Advances in Leishmania Vaccines: Current Development and Future Prospects
Abstract
1. Introduction
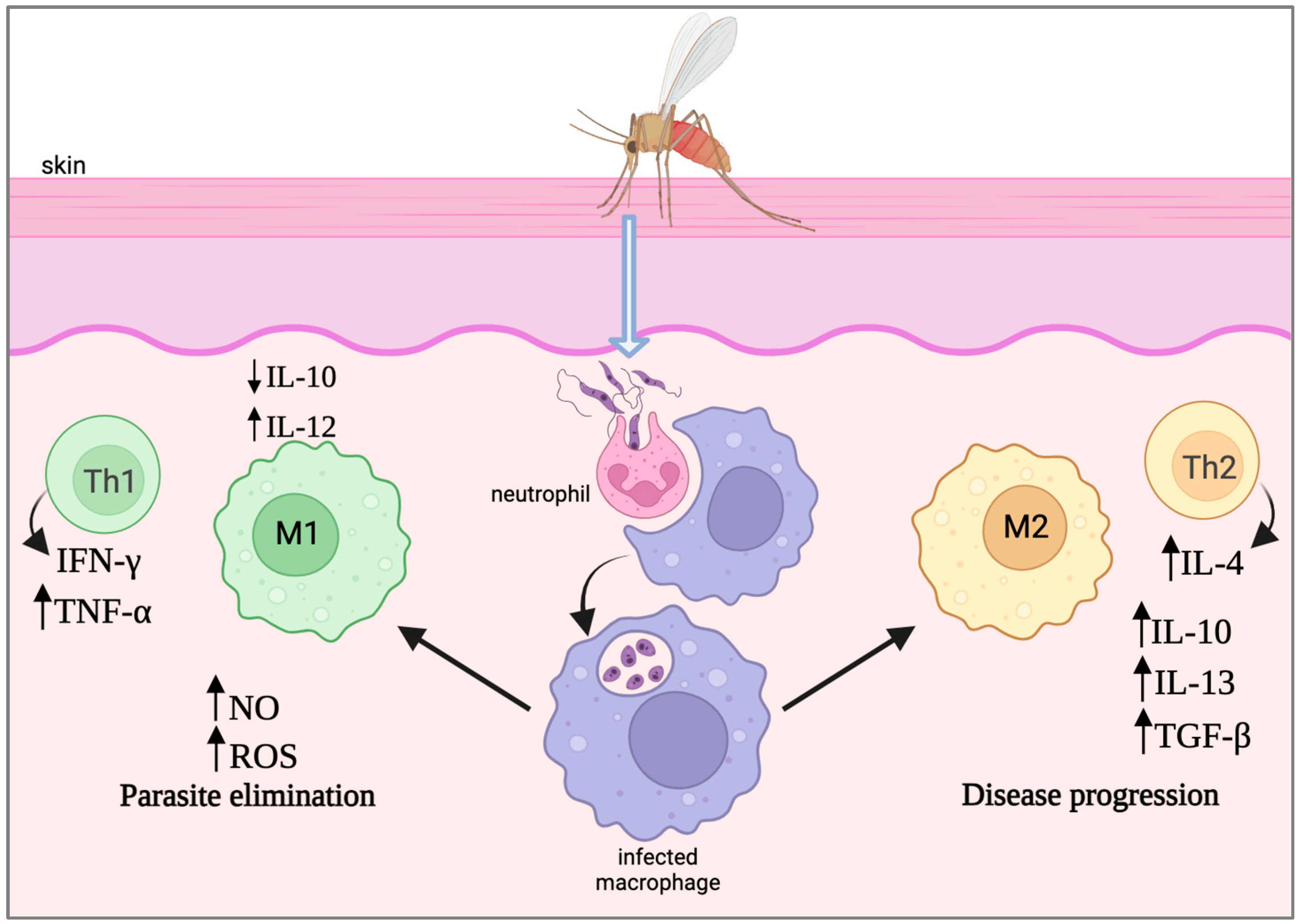
2. Immunological Landscape of Leishmania Infection
2.1. Host Immune Response against Leishmania
2.2. Mechanisms of Immune Evasion by Leishmania Parasites
2.3. Immune Dynamics of Leishmania–HIV Coinfection
3. First-Generation Antileishmanial Vaccines
3.1. Whole-Killed Parasites
3.2. Live-Attenuated Parasites
4. Second-Generation Antileishmanial Vaccines
5. Third-Generation Antileishmanial Vaccines
6. Conclusions: Limitations and Future Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Antinori, S.; Giacomelli, A. Leishmaniasis. In Encyclopedia of Infection and Immunity; Elsevier: Amsterdam, The Netherlands, 2022; pp. 622–643. [Google Scholar]
- Torres-Guerrero, E.; Quintanilla-Cedillo, M.R.; Ruiz-Esmenjaud, J.; Arenas, R. Leishmaniasis: A Review. F1000Research 2017, 6, 750. [Google Scholar] [CrossRef] [PubMed]
- Miramin-Mohammadi, A.; Javadi, A.; Eskandari, S.E.; Mortazavi, H.; Rostami, M.N.; Khamesipour, A. Immune Response in Cutaneous Leishmaniasis Patients with Healing vs. Non-Healing Lesions. Iran. J. Microbiol. 2020, 12, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Ronet, C.; Beverley, S.M.; Fasel, N. Muco-Cutaneous Leishmaniasis in the New World. Virulence 2011, 2, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Severino, P.; Santana, W.; Lisboa, E.S.; dos Santos, V.L.S.; Lima, E.T.d.S.; Cardoso, J.C.; de Albuquerque-Junior, R.L.C.; Naveros, B.C.; Santini, A.; Souto, E.B.; et al. Cutaneous/Mucocutaneous Leishmaniasis Treatment for Wound Healing: Classical versus New Treatment Approaches. Microbiol. Res. 2022, 13, 836–852. [Google Scholar] [CrossRef]
- Abadías-Granado, I.; Diago, A.; Cerro, P.A.; Palma-Ruiz, A.M.; Gilaberte, Y. Cutaneous and Mucocutaneous Leishmaniasis. Actas Dermo-Sifiliográficas (Engl. Ed.) 2021, 112, 601–618. [Google Scholar] [CrossRef]
- Pasha, F.; Saleem, S.; Nazir, T.; Tariq, J.; Qureshi, K. Visceral Leishmaniasis (Kala-Azar): A Triumph Against a Trickster Disease. Cureus 2022, 14, e25698. [Google Scholar] [CrossRef]
- Ready, P.D. Epidemiology of Visceral Leishmaniasis. Clin. Epidemiol. 2014, 6, 147–154. [Google Scholar] [CrossRef]
- Scarpini, S.; Dondi, A.; Totaro, C.; Biagi, C.; Melchionda, F.; Zama, D.; Pierantoni, L.; Gennari, M.; Campagna, C.; Prete, A.; et al. Visceral Leishmaniasis: Epidemiology, Diagnosis, and Treatment Regimens in Different Geographical Areas with a Focus on Pediatrics. Microorganisms 2022, 10, 1887. [Google Scholar] [CrossRef]
- Alvar, J.; Vélez, I.D.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; Jannin, J.; Boer, M. den Leishmaniasis Worldwide and Global Estimates of Its Incidence. PLoS ONE 2012, 7, e35671. [Google Scholar] [CrossRef]
- DebRoy, S.; Prosper, O.; Mishoe, A.; Mubayi, A. Challenges in Modeling Complexity of Neglected Tropical Diseases: A Review of Dynamics of Visceral Leishmaniasis in Resource Limited Settings. Emerg. Themes Epidemiol. 2017, 14, 10. [Google Scholar] [CrossRef]
- Chakravarty, J.; Sundar, S. Current and Emerging Medications for the Treatment of Leishmaniasis. Expert Opin. Pharmacother. 2019, 20, 1251–1265. [Google Scholar] [CrossRef] [PubMed]
- Ponte-Sucre, A.; Gamarro, F.; Dujardin, J.-C.; Barrett, M.P.; López-Vélez, R.; García-Hernández, R.; Pountain, A.W.; Mwenechanya, R.; Papadopoulou, B. Drug Resistance and Treatment Failure in Leishmaniasis: A 21st Century Challenge. PLoS Negl. Trop. Dis. 2017, 11, e0006052. [Google Scholar] [CrossRef] [PubMed]
- Knight, C.A.; Harris, D.R.; Alshammari, S.O.; Gugssa, A.; Young, T.; Lee, C.M. Leishmaniasis: Recent Epidemiological Studies in the Middle East. Front. Microbiol. 2023, 13, 1052478. [Google Scholar] [CrossRef]
- Kedzierski, L. Leishmaniasis Vaccine: Where Are We Today? J. Glob. Infect. Dis. 2010, 2, 177. [Google Scholar] [CrossRef]
- Zutshi, S.; Kumar, S.; Chauhan, P.; Bansode, Y.; Nair, A.; Roy, S.; Sarkar, A.; Saha, B. Anti-Leishmanial Vaccines: Assumptions, Approaches, and Annulments. Vaccines 2019, 7, 156. [Google Scholar] [CrossRef] [PubMed]
- Lacey, C.; Musa, A.; Khalil, E.T.; Younis, B.; Osman, M.; Wiggins, R.; Keding, A.; Kaye, P. LEISH2b—A Phase 2b Study to Assess the Safety, Efficacy, and Immunogenicity of the Leishmania Vaccine ChAd63-KH in Post-Kala Azar Dermal Leishmaniasis. Wellcome Open Res. 2022, 7, 200. [Google Scholar] [CrossRef]
