A Case of Dibothriocephalosis (Dibothriocephalus latus) from Iseo Lake (Northern Italy): An Update on a Persistent Sanitary Issue
Abstract
1. Introduction
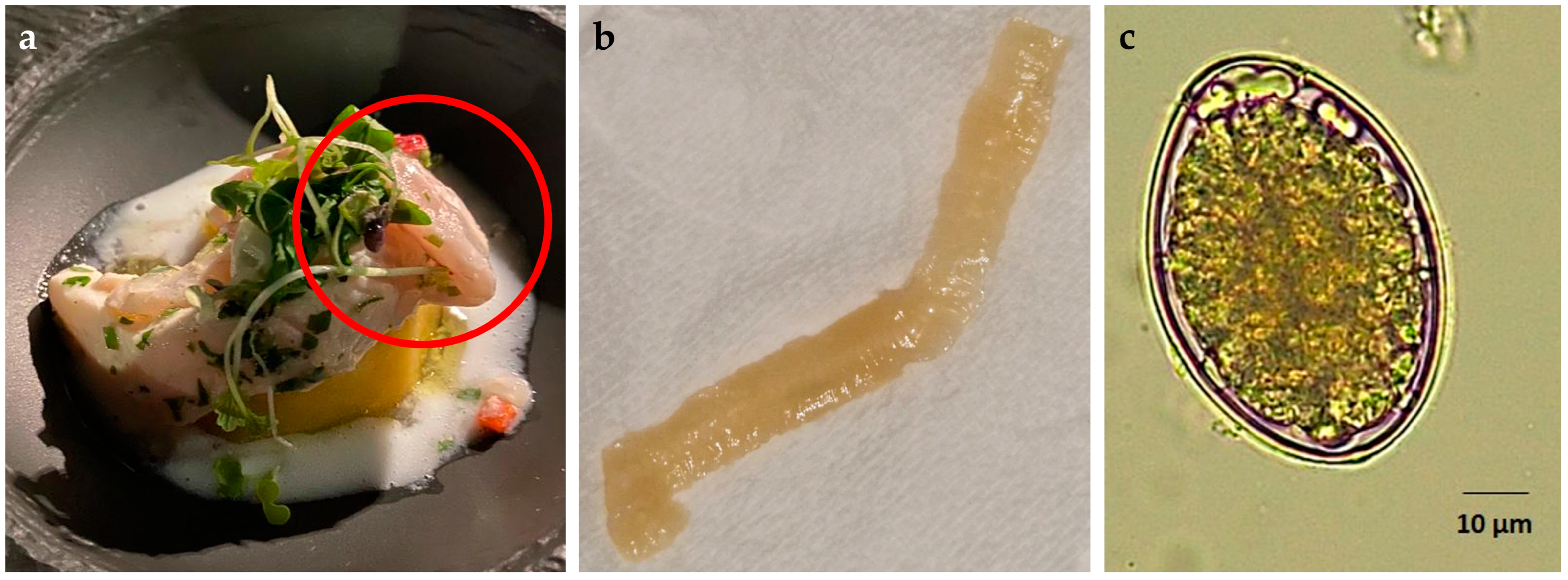
2. Case Report
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Menconi, V.; Lazzaro, E.; Bertola, M.; Guardone, L.; Mazzucato, M.; Prearo, M.; Bilska-Zajac, E.; Cortinovis, L.; Manfrin, A.; Arcangeli, G.; et al. The Occurrence of Freshwater Fish-Borne Zoonotic Helminths in Italy and Neighbouring Countries: A Systematic Review. Animals 2023, 13, 3793. [Google Scholar] [CrossRef] [PubMed]
- Radačovská, A.; Bazsalovicsová, E.; Costa, I.B.; Orosová, M.; Gustinelli, A.; Králová-Hromadová, I. Occurrence of Dibothriocephalus latus in European Perch from Alpine Lakes, an Important Focus of Diphyllobothriosis in Europe. Rev. Suisse De Zool. 2020, 126, 219–225. [Google Scholar] [CrossRef]
- Menconi, V.; Zoppi, S.; Pastorino, P.; Di Blasio, A.; Tedeschi, R.; Pizzul, E.; Mugetti, D.; Tomasoni, M.; Dondo, A.; Prearo, M. Relationship between the Prevalence of Dibothriocephalus latus (Cestoda: Diphyllobothriidea) and the Load of Escherichia Coli: New Findings in a Neglected Fish-Borne Parasitic Zoonosis. Zoonoses Public Health 2021, 68, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Gustinelli, A.; Menconi, V.; Prearo, M.; Caffara, M.; Righetti, M.; Scanzio, T.; Raglio, A.; Fioravanti, M.L. Prevalence of Diphyllobothrium latum (Cestoda: Diphyllobothriidae) Plerocercoids in Fish Species from Four Italian Lakes and Risk for the Consumers. Int. J. Food Microbiol. 2016, 235, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Peduzzi, R.; Boucher-Rodoni, R. Resurgence of Human Bothriocephalosis (Diphyllobothrium latum) in the Subalpine Lake Region. J. Limnol. 2001, 60, 41–44. [Google Scholar] [CrossRef]
- Dupouy-Camet, J.; Peduzzi, R. Current Situation of Human Diphyllobothriasis in Europe. Euro Surveill. 2004, 9, 5–6. [Google Scholar] [CrossRef] [PubMed]
- Wicht, B.; Yanagida, T.; Scholz, T.; Ito, A.; Jiménez, J.A.; Brabec, J. Multiplex PCR for Differential Identification of Broad Tapeworms (Cestoda: Diphyllobothrium) Infecting Humans. J. Clin. Microbiol. 2010, 48, 3111–3116. [Google Scholar] [CrossRef] [PubMed]
