Loss of Type 1 Pili and Flagella in Uropathogenic Escherichia coli Leads to Reduced Phagocytosis by Human and Murine Monocytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains, Plasmids, and Growth Media
2.2. Construction of the ΔfimA Single Mutant and ΔfimA ΔfliC Double Mutant
2.3. Hemagglutination Assay (HA)
2.4. Motility Assay
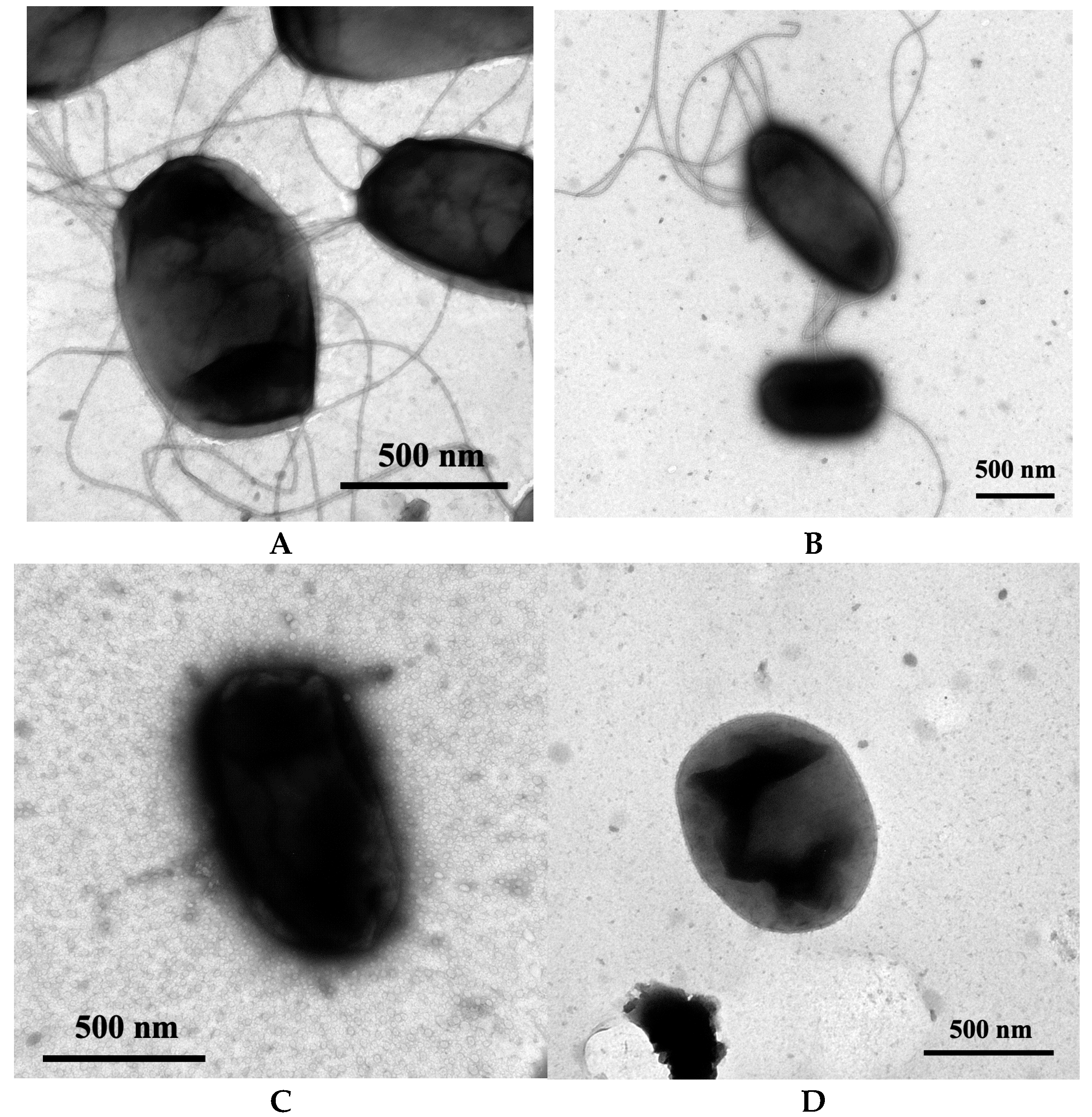
2.5. Transmission Electron Microscopy
2.6. Phagocytosis Assay
2.7. Statistics
3. Results
3.1. Confirming the Loss of Type 1 Pili and Flagella in the Strain NU149 Mutants
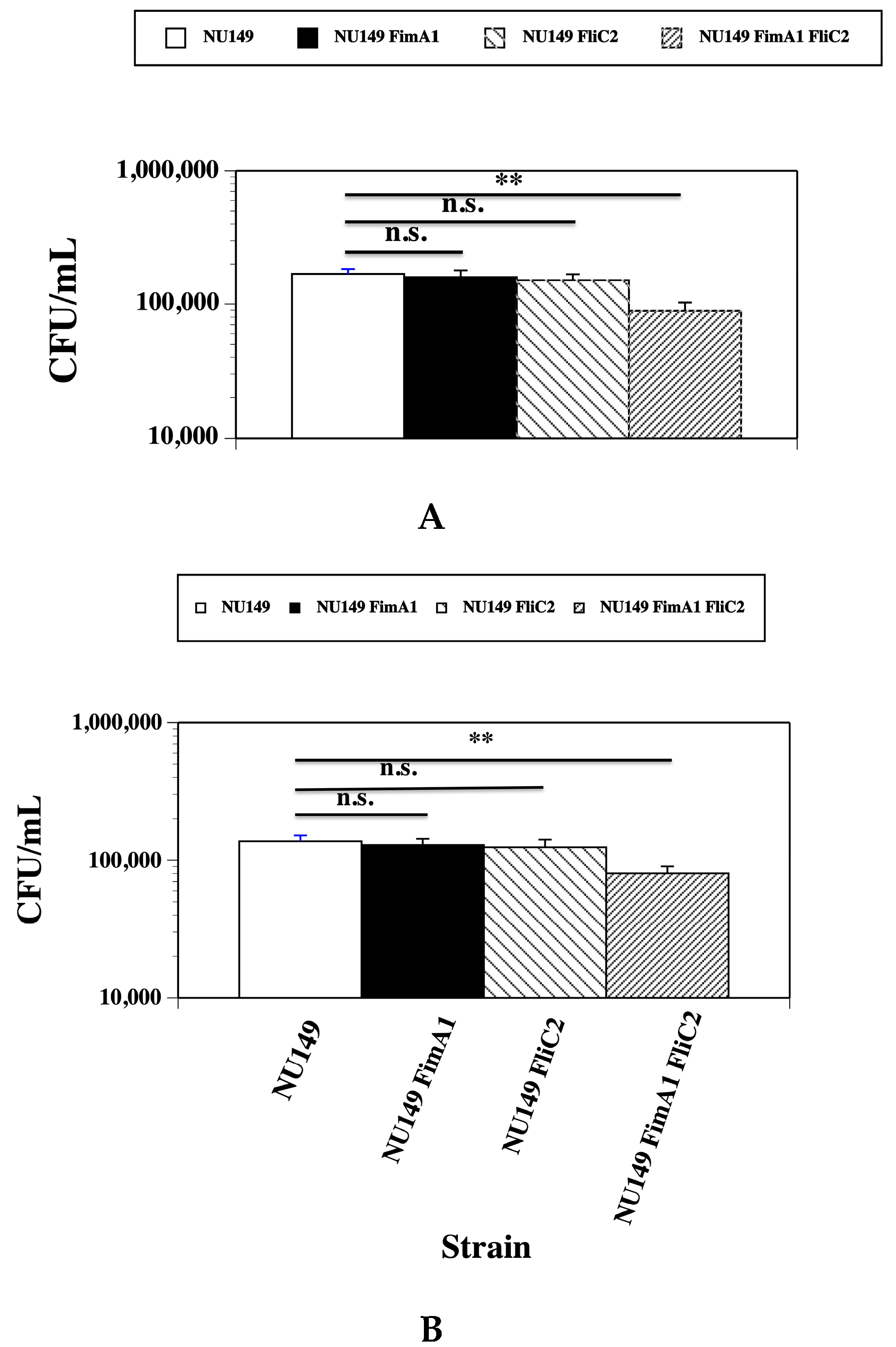
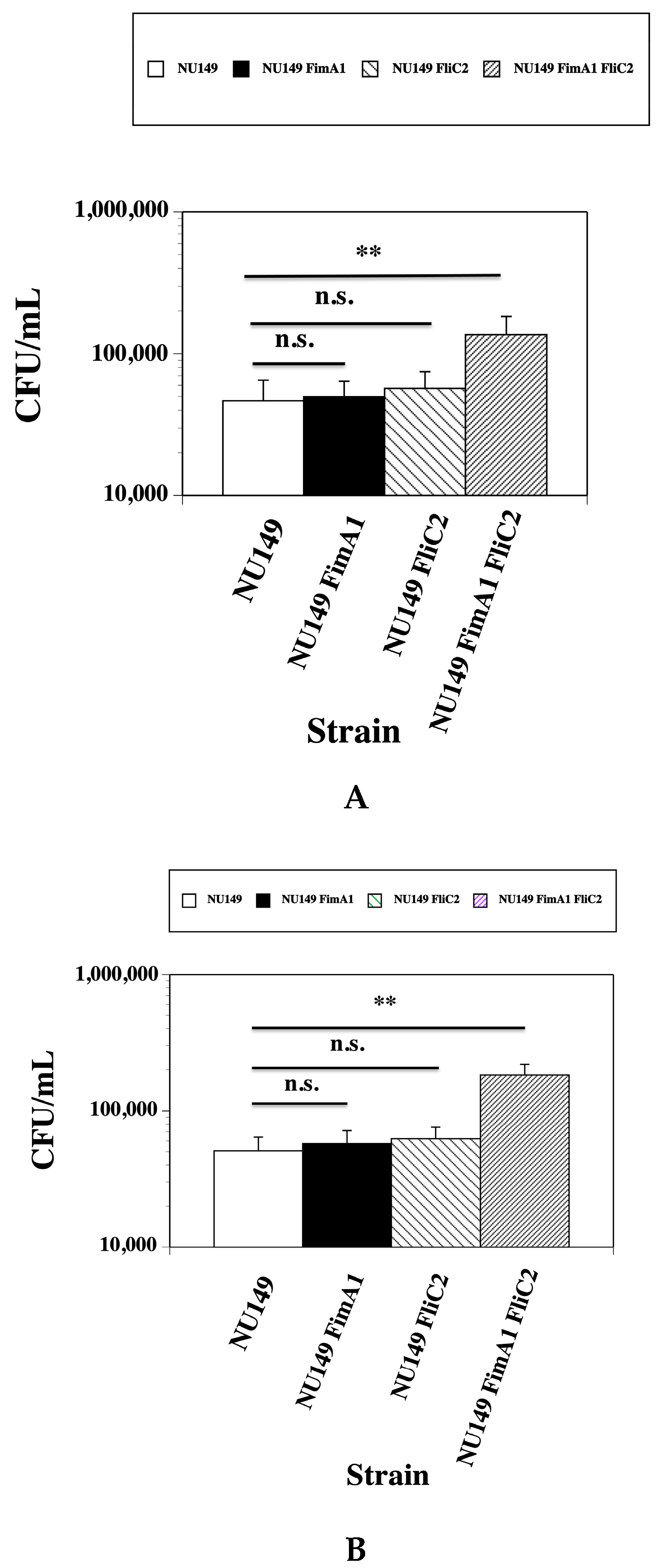
3.2. Fewer fimA fliC Double Mutant Cells Phagocytized by Human and Murine Monocytes
3.3. Loss of Type 1 Pili and Flagella Leads to Greater UPEC Survival in the Presence of Human or Murine Monocytes
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, X.; Chen, H.; Zheng, Y.; Qu, S.; Wang, H.; Yi, F. Disease burden and long-term trends of urinary tract infections: A worldwide report. Front. Public Health 2022, 10, 888205. [Google Scholar] [CrossRef]
- Dielubanza, E.J.; Schaeffer, A.J. Urinary tract infections in women. Med. Clin. N. Am. 2011, 95, 27–41. [Google Scholar] [CrossRef]
- Timm, M.R.; Russell, S.K.; Hultgren, S.J. Urinary tract infections: Pathogenesis, host susceptibility and emerging therapeutics. Nat. Rev. Microbiol. 2025, 23, 72–86. [Google Scholar] [CrossRef]
- Foxman, B. Urinary tract infection syndromes: Occurrence, recurrence, bacteriology, risk factors, and disease burden. Infect. Dis. Clin. N. Am. 2014, 28, 1–13. [Google Scholar] [CrossRef]
- Kallenius, G.; Mollby, R.; Winberg, J. In vitro adhesion of uropathogenic Escherichia coli to human periurethral cells. Infect. Immun. 1980, 28, 972–980. [Google Scholar] [CrossRef]
- Van der Bosch, J.F.; Verboom-Sohmer, U.; Postma, P.; de Graff, J.; MacLaren, D.M. Mannose-sensitive and mannose-resistant adherence to human uroepithelial cells and urinary virulence of Escherichia coli. Infect. Immun. 1980, 29, 226–233. [Google Scholar] [CrossRef]
