Z.; Chen, G.-Y.; McKnight, K.L.; Shirasaki, T.; Yonish, B.; Kim, H.; Fritch, E.J.; Sheahan, T.P.; Muramatsu, M.; et al. Hepatoviruses Promote Very-Long-Chain Fatty Acid and Sphingolipid Synthesis for Viral RNA Replication and Quasi-Enveloped Virus Release. Sci. Adv. 2023, 9, eadj4198. [Google Scholar] [CrossRef]
- Farías, M.A.; Diethelm-Varela, B.; Navarro, A.J.; Kalergis, A.M.; González, P.A. Interplay between Lipid Metabolism, Lipid Droplets, and DNA Virus Infections. Cells 2022, 11, 2224. [Google Scholar] [CrossRef]
- Scherer, G.F.E.; Ryu, S.B.; Wang, X.; Matos, A.R.; Heitz, T. Patatin-Related Phospholipase A: Nomenclature, Subfamilies and Functions in Plants. Trends Plant Sci. 2010, 15, 693–700. [Google Scholar] [CrossRef]
- Khan, S.A.; Ilies, M.A. The Phospholipase A2 Superfamily: Structure, Isozymes, Catalysis, Physiologic and Pathologic Roles. Int. J. Mol. Sci. 2023, 24, 1353. [Google Scholar] [CrossRef]
- Lulić, A.-M.; Katalinić, M. The PNPLA Family of Enzymes: Characterisation and Biological Role. Arh. Za Hig. Rada I Toksikol. 2023, 74, 75–89. [Google Scholar] [CrossRef]
- Li, S.; Gai, Z.; Tian, Y.; Wang, X.; Guo, J.; Zheng, Y.; Li, T. Dual Enzymatic Activities of Potato Patatin: Esterase and Lipase Characterization. J. Food Meas. Charact. 2025, 19, 1037–1047. [Google Scholar] [CrossRef]
- Strickland, J.A.; Orr, G.L.; Walsh, T.A. Inhibition of Diabrotica Larval Growth by Patatin, the Lipid Acyl Hydrolase from Potato Tubers. Plant Physiol. 1995, 109, 667–674. [Google Scholar] [CrossRef]
- Borgo, G.M.; Burke, T.P.; Tran, C.J.; Lo, N.T.N.; Engström, P.; Welch, M.D. A Patatin-like Phospholipase Mediates Rickettsia Parkeri Escape from Host Membranes. Nat. Commun. 2022, 13, 3656. [Google Scholar] [CrossRef]
- Li, M.; Markham, J.E.; Wang, X. Overexpression of Patatin-Related Phospholipase AIIIÎ2 Altered the Content and Composition of Sphingolipids in Arabidopsis. Front. Plant Sci. 2014, 5, 553. [Google Scholar] [CrossRef]
- Parveen, F.; Bender, D.; Law, S.-H.; Mishra, V.K.; Chen, C.-C.; Ke, L.-Y. Role of Ceramidases in Sphingolipid Metabolism and Human Diseases. Cells 2019, 8, 1573. [Google Scholar] [CrossRef]
- Quinville, B.M.; Deschenes, N.M.; Ryckman, A.E.; Walia, J.S. A Comprehensive Review: Sphingolipid Metabolism and Implications of Disruption in Sphingolipid Homeostasis. Int. J. Mol. Sci. 2021, 22, 5793. [Google Scholar] [CrossRef]
- Sheridan, M.; Ogretmen, B. The Role of Ceramide Metabolism and Signaling in the Regulation of Mitophagy and Cancer Therapy. Cancers 2021, 13, 2475. [Google Scholar] [CrossRef]
- Sharma, D.; Czarnota, G.J. Involvement of Ceramide Signalling in Radiation-Induced Tumour Vascular Effects and Vascular-Targeted Therapy. Int. J. Mol. Sci. 2022, 23, 6671. [Google Scholar] [CrossRef]
- Taha, T.A.; Mullen, T.D.; Obeid, L.M. A House Divided: Ceramide, Sphingosine, and Sphingosine-1-Phosphate in Programmed Cell Death. Biochim. Biophys. Acta 2006, 1758, 2027–2036. [Google Scholar] [CrossRef] [PubMed]
- Pilátová, M.B.; Solárová, Z.; Mezencev, R.; Solár, P. Ceramides and Their Roles in Programmed Cell Death. Adv. Med. Sci. 2023, 68, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Pettus, B.J.; Chalfant, C.E.; Hannun, Y.A. Ceramide in Apoptosis: An Overview and Current Perspectives. Biochim. Biophys. Acta 2002, 1585, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Perry, D.K.; Carton, J.; Shah, A.K.; Meredith, F.; Uhlinger, D.J.; Hannun, Y.A. Serine Palmitoyltransferase Regulates de Novo Ceramide Generation during Etoposide-Induced Apoptosis. J. Biol. Chem. 2000, 275, 9078–9084. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Han, T.Y.; Giuliano, A.E.; Cabot, M.C. Expression of Glucosylceramide Synthase, Converting Ceramide to Glucosylceramide, Confers Adriamycin Resistance in Human Breast Cancer Cells. J. Biol. Chem. 1999, 274, 1140–1146. [Google Scholar] [CrossRef]