- Fernández Cotrina, J.; Iniesta, V.; Monroy, I.; Baz, V.; Hugnet, C.; Marañon, F.; Fabra, M.; Gómez-Nieto, L.C.; Alonso, C. A Large-Scale Field Randomized Trial Demonstrates Safety and Efficacy of the Vaccine LetiFend® against Canine Leishmaniosis. Vaccine 2018, 36, 1972–1982. [Google Scholar] [CrossRef]
- Coelho, E.A.F.; Christodoulides, M. Vaccines for Canine Leishmaniasis. In Vaccines for Neglected Pathogens: Strategies, Achievements and Challenges; Springer International Publishing: Cham, Switzerland, 2023; pp. 281–306. [Google Scholar]
- Velez, R.; Gállego, M. Commercially Approved Vaccines for Canine Leishmaniosis: A Review of Available Data on Their Safety and Efficacy. Trop. Med. Int. Health 2020, 25, 540–557. [Google Scholar] [CrossRef]
- Genaro, O.; de Toledo, V.P.C.P.; Da Costa, C.A.; Hermeto, M.V.; Afonso, L.C.C.; Mayrink, W. Vaccine for Prophylaxis and Immunotherapy, Brazil. Clin. Dermatol. 1996, 14, 503–512. [Google Scholar] [CrossRef]
- Araújo, M.S.S.; de Andrade, R.A.; Vianna, L.R.; Mayrink, W.; Reis, A.B.; Sathler-Avelar, R.; Teixeira-Carvalho, A.; Andrade, M.C.; Mello, M.N.; Martins-Filho, O.A. Despite Leishvaccine and Leishmune® Trigger Distinct Immune Profiles, Their Ability to Activate Phagocytes and CD8+ T-Cells Support Their High-Quality Immunogenic Potential against Canine Visceral Leishmaniasis. Vaccine 2008, 26, 2211–2224. [Google Scholar] [CrossRef]
- Satti, I.N.; Osman, H.Y.; Daifalla, N.S.; Younis, S.A.; Khalil, E.A.G.; Zijlstra, E.E.; El Hassan, A.M.; Ghalib, H.W. Immunogenicity and Safety of Autoclaved Leishmania major plus BCG Vaccine in Healthy Sudanese Volunteers. Vaccine 2001, 19, 2100–2106. [Google Scholar] [CrossRef] [PubMed]
- Armijos, R.X.; Weigel, M.M.; Romero, L.; Garcia, V.; Salazar, J. Field Trial of a Vaccine against New World Cutaneous Leishmaniasis in an At-Risk Child Population: How Long Does Protection Last? J. Infect. Dis. 2003, 187, 1959–1961. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Convit, J.; Ulrich, M.; Zerpa, O.; Borges, R.; Aranzazu, N.; Valera, M.; Villarroel, H.; Zapata, Z.; Tomedes, I. Immunotherapy of American Cutaneous Leishmaniasis in Venezuela during the Period 1990–1999. Trans. R. Soc. Trop. Med. Hyg. 2003, 97, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Convit, J.; Ulrich, M.; Polegre, M.A.; Avila, A.; Rodríguez, N.; Mazzedo, M.I.; Blanco, B. Therapy of Venezuelan Patients with Severe Mucocutaneous or Early Lesions of Diffuse Cutaneous Leishmaniasis with a Vaccine Containing Pasteurized Leishmania Promastigotes and Bacillus Calmette-Guerin: Preliminary Report. Memórias Inst. Oswaldo Cruz 2004, 99, 57–62. [Google Scholar] [CrossRef]
- Dantas-Torres, F. Leishmune® Vaccine: The Newest Tool for Prevention and Control of Canine Visceral Leishmaniosis and Its Potential as a Transmission-Blocking Vaccine. Vet. Parasitol. 2006, 141, 1–8. [Google Scholar] [CrossRef]
- Lemesre, J.-L.; Holzmuller, P.; Gonçalves, R.B.; Bourdoiseau, G.; Hugnet, C.; Cavaleyra, M.; Papierok, G. Long-Lasting Protection against Canine Visceral Leishmaniasis Using the LiESAp-MDP Vaccine in Endemic Areas of France: Double-Blind Randomised Efficacy Field Trial. Vaccine 2007, 25, 4223–4234. [Google Scholar] [CrossRef]
- Velez, R.; Domenech, E.; Cairó, J.; Gállego, M. The Impact of Canine Leishmaniosis Vaccination with Canileish® in Leishmania infantum Infection Seroprevalence Studies. Acta Trop. 2020, 202, 105259. [Google Scholar] [CrossRef]
- Toepp, A.; Larson, M.; Wilson, G.; Grinnage-Pulley, T.; Bennett, C.; Leal-Lima, A.; Anderson, B.; Parrish, M.; Anderson, M.; Fowler, H.; et al. Randomized, Controlled, Double-Blinded Field Trial to Assess Leishmania Vaccine Effectiveness as Immunotherapy for Canine Leishmaniosis. Vaccine 2018, 36, 6433–6441. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, J.; Kumar, S.; Trivedi, S.; Rai, V.K.; Singh, A.; Ashman, J.A.; Laughlin, E.M.; Coler, R.N.; Kahn, S.J.; Beckmann, A.M.; et al. A Clinical Trial to Evaluate the Safety and Immunogenicity of the LEISH-F1+MPL-SE Vaccine for Use in the Prevention of Visceral Leishmaniasis. Vaccine 2011, 29, 3531–3537. [Google Scholar] [CrossRef]
- NCT01011309. A Study of the Efficacy and Safety of the LEISH-F2 + MPL-SE Vaccine for Treatment of Cutaneous Leishmaniasis. Available online: https://clinicaltrials.gov/study/NCT01011309 (accessed on 15 May 2024).
- Coler, R.N.; Duthie, M.S.; Hofmeyer, K.A.; Guderian, J.; Jayashankar, L.; Vergara, J.; Rolf, T.; Misquith, A.; Laurance, J.D.; Raman, V.S.; et al. From Mouse to Man: Safety, Immunogenicity and Efficacy of a Candidate Leishmaniasis Vaccine LEISH-F3+GLA-SE. Clin. Transl. Immunol. 2015, 4, e35. [Google Scholar] [CrossRef]