- Klemm, P. The fimA gene encoding the type-1 fimbrial subunit of Escherichia coli. Nucleotide sequence and primary structure of the protein. Eur. J. Biochem. 1984, 143, 395–399. [Google Scholar] [CrossRef]
- Orndorff, P.E.; Falkow, S. Nucleotide sequence of pilA, the gene encoding the structural component of type 1 pili in Escherichia coli. J. Bacteriol. 1985, 162, 454–457. [Google Scholar] [CrossRef]
- Abraham, S.N.; Goguen, J.D.; Beachey, E.H. Hyperadhesive mutant of type 1-fimbriated Escherichia coli associated with formation of FimH organelles (fimbriosomes). Infect. Immun. 1988, 56, 1023–1029. [Google Scholar] [CrossRef] [PubMed]
- Hanson, M.S.; Brinton, C.C. Identification and characterization of E. coli type-1 pilus tip adhesion protein. Nature 1988, 332, 265–268. [Google Scholar] [CrossRef]
- Krogfelt, K.A.; Bergmans, H.; Klemm, P. Direct evidence that the FimH protein is the mannose-specific adhesin of Escherichia coli type 1 fimbriae. Infect. Immun. 1990, 58, 1995–1998. [Google Scholar] [CrossRef]
- Iwahi, T.; Abe, Y.; Tsuchiya, K. Virulence of Escherichia coli in ascending urinary-tract infection in mice. J. Med. Microbiol. 1982, 15, 303–316. [Google Scholar] [CrossRef] [PubMed]
- Keith, B.R.; Maurer, L.; Spears, P.A.; Orndorff, P.E. Receptor-binding function of type 1 pili effects bladder colonization by a clinical isolate of Escherichia coli. Infect. Immun. 1986, 53, 693–696. [Google Scholar] [CrossRef] [PubMed]
- Virkola, R.; Westerlund, B.; Holthofer, H.; Parkkinen, J.; Kekomaki, M.; Korhonen, T.K. Binding characteristics of Escherichia coli adhesins in human urinary bladder. Infect. Immun. 1988, 56, 2615–2622. [Google Scholar] [CrossRef]
- Anderson, G.G.; Palermo, J.J.; Schilling, J.D.; Roth, R.; Heuser, J.; Hultgren, S.J. Intracellular bacterial biofilm-like pods in urinary tract infections. Science 2003, 301, 105–107. [Google Scholar] [CrossRef]
- Mulvey, M.A.; Schilling, J.D.; Hultgren, S.J. Establishment of a persistent Escherichia coli reservoir during the acute phase of a bladder infection. Infect. Immun. 2001, 69, 4572–4579. [Google Scholar] [CrossRef]
