Use of Tick Cell Lines in Co-Infection Studies with a Preliminary Study of Co-Culture of Borrelia burgdorferi and Anaplasma phagocytophilum
Abstract
1. Introduction
2. Materials and Methods
2.1. Tick Cell Line
2.2. Infection of Tick Cell Line with B. burgdorferi and A. phagocytophilum
2.3. RNA Isolation, Reverse Transcription, and Real-Time PCR
2.4. Statistical Analysis
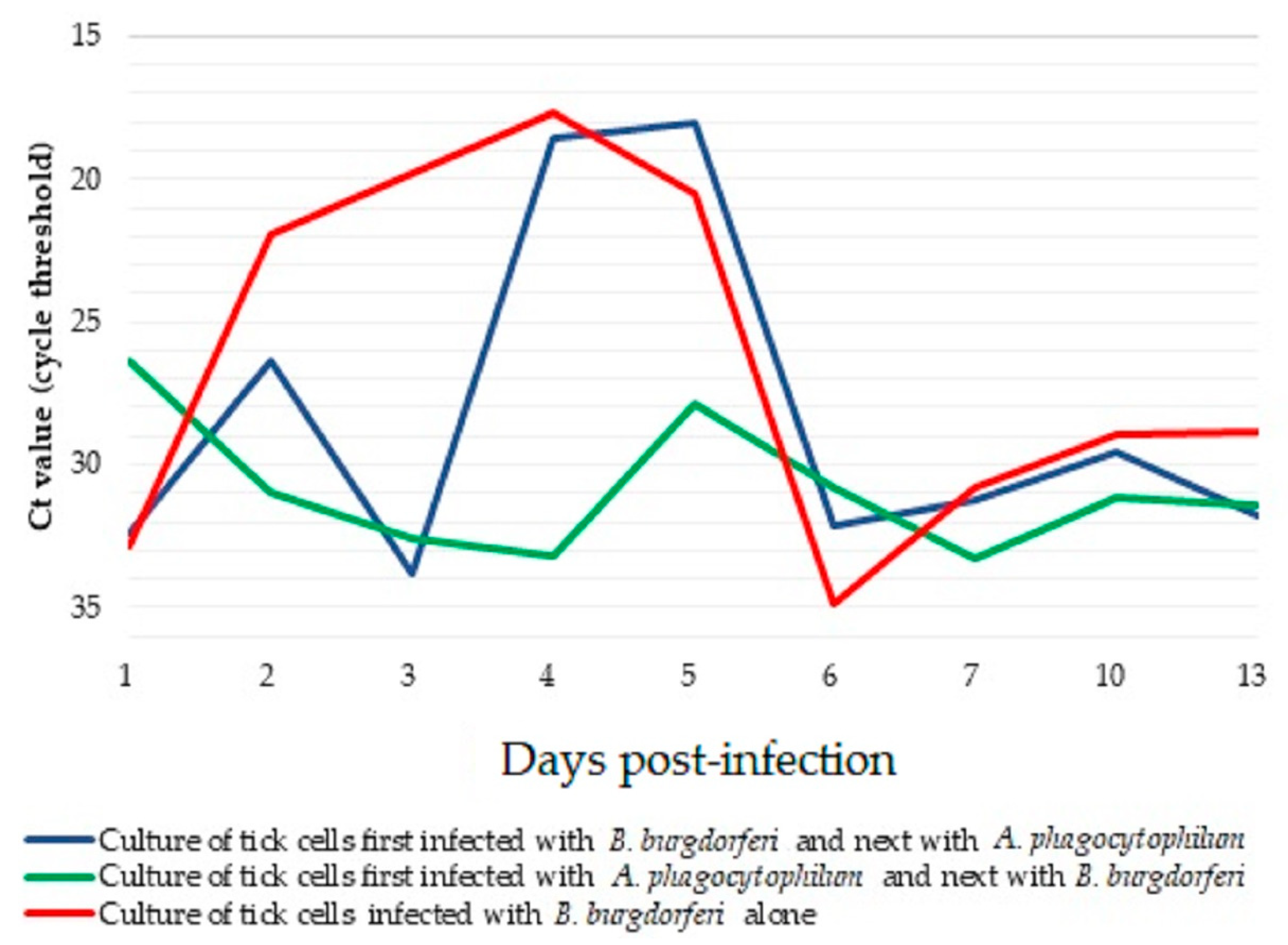
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kahl, O.; Gray, J.S. The Biology of Ixodes ricinus with Emphasis on Its Ecology. Ticks Tick-Borne Dis. 2023, 14, 102114. [Google Scholar] [CrossRef] [PubMed]
- Černý, J.; Lynn, G.; Hrnková, J.; Golovchenko, M.; Rudenko, N.; Grubhoffer, L. Management Options for Ixodes ricinus-Associated Pathogens: A Review of Prevention Strategies. Int. J. Environ. Res. Public Health 2020, 17, 1830. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, C.; Lynn, G.E.; Pedra, J.H.F.; Pal, U.; Narasimhan, S.; Fikrig, E. Interactions between Borrelia burgdorferi and Ticks. Nat. Rev. Microbiol. 2020, 18, 587–600. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Yan, M.; Liu, A.; Chen, T.; Luo, L.; Li, L.; Teng, Z.; Li, B.; Ji, Z.; Jian, M.; et al. The Seroprevalence of Anaplasma phagocytophilum in Global Human Populations: A Systematic Review and Meta-analysis. Transbound. Emerg. Dis. 2020, 67, 2050–2064. [Google Scholar] [CrossRef]
- Gray, J.; Kahl, O.; Zintl, A. Pathogens Transmitted by Ixodes ricinus. Ticks Tick-Borne Dis. 2024, 15, 102402. [Google Scholar] [CrossRef]
- Sprong, H.; Azagi, T.; Hoornstra, D.; Nijhof, A.M.; Knorr, S.; Baarsma, M.E.; Hovius, J.W. Control of Lyme Borreliosis and Other Ixodes ricinus-Borne Diseases. Parasites Vectors 2018, 11, 145. [Google Scholar] [CrossRef]
- Hodosi, R.; Kazimirova, M.; Soltys, K. What Do We Know about the Microbiome of I. ricinus? Front. Cell. Infect. Microbiol. 2022, 12, 990889. [Google Scholar] [CrossRef]
- de La Fuente, J.; Antunes, S.; Bonnet, S.; Cabezas-Cruz, A.; Domingos, A.G.; Estrada-Pena, A.; Johnson, N.; Kocan, K.M.; Mansfield, K.L.; Nijhof, A.M.; et al. Tick-Pathogen Interactions and Vector Competence: Identification of Molecular Drivers for Tick-Borne Diseases. Front. Cell. Infect. Microbiol. 2017, 7, 114. [Google Scholar] [CrossRef]
- Parveen, N.; Bhanot, P. Babesia microti—Borrelia burgdorferi Coinfection. Pathogens 2019, 8, 117. [Google Scholar] [CrossRef]