- Králová-Hromadová, I.; Radačovská, A.; Čisovská Bazsalovicsová, E.; Kuchta, R. Ups and downs of infections with broad fish tapeworm Dibothriocephalus latus in Europe from 1900 to 2020: Part I. Adv. Parasitol. 2021, 114, 75–166. [Google Scholar] [CrossRef]
- Scholz, T.; Garcia, H.H.; Kuchta, R.; Wicht, B. Update on the Human Broad Tapeworm (Genus Diphyllobothrium), Including Clinical Relevance. Clin. Microbiol. Rev. 2009, 22, 146. [Google Scholar] [CrossRef] [PubMed]
- Bowles, J.; Blair, D.; McManus, D.P. Genetic Variants within the Genus Echinococcus Identified by Mitochondrial DNA Sequencing. Mol. Biochem. Parasitol. 1992, 54, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, J.H. Images in Clinical Medicine. Diphyllobothrium latum during Colonoscopy. N. Engl. J. Med. 2010, 362, e40. [Google Scholar] [CrossRef]
- Nutman, T.B.; Weller, P.F. Diphyllobothriasis. In Harrison’s Principles of Internal Medicine, 14th ed.; Volume I: International edition; McGrow-Hell: New York, NY, USA, 1998; pp. 1226–1227. [Google Scholar]
- Mohan, S.; Halle-Ekane, G.; Konje, J.C. Intestinal Parasitic Infections in Pregnancy—A Review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 254, 59–63. [Google Scholar] [CrossRef]
- Wilcox, S.R.; Thomas, S.; Brown, D.F.M.; Nadel, E.S. Gastrointestinal Parasite. J. Emerg. Med. 2007, 33, 277–280. [Google Scholar] [CrossRef]
- Salminen, K. The Infestiveness of Heat and Cold Exposed Diphyllobothrium latum Plerocercoids on Golden Hamster. Acta Vet. Scand. 1970, 11, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A32002R0178 (accessed on 1 October 2024).
- Regulation (EC) No 853/2004 of the European Parliament and of the Council of 29 April 2004. Available online: https://eur-lex.europa.eu/eli/reg/2004/853/oj (accessed on 1 October 2024).
- Regulation (EC) No 2074/2005 of the European Parliament and of the Council of 5 December 2005. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A32005R2074 (accessed on 1 October 2024).
