A Ten-Year Retrospective Review of Medical Records of Patients Admitted with Meningitis or Encephalitis at Five Hospitals in the United States Highlights the Potential for Under-Ascertainment of Invasive Meningococcal Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Setting and Patients
2.2. Laboratory Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADEM | acute disseminated encephalitis and encephalomyelitis |
| CSF | cerebrospinal fluid |
| DRG | Diagnosis-related group |
| EMRs | electronic medical records |
| ICD-10 | International Classification of Diseases, Tenth Revision |
| IMD | invasive meningococcal disease |
| IV | intravenous |
| PCR | polymerase chain reaction |
| WHO | World Health Organization |
References
- Dwilow, R.; Fanella, S. Invasive meningococcal disease in the 21st century—An update for the clinician. Curr. Neurol. Neurosci. Rep. 2015, 15, 2. [Google Scholar] [CrossRef]
- Thompson, M.J.; Ninis, N.; Perera, R.; Mayon-White, R.; Phillips, C.; Bailey, L.; Harnden, A.; Mant, D.; Levin, M. Clinical recognition of meningococcal disease in children and adolescents. Lancet 2006, 367, 397–403. [Google Scholar] [CrossRef]
- Mbaeyi, S.; Duffy, J.; McNamara, L.A. Meningococcal disease. In Epidemiology and Prevention of Vaccine-Preventable Diseases, 14th ed.; Hall, E., Wodi, A.P., Hamborsky, J., Schillie, S., Eds.; Public Health Foundation: Washington, DC, USA, 2021. [Google Scholar]
- Deghmane, A.-E.; Taha, S.; Taha, M.-K. Global epidemiology and changing clinical presentations of invasive meningococcal disease: A narrative review. Infect. Dis. 2022, 54, 1–7. [Google Scholar] [CrossRef]
- Wang, B.; Santoreneos, R.; Giles, L.; Haji Ali Afzali, H.; Marshall, H. Case fatality rates of invasive meningococcal disease by serogroup and age: A systematic review and meta-analysis. Vaccine 2019, 37, 2768–2782. [Google Scholar] [CrossRef]
- Shen, J.; Begum, N.; Ruiz-Garcia, Y.; Martinon-Torres, F.; Bekkat-Berkani, R.; Meszaros, K. Range of invasive meningococcal disease sequelae and health economic application—A systematic and clinical review. BMC Public Health 2022, 22, 1078. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Manual for the Surveillance of Vaccine-Preventable Diseases: Chapter 8: Meningococcal Disease. Available online: https://www.cdc.gov/surv-manual/php/table-of-contents/chapter-8-meningococcal-disease.html?CDC_AAref_Val=https://www.cdc.gov/vaccines/pubs/surv-manual/chpt08-mening.html (accessed on 19 September 2023).
- Olcen, P.; Fredlund, H. Isolation, culture, and identification of meningococci from clinical specimens. Methods Mol. Med. 2001, 67, 9–21. [Google Scholar]
- McGill, F.; Heyderman, R.S.; Michael, B.D.; Defres, S.; Beeching, N.J.; Borrow, R.; Glennie, L.; Gaillemin, O.; Wyncoll, D.; Kaczmarski, E.; et al. The UK joint specialist societies guideline on the diagnosis and management of acute meningitis and meningococcal sepsis in immunocompetent adults. J. Infect. 2016, 72, 405–438. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Guidelines on Meningitis Diagnosis, Treatment and Care. Available online: https://iris.who.int/bitstream/handle/10665/381006/9789240108042-eng.pdf?sequence=1 (accessed on 9 May 2025).
- Tunkel, A.R.; Hartman, B.J.; Kaplan, S.L.; Kaufman, B.A.; Roos, K.L.; Scheld, W.M.; Whitley, R.J. Practice guidelines for the management of bacterial meningitis. Clin. Infect. Dis. An. Off. Publ. Infect. Dis. Soc. Am. 2004, 39, 1267–1284. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence. Meningitis (Bacterial) and Meningococcal Disease: Recognition, Diagnosis and Management. Available online: https://www.nice.org.uk/guidance/ng240/chapter/Recommendations#recognising-bacterial-meningitis-and-meningococcal-disease (accessed on 12 May 2025).