- Taniguchi, M.; Nagaya, S.; Yuyama, K.; Kotani, A.; Igarashi, Y.; Okazaki, T. Ceramide Metabolism Regulated by Sphingomyelin Synthase 2 Is Associated with Acquisition of Chemoresistance via Exosomes in Human Leukemia Cells. Int. J. Mol. Sci. 2022, 23, 10648. [Google Scholar] [CrossRef]
- Senchenkov, A.; Litvak, D.A.; Cabot, M.C. Targeting Ceramide Metabolism--a Strategy for Overcoming Drug Resistance. J. Natl. Cancer Inst. 2001, 93, 347–357. [Google Scholar] [CrossRef]
- Gulbins, E.; Kolesnick, R. Raft Ceramide in Molecular Medicine. Oncogene 2003, 22, 7070–7077. [Google Scholar] [CrossRef]
- Birbes, H.; El Bawab, S.; Obeid, L.M.; Hannun, Y. Mitochondria Ceramide: Interactions Apoptosis. Biochim. Biophys. Acta 2005, 1758, 494–499. [Google Scholar]
- Pastorino, J.G.; Tafani, M.; Rothman, R.J.; Marcinkeviciute, A.; Hoek, J.B.; Farber, J.L. Functional Consequences of the Sustained or Transient Activation by Bax of the Mitochondrial Permeability Transition Pore. J. Biol. Chem. 1999, 274, 31734–31739. [Google Scholar] [CrossRef] [PubMed]
- Gudz, T.I.; Tserng, K.Y.; Hoppel, C.L. Direct Inhibition of Mitochondrial Respiratory Chain Complex III by Cell-Permeable Ceramide. J. Biol. Chem. 1997, 272, 24154–24158. [Google Scholar] [CrossRef]
- Siskind, L.J.; Kolesnick, R.N.; Colombini, M. Ceramide Channels Increase the Permeability of the Mitochondrial Outer Membrane to Small Proteins. J. Biol. Chem. 2002, 277, 26796–26803. [Google Scholar] [CrossRef]
- Paola, D.; Cocco, M.; Lorusso, T. Ceramide Interaction Respiratory Chain Heart Mitochondria. Biochemistry 2004, 43, 15384–15392. [Google Scholar] [CrossRef]
- Siskind, L.J. Mitochondrial Ceramide and the Induction of Apoptosis. J. Bioenerg. Biomembr. 2005, 37, 143–153. [Google Scholar] [CrossRef]
- McCracken, A.N.; Lipkin, E.W.; Obeid, L.M. Sphingosine Inhibits Akt/PKB Activation and Induces Apoptosis in PC12 Cells. J. Neurochem. 2003, 84, 1300–1310. [Google Scholar]
- Chang, Y.; Lee, J.J.; Kim, Y.J.; Lee, S.Y.; Kim, H.J.; Kim, J.H. Sphingosine Induces Apoptosis through Inhibition of Protein Kinase C and Activation of Caspases in Human Leukemia Cells. Exp. Mol. Med. 2001, 33, 354–362. [Google Scholar]
- Cuvillier, O.; Pirianov, G.; Kleuser, B.; Vanek, P.G.; Coso, O.A.; Gutkind, S.; Spiegel, S. Suppression of Ceramide-Mediated Programmed Cell Death by Sphingosine-1-Phosphate. Nature 1996, 381, 800–803. [Google Scholar] [CrossRef] [PubMed]
- Cuvillier, O. Sphingosine in Apoptosis Signaling. Biochim. Biophys. Acta 2002, 1585, 153–162. [Google Scholar] [CrossRef]
- Hung, W.C.; Chang, H.C.; Chuang, L.Y. Activation of Caspase-3-like Proteases in Apoptosis Induced by Sphingosine and Other Long-Chain Bases in Hep3B Hepatoma Cells. Biochem. J. 1999, 338 Pt 1, 161–166. [Google Scholar] [CrossRef]
- Kågedal, K.; Zhao, M.; Svensson, I.; Brunk, U.T. Sphingosine-Induced Apoptosis Is Dependent on Lysosomal Proteases. Biochem. J. 2001, 359, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Tallima, H. Clarification of Arachidonic Acid Metabolic Pathway Intricacies. ACS Omega 2021, 6, 15559–15563. [Google Scholar] [CrossRef]
- Di, Y.P.; Zhao, J.; Harper, R.; Gibson, L.F.; Pang, Y.Y.; Haddad, I.Y. Arachidonic Acid Induces Apoptosis Human Lung Epithelial Cells. Am. J. Respir. Cell Mol. Biol 2000, 23, 661–668. [Google Scholar]
- Garrido, C.; Brunet, M.; Didelot, C.; Zermati, Y.; Schmitt, E.; Kroemer, G. Heat Shock Proteins 27 and 70: Anti-Apoptotic Proteins with Tumorigenic Properties. Cell Cycle 2006, 5, 2592–2601. [Google Scholar] [CrossRef]
- Ko, S.; Woo, T.G.; Lee, S.H.; Ha, N.C.; Park, B.J. Arachidonic Acid Induces Apoptosis Under Serum-Free Conditions by Blocking PAK1-Mediated PUMA Suppression 2024. Clin. Oncol. 2024, 9, 2053. [Google Scholar]
- Jayadev, S.; Liu, B.; Bielawska, A.E.; Lee, J.Y.; Nazaire, F.; Pushkareva, M.Y.; Obeid, L.M.; Hannun, Y.A. Role for Ceramide in Cell Cycle Arrest. J. Biol. Chem. 1995, 270, 2047–2052. [Google Scholar] [CrossRef]
