From Bioinformatic Modeling to Clinical Observation: Potential Implications of Ribosomal RNA Folding in Blastocystis sp. Isolates from Symptomatic and Asymptomatic Carriers
Abstract
1. Introduction
2. Materials and Methods
2.1. Sequence Data
2.2. Sequence Alignments
2.3. Phylogeny and Haplotype Network Analysis
2.4. Population Structure Analysis
2.5. RNA Secondary Structure and Folding
2.6. Prediction of RNA–Protein Interactions
2.7. Statistical Analysis
3. Results
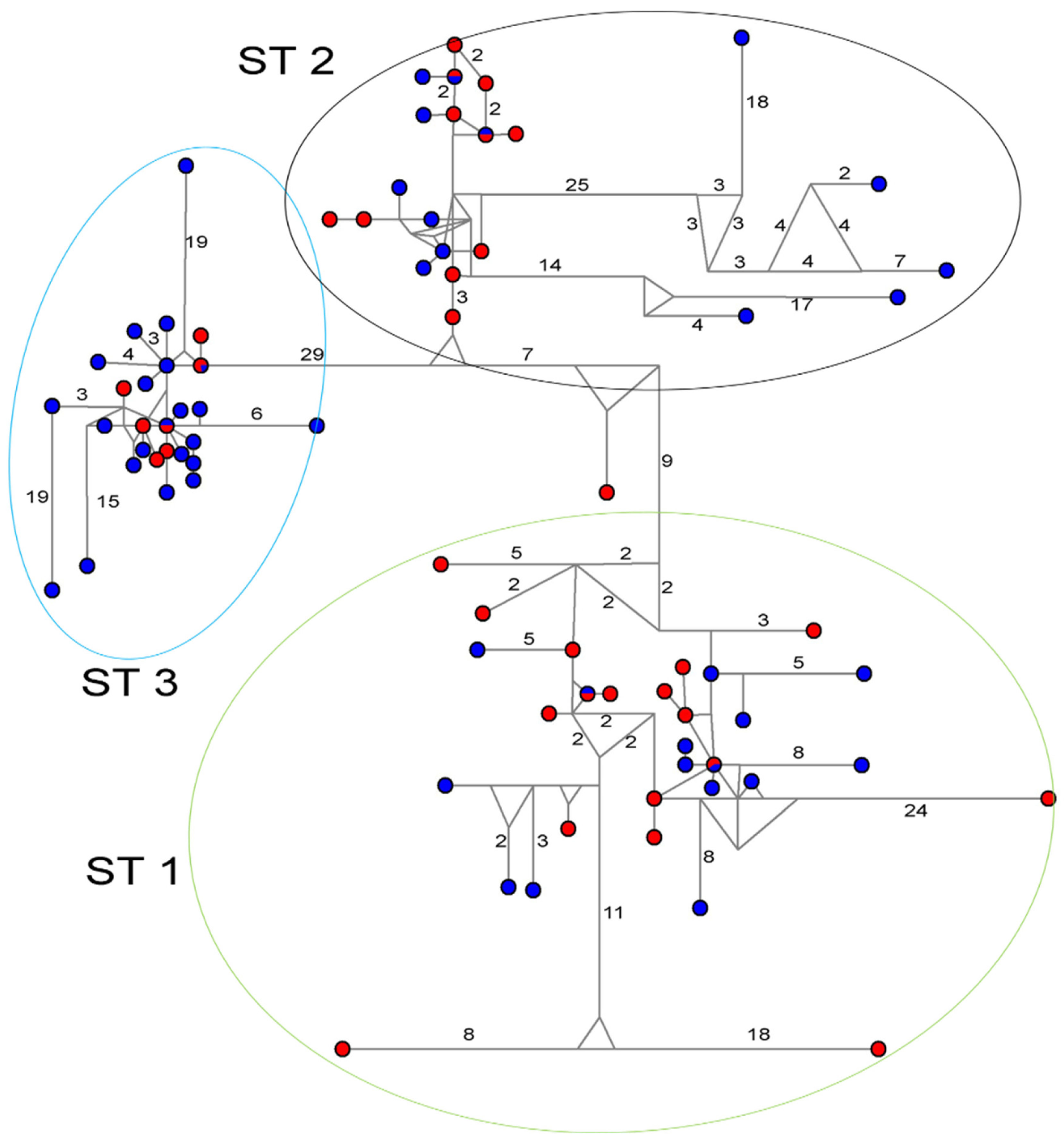
3.1. Phylogenies and Population Structure Analysis
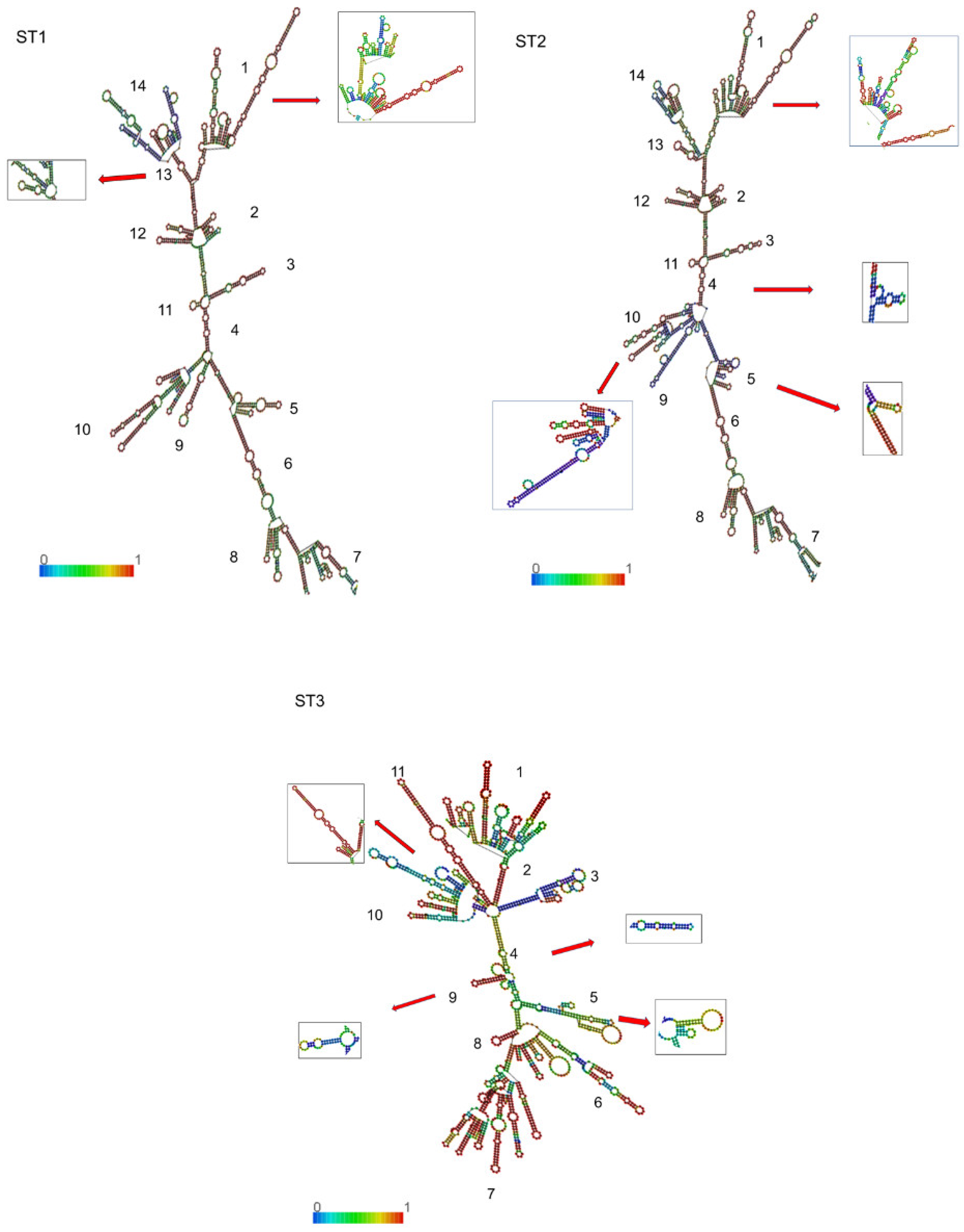
3.2. 18S-rRNA Secondary Structure and Conformational Analysis
3.3. RNA–Protein Interaction Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 18S-rDNA | 18S ribosomal DNA |
| 18S-rRNA | 18S ribosomal RNA |
| rRNA | Ribosomal RNA |
| ST | Subtype |
| RP | Ribosomal protein |
References
- Tan, K.S. New insights on classification, identification, and clinical relevance of Blastocystis spp. Clin. Microbiol. Rev. 2008, 21, 639–665. [Google Scholar] [CrossRef]
- Kwon, J.Y.; Choi, J.H.; Lee, H.I.; Ju, J.W.; Lee, M.R. Molecular Prevalence of Blastocystis sp. from Patients with Diarrhea in the Republic of Korea. Microorganisms 2024, 12, 523. [Google Scholar] [CrossRef]
- Olyaiee, A.; Sadeghi, A.; Yadegar, A.; Mirsamadi, E.S.; Mirjalali, H. Gut Microbiota Shifting in Irritable Bowel Syndrome: The Mysterious Role of Blastocystis sp. Front. Med. 2022, 9, 890127. [Google Scholar] [CrossRef]
- Stensvold, C.R.; Tan, K.S.W.; Clark, C.G. Blastocystis. Trends Parasitol. 2020, 36, 315–316. [Google Scholar] [CrossRef]
- Santin, M.; Figueiredo, A.; Molokin, A.; George, N.S.; Köster, P.C.; Dashti, A.; González-Barrio, D.; Carmena, D.; Maloney, J.G. Division of Blastocystis ST10 into three new subtypes: ST42–ST44. J. Eukaryot. Microbiol. 2024, 71, e12998. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Chao, W.; Zhong, S.; Ren, A.J. Eukaryotic Ribosomal Protein S5 of the 40S Subunit: Structure and Function. Int. J. Mol. Sci. 2023, 24, 3386. [Google Scholar] [CrossRef]