- Younis, B.M.; Osman, M.; Khalil, E.A.G.; Santoro, F.; Furini, S.; Wiggins, R.; Keding, A.; Carraro, M.; Musa, A.E.A.; Abdarahaman, M.A.A.; et al. Safety and Immunogenicity of ChAd63-KH Vaccine in Post-Kala-Azar Dermal Leishmaniasis Patients in Sudan. Mol. Ther. 2021, 29, 2366–2377. [Google Scholar] [CrossRef] [PubMed]
- Elmahallawy, E.K.; Alkhaldi, A.A.M.; Saleh, A.A. Host Immune Response against Leishmaniasis and Parasite Persistence Strategies: A Review and Assessment of Recent Research. Biomed. Pharmacother. 2021, 139, 111671. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Uzonna, J.E. The Early Interaction of Leishmania with Macrophages and Dendritic Cells and Its Influence on the Host Immune Response. Front. Cell Infect. Microbiol. 2012, 2, 83. [Google Scholar] [CrossRef] [PubMed]
- Young, J.; Kima, P.E. The Leishmania Parasitophorous Vacuole Membrane at the Parasite-Host Interface. Yale J. Biol. Med. 2019, 92, 511–521. [Google Scholar]
- Scott, P.; Novais, F.O. Cutaneous Leishmaniasis: Immune Responses in Protection and Pathogenesis. Nat. Rev. Immunol. 2016, 16, 581–592. [Google Scholar] [CrossRef]
- Costa-da-Silva, A.C.; Nascimento, D.d.O.; Ferreira, J.R.M.; Guimarães-Pinto, K.; Freire-de-Lima, L.; Morrot, A.; Decote-Ricardo, D.; Filardy, A.A.; Freire-de-Lima, C.G. Immune Responses in Leishmaniasis: An Overview. Trop. Med. Infect. Dis. 2022, 7, 54. [Google Scholar] [CrossRef]
- Mills, C. M1 and M2 Macrophages: Oracles of Health and Disease. Crit. Rev. Immunol. 2012, 32, 463–488. [Google Scholar] [CrossRef]
- Vacas, A.; Fernández-Rubio, C.; Larrea, E.; Peña-Guerrero, J.; Nguewa, P.A. LmjF.22.0810 from Leishmania major Modulates the Th2-Type Immune Response and Is Involved in Leishmaniasis Outcome. Biomedicines 2020, 8, 452. [Google Scholar] [CrossRef] [PubMed]
- Tomiotto-Pellissier, F.; Bortoleti, B.T.d.S.; Assolini, J.P.; Gonçalves, M.D.; Carloto, A.C.M.; Miranda-Sapla, M.M.; Conchon-Costa, I.; Bordignon, J.; Pavanelli, W.R. Macrophage Polarization in Leishmaniasis: Broadening Horizons. Front. Immunol. 2018, 9, 2529. [Google Scholar] [CrossRef]
- Colmenares, M.; Kima, P.E.; Samoff, E.; Soong, L.; McMahon-Pratt, D. Perforin and Gamma Interferon Are Critical CD8 + T-Cell-Mediated Responses in Vaccine-Induced Immunity against Leishmania amazonensis Infection. Infect. Immun. 2003, 71, 3172–3182. [Google Scholar] [CrossRef][Green Version]
- Belkaid, Y.; Von Stebut, E.; Mendez, S.; Lira, R.; Caler, E.; Bertholet, S.; Udey, M.C.; Sacks, D. CD8+ T Cells Are Required for Primary Immunity in C57BL/6 Mice Following Low-Dose, Intradermal Challenge with Leishmania major. J. Immunol. 2002, 168, 3992–4000. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, H.; Bras-Gonçalves, R.; Negi, N.S.; Lemesre, J.-L.; Papierok, G.; Salotra, P. Role of CD8+ T Cells in Protection against Leishmania donovani Infection in Healed Visceral Leishmaniasis Individuals. BMC Infect. Dis. 2014, 14, 653. [Google Scholar] [CrossRef] [PubMed]
- Müller, I.; Kropf, P.; Etges, R.J.; Louis, J.A. Gamma Interferon Response in Secondary Leishmania major Infection: Role of CD8+ T Cells. Infect. Immun. 1993, 61, 3730–3738. [Google Scholar] [CrossRef] [PubMed]
- McMahon-Pratt, D.; Alexander, J. Does the Leishmania major Paradigm of Pathogenesis and Protection Hold for New World Cutaneous Leishmaniases or the Visceral Disease? Immunol. Rev. 2004, 201, 206–224. [Google Scholar] [CrossRef]
- Bosque; Saravia; Valderrama; Milon. Distinct Innate and Acquired Immune Responses to Leishmania in Putative Susceptible and Resistant Human Populations Endemically Exposed to L. (Viannia) panamensis Infection. Scand. J. Immunol. 2000, 51, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Inchaustegui, D.A.; Xin, L.; Soong, L. Leishmania braziliensis Infection Induces Dendritic Cell Activation, ISG15 Transcription, and the Generation of Protective Immune Responses. J. Immunol. 2008, 180, 7537–7545. [Google Scholar] [CrossRef]
- Tuon, F.F.; Gomes-Silva, A.; Da-Cruz, A.M.; Duarte, M.I.S.; Neto, V.A.; Amato, V.S. Local Immunological Factors Associated with Recurrence of Mucosal Leishmaniasis. Clin. Immunol. 2008, 128, 442–446. [Google Scholar] [CrossRef]
- Da-Cruz, A.M.; Conceição-Silva, F.; Bertho, A.L.; Coutinho, S.G. Leishmania-Reactive CD4+ and CD8+ T Cells Associated with Cure of Human Cutaneous Leishmaniasis. Infect. Immun. 1994, 62, 2614–2618. [Google Scholar] [CrossRef]