- Bryant, P.A.; Li, H.Y.; Zaia, A.; Griffith, J.; Hogg, G.; Curtis, N.; Carapetis, J.R. Prospective study of a real-time PCR that is highly sensitive, specific, and clinically useful for diagnosis of meningococcal disease in children. J. Clin. Microbiol. 2004, 42, 2919–2925. [Google Scholar] [CrossRef]
- Wu, H.M.; Cordeiro, S.M.; Harcourt, B.H.; Carvalho, M.; Azevedo, J.; Oliveira, T.Q.; Leite, M.C.; Salgado, K.; Reis, M.G.; Plikaytis, B.D.; et al. Accuracy of real-time PCR, Gram stain and culture for Streptococcus pneumoniae, Neisseria meningitidis and Haemophilus influenzae meningitis diagnosis. BMC Infect. Dis. 2013, 13, 26. [Google Scholar] [CrossRef]
- Crosswell, J.M.; Nicholson, W.R.; Lennon, D.R. Rapid sterilisation of cerebrospinal fluid in meningococcal meningitis: Implications for treatment duration. J. Paediatr. Child. Health 2006, 42, 170–173. [Google Scholar] [CrossRef]
- Sacchi, C.T.; Fukasawa, L.O.; Goncalves, M.G.; Salgado, M.M.; Shutt, K.A.; Carvalhanas, T.R.; Ribeiro, A.F.; Kemp, B.; Gorla, M.C.; Albernaz, R.K.; et al. Incorporation of real-time PCR into routine public health surveillance of culture negative bacterial meningitis in Sao Paulo, Brazil. PLoS ONE 2011, 6, e20675. [Google Scholar] [CrossRef]
- Drew, R.J.; Maoldomhnaigh, C.Ó.; Gavin, P.J.; O’ Sullivan, N.; Butler, K.M.; Cafferkey, M. The impact of meningococcal polymerase chain reaction testing on laboratory confirmation of invasive meningococcal disease. Pediatr. Infect. Dis. J. 2012, 31, 316–318. [Google Scholar] [CrossRef]
- Munoz-Almagro, C.; Rodriguez-Plata, M.T.; Marin, S.; Esteva, C.; Esteban, E.; Gene, A.; Gelabert, G.; Jordan, I. Polymerase chain reaction for diagnosis and serogrouping of meningococcal disease in children. Diagn. Microbiol. Infect. Dis. 2009, 63, 148–154. [Google Scholar] [CrossRef]
- Vazquez, J.A.; Taha, M.K.; Findlow, J.; Gupta, S.; Borrow, R. Global Meningococcal Initiative: Guidelines for diagnosis and confirmation of invasive meningococcal disease. Epidemiol. Infect. 2016, 144, 3052–3057. [Google Scholar] [CrossRef]
- Skoczynska, A.; Wasko, I.; Kuch, A.; Kadlubowski, M.; Golebiewska, A.; Forys, M.; Markowska, M.; Ronkiewicz, P.; Wasiak, K.; Kozinska, A.; et al. A decade of invasive meningococcal disease surveillance in Poland. PLoS ONE 2013, 8, e71943. [Google Scholar] [CrossRef]
- Fernandez-San Jose, C.; Moraga-Llop, F.A.; Codina, G.; Soler-Palacin, P.; Espiau, M.; Figueras, C. The use of polymerase chain reaction in the diagnosis of invasive meningococcal disease. An. Pediatría 2015, 82, 139–143. [Google Scholar] [CrossRef]
- US Centers for Disease Control and Prevention. Best Practice Guidelines for Diagnosis of Haemophilus influenzae and Neisseria meningitidis Disease. Available online: https://www.cdc.gov/meningococcal/php/guidance/index.html#cdc_generic_section_4-pcr-availability-and-capabilities (accessed on 26 February 2025).