- Black, J.J.; Johnson, A.W. Release of the ribosome biogenesis factor Bud23 from small subunit precursors in yeast. RNA 2022, 28, 371–389. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, M.L.; Woodson, S.A. A roadmap for rRNA folding and assembly during transcription. Trends Biochem. Sci. 2021, 46, 889–901. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Cerca, S.; Pöll, G.; Gleizes, P.E.; Tschochner, H.; Milkereit, P. Roles of eukaryotic ribosomal proteins in maturation and transport of pre-18S rRNA and ribosome function. Mol. Cell 2005, 20, 2632–2675. [Google Scholar] [CrossRef]
- Gillespie, J.J.; McKenna, C.H.; Yoder, M.J.; Gutell, R.R.; Johnston, J.S.; Kathirithamby, J.; Cognato, A.I. Assessing the odd secondary structural properties of nuclear small subunit ribosomal RNA sequences (18S) of the twisted-wing parasites (Insecta: Strepsiptera). Insect Mol. Biol. 2005, 14, 625–643. [Google Scholar] [CrossRef]
- Keller, A.; Förster, F.; Müller, T.; Dandekar, T.; Schultz, J.; Wolf, M. Including RNA secondary structures improves accuracy and robustness in reconstruction of phylogenetic trees. Biol. Direct 2010, 5, 4. [Google Scholar] [CrossRef]
- Qi, Y.; Zhang, Y.; Mu, Q.; Zheng, G.; Zhang, M.; Chen, B.; Huang, J.; Ma, C.; Wang, X. RNA Secondary Structurome Revealed Distinct Thermoregulation in Plasmodium falciparum. Front. Cell Dev. Biol. 2022, 9, 766532. [Google Scholar] [CrossRef] [PubMed]
- Black, J.J.; Sardana, R.; Elmir, E.W.; Johnson, A.W. Bud23 promotes the final disassembly of the small subunit processome in Saccharomyces cerevisiae. PLoS Genet. 2020, 16, e1009215. [Google Scholar] [CrossRef] [PubMed]
- Matragkou, C.N.; Papachristou, E.T.; Tezias, S.S.; Tsiftsoglou, A.S.; Choli-Papadopoulou, T.; Vizirianakis, I.S. The potential role of ribosomal protein S5 on cell cycle arrest and initiation of murine erythroleukemia cell differentiation. J. Cell. Biochem. 2008, 104, 1477–1490. [Google Scholar] [CrossRef]
- Xu, W.H.; Hu, H.G.; Tian, Y.; Wang, S.Z.; Li, J.; Li, J.Z.; Deng, X.; Qian, H.; Qiu, L.; Hu, Z.L.; et al. Bioactive compound reveals a novel function for ribosomal protein S5 in hepatic stellate cell activation and hepatic fibrosis. Hepatology 2014, 60, 648–660. [Google Scholar] [CrossRef] [PubMed]