- Coutinho, S.G.; Oliveira, M.P.; Da-Cruz, A.M.; De Luca, P.M.; Mendonça, S.C.F.; Bertho, A.L.; Soong, L.; McMahon-Pratt, D. T-Cell Responsiveness of American Cutaneous Leishmaniasis Patients to Purified Leishmania pifanoi Amastigote Antigens And Leishmania braziliensis Promastigote Antigens: Immunologic Patterns Associated with Cure. Exp. Parasitol. 1996, 84, 144–155. [Google Scholar] [CrossRef]
- Jayakumar, A.; Castilho, T.M.; Park, E.; Goldsmith-Pestana, K.; Blackwell, J.M.; McMahon-Pratt, D. TLR1/2 Activation during Heterologous Prime-Boost Vaccination (DNA-MVA) Enhances CD8+ T Cell Responses Providing Protection against Leishmania (Viannia). PLoS Negl. Trop. Dis. 2011, 5, e1204. [Google Scholar] [CrossRef]
- Stäger, S.; Rafati, S. CD8+ T Cells in Leishmania Infections: Friends or Foes? Front. Immunol. 2012, 3, 5. [Google Scholar] [CrossRef] [PubMed]
- Ikeogu, N.M.; Akaluka, G.N.; Edechi, C.A.; Salako, E.S.; Onyilagha, C.; Barazandeh, A.F.; Uzonna, J.E. Leishmania Immunity: Advancing Immunotherapy and Vaccine Development. Microorganisms 2020, 8, 1201. [Google Scholar] [CrossRef]
- Filho, A.A.P.; Nascimento, A.A.d.S.; Saab, N.A.A.; Fugiwara, R.T.; D’Ávila Pessoa, G.C.; Koerich, L.B.; Pereira, M.H.; Araújo, R.N.; Sant’Anna, M.R.V.; Gontijo, N.F. Evasion of the Complement System by Leishmania through the Uptake of Factor H, a Complement Regulatory Protein. Acta Trop. 2021, 224, 106152. [Google Scholar] [CrossRef]
- Hermoso, T.; Fishelson, Z.; Becker, S.I.; Hirschberg, K.; Jaffe, C.L. Leishmanial Protein Kinases Phosphorylate Components of the Complement System. EMBO J. 1991, 10, 4061–4067. [Google Scholar] [CrossRef]
- Chan, A.; Ayala, J.-M.; Alvarez, F.; Piccirillo, C.; Dong, G.; Langlais, D.; Olivier, M. The Role of Leishmania GP63 in the Modulation of Innate Inflammatory Response to Leishmania major Infection. PLoS ONE 2021, 16, e0262158. [Google Scholar] [CrossRef] [PubMed]
- El-Zayat, S.R.; Sibaii, H.; Mannaa, F.A. Toll-like Receptors Activation, Signaling, and Targeting: An Overview. Bull. Natl. Res. Cent. 2019, 43, 187. [Google Scholar] [CrossRef]
- Chauhan, P.; Shukla, D.; Chattopadhyay, D.; Saha, B. Redundant and Regulatory Roles for Toll-like Receptors in Leishmania Infection. Clin. Exp. Immunol. 2017, 190, 167–186. [Google Scholar] [CrossRef]
- Srivastava, S.; Pandey, S.P.; Jha, M.K.; Chandel, H.S.; Saha, B. Leishmania Expressed Lipophosphoglycan Interacts with Toll-like Receptor (TLR)-2 to Decrease TLR-9 Expression and Reduce Anti-Leishmanial Responses. Clin. Exp. Immunol. 2013, 172, 403–409. [Google Scholar] [CrossRef]
- Faria, M.S.; Reis, F.C.G.; Azevedo-Pereira, R.L.; Morrison, L.S.; Mottram, J.C.; Lima, A.P.C.A. Leishmania Inhibitor of Serine Peptidase 2 Prevents TLR4 Activation by Neutrophil Elastase Promoting Parasite Survival in Murine Macrophages. J. Immunol. 2011, 186, 411–422. [Google Scholar] [CrossRef]
- Shweash, M.; Adrienne McGachy, H.; Schroeder, J.; Neamatallah, T.; Bryant, C.E.; Millington, O.; Mottram, J.C.; Alexander, J.; Plevin, R. Leishmania mexicana Promastigotes Inhibit Macrophage IL-12 Production via TLR-4 Dependent COX-2, INOS and Arginase-1 Expression. Mol. Immunol. 2011, 48, 1800–1808. [Google Scholar] [CrossRef]
- Desjardins, M.; Descoteaux, A. Inhibition of Phagolysosomal Biogenesis by the Leishmania Lipophosphoglycan. J. Exp. Med. 1997, 185, 2061–2068. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, S.; Ghosh, S.; Hussain, S.; Paul, J.; Mukherjee, B. Linking Membrane Fluidity with Defective Antigen Presentation in Leishmaniasis. Parasite Immunol. 2021, 43, e12835. [Google Scholar] [CrossRef]
- Shio, M.T.; Hassani, K.; Isnard, A.; Ralph, B.; Contreras, I.; Gomez, M.A.; Abu-Dayyeh, I.; Olivier, M. Host Cell Signalling and Leishmania Mechanisms of Evasion. J. Trop. Med. 2012, 2012, 819512. [Google Scholar] [CrossRef]
- Steigerwald, M.; Moll, H. Leishmania major Modulates Chemokine and Chemokine Receptor Expression by Dendritic Cells and Affects Their Migratory Capacity. Infect. Immun. 2005, 73, 2564–2567. [Google Scholar] [CrossRef]
- Seyed, N.; Taheri, T.; Rafati, S. Live Attenuated-Nonpathogenic Leishmania and DNA Structures as Promising Vaccine Platforms against Leishmaniasis: Innovations Can Make Waves. Front. Microbiol. 2024, 15, 6369. [Google Scholar] [CrossRef] [PubMed]
- Nateghi-Rostami, M.; Sohrabi, Y. Memory T Cells: Promising Biomarkers for Evaluating Protection and Vaccine Efficacy against Leishmaniasis. Front. Immunol. 2024, 15, 4696. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-W.; Karmakar, S.; Gannavaram, S.; Dey, R.; Lypaczewski, P.; Ismail, N.; Siddiqui, A.; Simonyan, V.; Oliveira, F.; Coutinho-Abreu, I.V.; et al. A Second Generation Leishmanization Vaccine with a Markerless Attenuated Leishmania major Strain Using CRISPR Gene Editing. Nat. Commun. 2020, 11, 3461. [Google Scholar] [CrossRef]