- Guiducci, S.; Moriondo, M.; Nieddu, F.; Ricci, S.; De Vitis, E.; Casini, A.; Poggi, G.M.; Indolfi, G.; Resti, M.; Azzari, C. Culture and real-time polymerase chain reaction sensitivity in the diagnosis of invasive meningococcal disease: Does culture miss less severe cases? PLoS ONE 2019, 14, e0212922. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.A.; Campbell, H.; Ribeiro, S.; Bertran, M.; Walsh, L.; Walker, A.; Willerton, L.; Lekshmi, A.; Bai, X.; Lucidarme, J.; et al. Epidemiological and strain characteristics of invasive meningococcal disease prior to, during and after COVID-19 pandemic restrictions in England. J. Infect. 2023, 87, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Asturias, E.J.; Bai, X.; Bettinger, J.A.; Borrow, R.; Castillo, D.N.; Caugant, D.A.; Chacon, G.C.; Dinleyici, E.C.; Echaniz-Aviles, G.; Garcia, L.; et al. Meningococcal disease in North America: Updates from the Global Meningococcal Initiative. J. Infect. 2022, 85, 611–622. [Google Scholar] [CrossRef]
- Parikh, S.R.; Campbell, H.; Bettinger, J.A.; Harrison, L.H.; Marshall, H.S.; Martinon-Torres, F.; Safadi, M.A.; Shao, Z.; Zhu, B.; von Gottberg, A.; et al. The everchanging epidemiology of meningococcal disease worldwide and the potential for prevention through vaccination. J. Infect. 2020, 81, 483–498. [Google Scholar] [CrossRef] [PubMed]
- Ladhani, S.N.; Waight, P.A.; Ribeiro, S.; Ramsay, M.E. Invasive meningococcal disease in England: Assessing disease burden through linkage of multiple national data sources. BMC Infect. Dis. 2015, 15, 551. [Google Scholar] [CrossRef] [PubMed]
- Pezzotti, P.; Bellino, S.; Riccardo, F.; Lucaroni, F.; Cerquetti, M.; Pantosti, A.; Rezza, G.; Stefanelli, P. Vaccine preventable invasive bacterial diseases in Italy: A comparison between the national surveillance system and recorded hospitalizations, 2007–2016. Vaccine 2019, 37, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Negi, S.; Grover, S.; Rautela, S.; Rawat, D.; Gupta, S.; Khare, S.; Lal, S.; Rai, A. Direct detection and serogroup characterization of Neisseria meningitidis from outbreak of meningococcal meningitis in Delhi. Iran. J. Microbiol. 2010, 2, 73–79. [Google Scholar]
- Papavasileiou, K.; Papavasileiou, E.; Tzanakaki, G.; Voyatzi, A.; Kremastinou, J.; Chatzipanagiotou, S. Acute bacterial meningitis cases diagnosed by culture and PCR in a children’s hospital throughout a 9-Year period (2000–2008) in Athens, Greece. Mol. Diagn. Ther. 2011, 15, 109–113. [Google Scholar] [CrossRef]