- Ramanadham, S.; Hsu, F.-F.; Zhang, S.; Jin, C.; Bohrer, A.; Song, H.; Bao, S.; Ma, Z.; Turk, J. Apoptosis of Insulin-Secreting Cells Induced by Endoplasmic Reticulum Stress Is Amplified by Overexpression of Group VIA Calcium-Independent Phospholipase A2 (iPLA2 Beta) and Suppressed by Inhibition of iPLA2 Beta. Biochemistry 2004, 43, 918–930. [Google Scholar] [CrossRef]
- Andrés, C.M.C.; Pérez de la Lastra, J.M.; Juan, C.A.; Plou, F.J.; Pérez-Lebeña, E. Chemistry of Hydrogen Peroxide Formation and Elimination in Mammalian Cells, and Its Role in Various Pathologies. Stresses 2022, 2, 256–274. [Google Scholar] [CrossRef]
- Pryor, W.A.; Squadrito, G.L.; Friedman, M. The Cascade Mechanism to Explain Ozone Toxicity: The Role of Lipid Ozonation Products. Free Radic. Biol. Med. 1995, 19, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Menzel, J.P.; Young, R.S.E.; Benfield, A.H.; Scott, J.S.; Wongsomboon, P.; Cudlman, L.; Cvačka, J.; Butler, L.M.; Henriques, S.T.; Poad, B.L.J.; et al. Ozone-Enabled Fatty Acid Discovery Reveals Unexpected Diversity in the Human Lipidome. Nat. Commun. 2023, 14, 3940. [Google Scholar] [CrossRef]
- Park, J.T.; Lee, Y.-S.; Cho, K.A.; Park, S.C. Adjustment of the Lysosomal-Mitochondrial Axis for Control of Cellular Senescence. Ageing Res. Rev. 2018, 47, 176–182. [Google Scholar] [CrossRef]
- Voronina, M.V.; Frolova, A.S.; Kolesova, E.P.; Kuldyushev, N.A.; Parodi, A.; Zamyatnin, A.A., Jr. The Intricate Balance between Life and Death: ROS, Cathepsins, and Their Interplay in Cell Death and Autophagy. Int. J. Mol. Sci. 2024, 25, 4087. [Google Scholar] [CrossRef]
- Dorstyn, L.; Akey, C.W.; Kumar, S. New Insights into Apoptosome Structure and Function. Cell Death Differ. 2018, 25, 1194–1208. [Google Scholar] [CrossRef]
- Yang, M.; Wei, X.; Yi, X.; Jiang, D.-S. Mitophagy-Related Regulated Cell Death: Molecular Mechanisms and Disease Implications. Cell Death Dis. 2024, 15, 505. [Google Scholar] [CrossRef]
- Küng, C.; Lazarou, M.; Nguyen, T.N. Advances in Mitophagy Initiation Mechanisms. Curr. Opin. Cell Biol. 2025, 94, 102493. [Google Scholar] [CrossRef]
- Draeger, A.; Babiychuk, E.B. Ceramide in Plasma Membrane Repair. Handb. Exp. Pharmacol. 2013, 216, 341–353. [Google Scholar] [CrossRef]
- Kaltenegger, M.; Kremser, J.; Frewein, M.P.K.; Ziherl, P.; Bonthuis, D.J.; Pabst, G. Intrinsic Lipid Curvatures of Mammalian Plasma Membrane Outer Leaflet Lipids and Ceramides. Biochim. Biophys. Acta Biomembr. 2021, 1863, 183709. [Google Scholar] [CrossRef] [PubMed]
- Beckmann, N.; Becker, K.A. Ceramide and Related Molecules in Viral Infections. Int. J. Mol. Sci. 2021, 22, 5676. [Google Scholar] [CrossRef]
- Lee, S.H.; Kho, A.R.; Lee, S.H.; Hong, D.K.; Kang, B.S.; Park, M.K.; Lee, C.J.; Yang, H.W.; Woo, S.Y.; Park, S.W.; et al. Acid Sphingomyelinase Inhibitor, Imipramine, Reduces Hippocampal Neuronal Death after Traumatic Brain Injury. Int. J. Mol. Sci. 2022, 23, 14749. [Google Scholar] [CrossRef] [PubMed]
- Kho, A.R.; Choi, B.Y.; Lee, S.H.; Hong, D.K.; Kang, B.S.; Lee, S.H.; Suh, S.W. Administration of an Acidic Sphingomyelinase (ASMase) Inhibitor, Imipramine, Reduces Hypoglycemia-Induced Hippocampal Neuronal Death. Cells 2022, 11, 667. [Google Scholar] [CrossRef]
- Lee, S.H.; Yang, H.W.; Kang, B.S.; Park, M.K.; Kim, D.Y.; Song, H.K.; Choi, H.C.; Lee, M.; Choi, B.Y.; Son, D.S.; et al. Imipramine, an Acid Sphingomyelinase Inhibitor, Promotes Newborn Neuron Survival in the Hippocampus After Seizure. Cells 2025, 14, 281. [Google Scholar] [CrossRef] [PubMed]
- Havranek, K.E.; Reyes Ballista, J.M.; Hines, K.M.; Brindley, M.A. Untargeted Lipidomics of Vesicular Stomatitis Virus-Infected Cells and Viral Particles. Viruses 2022, 14, 3. [Google Scholar] [CrossRef]
- Girik, V.; Klymenko, T.; Kovalchuk, O.; Dey, P.; Kovalchuk, I. Genetically Encoded Fluorescent Ceramide Probes Reveal Ceramide Dynamics during Phagocytosis and Autophagy. Int. J. Mol. Sci. 2024, 25, 2996. [Google Scholar] [CrossRef]
- Unniyampurath, U.; Pilankatta, R.; Krishnan, M.N. RNA Interference in the Age of CRISPR: Will CRISPR Interfere with RNAi? Int. J. Mol. Sci. 2016, 17, 291. [Google Scholar] [CrossRef]