- Maksoud, S.; El Hokayem, J. The Cytokine/Chemokine Response in Leishmania/HIV Infection and Co-Infection. Heliyon 2023, 9, e15055. [Google Scholar] [CrossRef]
- Bernier, R.; Turco, S.J.; Olivier, M.; Tremblay, M. Activation of Human Immunodeficiency Virus Type 1 in Monocytoid Cells by the Protozoan Parasite Leishmania donovani. J. Virol. 1995, 69, 7282–7285. [Google Scholar] [CrossRef]
- Wolday, D.; Akuffo, H.; Britton, S.; Hathaway, A.; Sander, B. HIV-1 Inhibits Leishmania-Induced Cell Proliferation but Not Production of Interleukin-6 and Tumour Necrosis Factor Alpha. Scand. J. Immunol. 1994, 39, 380–386. [Google Scholar] [CrossRef]
- Bentwich, Z. Concurrent Infections That Rise the HIV Viral Load. J. HIV Ther. 2003, 8, 72–75. [Google Scholar] [PubMed]
- Wolday, D.; Akuffo, H.; Demissie, A.; Britton, S. Role of Leishmania donovani and Its Lipophosphoglycan in CD4+ T-Cell Activation-Induced Human Immunodeficiency Virus Replication. Infect. Immun. 1999, 67, 5258–5264. [Google Scholar] [CrossRef]
- Cacopardo, B.; Nigro, L.; Preiser, W.; Famà, A.; Satariano, M.I.; Braner, J.; Celesia, B.M.; Weber, B.; Russo, R.; Doerr, H.W. Prolonged Th2 Cell Activation and Increased Viral Replication in HIV-Leishmania Co-Infected Patients despite Treatment. Trans. R. Soc. Trop. Med. Hyg. 1996, 90, 434–435. [Google Scholar] [CrossRef] [PubMed]
- Barreto-de-Souza, V.; Pacheco, G.J.; Silva, A.R.; Castro-Faria-Neto, H.C.; Bozza, P.T.; Saraiva, E.M.; Bou-Habib, D.C. Increased Leishmania Replication in HIV-1–Infected Macrophages Is Mediated by Tat Protein through Cyclooxygenase-2 Expression and Prostaglandin E 2 Synthesis. J. Infect. Dis. 2006, 194, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Alvar, J.; Aparicio, P.; Aseffa, A.; Den Boer, M.; Cañavate, C.; Dedet, J.-P.; Gradoni, L.; Ter Horst, R.; López-Vélez, R.; Moreno, J. The Relationship between Leishmaniasis and AIDS: The Second 10 Years. Clin. Microbiol. Rev. 2008, 21, 334–359. [Google Scholar] [CrossRef]
- Crum-Cianflone, N.F.; Wallace, M.R. Vaccination in HIV-Infected Adults. AIDS Patient Care STDS 2014, 28, 397–410. [Google Scholar] [CrossRef] [PubMed]
- Rubin, L.G.; Levin, M.J.; Ljungman, P.; Davies, E.G.; Avery, R.; Tomblyn, M.; Bousvaros, A.; Dhanireddy, S.; Sung, L.; Keyserling, H.; et al. Executive Summary: 2013 IDSA Clinical Practice Guideline for Vaccination of the Immunocompromised Host. Clin. Infect. Dis. 2014, 58, 309–318. [Google Scholar] [CrossRef]
- Khalil, E.A.G. Vaccines for Visceral Leishmaniasis: Hopes and Hurdles. In Leishmaniases as Re-emerging Diseases; InTech: London, UK, 2018. [Google Scholar]
- Noazin, S.; Khamesipour, A.; Moulton, L.H.; Tanner, M.; Nasseri, K.; Modabber, F.; Sharifi, I.; Khalil, E.A.G.; Bernal, I.D.V.; Antunes, C.M.F.; et al. Efficacy of Killed Whole-Parasite Vaccines in the Prevention of Leishmaniasis—A Meta-Analysis. Vaccine 2009, 27, 4747–4753. [Google Scholar] [CrossRef] [PubMed]
- Kamil, A.A.; Khalil, E.A.G.; Musa, A.M.; Modabber, F.; Mukhtar, M.M.; Ibrahim, M.E.; Zijlstra, E.E.; Sacks, D.; Smith, P.G.; Zicker, F.; et al. Alum-Precipitated Autoclaved Leishmania major plus Bacille Calmette-Guérrin, a Candidate Vaccine for Visceral Leishmaniasis: Safety, Skin-Delayed Type Hypersensitivity Response and Dose Finding in Healthy Volunteers. Trans. R. Soc. Trop. Med. Hyg. 2003, 97, 365–368. [Google Scholar] [CrossRef]
- Schetters, T.H.P.M.; Gravendyck, M. Regulations and Procedures in Parasite Vaccine Development. Parasitology 2006, 133, S189–S195. [Google Scholar] [CrossRef]
- Bertholet, S.; Goto, Y.; Carter, L.; Bhatia, A.; Howard, R.F.; Carter, D.; Coler, R.N.; Vedvick, T.S.; Reed, S.G. Optimized Subunit Vaccine Protects against Experimental Leishmaniasis. Vaccine 2009, 27, 7036–7045. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.C.; Kumar, A.; Samant, M. Genetically Modified Live Attenuated Vaccine: A Potential Strategy to Combat Visceral Leishmaniasis. Parasite Immunol. 2020, 42, e12732. [Google Scholar] [CrossRef]
- Dey, R.; Dagur, P.K.; Selvapandiyan, A.; McCoy, J.P.; Salotra, P.; Duncan, R.; Nakhasi, H.L. Live Attenuated Leishmania donovani P27 Gene Knockout Parasites Are Nonpathogenic and Elicit Long-Term Protective Immunity in BALB/c Mice. J. Immunol. 2013, 190, 2138–2149. [Google Scholar] [CrossRef]