- Diuk-Wasser, M.A.; Vannier, E.; Krause, P.J. Coinfection by Ixodes Tick-Borne Pathogens: Ecological, Epidemiological, and Clinical Consequences. Trends Parasitol. 2016, 32, 30–42. [Google Scholar] [CrossRef]
- Cao, W.-C.; Zhao, Q.-M.; Zhang, P.-H.; Yang, H.; Wu, X.-M.; Wen, B.-H.; Zhang, X.-T.; Habbema, J.D.F. Prevalence of Anaplasma phagocytophila and Borrelia burgdorferi in Ixodes persulcatus ticks from northeastern China. Am. J. Trop. Med. Hyg. 2003, 68, 547–550. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rocha, S.C.; Velásquez, C.V.; Aquib, A.; Al-Nazal, A.; Parveen, N. Transmission Cycle of Tick-Borne Infections and Co-Infections, Animal Models and Diseases. Pathogens 2022, 11, 1309. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Zhou, G.; Cao, W.; Xu, X.; Zhang, Y.; Ji, Z.; Yang, J.; Chen, J.; Liu, M.; Fan, Y.; et al. Global Seroprevalence and Sociodemographic Characteristics of Borrelia burgdorferi sensu lato in Human Populations: A Systematic Review and Meta-Analysis. BMJ Glob. Health 2022, 7, e007744. [Google Scholar] [CrossRef] [PubMed]
- Moss, W.J.; Dumler, J.S. Simultaneous Infection with Borrelia burgdorferi and Human Granulocytic Ehrlichiosis. Pediatr. Infect. Dis. J. 2003, 22, 91–92. [Google Scholar] [CrossRef]
- Loebermann, M.; Fingerle, V.; Lademann, M.; Fritzsche, C.; Reisinger, E.C. Borrelia burgdorferi and Anaplasma phagocytophilum Coinfection. Emerg. Infect. Dis. 2006, 12, 353–355. [Google Scholar] [CrossRef]
- Bell-Sakyi, L.; Zweygarth, E.; Blouin, E.F.; Gould, E.A.; Jongejan, F. Tick Cell Lines: Tools for Tick and Tick-Borne Disease Research. Trends Parasitol. 2007, 23, 450–457. [Google Scholar] [CrossRef]
- Bell-Sakyi, L.; Darby, A.; Baylis, M.; Makepeace, B.L. The Tick Cell Biobank: A Global Resource for in Vitro Research on Ticks, Other Arthropods and the Pathogens They Transmit. Ticks Tick-Borne Dis. 2018, 9, 1364–1371. [Google Scholar] [CrossRef]
- De La Fuente, J.; Garcia-Garcia, J.C.; Blouin, E.F.; Saliki, J.T.; Kocan, K.M. Infection of Tick Cells and Bovine Erythrocytes with One Genotype of the Intracellular Ehrlichia Anaplasma marginale Excludes Infection with Other Genotypes. Clin. Vaccine Immunol. 2002, 9, 658–668. [Google Scholar] [CrossRef]
- Mattila, J.T.; Munderloh, U.G.; Kurtti, T.J. Phagocytosis of the Lyme Disease Spirochete, Borrelia burgdorferi, by Cells from the Ticks, Ixodes scapularis and Dermacentor andersoni, Infected with An Endosymbiont, Rickettsia peacockii. J. Insect Sci. 2007, 7, 58. [Google Scholar] [CrossRef]