- Regulation (EC) No 1276/2011 of the European Parliament and of the Council of 8 December 2011. Available online: https://eur-lex.europa.eu/eli/reg/2011/1276/oj (accessed on 1 October 2024).
- Yera, H.; Estran, C.; Delaunay, P.; Gari-Toussaint, M.; Dupouy-Camet, J.; Marty, P. Putative Diphyllobothrium nihonkaiense Acquired from a Pacific Salmon (Oncorhynchus Keta) Eaten in France; Genomic Identification and Case Report. Parasitol. Int. 2006, 55, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Wicht, B.; de Marval, F.; Peduzzi, R. Diphyllobothrium nihonkaiense (Yamane et al., 1986) in Switzerland: First Molecular Evidence and Case Reports. Parasitol. Int. 2007, 56, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Greigert, V.; Brunet, J.; Pfaff, A.W.; Lemoine, J.P.; Candolfi, E.; Abou-Bacar, A. Locally Acquired Infection with Dibothriocephalus Nihonkaiense (=Diphyllobothrium nihonkaiense) in France: The Importance of Molecular Diagnosis. Parasitol. Res. 2020, 119, 513–518. [Google Scholar] [CrossRef] [PubMed]
- De Marval, F.; Gottstein, B.; Weber, M.; Wicht, B. Imported Diphyllobothriasis in Switzerland: Molecular Methods to Define a Clinical Case of Diphyllobothrium Infection as Diphyllobothrium Dendriticum, August 2010. Eurosurveillance 2013, 18, 20355. [Google Scholar] [CrossRef]
- Esteban, J.G.; Muñoz-Antoli, C.; Borras, M.; Colomina, J.; Toledo, R. Human Infection by a “Fish Tapeworm”, Diphyllobothrium latum, in a Non-Endemic Country. Infection 2014, 42, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Dupouy-Camet, J.; Gay, M.; Houin, R. New Eating Habits, New Parasitic Risks: The Example of Fish. Bull. Acad. Natl. Med. 2020, 204, 1010–1016. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menconi, V.; Guardone, L.; Lazzaro, E.; Bottazzo, R.; Besutti, V.; Danesi, P.; Manfrin, A.; Basso, A.; Arcangeli, G.; Cortinovis, L.; et al. A Case of Dibothriocephalosis (Dibothriocephalus latus) from Iseo Lake (Northern Italy): An Update on a Persistent Sanitary Issue. Pathogens 2025, 14, 100. https://doi.org/10.3390/pathogens14010100
Menconi V, Guardone L, Lazzaro E, Bottazzo R, Besutti V, Danesi P, Manfrin A, Basso A, Arcangeli G, Cortinovis L, et al. A Case of Dibothriocephalosis (Dibothriocephalus latus) from Iseo Lake (Northern Italy): An Update on a Persistent Sanitary Issue. Pathogens. 2025; 14(1):100. https://doi.org/10.3390/pathogens14010100
Chicago/Turabian StyleMenconi, Vasco, Lisa Guardone, Elena Lazzaro, Romina Bottazzo, Valeria Besutti, Patrizia Danesi, Amedeo Manfrin, Andrea Basso, Giuseppe Arcangeli, Luana Cortinovis, and et al. 2025. "A Case of Dibothriocephalosis (Dibothriocephalus latus) from Iseo Lake (Northern Italy): An Update on a Persistent Sanitary Issue" Pathogens 14, no. 1: 100. https://doi.org/10.3390/pathogens14010100
APA StyleMenconi, V., Guardone, L., Lazzaro, E., Bottazzo, R., Besutti, V., Danesi, P., Manfrin, A., Basso, A., Arcangeli, G., Cortinovis, L., Bilska-Zając, E., & Angeloni, G. (2025). A Case of Dibothriocephalosis (Dibothriocephalus latus) from Iseo Lake (Northern Italy): An Update on a Persistent Sanitary Issue. Pathogens, 14(1), 100. https://doi.org/10.3390/pathogens14010100