- Dunne, E.M.; Mantanitobua, S.; Singh, S.P.; Reyburn, R.; Tuivaga, E.; Rafai, E.; Tikoduadua, L.; Porter, B.; Satzke, C.; Strachan, J.E.; et al. Real-time qPCR improves meningitis pathogen detection in invasive bacterial-vaccine preventable disease surveillance in Fiji. Sci. Rep. 2016, 6, 39784. [Google Scholar] [CrossRef]

| Code | Definition |
|---|---|
| Meningitis | |
| A17.0 | Tuberculous meningitis |
| A20.3 | Plague meningitis |
| A32.11 | Listeria meningitis |
| A39.0 | Meningococcal meningitis |
| A69.21 | Meningitis due to Lyme disease |
| A87 * | Enteroviral meningitis; adenoviral meningitis; lymphocytic choriomeningitis; other viral meningitis; viral meningitis, unspecified |
| B01.0 | Varicella meningitis |
| B02.1 | Zoster meningitis |
| B27.02 | Gamma herpes viral mononucleosis with meningitis |
| B27.92 | Infectious mononucleosis, unspecified with meningitis |
| B37.5 | Candidal meningitis |
| B38.4 | Coccidioidomycosis meningitis |
| D86.81 | Sarcoid meningitis |
| G00 * | Hemophilus meningitis; pneumococcal meningitis; streptococcal meningitis; staphylococcal meningitis; other bacterial meningitis; bacterial meningitis, unspecified |
| G01 * | Meningitis in bacterial diseases classified elsewhere |
| G02 * | Meningitis in other infectious and parasitic diseases classified elsewhere |
| G03.0 | Nonpyogenic meningitis |
| G03.8 | Meningitis due to other specified causes |
| G03.9 | Meningitis, unspecified |
| Encephalitis | |
| A39.81 | Meningococcal encephalitis |
| A83 * | Japanese encephalitis; western equine encephalitis; eastern equine encephalitis; St Louis encephalitis; Australian encephalitis; California encephalitis; Rocio virus disease; other mosquito-borne viral encephalitis; mosquito-borne viral encephalitis, unspecified |
| A84 * | Far eastern tick-borne encephalitis [Russian spring-summer encephalitis]; central European tick-borne encephalitis; Powassan virus disease; other tick-borne viral encephalitis; tick-borne viral encephalitis, unspecified |
| A85 * | Enteroviral encephalitis; adenoviral encephalitis; arthropod-borne viral encephalitis, unspecified; other specified viral encephalitis |
| A86 | Unspecified viral encephalitis |