- Abbaszadeh Afshar, M.J.; Elikaee, S.; Saberi, R.; Mohtasebi, S.; Mohebali, M. Expression Analysis of Centrin Gene in Promastigote and Amastigote Forms of Leishmania infantum Iranian Isolates: A Promising Target for Live Attenuated Vaccine Development against Canine Leishmaniasis. BMC Vet. Res. 2021, 17, 162. [Google Scholar] [CrossRef] [PubMed]
- Selvapandiyan, A.; Dey, R.; Gannavaram, S.; Solanki, S.; Salotra, P.; Nakhasi, H.L. Generation of Growth Arrested Leishmania Amastigotes: A Tool to Develop Live Attenuated Vaccine Candidates against Visceral Leishmaniasis. Vaccine 2014, 32, 3895–3901. [Google Scholar] [CrossRef] [PubMed]
- Volpedo, G.; Pacheco-Fernandez, T.; Holcomb, E.A.; Zhang, W.-W.; Lypaczewski, P.; Cox, B.; Fultz, R.; Mishan, C.; Verma, C.; Huston, R.H.; et al. Centrin-Deficient Leishmania mexicana Confers Protection against New World Cutaneous Leishmaniasis. NPJ Vaccines 2022, 7, 32. [Google Scholar] [CrossRef]
- Sharma, R.; Avendaño Rangel, F.; Reis-Cunha, J.L.; Marques, L.P.; Figueira, C.P.; Borba, P.B.; Viana, S.M.; Beneke, T.; Bartholomeu, D.C.; de Oliveira, C.I. Targeted Deletion of Centrin in Leishmania braziliensis Using CRISPR-Cas9-Based Editing. Front. Cell Infect. Microbiol. 2022, 11, 790418. [Google Scholar] [CrossRef]
- Selvapandiyan, A.; Debrabant, A.; Duncan, R.; Muller, J.; Salotra, P.; Sreenivas, G.; Salisbury, J.L.; Nakhasi, H.L. Centrin Gene Disruption Impairs Stage-Specific Basal Body Duplication and Cell Cycle Progression in Leishmania. J. Biol. Chem. 2004, 279, 25703–25710. [Google Scholar] [CrossRef] [PubMed]
- Plitnick, L.M. Global Regulatory Guidelines for Vaccines. In Nonclinical Development of Novel Biologics, Biosimilars, Vaccines and Specialty Biologics; Elsevier: Amsterdam, The Netherlands, 2013; pp. 225–241. [Google Scholar]
- Avendaño-Rangel, F.; Agra-Duarte, G.; Borba, P.B.; Moitinho, V.; Avila, L.T.; da Silva, L.O.; Viana, S.M.; Sharma, R.; Gannavaram, S.; Nakhasi, H.L.; et al. Immunization with Centrin-Deficient Leishmania braziliensis Does Not Protect against Homologous Challenge. Vaccines 2024, 12, 310. [Google Scholar] [CrossRef]
- Datta, S.; Roy, S.; Manna, M. Therapy with Radio-Attenuated Vaccine in Experimental Murine Visceral Leishmaniasis Showed Enhanced T Cell and Inducible Nitric Oxide Synthase Levels, Suppressed Tumor Growth Factor-Beta Production with Higher Expression of Some Signaling Molecules. Braz. J. Infect. Dis. 2015, 19, 36–42. [Google Scholar] [CrossRef]
- Daneshvar, H.; Coombs, G.H.; Hagan, P.; Phillips, R.S. Leishmania mexicana and Leishmania major: Attenuation of Wild-Type Parasites and Vaccination with the Attenuated Lines. J. Infect. Dis. 2003, 187, 1662–1668. [Google Scholar] [CrossRef] [PubMed]
- Daneshvar, H.; Namazi, M.J.; Kamiabi, H.; Burchmore, R.; Cleaveland, S.; Phillips, S. Gentamicin-Attenuated Leishmania infantum Vaccine: Protection of Dogs against Canine Visceral Leishmaniosis in Endemic Area of Southeast of Iran. PLoS Negl. Trop. Dis. 2014, 8, e2757. [Google Scholar] [CrossRef]
- Saljoughian, N.; Taheri, T.; Rafati, S. Live Vaccination Tactics: Possible Approaches for Controlling Visceral Leishmaniasis. Front. Immunol. 2014, 5, 134. [Google Scholar] [CrossRef] [PubMed]
- Breton, M.; Tremblay, M.J.; Ouellette, M.; Papadopoulou, B. Live Nonpathogenic Parasitic Vector as a Candidate Vaccine against Visceral Leishmaniasis. Infect. Immun. 2005, 73, 6372–6382. [Google Scholar] [CrossRef]
- Saljoughian, N.; Taheri, T.; Zahedifard, F.; Taslimi, Y.; Doustdari, F.; Bolhassani, A.; Doroud, D.; Azizi, H.; Heidari, K.; Vasei, M.; et al. Development of Novel Prime-Boost Strategies Based on a Tri-Gene Fusion Recombinant L. tarentolae Vaccine against Experimental Murine Visceral Leishmaniasis. PLoS Negl. Trop. Dis. 2013, 7, e2174. [Google Scholar] [CrossRef]
- Topuz Ata, D.; Hussain, M.; Jones, M.; Best, J.; Wiese, M.; Carter, K.C. Immunisation with Transgenic L. tarentolae Expressing Gamma Glutamyl Cysteine Synthetase from Pathogenic Leishmania Species Protected against L. major and L. donovani Infection in a Murine Model. Microorganisms 2023, 11, 1322. [Google Scholar] [CrossRef]
- Coler, R.; Reed, S. Second-Generation Vaccines against Leishmaniasis. Trends Parasitol. 2005, 21, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Morales-Yuste, M.; Martín-Sánchez, J.; Corpas-Lopez, V. Canine Leishmaniasis: Update on Epidemiology, Diagnosis, Treatment, and Prevention. Vet. Sci. 2022, 9, 387. [Google Scholar] [CrossRef]
- Dantas-Torres, F. Canine Leishmaniasis in the Americas: Etiology, Distribution, and Clinical and Zoonotic Importance. Parasit. Vectors 2024, 17, 198. [Google Scholar] [CrossRef]