- Costa, G.C.A.; Silva, F.A.A.; Torquato, R.J.S.; Silva Vaz, I.; Parizi, L.F.; Tanaka, A.S. Evaluation of the biological function of ribosomal protein S18 from cattle tick Rhipicephalus microplus. Ticks Tick-Borne Dis. 2024, 15, 102333. [Google Scholar] [CrossRef]
- Malygin, A.A.; Karpova, G.G. Structural motifs of the bacterial ribosomal proteins S20, S18 and S16 that contact rRNA present in the eukaryotic ribosomal proteins S25, S26 and S27A, respectively. Nucleic Acids Res. 2010, 38, 2089–2098. [Google Scholar] [CrossRef]
- Vargas-Sanchez, G.B.; Romero-Valdovinos, M.; Ramirez-Guerrero, C.; Vargas-Hernandez, I.; Ramirez-Miranda, M.E.; Martinez-Ocaña, J.; Valadez, A.; Ximenez, C.; Lopez-Escamilla, E.; Hernandez-Campos, M.E.; et al. Blastocystis Isolates from Patients with Irritable Bowel Syndrome and from Asymptomatic Carriers Exhibit Similar Parasitological Loads, but Significantly Different Generation Times and Genetic Variability across Multiple Subtypes. PLoS ONE 2015, 10, e0124006. [Google Scholar] [CrossRef]
- Nawrocki, E.P. Structural RNA Homology Search and Alignment Using Covariance Models. Ph.D. Thesis, School of Medicine, Washington University in Saint Louis, St. Louis, MO, USA, 2009. [Google Scholar]
- Stensvold, C.R.; Clark, C.G. Pre-empting Pandora’s Box: Blastocystis Subtypes Revisited. Trends Parasitol. 2020, 36, 229–232. [Google Scholar] [CrossRef]
- Gruber, A.R.; Lorenz, R.; Bernhart, S.H.; Neuböck, R.; Hofacker, I.L. The Vienna RNA website. Nucleic Acids Res. 2008, 36, W70–W74. [Google Scholar] [CrossRef]
- Bandelt, H.J.; Forster, P.; Röhl, A. Median-joining networks for inferring intraspecific phylogenies. Mol. Biol. Evol. 1999, 16, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Ronquis, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar] [CrossRef]
- Evanno, G.; Regnaut, S.; Goudet, J. Detecting the number of clusters of individuals using the software structure: A simulation study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef]
- Spahn, C.M.; Beckmann, R.; Eswar, N.; Penczek, P.A.; Sali, A.; Blobel, G.; Frank, J. Structure of the 80S ribosome from Saccharomyces cerevisiae—tRNA-ribosome and subunit-subunit interactions. Cell 2001, 107, 373–386. [Google Scholar] [CrossRef]