- Paz, M.; Moratorio, G. Deep Mutational Scanning and CRISPR-Engineered Viruses: Tools for Evolutionary and Functional Genomics Studies. mSphere 2025, 10, e00508–e00524. [Google Scholar] [CrossRef]
- Zhang, L.; Qin, Y.; Chen, M. Viral Strategies for Triggering and Manipulating Mitophagy. Autophagy 2018, 14, 1665–1673. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Wang, Y.; Zou, W.; Wang, Z.; Cao, W.; Liang, M.; Li, F.; Zeng, Q.; Ren, Z.; Wang, Y.; et al. Inhibition of Mitophagy via the EIF2S1-ATF4-PRKN Pathway Contributes to Viral Encephalitis. J. Adv. Res. 2025, 73, 199–217. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Zhang, Y.; Zhang, X.; Zhao, Y.; Liu, J.; Chen, Y.; Liu, H.; Zhou, S. The Impact of Mitochondrial Dysfunction in Amyotrophic Lateral Sclerosis. Cells 2022, 11, 2049. [Google Scholar] [CrossRef]
- Li, Y.; Wu, K.; Zeng, S.; Zou, L.; Li, X.; Xu, C.; Li, B.; Liu, X.; Li, Z.; Zhu, W.; et al. The Role of Mitophagy in Viral Infection. Cells 2022, 11, 711. [Google Scholar] [CrossRef]
- Elefantova, K.; Lakatos, B.; Kubickova, J.; Sulova, Z.; Breier, A. Detection of the Mitochondrial Membrane Potential by the Cationic Dye JC-1 in L1210 Cells with Massive Overexpression of the Plasma Membrane ABCB1 Drug Transporter. Int. J. Mol. Sci. 2018, 19, 1985. [Google Scholar] [CrossRef]
- Xie, F.; Zhou, J.; Liu, B.; Zhao, L.; Lv, C.; Zhang, Q.; Yuan, L.; Sun, D.; Wei, W. Low Fluoride Regulates Macrophage Polarization Through Mitochondrial Autophagy Mediated by PINK1/Parkin Axis. Biomolecules 2025, 15, 647. [Google Scholar] [CrossRef]
- Lara-Hernandez, I.; Muñoz-Escalante, J.C.; Bernal-Silva, S.; Noyola, D.E.; Wong-Chew, R.M.; Comas-García, A.; Comas-Garcia, M. Ultrastructural and Functional Characterization of Mitochondrial Dynamics Induced by Human Respiratory Syncytial Virus Infection in HEp-2 Cells. Viruses 2023, 15, 1518. [Google Scholar] [CrossRef]
- Gong, Y.; Chen, R.; Yang, C.; Lu, Y.; Fu, Z.; Feng, Y.; Li, X.; Li, L.; Li, X. Genomic-Encoded Mitovirus RdRp Is Required for Embryo Development and Maintaining Mitochondrial Dynamics in Arabidopsis. Int. J. Mol. Sci. 2025, 26, 9035. [Google Scholar] [CrossRef]
- Demirbolat, G.M.; Coskun, G.P.; Erdogan, O.; Cevik, O. Long Chain Fatty Acids Can Form Aggregates and Affect the Membrane Integrity. Colloids Surf. B Biointerfaces 2021, 204, 111795. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, Y.; Wang, Q.; Wu, M.; Wang, R.; Han, X.; Liu, L.; Liu, T.; Shi, C.; Zhong, L.; et al. VAP27-1 Interacts with KCS6 and CER2 to Facilitate the Biosynthesis of Very- Long-Chain Fatty Acids. Plant Sci. 2025, 355, 112489. [Google Scholar] [CrossRef] [PubMed]
- Gasmi, L.; Sieminska, E.; Okuno, S.; Ohta, R.; Coutu, C.; Vatanparast, M.; Harris, S.; Baldwin, D.; Hegedus, D.D.; Theilmann, D.A.; et al. Horizontally Transmitted Parasitoid Killing Factor Shapes Insect Defense to Parasitoids. Science 2021, 373, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Yang, C.-J.; Li, N.; Zhao, Y.; Chen, Z.-M.; Yi, S.-J.; Li, Z.-Q.; Adang, M.J.; Huang, G.-H. Novel Strategies for the Biocontrol of Noctuid Pests (Lepidoptera) Based on Improving Ascovirus Infectivity Using Bacillus thuringiensis. Insect Sci. 2020, 28, 1452–1467. [Google Scholar] [CrossRef]
- Mohanty, P.; Rajadurai, G.; Mohankumar, S.; Balakrishnan, N.; Raghu, R.; Balasubramani, V.; Sivakumar, U. Interactions between insecticidal Cry toxins and their receptors. Curr. Genet. 2025, 71, 9. [Google Scholar] [CrossRef]
- Salehi Jouzani, G.; Sharafi, R.; Argentel-Martínez, L.; Peñuelas-Rubio, O.; Ozkan, C.; Incegul, B.; Goksu, R.; Hayta, Z.; Yilki, D.; Yazici, B.; et al. Novel insights into Bacillus thuringiensis: Beyond its role as a bioinsecticide. Res. Microbiol. 2025, 176, 104264. [Google Scholar] [CrossRef]