- Moniuszko, A.; Rückert, C.; Alberdi, M.P.; Barry, G.; Stevenson, B.; Fazakerley, J.K.; Kohl, A.; Bell-Sakyi, L. Coinfection of Tick Cell Lines Has Variable Effects on Replication of Intracellular Bacterial and Viral Pathogens. Ticks Tick-Borne Dis. 2014, 5, 415–422. [Google Scholar] [CrossRef]
- Cull, B.; Burkhardt, N.Y.; Wang, X.-R.; Thorpe, C.J.; Oliver, J.D.; Kurtti, T.J.; Munderloh, U.G. The Ixodes scapularis Symbiont Rickettsia buchneri Inhibits Growth of Pathogenic Rickettsiaceae in Tick Cells: Implications for Vector Competence. Front. Vet. Sci. 2022, 8, 748427. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.-S.; Cui, X.-M.; Du, L.-F.; Xia, L.-Y.; Du, C.-H.; Bell-Sakyi, L.; Zhang, M.-Z.; Zhu, D.-Y.; Dong, Y.; Wei, W.; et al. Coinfection of Two Rickettsia Species in a Single Tick Species Provides New Insight into Rickettsia—Rickettsia and Rickettsia -Vector Interactions. Microbiol. Spectr. 2022, 10, e02323-22. [Google Scholar] [CrossRef] [PubMed]
- Skinner, K.M.; Underwood, J.; Ghosh, A.; Oliva Chavez, A.S.; Brelsfoard, C.L. Wolbachia Impacts Anaplasma Infection in Ixodes scapularis Tick Cells. Int. J. Environ. Res. Public Health 2022, 19, 1051. [Google Scholar] [CrossRef] [PubMed]
- Mazuecos, L.; Alberdi, P.; Hernández-Jarguín, A.; Contreras, M.; Villar, M.; Cabezas-Cruz, A.; Simo, L.; González-García, A.; Díaz-Sánchez, S.; Neelakanta, G.; et al. Frankenbacteriosis Targeting Interactions between Pathogen and Symbiont to Control Infection in the Tick Vector. iScience 2023, 26, 106697. [Google Scholar] [CrossRef]
- Kotsarenko, K.; Vechtova, P.; Lieskovska, J.; Füssy, Z.; Cabral-de-Mello, D.C.; Rego, R.O.M.; Alberdi, P.; Collins, M.; Bell-Sakyi, L.; Sterba, J.; et al. Karyotype changes in long-term cultured tick cell lines. Sci. Rep. 2020, 10, 13443. [Google Scholar] [CrossRef]
- Scott, G.R.; Horsburgh, D. New Rickettsial Isolates. Centre for Tropical Veterinary Medicine Annual Report 1982–1983; University of Edinburgh: Edinburgh, UK, 1983. [Google Scholar]
- Bown, K.J.; Lambin, X.; Ogden, N.H.; Petrovec, M.; Shaw, S.E.; Woldehiwet, Z.; Birtles, R.J. High-Resolution Genetic Fingerprinting of European Strains of Anaplasma phagocytophilum by Use of Multilocus Variable-Number Tandem-Repeat Analysis. J. Clin. Microbiol. 2007, 45, 1771–1776. [Google Scholar] [CrossRef]
- Woldehiwet, Z. The Natural History of Anaplasma phagocytophilum. Vet. Parasitol. 2010, 167, 108–122. [Google Scholar] [CrossRef]