- Granneman, S.; Petfalski, E.; Swiatkowska, A.; Tollervey, D. Cracking pre-40S ribosomal subunit structure by systematic analyses of RNA-protein cross-linking. EMBO J. 2010, 29, 2026–2036. [Google Scholar] [CrossRef]
- Muppirala, U.K.; Honavar, V.G.; Dobbs, D. Predicting RNA-Protein Interactions Using Only Sequence Information. BMC Bioinform. 2011, 12, 489. [Google Scholar] [CrossRef]
- Van de Peer, Y.; De Rijk, P.; Wuyts, J.; Winkelmans, T.; De Wachter, R. The European small subunit ribosomal RNA database. Nucleic Acids Res. 2000, 28, 175–176. [Google Scholar] [CrossRef] [PubMed]
- Wuyts, J.; De Rijk, P.; Van de Peer, Y.; Winkelmans, T.; De Wachter, R. The European Large Subunit Ribosomal RNA Database. Nucleic Acids Res. 2001, 29, 175–177. [Google Scholar] [CrossRef] [PubMed]
- Jacob, A.S.; Busby, E.J.; Levy, A.D.; Komm, N.; Clark, C.G. Expanding the Entamoeba Universe: New Hosts Yield Novel Ribosomal Lineages. J. Eukaryot. Microbiol. 2016, 63, 69–78. [Google Scholar] [CrossRef]
- Smirnov, E.; Kalmárová, M.; Koberna, K.; Zemanová, Z.; Malínský, J.; Masata, M.; Cvacková, Z.; Michalová, K.; Raska, I. NORs and their transcription competence during the cell cycle. Folia Biol. 2006, 52, 59–70. [Google Scholar] [CrossRef]
- Poirier, P.; Meloni, D.; Nourrisson, C.; Wawrzyniak, I.; Viscogliosi, E.; Livrelli, V.; Delbac, F. Molecular subtyping of Blastocystis spp. using a new rDNA marker from the mitochondria-like organelle genome. Parasitology 2014, 141, 670–681. [Google Scholar] [CrossRef]
- Liao, D. Concerted evolution: Molecular mechanism and biological implications. Am. J. Hum. Genet. 1999, 64, 24–30. [Google Scholar] [CrossRef]
- Gong, L.; Shi, W.; Yang, M.; Kong, X. Characterization of 18S-ITS1-5.8S rDNA in eleven species in Soleidae: Implications for phylogenetic analysis. Hydrobiologia 2018, 819, 161–175. [Google Scholar] [CrossRef]
- Coen, E.; Strachan, T.; Dover, G. Dynamics of concerted evolution of ribosomal DNA and histone gene families in the melanogaster species subgroup of Drosophila. J. Mol. Biol. 1982, 158, 17–35. [Google Scholar] [CrossRef]