- Rudd, S.R.; Miranda, L.S.; Curtis, H.R.; Bigot, Y.; Diaz-Mendoza, M.; Hice, R.; Nizet, V.; Park, H.W.; Blaha, G.; Federici, B.A.; et al. The parasporal body of Bacillus thuringiensis subsp. israelensis: A unique phage capsid-associated prokaryotic insecticidal organelle. Biology 2023, 12, 1421. [Google Scholar] [CrossRef]
- Miranda, L.S.; Rudd, S.R.; Mena, O.; Hudspeth, P.E.; Barboza-Corona, J.E.; Park, H.W.; Bideshi, D.K. The perpetual vector mosquito threat and its eco-friendly nemeses. Biology 2024, 13, 182. [Google Scholar] [CrossRef]
- Martínez-Balerdi, M.; Caballero, J.; Aguirre, E.; Caballero, P.; Beperet, I. Baculoviruses as Microbial Pesticides: Potential, Challenges, and Market Overview. Viruses 2025, 17, 917. [Google Scholar] [CrossRef]
- Kyle, J.E. How lipidomics can transform our understanding of virus infections. Expert Rev. Proteom. 2021, 18, 329–332. [Google Scholar] [CrossRef]
- Hsu, P.-C.; Tsai, C.-C.; Lin, Y.-H.; Kuo, C.-Y. Therapeutic Targeting of Apoptosis, Autophagic Cell Death, Necroptosis, Pyroptosis, and Ferroptosis Pathways in Oral Squamous Cell Carcinoma: Molecular Mechanisms and Potential Strategies. Biomedicines 2025, 13, 1745. [Google Scholar] [CrossRef]
- Su, L.-J.; Zhang, J.-H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.-Y. Reactive Oxygen Species-Induced Lipid Peroxidation in Apoptosis, Autophagy, and Ferroptosis. Oxidative Med. Cell. Longev. 2019, 2019, 5080843. [Google Scholar] [CrossRef] [PubMed]
- Wiernicki, B.; Dubois, H.; Tyurina, Y.Y.; Hassannia, B.; Bayir, H.; Kagan, V.E.; Vandenabeele, P.; Wullaert, A.; Vanden Berghe, T. Excessive phospholipid peroxidation distinguishes ferroptosis from other cell death modes. Nat Commun. 2020, 11, 5454. [Google Scholar] [CrossRef]
- Khawar, M.B.; Gao, H.; Li, W. Autophagy and Lipid Metabolism. In Autophagy: Biology and Diseases. Advances in Experimental Medicine and Biology; Qin, Z.H., Ed.; Springer: Singapore, 2019; Volume 1206, pp. 359–374. [Google Scholar] [CrossRef]
- Yin, C.; Heit, B. Cellular Responses to the Efferocytosis of Apoptotic Cells. Front. Immunol. 2021, 12, 631714. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kim, N.S.; Haince, J.F.; Kang, H.C.; David, K.K.; Andrabi, S.A.; Poirier, G.G.; Dawson, V.L.; Dawson, T.M. Poly(ADP-ribose) (PAR) binding to apoptosis-inducing factor is critical for PAR polymerase-1-dependent cell death (parthanatos). Sci. Signal. 2011, 4, ra20. [Google Scholar] [CrossRef]
- Zhang, X.; Matsuda, M.; Yaegashi, N.; Nabe, T.; Kitatani, K. Regulation of Necroptosis by Phospholipids and Sphingolipids. Cells 2020, 9, 627. [Google Scholar] [CrossRef]
- Zhou, J.; Huang, R.; Aimaiti, M.; Zhou, Q.; Wu, X.; Zhu, J.; Ma, X.; Qian, K.; Zhou, Q.; Hu, L.; et al. Mechanism of RCD and the Role of Different Death Signaling Pathways in Cancer. Biomedicines 2025, 13, 1880. [Google Scholar] [CrossRef]
- Djambazova, K.V.; Klein, D.R.; Migas, L.G.; Neumann, E.K.; Rivera, E.S.; Van de Plas, R.; Caprioli, R.M.; Spraggins, J.M. Resolving the Complexity of Spatial Lipidomics Using MALDI TIMS Imaging Mass Spectrometry. Anal. Chem. 2020, 92, 13290–13297. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Smagghe, G.; Swevers, L.; Lu, Y. Metabolomics-Based Approaches in Unraveling Virus Infections in Insects: Revealing Unknown Hidden Interactions. Entomol. Gen. 2024, 44, 511–524. [Google Scholar] [CrossRef]
- Louie, K.B.; Faltane, M.; Lynde, M.; Bowen, B.P.; Northen, T. JGI/LBNL Metabolomics–Standard LC-MS/MS ESI Method–Lipids. Protocols.io 2024. [Google Scholar] [CrossRef]
- Musselman, L.P.; Cedden, D.; Güney, G.; Toprak, U. Insect Lipidomics: Advances, Applications, and Physiological Insights. In Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2025; pp. 1–31. [Google Scholar] [CrossRef]


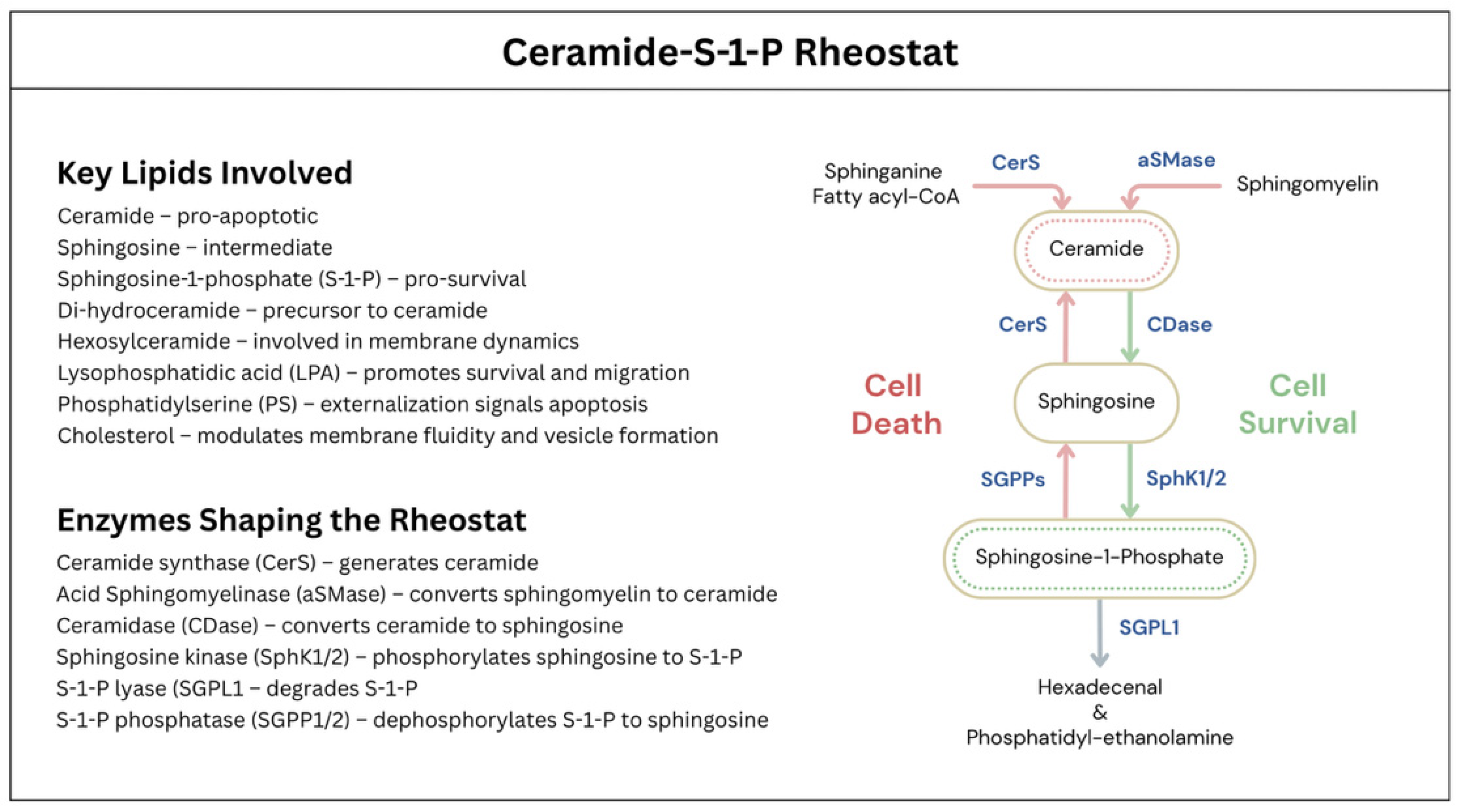
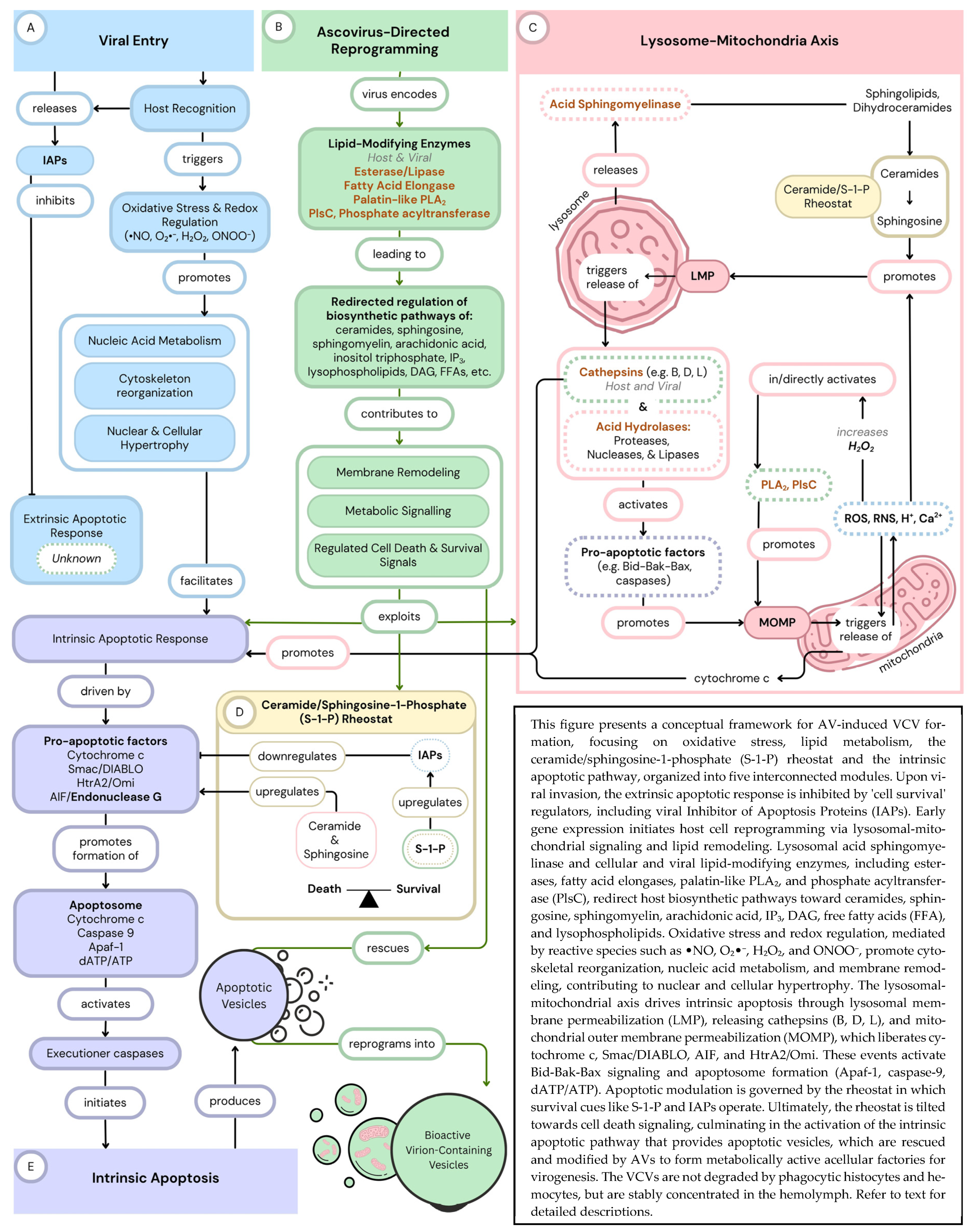
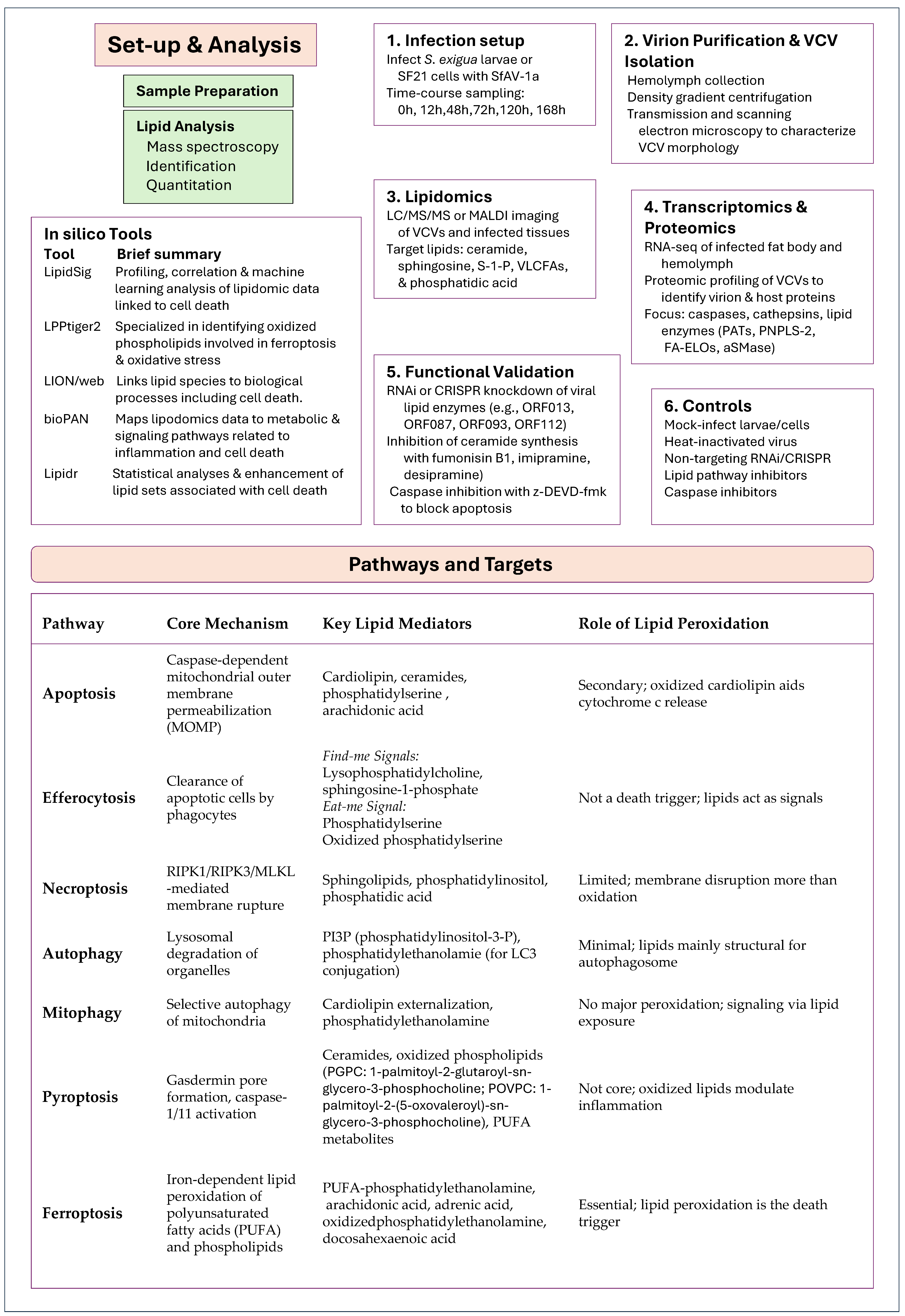
| Genus | Accession | Genome | G+C | Coding |
|---|---|---|---|---|
| Number | Length | (%) | Sequences * | |
| Ascovirus | ||||
| Spodoptera frugiperda ascovirus 1a (SfAV-1a) | NC_008361.1 | 156,922 | 49.26 | 123 |