- Moreno, J. Assessment of Vaccine-Induced Immunity Against Canine Visceral Leishmaniasis. Front. Vet. Sci. 2019, 6, 168. [Google Scholar] [CrossRef]
- Borja-Cabrera, G.P.; Santos, F.N.; Bauer, F.S.; Parra, L.E.; Menz, I.; Morgado, A.A.; Soares, I.S.; Batista, L.M.M.; Palatnik-de-Sousa, C.B. Immunogenicity Assay of the Leishmune® Vaccine against Canine Visceral Leishmaniasis in Brazil. Vaccine 2008, 26, 4991–4997. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, G.; Teva, A.; dos-Santos, C.B.; Santos, F.N.; Pinto, I.d.-S.; Fux, B.; Leite, G.R.; Falqueto, A. Field Trial of Efficacy of the Leish-Tec® Vaccine against Canine Leishmaniasis Caused by Leishmania infantum in an Endemic Area with High Transmission Rates. PLoS ONE 2017, 12, e0185438. [Google Scholar] [CrossRef] [PubMed]
- Bhowmick, S.; Ravindran, R.; Ali, N. Gp63 in Stable Cationic Liposomes Confers Sustained Vaccine Immunity to Susceptible BALB/c Mice Infected with Leishmania donovani. Infect. Immun. 2008, 76, 1003–1015. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Sundaram, S.; Singh, A.P.; Tripathi, A. A Gp63 Based Vaccine Candidate against Visceral Leishmaniasis. Bioinformation 2011, 5, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Kemp, M.; Theander, T.G.; Handman, E.; Hey, A.S.; Kurtzhals, J.A.L.; Hviid, L.; Sørensen, A.L.; Were, J.O.B.; Koech, D.K.; Kharazmi, A. Activation of Human T Lymphocytes by Leishmania Lipophosphoglycan. Scand. J. Immunol. 1991, 33, 219–224. [Google Scholar] [CrossRef]
- Mendoça, S.C.F.; Russell, D.G.; Coutinho, S.G. Analysis of the Human T Cell Responsiveness to Purified Antigens of Leishmania: Lipophosphoglycan (LPG) and Glycoprotein 63 (Gp 63). Clin. Exp. Immunol. 2008, 83, 472–478. [Google Scholar] [CrossRef]
- Jaffe, C.L.; Shor, R.; Trau, H.; Passwell, J.H. Parasite Antigens Recognized by Patients with Cutaneous Leishmaniasis. Clin. Exp. Immunol. 2008, 80, 77–82. [Google Scholar] [CrossRef]
- Garde, E.; Ramírez, L.; Corvo, L.; Solana, J.C.; Martín, M.E.; González, V.M.; Gómez-Nieto, C.; Barral, A.; Barral-Netto, M.; Requena, J.M.; et al. Analysis of the Antigenic and Prophylactic Properties of the Leishmania Translation Initiation Factors EIF2 and EIF2B in Natural and Experimental Leishmaniasis. Front. Cell Infect. Microbiol. 2018, 8, 112. [Google Scholar] [CrossRef]
- de Mendonça, S.C.F.; Cysne-Finkelstein, L.; Matos, D.C.d.S. Kinetoplastid Membrane Protein-11 as a Vaccine Candidate and a Virulence Factor in Leishmania. Front. Immunol. 2015, 6, 524. [Google Scholar] [CrossRef]
- Almeida, A.P.M.M.; Machado, L.F.M.; Doro, D.; Nascimento, F.C.; Damasceno, L.; Gazzinelli, R.T.; Fernandes, A.P.; Junqueira, C. New Vaccine Formulations Containing a Modified Version of the Amastigote 2 Antigen and the Non-Virulent Trypanosoma cruzi CL-14 Strain Are Highly Antigenic and Protective against Leishmania infantum Challenge. Front. Immunol. 2018, 9, 465. [Google Scholar] [CrossRef]
- Buxbaum, L.U.; Denise, H.; Coombs, G.H.; Alexander, J.; Mottram, J.C.; Scott, P. Cysteine Protease B of Leishmania mexicana Inhibits Host Th1 Responses and Protective Immunity. J. Immunol. 2003, 171, 3711–3717. [Google Scholar] [CrossRef] [PubMed]
- Mortazavidehkordi, N.; Fallah, A.; Abdollahi, A.; Kia, V.; Khanahmad, H.; Najafabadi, Z.G.; Hashemi, N.; Estiri, B.; Roudbari, Z.; Najafi, A.; et al. A Lentiviral Vaccine Expressing KMP11-HASPB Fusion Protein Increases Immune Response to Leishmania major in BALB/C. Parasitol. Res. 2018, 117, 2265–2273. [Google Scholar] [CrossRef]
- Lopes Valentim Di Paschoale Ostolin, T.; Rodrigues Gusmão, M.; Augusto Siqueira Mathias, F.; Mirelle de Oliveira Cardoso, J.; Mendes Roatt, B.; Dian de Oliveira Aguiar-Soares, R.; Conceição Ruiz, J.; de Melo Resende, D.; Cristiane Fortes de Brito, R.; Barbosa Reis, A. A Specific Leishmania infantum Polyepitope Vaccine Triggers Th1-Type Immune Response and Protects against Experimental Visceral Leishmaniasis. Cell Immunol. 2022, 380, 104592. [Google Scholar] [CrossRef] [PubMed]
- Joshi, J.; Kaur, S. Studies on the Protective Efficacy of Second-Generation Vaccine along with Standard Antileishmanial Drug in Leishmania donovani Infected BALB/c Mice. Parasitology 2014, 141, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Melo, A.R.d.S.; de Macêdo, L.S.; Invenção, M.d.C.V.; de Moura, I.A.; da Gama, M.A.T.M.; de Melo, C.M.L.; Silva, A.J.D.; Batista, M.V.d.A.; Freitas, A.C.d. Third-Generation Vaccines: Features of Nucleic Acid Vaccines and Strategies to Improve Their Efficiency. Genes 2022, 13, 2287. [Google Scholar] [CrossRef]