- Schlötterer, C.; Tautz, D. Chromosomal homogeneity of Drosophila ribosomal DNA arrays suggests intrachromosomal exchanges drive concerted evolution. Curr. Biol. 1994, 4, 777–783. [Google Scholar] [CrossRef]
- Zierdt, C.H.; Swan, J.C. Generation time and growth rate of the human intestinal parasite Blastocystis hominis. J. Protozool. 1981, 28, 483–485. [Google Scholar] [CrossRef] [PubMed]
- Eickbush, T.H.; Eickbush, D.G. Finely orchestrated movements: Evolution of the ribosomal RNA genes. Genetics 2007, 175, 477–485. [Google Scholar] [CrossRef]
- Vogelberg, C.; Stensvold, C.R.; Monecke, S.; Ditzen, A.; Stopsack, K.; Heinrich-Gräfe, U.; Pöhlmann, C. Blastocystis sp. subtype 2 detection during recurrence of gastrointestinal and urticarial symptoms. Parasitol. Int. 2010, 59, 469–471. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.C.; Suresh, K.G.; Smith, H.V. Phenotypic and genotypic characterisation of Blastocystis hominis isolates implicates subtype 3 as a subtype with pathogenic potential. Parasitol. Res. 2008, 104, 85–93. [Google Scholar] [CrossRef]
- Gonzalez-Arenas, N.R.; Villalobos, G.; Vargas-Sanchez, G.B.; Avalos-Galarza, C.A.; Marquez-Valdelamar, L.M.; Ramirez-Miranda, M.E.; Olivo-Diaz, A.; Romero-Valdovinos, M.; Martinez-Hernandez, F.; Maravilla, P. Is the genetic variability of Cathepsin B important in the pathogenesis of Blastocystis spp.? Parasitol. Res. 2018, 117, 3935–3943. [Google Scholar] [CrossRef] [PubMed]
- Wawrzyniak, I.; Poirier, P.; Viscogliosi, E.; Dionigia, M.; Texier, C.; Delbac, F.; Alaoui, H.E. Blastocystis, an unrecognized parasite: An overview of pathogenesis and diagnosis. Ther. Adv. Infect. Dis. 2013, 1, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Nourrisson, C.; Wawrzyniak, I.; Cian, A.; Livrelli, V.; Viscogliosi, E.; Delbac, F.; Poirier, P. Blastocystis secreted cysteine proteases: A legumain-activated cathepsin B increases paracellular permeability of intestinal Caco-2 cell monolayers. Parasitology 2016, 143, 1713–1722. [Google Scholar] [CrossRef] [PubMed]

| Cases (n = 46) | Controls (n = 45) | Mean Difference 95%IC | Level of Significance b Cases vs. Controls | ||||
|---|---|---|---|---|---|---|---|