| Trichoplusia ni ascovirus 2c (TnAV-2c) | NC_008518.1 | 174,059 | 35.24 | 165 |
| Trichoplusia ni ascovirus 6b (TnAV-6b) | KY434117.1 | 185,664 | 35.43 | 178 |
| Heliothis virescens ascovirus 3e (HvAV-3e) | NC_009233.1 | 186,262 | 45.88 | 178 |
| Heliothis virescens ascovirus 3f (HvAV-3f) | NC_044938.1 | 198,157 | 46.0 | 190 |
| Heliothis virescens ascovirus 3g (HvAV-3g) | JX491653.1 | 199,721 | 45.85 | 194 |
| Heliothis virescens ascovirus 3h (HvAV-3h) | KU170628.1 | 190,519 | 45.50 | 185 |
| Heliothis virescens ascovirus 3i (HvAV-3i) | MF781070.1 | 185,650 | 45.42 | 181 |
| Heliothis virescens ascovirus 3j (HvAV-3j) | LC332918.1 | 191,718 | 45.62 | 189 |
| Toursvirus | ||||
| Diadromus pulchellus toursvirus (DpTV-1a) | NC_011335.1 | 119,343 | 48.16 | 119 |
| Dasineura jujubifolia tourvirus (DpTV-2a) | MK867691.1 | 142,600 | 45.97 | 141 |
| Protein Name | Function Summary | References |
|---|---|---|
| Major capsid protein (MCP) | Forms the viral capsid; facilitates host cell attachment, virion entry, assembly, and immune evasion; highly abundant in SfAV-1a, HvAV-3a, and TnAV-6a. | [75,76,77] |
| P64 family of DNA-binding proteins | Homologous to neurofilament H1-like proteins; condenses AV gDNA for encapsidation; contains virus-specific motifs and basic tandem repeats; cationic protein with predicted isoelectric point (pI) = 12.1; abundant and structurally unique. | [77,78,79,80,81,82,83,84,85,86] |
| Serine/threonine kinase (S/T-K) | Phosphorylates proteins to regulate viral replication, immune evasion, and virion assembly; may phosphorylate P64 to disrupt viral DNA complexes. | [45,77,78,82,83,84,85,86,87] |
| CTD phosphatase transcription factor | Dephosphorylates RNA polymerase II; manipulates host transcription to enhance viral gene expression and replication. | [88,89] |
| Inhibitor of apoptosis (IAP)-like protein | Suppresses host cell death; inhibits caspases, modulates ubiquitination, and disrupts immune signaling to prolong cell survival. | [90,91] |
| SNF2 DEAD-like helicase | Unwinds DNA/RNA using ATP hydrolysis; essential for viral genome replication, repair, and recombination. | [92,93] |
| Myristylated membrane protein-like protein | Targets viral proteins to membranes via lipid modification; involved in viral entry, assembly, budding, and egress. | [94,95,96] |
| Sulfhydryl oxidase Erv1-like protein | Catalyzes disulfide bond formation; ensures proper folding and stability of viral proteins and virion integrity. | [97,98,99] |
| S1/P1 nuclease | Degrades single-stranded DNA/RNA; facilitates genome processing, nucleic acid maturation, and host defense evasion. | [100,101,102] |
| Dynein-like β chain | Motor protein subunit; drives microtubule sliding, mitotic spindle positioning, chromosome segregation, and cargo transport. | [103,104,105,106,107,108] |
| SfAV-1a Gene | Virus | Protein Description |
|---|---|---|
| DNA metabolism | ||
| ORF001 | H, T, D | DNA polymerase |
| ORF037 | T, D | GIY_YIG-like endonuclease |
| ORF066 | H, T | FLAP-like endonuclease |
| ORF059 | H, T, D | DNA repair exonuclease |
| ORF103 | H, T, D | ATPase involved in DNA repair |
| ORF110 | H, T, D | ATPase |
| ORF099 | H, T, D | Primase |
| ORF095 | H, T | Helicase 2 |
| ORF086 | H, D | Uvr/REP helicase |
| RNA metabolism | ||
| ORF008 | H, T, D | DNA-directed RNA polymerase largest subunit, N-terminal domain |
| ORF052 | H, T, D | DNA-directed RNA polymerase subunit B |
| ORF067 | H, T, D | DNA-directed RNA polymerase largest subunit, C-terminal domain |
| ORF022 | H, T, D | RNase III |
| ORF113 | H, T, D | VLTF2-like late transcription factor/Zn finger DNA binding protein |
| Nucleotide metabolism | ||
| ORF040 | H, T, D | Thymidine kinase |
| ORF090 | H, T, D | Putative lipopolysaccharide-modifying enzyme/tyrosine protein kinase |
| ORF104 | H, T, D | Serine/threonine kinase-like protein |
| Inhibitor of Apoptosis-Like Proteins | ||
| ORF016 | H, T, D | RING Finger, E3 ubiquitin ligase |