- Iyer, R.; Mukherjee, P.; Wang, K.; Simons, J.; Wormser, G.P.; Schwartz, I. Detection of Borrelia burgdorferi Nucleic Acids after Antibiotic Treatment Does Not Confirm Viability. J. Clin. Microbiol. 2013, 51, 857–862. [Google Scholar] [CrossRef]
- Liveris, D.; Schwartz, I.; McKenna, D.; Nowakowski, J.; Nadelman, R.B.; DeMarco, J.; Iyer, R.; Cox, M.E.; Holmgren, D.; Wormser, G.P. Quantitation of Cell-Associated Borrelial DNA in the Blood of Lyme Disease Patients with Erythema Migrans. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 791–795. [Google Scholar] [CrossRef]
- Silaghi, C.; Kauffmann, M.; Passos, L.M.F.; Pfister, K.; Zweygarth, E. Isolation, Propagation and Preliminary Characterisation of Anaplasma phagocytophilum from Roe Deer (Capreolus capreolus) in the Tick Cell Line IDE8. Ticks Tick-Borne Dis. 2011, 2, 204–208. [Google Scholar] [CrossRef]
- Courtney, J.W.; Kostelnik, L.M.; Zeidner, N.S.; Massung, R.F. Multiplex Real-Time PCR for Detection of Anaplasma phagocytophilum and Borrelia burgdorferi. J. Clin. Microbiol. 2004, 42, 3164–3168. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Bao, F.; Wu, H.; Ma, W.; Zhu, L.; Huang, X.; Yang, R.; Peng, L.; Gao, L.; Wu, X.; et al. Global Prevalence of Borrelia burgdorferi and Anaplasma phagocytophilum Coinfection in Ixodes Tick Populations: Protocol for a Systematic Review and Meta-Analysis. BMJ Open 2024, 14, e083052. [Google Scholar] [CrossRef] [PubMed]
- Obonyo, M.; Munderloh, U.G.; Fingerle, V.; Wilske, B.; Kurtti, T.J. Borrelia burgdorferi in Tick Cell Culture Modulates Expression of Outer Surface Proteins A and C in Response to Temperature. J. Clin. Microbiol. 1999, 37, 2137–2141. [Google Scholar] [CrossRef] [PubMed]
- Kurtti, T.J.; Munderloh, U.G.; Ahlstrand, G.G.; Johnson, R.C. Borrelia burgdorferi in Tick Cell Culture: Growth and Cellular Adherence. J. Med. Entomol. 1988, 25, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Rezende, J.; Rangel, C.; Cunha, N.; Fonseca, A. Primary Embryonic Cells of Rhipicephalus microplus and Amblyomma cajennense Ticks as a Substrate for the Development of Borrelia burgdorferi (Strain G39/40). Braz. J. Biol. 2012, 72, 577–582. [Google Scholar] [CrossRef]
- Woldehiwet, Z. Anaplasma phagocytophilum in ruminants in Europe. Ann. N. Y. Acad. Sci. 2006, 1078, 446–460. [Google Scholar] [CrossRef]