| G04 * | Acute disseminated encephalitis and encephalomyelitis, unspecified; postinfectious acute disseminated encephalitis and encephalomyelitis (postinfectious ADEM); postimmunization acute disseminated encephalitis, myelitis and encephalomyelitis; tropical spastic paraplegia; bacterial meningoencephalitis and meningomyelitis, not elsewhere classified; acute necrotizing hemorrhagic encephalopathy, unspecified; postinfectious acute necrotizing hemorrhagic encephalopathy; postimmunization acute necrotizing hemorrhagic encephalopathy; other acute necrotizing hemorrhagic encephalopathy; other encephalitis and encephalomyelitis; acute flaccid myelitis; other myelitis; encephalitis and encephalomyelitis, unspecified; myelitis, unspecified |
| G05 * | Encephalitis and encephalomyelitis in diseases classified elsewhere; myelitis in diseases classified elsewhere |
| Characteristic, n (%) | <18 Years of Age (n = 560) | 18–64 Years of Age (n = 330) | ≥65 Years of Age (n = 98) | Total (n = 988) |
|---|---|---|---|---|
| Sex | ||||
| Male | 298 (53.2) | 145 (43.9) | 44 (44.9) | 487 (49.3) |
| Female | 262 (46.8) | 185 (56.1) | 54 (55.1) | 501 (50.7) |
| Race | ||||
| White | 353 (63.0) | 187 (56.7) | 48 (49.0) | 588 (59.5) |
| Black | 70 (12.5) | 68 (20.6) | 8 (8.2) | 146 (14.8) |
| Asian | 6 (1.1) | 6 (1.8) | 3 (3.1) | 15 (1.5) |
| Other | 9 (1.6) | 5 (1.5) | 1 (1.0) | 15 (1.5) |
| Unknown/not reported | 122 (21.8) | 64 (19.4) | 38 (38.8) | 224 (22.7) |
| Ethnicity | ||||
| Hispanic/Latino | 26 (4.6) | 8 (2.4) | 1 (1.0) | 35 (3.5) |
| Not Hispanic/Latino | 431 (77.0) | 260 (78.8) | 59 (60.2) | 750 (75.9) |
| Unknown/not reported | 103 (18.4) | 62 (18.8) | 38 (38.8) | 203 (20.5) |
| One or more underlying medical conditions | 9 (1.6) | 63 (19.1) | 51 (52.0) | 123 (12.4) |
| Heart Failure | 2 (0.4) | 10 (3.0) | 14 (14.3) | 26 (2.6) |
| Cerebrovascular disease | 3 (0.5) | 7 (2.1) | 8 (8.2) | 18 (1.8) |
| Renal disease | 1 (0.2) | 9 (2.7) | 11 (11.2) | 21 (2.1) |
| Liver disease | 0 | 7 (2.1) | 0 | 7 (0.7) |
| Diabetes | 3 (0.5) | 29 (8.8) | 19 (19.4) | 51 (5.2) |
| Asplenia | 0 | 1 (0.3) | 0 | 1 (0.1) |
| Chronic obstructive pulmonary disease | 0 | 10 (3.0) | 12 (12.2) | 22 (2.2) |
| HIV infection | 0 | 7 (2.1) | 1 (1.0) | 8 (0.8) |
| Cancer | 2 (0.4) | 14 (4.2) | 17 (17.3) | 33 (3.3) |
| Two or more underlying medical conditions | 1 (0.2) | 23 (7.0) | 24 (24.5) | 48 (4.9) |
| Characteristic, n (%) | <18 Years (n = 560) | 18–64 Years (n = 330) | ≥65 Years (n = 98) | Total (n = 988) |
|---|---|---|---|---|
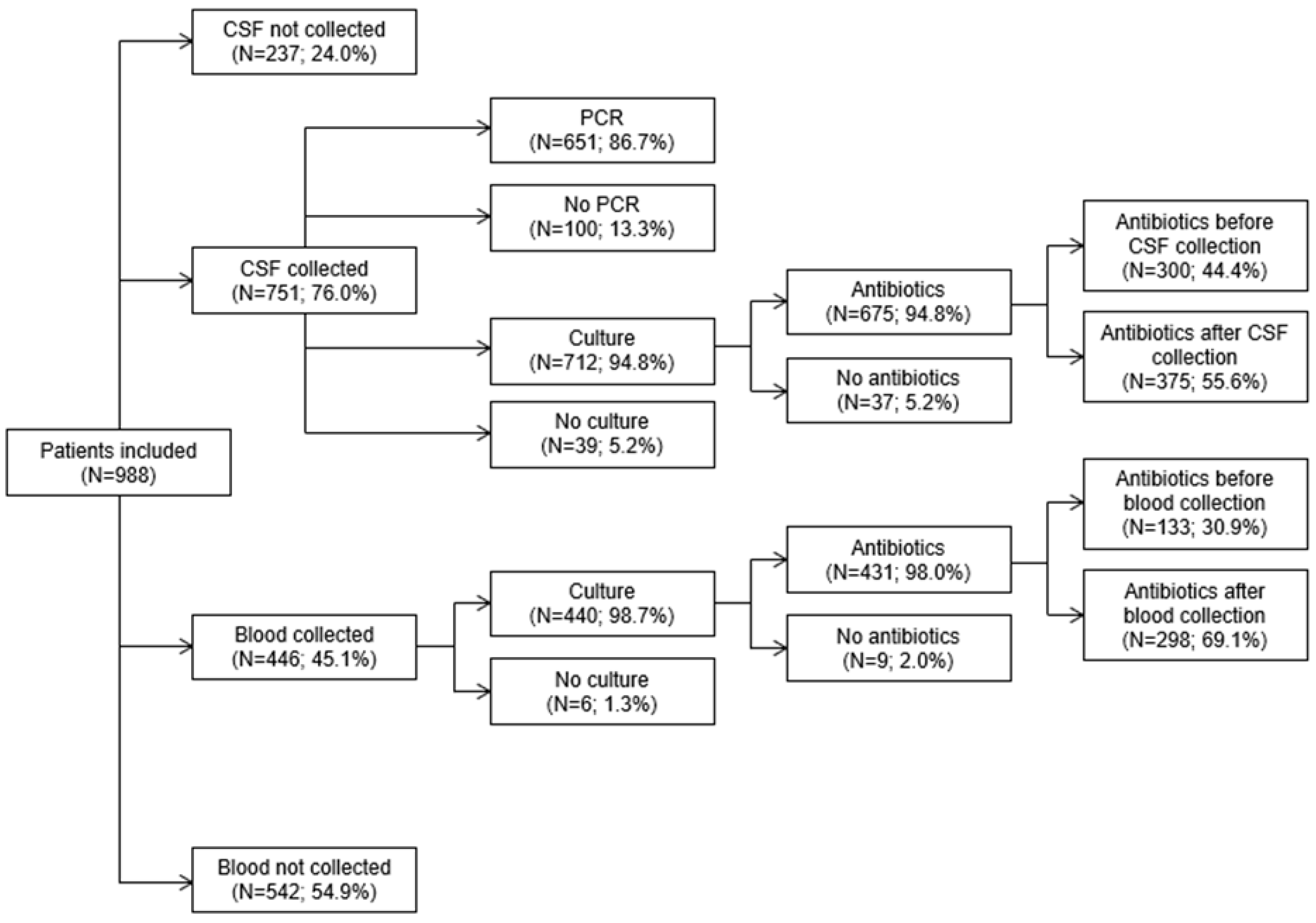
| Antibiotics | 428 (76.4) | 243 (73.6) | 57 (58.2) | 728 (73.7) |
| CSF collected | 440 (78.6) | 254 (77.0) | 57 (58.2) | 751 (76.0) |
| CSF PCR for N. meningitidis | 415 (74.1) | 186 (56.4) | 50 (51.0) | 651 (65.9) |