| ORF025 | H, T, D | RING Finger, E3 ubiquitin ligase |
| ORF074 | H, T | RING Finger, E3 ubiquitin ligase |
| Apoptosis | ||
| ORF073 | H, T | Caspase or Caspase-like/Interleukin-1-β–converting enzyme |
| ORF114 | H, T, D | Cathepsin B |
| Lipid Metabolism | ||
| ORF013 | H, D | Esterase/Lipase |
| ORF087 | H, T, D | Fatty acid elongase |
| ORF093 | H, T, D | Patatin-like phospholipase A2 (PLA2) |
| ORF112 | H, T, D | Phosphate acetytransferase |
| Enzyme | Selected Ascoviruses (ORF/Protein ID) | Cellular Function | Role in Cell Death and VCV Biogenesis |
|---|---|---|---|
| Esterase/Lipase | SfAV-1a: 013/YP_762368.1 TnAV-6b: 139/AUS94238.1 HvAV-3i: 018/AXN77201.1 | Hydrolyzes ester bonds in lipids, releasing fatty acids | May generate free fatty acids that undergo lipid peroxidation, a key trigger of ferroptosis. Also supports viral replication, cell cleavage, and VCV formation, potentially enhance infectivity through membrane remodeling. |
| Fatty Acid Elongase (FA-ELO) | SfAV-1a: 087/YP_762442.1 TnAV-6b: 053/AUS94152.1 HvAV-3i: 141/AXN77324.1 | Extends fatty acid chains to produce very-long-chain fatty acids (VLCFAs) | VLCFAs can disrupt membrane integrity via interdigitation, promoting necroptosis. May alter host membrane composition, aiding immune evasion and viral replication. VLCFAs may also contribute to VCV biogenesis and stability. |
| Patatin-like Phospholipase A2 (PNPLA) | SfAV-1a: 093/YP_762448.1 TnAV-6b: 073/AUS94172.1 HvAV-3i: 128/AXN77311.1 | Hydrolyzes phospholipids, releasing lysophospholipids and fatty acids | Facilitates organelle interactions and intercellular spread, possibly contributing to host cell remodeling, including membrane curvature and VCV biogenesis. May induce ER stress and disrupt mitochondrial integrity. Reactive oxygen and nitrogen species released from mitochondria can promote lysosomal membrane permealization leading to the release of acid hydrolases including cathepsin and acid sphingomyelinase (aSMase) which can activate the intrinsic apoptotic response. |
| PlcS, Phosphate Acyltransferase (PAT) | SfAV-1a: 112/YP_762467.1 TnAV-6b: 105/AUS94204.1 HvAV-3i: 107/AXN77290.1 | Transfers acyl groups to phospholipids, modifying membrane composition | Regulates incorporation of polyunsaturated fatty acids (PUFAs) into membranes which can either promote or protect against ferroptosis, depending on lipid context. May be involved in modulating membrane fluidity and viral envelope and VCV biogenesis. |
| Caspase/Caspase-like protein | SfAV-1a: 073/YP_762428.1 TnAV-6b: 79/AUS94178.1 HvAV-3i: 167/AXN77350.1 | Proteolytic enzymes that cleave substrates during apoptosis | Central regulators of apoptosis. May assist in host cell remodeling and viral protein processing, and precursor events leading to VCV biogenesis. |
| Cathepsin B | SfAV-1a: 114/YP_762469.1 TnAV-6b: 109/AUS94208.1 HvAV-3i: 103/AXN77286.1 | Lysosomal protease involved in protein degradation | May trigger controlled cell death under stress. Potentially activates viral caspase and contributes to cellular degradation. |
| Enzyme/Class | Catalytic Domain/Family | SfAV-1a ORF | TnAV-6a ORF | HvAV-3h ORF | Predicted Role in VCV Formation |
|---|---|---|---|---|---|
| Acid sphingomyelinase (aSMase) | Sphingomyelinase, (SMPD) | ORF085 | ORF38 | ORF29 | Ceramide microdomain formation, membrane curvature |
| Sphingosine kinase (SphK) | Sphingosine kinase, (SphK) | ORF091 | ORF50 | ORF47 | S-1-P production, regulation of apoptosis/survival |
| Fatty acid elongase (FA-ELO) | FA elongase (GNS1/SUR4) | ORF033 | ORF12 | ORF04 | VLCFA synthesis, ceramide and lipid precursors |
| Patatin-like phospholipase (PNPLA-2) | PNPLA (Patatin) | ORF017 | ORF11 | ORF16 | Phospholipid hydrolysis, membrane remodeling |
| Esterase/Lipase | Lipase/esterase | ORF019 | ORF15 | ORF19 | Lipid turnover, VCV cleavage, virion assembly |
| Ceramide synthase | Ceramide synthase | ORF079 | ORF41 | ORF05 | Ceramide production, membrane biogenesis |