- O’Hanley, P.; Low, D.; Romro, I.; Lark, D.; Vosti, K.; Falkow, S.; Schoolnik, G. Gal-Gal binding and hemolysin phenotypes and genotypes associated with uropathogenic Escherichia coli. N. Engl. J. Med. 1985, 313, 414–420. [Google Scholar] [CrossRef]
- Schreiber, H.L.; Conover, M.S.; Chou, W.C.; Hibbing, M.E.; Manson, A.L.; Dodson, K.W.; Hannan, T.J.; Roberts, P.L.; Stapleton, A.E.; Hooton, T.M.; et al. Bacterial virulence phenotypes of Escherichia coli and host susceptibility determine risk for urinary tract infections. Sci. Transl. Med. 2017, 9, eaaf1283. [Google Scholar] [CrossRef]
- Namba, K.; Vonderviszt, F. Molecular architecture of bacterial flagellum. Q. Rev. Biophys. 1997, 30, 1–65. [Google Scholar] [CrossRef]
- Berg, H.C. The rotary motor of bacterial flagella. Annu. Rev. Biochem. 2003, 72, 19–54. [Google Scholar] [CrossRef]
- De Souza, G.M.; Neto, E.R.D.S.; Da Silva, A.M.; Iacia, M.V.M.S.; Rodrigues, M.V.P.; Cataneli Pereira, V.; Winkelstroter, L.K. Comparative study of diversity, virulence genotypes, biofilm formation and antimicrobial resistance of uropathogenic Escherichia coli (UPEC) isolated from nosocomial and community acquired urinary tract infections. Infect. Drug Resist. 2019, 12, 3595–3606. [Google Scholar] [CrossRef]
- Lane, M.C.; Alteri, C.J.; Smith, S.N.; Mobley, H.L. Expression of flagella is coincident with uropathogenic Escherichia coli ascension to the upper urinary tract. Proc. Natl. Acad. Sci. USA 2007, 104, 16669–16674. [Google Scholar] [CrossRef]
- Lane, M.C.; Lockatell, V.; Monterosso, G.; Lamphier, D.; Weinert, J.; Hebel, J.R.; Johnson, D.E.; Mobley, H.L. Role of motility in the colonization of uropathogenic Escherichia coli in the urinary tract. Infect. Immun. 2005, 73, 7644–7656. [Google Scholar] [CrossRef]
- Schwan, W.R. Flagella allow uropathogenic Escherichia coli ascension into murine kidneys. Int. J. Med. Microbiol. 2008, 298, 441–447. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wright, K.J.; Seed, P.C.; Hultgren, S.J. Uropathogenic Escherichia coli flagella aid in efficient urinary tract colonization. Infect. Immun. 2005, 73, 7657–7668. [Google Scholar] [CrossRef] [PubMed]