- Cabezas-Cruz, A.; Alberdi, P.; Ayllón, N.; Valdés, J.J.; Pierce, R.; Villar, M.; de la Fuente, J. Anaplasma phagocytophilum increases the levels of histone modifying enzymes to inhibit cell apoptosis and facilitate pathogen infection in the tick vector Ixodes scapularis. Epigenetics 2016, 11, 303–319. [Google Scholar] [CrossRef]
- Abraham, N.M.; Liu, L.; Jutras, B.L.; Yadav, A.K.; Narasimhan, S.; Gopalakrishnan, V.; Ansari, J.M.; Jefferson, K.K.; Cava, F.; Jacobs-Wagner, C.; et al. Pathogen-mediated manipulation of arthropod microbiota to promote infection. Proc. Natl. Acad. Sci. USA 2017, 114, E781–E790. [Google Scholar] [CrossRef]
- Holden, K.; Hodzic, E.; Feng, S.; Freet, K.J.; Lefebvre, R.B.; Barthold, S.W. Coinfection with Anaplasma phagocytophilum Alters Borrelia burgdorferi Population Distribution in C3H/HeN Mice. Infect. Immun. 2005, 73, 3440–3444. [Google Scholar] [CrossRef]
- Thomas, V.; Anguita, J.; Barthold, S.W.; Fikrig, E. Coinfection with Borrelia burgdorferi and the Agent of Human Granulocytic Ehrlichiosis Alters Murine Immune Responses, Pathogen Burden, and Severity of Lyme Arthritis. Infect. Immun. 2001, 69, 3359–3371. [Google Scholar] [CrossRef]
- Munderloh, U.G.; Jauron, S.D.; Fingerle, V.; Leitritz, L.; Hayes, S.F.; Hautman, J.M.; Nelson, C.M.; Huberty, B.W.; Kurtti, T.J.; Ahlstrand, G.G.; et al. Invasion and Intracellular Development of the Human Granulocytic Ehrlichiosis Agent in Tick Cell Culture. J. Clin. Microbiol. 1999, 37, 2518–2524. [Google Scholar] [CrossRef] [PubMed]
- Dyachenko, V.; Geiger, C.; Pantchev, N.; Majzoub, M.; Bell-Sakyi, L.; Krupka, I.; Straubinger, R.K. Isolation of Canine Anaplasma phagocytophilum Strains from Clinical Blood Samples Using the Ixodes ricinus Cell Line IRE/CTVM20. Vet. Microbiol. 2013, 162, 980–986. [Google Scholar] [CrossRef] [PubMed]
- Alberdi, P.; Ayllón, N.; Cabezas-Cruz, A.; Bell-Sakyi, L.; Zweygarth, E.; Stuen, S.; De La Fuente, J. Infection of Ixodes spp. Tick Cells with Different Anaplasma phagocytophilum Isolates Induces the Inhibition of Apoptotic Cell Death. Ticks Tick-Borne Dis. 2015, 6, 758–767. [Google Scholar] [CrossRef] [PubMed]
- Goodman, J.L.; Nelson, C.; Vitale, B.; Madigan, J.E.; Dumler, J.S.; Kurtti, T.J.; Munderloh, U.G. Direct Cultivation of the Causative Agent of Human Granulocytic Ehrlichiosis. N. Engl. J. Med. 1996, 334, 209–215. [Google Scholar] [CrossRef]
- Munderloh, U.G.; Madigan, J.E.; Dumler, J.S.; Goodman, J.L.; Hayes, S.F.; Barlough, J.E.; Nelson, C.M.; Kurtti, T.J. Isolation of the Equine Granulocytic Ehrlichiosis Agent, Ehrlichia equi, in Tick Cell Culture. J. Clin. Microbiol. 1996, 34, 664–670. [Google Scholar] [CrossRef]