| Mean 95%IC a Median | Mean 95%IC Median | All Participants | ST1 | ST2 | ST3 | ||
| RPS18 c | 0.940 0.935 to 0.945 0.940 | 0.947 0.942 to 953 0.960 | −0.00734 −0.0146 to −0.0003 | 0.024 | 0.543 | 0.686 | 1 |
| RPS5 d | 0.923 0.914 to 0.932 0.920 | 0.934 0.924 to 0.943 0.90 | −0.01030 −0.0231 to 0.00251 | 0.057 | 0.386 | 0.713 | 0.467 |
| BUD23 e | 0.984 0.983 to 0.986 0.990 | 0.986 0.985 to 0.988 0.990 | −0.00211 −0.0044 to 0.00028 | 0.061 | 0.576 | 0.485 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Hernández, F.; Martínez, A.; Zampedri, C.; Romero-Valdovinos, M.; Jiménez-Gutiérrez, C.; Flores-Martínez, K.; Trejo-Chávez, A.; Villalobos, G.; Maravilla, P. From Bioinformatic Modeling to Clinical Observation: Potential Implications of Ribosomal RNA Folding in Blastocystis sp. Isolates from Symptomatic and Asymptomatic Carriers. Pathogens 2025, 14, 1009. https://doi.org/10.3390/pathogens14101009
Martínez-Hernández F, Martínez A, Zampedri C, Romero-Valdovinos M, Jiménez-Gutiérrez C, Flores-Martínez K, Trejo-Chávez A, Villalobos G, Maravilla P. From Bioinformatic Modeling to Clinical Observation: Potential Implications of Ribosomal RNA Folding in Blastocystis sp. Isolates from Symptomatic and Asymptomatic Carriers. Pathogens. 2025; 14(10):1009. https://doi.org/10.3390/pathogens14101009
Chicago/Turabian StyleMartínez-Hernández, Fernando, Arony Martínez, Cecilia Zampedri, Mirza Romero-Valdovinos, Carlos Jiménez-Gutiérrez, Karina Flores-Martínez, Armando Trejo-Chávez, Guiehdani Villalobos, and Pablo Maravilla. 2025. "From Bioinformatic Modeling to Clinical Observation: Potential Implications of Ribosomal RNA Folding in Blastocystis sp. Isolates from Symptomatic and Asymptomatic Carriers" Pathogens 14, no. 10: 1009. https://doi.org/10.3390/pathogens14101009
APA StyleMartínez-Hernández, F., Martínez, A., Zampedri, C., Romero-Valdovinos, M., Jiménez-Gutiérrez, C., Flores-Martínez, K., Trejo-Chávez, A., Villalobos, G., & Maravilla, P. (2025). From Bioinformatic Modeling to Clinical Observation: Potential Implications of Ribosomal RNA Folding in Blastocystis sp. Isolates from Symptomatic and Asymptomatic Carriers. Pathogens, 14(10), 1009. https://doi.org/10.3390/pathogens14101009