- Greene, S.E.; Hibbing, M.E.; Janetka, J.; Chen, S.L.; Hultgren, S.J. Human urine decreases function and expression of type 1 pili in uropathogenic Escherichia coli. mBio 2015, 6, e00820-15. [Google Scholar] [CrossRef]
- Hultgren, S.J.; Porter, T.N.; Schaeffer, A.J.; Duncan, J.L. Role of type 1 pili and effects of phase variation on lower urinary tract infections produced by Escherichia coli. Infect. Immun. 1985, 50, 370–377. [Google Scholar] [CrossRef]
- Schaeffer, A.J.; Schwan, W.R.; Hultgren, S.J.; Duncan, D.L. Relationship of type 1 pilus expression in Escherichia coli to ascending urinary tract infections in mice. Infect. Immun. 1987, 55, 373–380. [Google Scholar] [CrossRef]
- Snyder, J.A.; Haugen, B.J.; Buckles, E.L.; Lockatell, C.V.; Johnson, D.E.; Donnenberg, M.S.; Welch, R.A.; Mobley, H.L. Transcriptome of uropathogenic Escherichia coli during urinary tract infection. Infect. Immun. 2004, 72, 6373–6381. [Google Scholar] [CrossRef]
- Subashchandrabose, S.; Hazen, T.H.; Brumbaugh, A.R.; Himpsl, S.D.; Smith, S.N.; Ernst, R.E.; Rasko, D.A.; Mobley, H.L.T. Host-specific induction of Escherichia coli fitness genes during human urinary tract infection. Proc. Natl. Acad. Sci. USA 2014, 111, 18327–18332. [Google Scholar] [CrossRef] [PubMed]
- Hultgren, S.J.; Schwan, W.R.; Schaeffer, A.J.; Duncan, D.L. Regulation of production of type 1 pili among urinary tract isolates of Escherichia coli. Infect. Immun. 1986, 54, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Datsenko, K.A.; Wanner, B.L. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 2000, 97, 6640–6645. [Google Scholar] [CrossRef] [PubMed]
- Schwan, W.R.; Lee, J.L.; Lenard, F.A.; Matthews, B.T.; Beck, M.T. Osmolarity and pH growth conditions regulate fim gene transcription and type 1 pilus expression in uropathogenic. Escherichia coli. Infect. Immun. 2002, 70, 1391–1402. [Google Scholar] [CrossRef]