- Sultana, H.; Neelakanta, G.; Kantor, F.S.; Malawista, S.E.; Fish, D.; Montgomery, R.R.; Fikrig, E. Anaplasma phagocytophilum Induces Actin Phosphorylation to Selectively Regulate Gene Transcription in Ixodes Scapularis Ticks. J. Exp. Med. 2010, 207, 1727–1743. [Google Scholar] [CrossRef]
- Gomez-Chamorro, A.; Hodžić, A.; King, K.C.; Cabezas-Cruz, A. Ecological and Evolutionary Perspectives on Tick-Borne Pathogen Co-Infections. Curr. Res. Parasitol. Vector-Borne Dis. 2021, 1, 100049. [Google Scholar] [CrossRef]
- Xiang, X.; Yang, Y.; Du, J.; Lin, T.; Chen, T.; Yang, X.F.; Lou, Y. Investigation of ospC Expression Variation among Borrelia burgdorferi Strains. Front. Cell. Infect. Microbiol. 2017, 7, 131. [Google Scholar] [CrossRef]
- Munderloh, U.G.; Park, Y.J.; Dioh, J.M.; Fallon, A.M.; Kurtti, T.J. Plasmid modifications in a tick-borne pathogen, Borrelia burgdorferi, cocultured with tick cells. Insect Mol. Biol. 1993, 1, 195–203. [Google Scholar] [CrossRef]
- Pothineni, V.R.; Wagh, D.; Babar, M.M.; Inayathullah, M.; Solow-Cordero, D.; Kim, K.M.; Samineni, A.V.; Parekh, M.B.; Tayebi, L.; Rajadas, J. Identification of new drug candidates against Borrelia burgdorferi using high-throughput screening. Drug Des. Dev. Ther. 2016, 10, 1307–1322. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zając, V.; Bell-Sakyi, L.; Wójcik-Fatla, A. Use of Tick Cell Lines in Co-Infection Studies with a Preliminary Study of Co-Culture of Borrelia burgdorferi and Anaplasma phagocytophilum. Pathogens 2025, 14, 78. https://doi.org/10.3390/pathogens14010078
Zając V, Bell-Sakyi L, Wójcik-Fatla A. Use of Tick Cell Lines in Co-Infection Studies with a Preliminary Study of Co-Culture of Borrelia burgdorferi and Anaplasma phagocytophilum. Pathogens. 2025; 14(1):78. https://doi.org/10.3390/pathogens14010078
Chicago/Turabian StyleZając, Violetta, Lesley Bell-Sakyi, and Angelina Wójcik-Fatla. 2025. "Use of Tick Cell Lines in Co-Infection Studies with a Preliminary Study of Co-Culture of Borrelia burgdorferi and Anaplasma phagocytophilum" Pathogens 14, no. 1: 78. https://doi.org/10.3390/pathogens14010078
APA StyleZając, V., Bell-Sakyi, L., & Wójcik-Fatla, A. (2025). Use of Tick Cell Lines in Co-Infection Studies with a Preliminary Study of Co-Culture of Borrelia burgdorferi and Anaplasma phagocytophilum. Pathogens, 14(1), 78. https://doi.org/10.3390/pathogens14010078