| CSF cultured | 415 (74.1) | 243 (73.6) | 54 (55.1) | 712 (72.1) |
| Antibiotics before | 162 (28.9) | 99 (30.0) | 39 (39.8) | 300 (30.4) |
| Antibiotics after | 237 (42.3) | 125 (37.9) | 13 (13.3) | 375 (38.0) |
| Blood collected | 307 (54.8) | 113 (34.2) | 26 (26.5) | 446 (45.1) |
| Blood cultured | 301 (53.8) | 113 (34.2) | 26 (26.5) | 440 (44.5) |
| Antibiotics before | 93 (16.6) | 29 (8.8) | 11 (11.2) | 133 (13.5) |
| Antibiotics after | 204 (36.4) | 79 (23.9) | 15 (15.3) | 298 (30.2) |
| Both CSF and blood collected | 306 (54.6) | 109 (33.0) | 26 (26.5) | 441 (44.6) |
| Neither CSF nor blood collected | 119 (21.3) | 72 (21.8) | 41 (41.8) | 232 (23.5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramirez, J.; Furmanek, S.; Chandler, T.; Prado, J.; Harper, L.R.; Shen, S.; Iantomasi, R.; Presa, J.V.; Ali, M.; Findlow, J.; et al. A Ten-Year Retrospective Review of Medical Records of Patients Admitted with Meningitis or Encephalitis at Five Hospitals in the United States Highlights the Potential for Under-Ascertainment of Invasive Meningococcal Disease. Pathogens 2025, 14, 962. https://doi.org/10.3390/pathogens14100962
Ramirez J, Furmanek S, Chandler T, Prado J, Harper LR, Shen S, Iantomasi R, Presa JV, Ali M, Findlow J, et al. A Ten-Year Retrospective Review of Medical Records of Patients Admitted with Meningitis or Encephalitis at Five Hospitals in the United States Highlights the Potential for Under-Ascertainment of Invasive Meningococcal Disease. Pathogens. 2025; 14(10):962. https://doi.org/10.3390/pathogens14100962
Chicago/Turabian StyleRamirez, Julio, Stephen Furmanek, Thomas Chandler, Josue Prado, Lisa R. Harper, Steven Shen, Raffaella Iantomasi, Jessica V. Presa, Mohammad Ali, Jamie Findlow, and et al. 2025. "A Ten-Year Retrospective Review of Medical Records of Patients Admitted with Meningitis or Encephalitis at Five Hospitals in the United States Highlights the Potential for Under-Ascertainment of Invasive Meningococcal Disease" Pathogens 14, no. 10: 962. https://doi.org/10.3390/pathogens14100962
APA StyleRamirez, J., Furmanek, S., Chandler, T., Prado, J., Harper, L. R., Shen, S., Iantomasi, R., Presa, J. V., Ali, M., Findlow, J., Moïsi, J. C., & Angulo, F. J. (2025). A Ten-Year Retrospective Review of Medical Records of Patients Admitted with Meningitis or Encephalitis at Five Hospitals in the United States Highlights the Potential for Under-Ascertainment of Invasive Meningococcal Disease. Pathogens, 14(10), 962. https://doi.org/10.3390/pathogens14100962