- Schwan, W.R.; Seifert, H.S.; Duncan, J.L. Growth conditions mediate differential transcription of fim genes involved in phase variation of type 1 pili. J. Bacteriol. 1992, 174, 2367–2375. [Google Scholar] [CrossRef]
- Craven, R.C.; Montie, T.C. Motility and chemotaxis of three strains of Pseudomonas aeruginosa used for virulence studies. Can. J. Microbiol. 1981, 27, 458–460. [Google Scholar] [CrossRef] [PubMed]
- Hultgren, S.; Hidvegi, D.F. Improved transmission electron microscopy technique for the study of cytologic material. Acta Cytol. 1985, 29, 179–183. [Google Scholar]
- Kuby, J. Immunology, 3rd ed.; W.H. Freemen and Company: New York, NY, USA, 1997. [Google Scholar]
- Acharya, D.; Ulett, G.C. Rapid bladder interleukin-10 synthesis in response to uropathogenic Escherichia coli is part of a defense strategy triggered by the major bacterial flagellar filament FliC and contingent on TLR5. mSphere 2019, 4, e00545-19. [Google Scholar] [CrossRef]
- Vega-Hernandez, R.; Ochoa, S.A.; Valle-Rios, R.; Xicohtencatl-Cortes, J. Flagella, Type I fimbriae and curli of uropathogenic Escherichia coli promote the release of proinflammatory cytokines in a coculture system. Microorganisms 2021, 9, 2233. [Google Scholar] [CrossRef]
- Tan, A.; Alsenani, Q.; Lanz, M.; Birchll, C.; Drage, L.K.L.; Picton, D.; Mowbray, C.; Ali, A.; Harding, C.; Picard, R.S.; et al. Evasion of toll-like receptor recognition by Escherichia coli is mediated via population level regulation of flagellin production. Front. Microbiol. 2023, 23, 1093922. [Google Scholar] [CrossRef]
- Carbone, M.; Hasty, D.L.; Yi, K.C.; Rue, J.; Fera, M.T.; Torre, F.; Giannone, M.; Losi, E. Cytokine induction in murine bladder tissue by type 1 fimbriated Escherichia coli. Ann. N. Y. Acad. Sci. 2006, 963, 332–335. [Google Scholar] [CrossRef]
- Schilling, J.D.; Martin, S.M.; Hunstad, D.A.; Patel, K.P.; Mulvey, M.A.; Justice, S.S.; Lorenz, R.G.; Hultgren, S.J. CD14- and Toll-like receptor-dependent activation of bladder epithelial cells by lipopolysaccharide and type 1 piliated Escherichia coli. Infect. Immun. 2003, 71, 1470–1480. [Google Scholar] [CrossRef]