| Chitinase/Cathepsin Suppressor | Cathepsin inhibitor, Chitinase suppressor | ORF32 | ORF26 | ORF31 | Limits host tissue liquefaction to support VCV persistence |
| Phosphate acyltransferase (PAT) | PlsC family (GPAT/AGPAT) | ORF029 | ORF20 | ORF21 | Glycerophospholipid biosynthesis |
| Hypothesis | Falsifiable Test | Experimental Approach |
|---|---|---|
| VCV biogenesis is dependent on host-derived aSMase activity and ceramide microdomain formation | Inhibiting aSMase function will reduce VCV formation and alter vesicle membrane composition | Treat infected insect cells/larvae with imipramine or desipramine (aSMase inhibitors); quantify VCVs and analyze membrane lipids via LC-MS/MS |
| Sphingosine-1-phosphate (S-1-P) balance modulates VCV structure/function via SphK activity | SphK inhibitor SKI-II disrupts normal ceramide/S-1-P ratio, resulting in abnormal VCV morphology or reduced virus yield | Apply SKI-II to infected cultures; quantify S-1-P and ceramides by lipidomics; assess VCV integrity and infectious output |
| Caspase inhibition at late stages impairs VCV cleavage and virion dissemination | Pharmacological or RNAi-mediated inhibition of executioner caspases at late infection reduces VCV partitioning and virion release | Apply caspase inhibitors or RNAi at different time points; monitor VCV biogenesis and release by microscopy and titration |
| Functional mitophagy supports proper VCV formation | Interfering with mitophagy pathways alters mitochondrial positioning or reduces VCV biogenesis | Use mitophagy probes (mito-Keima, mCherry-GFP-Atg8) or Parkin/FUNDC1 immunostaining; assess changes in mitochondrial localization and VCV cleavage |
| VCVs evade efferocytosis by lacking surface “eat-me” signals or overwhelming clearance machinery | VCVs exposed to annexin V show altered hemocyte uptake kinetics; blocking PS exposure impairs VCV clearance | Flow cytometry for PS exposure (Annexin V) on VCVs; track VCV uptake by hemocytes in vitro with/without masking treatments |
| Lipidomic shifts in infected tissues predict changes in VCV composition and infectivity | Time-course analysis reveals ceramide and S-1-P enrichment in VCVs compared to uninfected controls | Extract and analyze lipids from infected tissues and isolated VCVs (LC-MS/MS) at multiple infection time points |
| Cathepsin/chitinase suppression by viral ORFs modulates host tissue dissolution and VCV yield | Overexpression or knockout of HvAV-3h ORF31 changes substrate breakdown and the abundance of VCVs | Use gene perturbation assays; quantify protease activity and VCVs |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rudd, S.R.; Miranda, L.S.; Asariah, S.J.; Rodgers, C.S.; Estrada, J.T.; Alonzo, M.A.; Bideshi, D.K. Pathogens That Rewrite the Rules: Ascoviruses, Elegant Manipulators of Cell Death Pathways and Architects of the Extracellular Viral Paradigm. Pathogens 2025, 14, 1094. https://doi.org/10.3390/pathogens14111094
Rudd SR, Miranda LS, Asariah SJ, Rodgers CS, Estrada JT, Alonzo MA, Bideshi DK. Pathogens That Rewrite the Rules: Ascoviruses, Elegant Manipulators of Cell Death Pathways and Architects of the Extracellular Viral Paradigm. Pathogens. 2025; 14(11):1094. https://doi.org/10.3390/pathogens14111094
Chicago/Turabian StyleRudd, Sarah R., Leticia S. Miranda, Sharon J. Asariah, Chloe S. Rodgers, Jenive T. Estrada, Michael A. Alonzo, and Dennis K. Bideshi. 2025. "Pathogens That Rewrite the Rules: Ascoviruses, Elegant Manipulators of Cell Death Pathways and Architects of the Extracellular Viral Paradigm" Pathogens 14, no. 11: 1094. https://doi.org/10.3390/pathogens14111094
APA StyleRudd, S. R., Miranda, L. S., Asariah, S. J., Rodgers, C. S., Estrada, J. T., Alonzo, M. A., & Bideshi, D. K. (2025). Pathogens That Rewrite the Rules: Ascoviruses, Elegant Manipulators of Cell Death Pathways and Architects of the Extracellular Viral Paradigm. Pathogens, 14(11), 1094. https://doi.org/10.3390/pathogens14111094