- Ebersole, J.L.; Molinari, J.A. Gastrointestinal antibody responses in axenic mice to topically administered Escherichia coli. Infect. Immun. 1977, 16, 938–946. [Google Scholar] [CrossRef]
- Su, S.; Li, Z.; Sun, Y.; Gao, S.; Gao, Q. The multifaceted role of TolA protein in promoting survival, biofilm formation and virulence of avian pathogenic Escherichia coli. Poult. Sci. 2024, 103, 104142. [Google Scholar] [CrossRef]
- Wu, X.B.; Tian, L.H.; Zou, H.J.; Wang, C.Y.; Yu, Z.Q.; Tang, C.H.; Zhao, F.K.; Pan, J.Y. Outer membrane protein OmpW of Escherichia coli is required for resistance to phagocytosis. Res. Microbiol. 2013, 164, 848–855. [Google Scholar] [CrossRef]
- Sharon, N.; Eshdat, Y.; Silverblatt, F.J.; Ofek, I. Bacterial adherence to cell surface sugars. Ciba Found. Symp. 1981, 80, 119–141. [Google Scholar] [PubMed]
- Burns, S.M.; Hull, S.I. Loss of resistance to ingestion and phagocytic killing by O(-) and K(-) mutants of a uropathogenic Escherichia coli O75:K5 strain. Infect. Immun. 1999, 67, 3757–3762. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Ulett, G.C.; Ttotsika, M.; Phan, M.D.; Schembri, M.A. Role of capsule and O antigen in the virulence of uropathogenic Escherichia coli. PLoS ONE 2014, 9, e94786. [Google Scholar] [CrossRef] [PubMed]
- Schwan, W.R.; Ding, H. Temporal regulation of fim genes in uropathogenic Escherichia coli during infection of the murine urinary tract. J. Pathog. 2017, 2017, 8694356. [Google Scholar] [CrossRef]
- Loeb, W.F.; Quimby, F.W. The Clinical Chemistry of Laboratory Animals; Pergamon Press: New York, NY, USA, 1989. [Google Scholar]
- Ross, D.L.; Neely, A.E. Textbook of Urinalysis and Bodily Fluids; Appleton-Century-Crofts: Norwalk, CT, USA, 1983. [Google Scholar]
- Schwan, W.R.; Flohr, N.L.; Multerer, A.R.; Starkey, J.C. GadE regulates fliC gene transcription and motility in Escherichia coli. World J. Clin. Infect. Dis. 2020, 10, 14–23. [Google Scholar] [CrossRef]
- Bokil, N.J.; Sweet, M.J. Intramacrophage survival of uropathogenic Escherichia coli differences between diverse clinical isolates and between mouse and human macrophages. Immunobiology 2011, 216, 1164–1171. [Google Scholar] [CrossRef]
- Peterson, E.; Soderstrom, B.; Prins, N.; Le, G.H.B.; Hartley-Tassell, L.; Evenhuis, C.; Gronnemose, R.B.; Andersen, T.E.; Moller-Jesnsen, J.; Iosifidis, G.; et al. The role of bacterial size, shape and surface in macrophage engulfment of uropathogenic E. coli cells. PLoS Pathog. 2024, 20, e1012458. [Google Scholar] [CrossRef] [PubMed]
| Strain or Plasmid | Description | Source |
|---|---|---|
| Strain | ||
| DH5α | General cloning strain | Gibco/BBL |
| NU149 | E. coli cystitis isolate | [28] |
| NU149 FimA1 | NU149 ΔfimA | This study |
| NU149 FliC2 | NU149 ΔfliC | [24] |
| NU149 FimA1 FliC2 | NU149 ΔfimA ΔfliC | This study |
| Plasmid | ||
| pKD4 | Flp recombinase sites | [32] |
| pKD46 | λ Red recombinase | [32] |
| pCP20 | Flp recombinase | [32] |
| Strain | Mutation | HA Titer a | Motility b |
|---|---|---|---|
| NU149 | Wild type | 512 c | 42 |
| NU149 FimA1 | ΔfimA | 0 | 39 |
| NU149 FliC2 | ΔfliC | 512 | 5 |
| NU149 FimA1 FliC2 | ΔfimA ΔfliC | 0 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schwan, W.R. Loss of Type 1 Pili and Flagella in Uropathogenic Escherichia coli Leads to Reduced Phagocytosis by Human and Murine Monocytes. Pathogens 2025, 14, 968. https://doi.org/10.3390/pathogens14100968
Schwan WR. Loss of Type 1 Pili and Flagella in Uropathogenic Escherichia coli Leads to Reduced Phagocytosis by Human and Murine Monocytes. Pathogens. 2025; 14(10):968. https://doi.org/10.3390/pathogens14100968
Chicago/Turabian StyleSchwan, William R. 2025. "Loss of Type 1 Pili and Flagella in Uropathogenic Escherichia coli Leads to Reduced Phagocytosis by Human and Murine Monocytes" Pathogens 14, no. 10: 968. https://doi.org/10.3390/pathogens14100968
APA StyleSchwan, W. R. (2025). Loss of Type 1 Pili and Flagella in Uropathogenic Escherichia coli Leads to Reduced Phagocytosis by Human and Murine Monocytes. Pathogens, 14(10), 968. https://doi.org/10.3390/pathogens14100968