- Walker, P.S.; Scharton-Kersten, T.; Rowton, E.D.; Hengge, U.; Bouloc, A.; Udey, M.C.; Vogel, J.C. Genetic Immunization with Glycoprotein 63 cDNA Results in a Helper T Cell Type 1 Immune Response and Protection in a Murine Model of Leishmaniasis. Hum. Gene Ther. 1998, 9, 1899–1907. [Google Scholar] [CrossRef]
- Dumonteil, E. DNA Vaccines Induce Partial Protection against Leishmania mexicana. Vaccine 2003, 21, 2161–2168. [Google Scholar] [CrossRef]
- Jorjani, O.; Ghaffarifar, F.; Sharifi, Z.; Dalimi, A.; Ziaei-Hezarjaribi, H.; Talebi, B. LACK Gene’s Immune Response Induced by Cocktail DNA Vaccine with IL-12 Gene Against Cutaneous Leishmaniasis in BALB/c Mice. Avicenna J. Med. Biotechnol. 2018, 10, 134–140. [Google Scholar]
- Gomes, D.C.O.; Souza, B.L.d.S.C.; Schwedersky, R.P.; Covre, L.P.; de Matos Guedes, H.L.; Lopes, U.G.; Ré, M.I.; Rossi-Bergmann, B. Intranasal Immunization with Chitosan Microparticles Enhances LACK-DNA Vaccine Protection and Induces Specific Long-Lasting Immunity against Visceral Leishmaniasis. Microbes Infect. 2022, 24, 104884. [Google Scholar] [CrossRef]
- Kardani, K.; Bolhassani, A.; Shahbazi, S. Prime-Boost Vaccine Strategy against Viral Infections: Mechanisms and Benefits. Vaccine 2016, 34, 413–423. [Google Scholar] [CrossRef]
- Mazumder, S.; Maji, M.; Das, A.; Ali, N. Potency, Efficacy and Durability of DNA/DNA, DNA/Protein and Protein/Protein Based Vaccination Using Gp63 Against Leishmania donovani in BALB/c Mice. PLoS ONE 2011, 6, e14644. [Google Scholar] [CrossRef] [PubMed]
- Zanin, F.H.C.; Coelho, E.A.F.; Tavares, C.A.P.; Marques-da-Silva, E.A.; Silva Costa, M.M.; Rezende, S.A.; Gazzinelli, R.T.; Fernandes, A.P. Evaluation of Immune Responses and Protection Induced by A2 and Nucleoside Hydrolase (NH) DNA Vaccines against Leishmania chagasi and Leishmania amazonensis Experimental Infections. Microbes Infect. 2007, 9, 1070–1077. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaie, F.; Mahdavi, M.; Faezi, S.; Dalimi, A.; Sharifi, Z.; Akhlaghi, L.; Ghaffarifar, F. Th1 Platform Immune Responses Against Leishmania major Induced by Thiol-Specific Antioxidant-Based DNA Vaccines. Jundishapur J. Microbiol. 2014, 7. [Google Scholar] [CrossRef]
- Martínez-Rodrigo, A.; Dias, D.S.; Ribeiro, P.A.F.; Roatt, B.M.; Mas, A.; Carrión, J.; Coelho, E.A.F.; Domínguez-Bernal, G. Immunization with the HisAK70 DNA Vaccine Induces Resistance against Leishmania amazonensis Infection in BALB/c Mice. Vaccines 2019, 7, 183. [Google Scholar] [CrossRef] [PubMed]
- Osman, M.; Mistry, A.; Keding, A.; Gabe, R.; Cook, E.; Forrester, S.; Wiggins, R.; Di Marco, S.; Colloca, S.; Siani, L.; et al. A Third Generation Vaccine for Human Visceral Leishmaniasis and Post Kala Azar Dermal Leishmaniasis: First-in-Human Trial of ChAd63-KH. PLoS Negl. Trop. Dis. 2017, 11, e0005527. [Google Scholar] [CrossRef]
- Qin, F.; Xia, F.; Chen, H.; Cui, B.; Feng, Y.; Zhang, P.; Chen, J.; Luo, M. A Guide to Nucleic Acid Vaccines in the Prevention and Treatment of Infectious Diseases and Cancers: From Basic Principles to Current Applications. Front. Cell Dev. Biol. 2021, 9, 633776. [Google Scholar] [CrossRef]
- Dinc, R. Leishmania Vaccines: The Current Situation with Its Promising Aspect for the Future. Korean J. Parasitol. 2022, 60, 379–391. [Google Scholar] [CrossRef]
- You, H.; Jones, M.K.; Gordon, C.A.; Arganda, A.E.; Cai, P.; Al-Wassiti, H.; Pouton, C.W.; McManus, D.P. The MRNA Vaccine Technology Era and the Future Control of Parasitic Infections. Clin. Microbiol. Rev. 2023, 36, e00241-21. [Google Scholar] [CrossRef]
- Duthie, M.S.; Van Hoeven, N.; MacMillen, Z.; Picone, A.; Mohamath, R.; Erasmus, J.; Hsu, F.-C.; Stinchcomb, D.T.; Reed, S.G. Heterologous Immunization with Defined RNA and Subunit Vaccines Enhances T Cell Responses That Protect Against Leishmania donovani. Front. Immunol. 2018, 9, 2420. [Google Scholar] [CrossRef]
- Thakur, A.; Mikkelsen, H.; Jungersen, G. Intracellular Pathogens: Host Immunity and Microbial Persistence Strategies. J. Immunol. Res. 2019, 2019, 1356540. [Google Scholar] [CrossRef]
| Vaccine | Classification | Vaccine Antigen | Adjuvant | Target | Phase Reached | Major Findings | References |
|---|---|---|---|---|---|---|---|
| Leishvaccine | First generation | Whole-killed promastigotes of L. amazonensis | BCG | Dogs | III | Induced modifications in monocytes, activation of CD4+ T cells, CD8+ T cells, and B lymphocytes. It also prompted a mixed cytokine profile including IFN-γ and IL-4. | [21,22] |
| Autoclaved Leishmania | First generation | Killed Leishmania spp. | BCG | Humans | III | Minimal LST conversion in participants and significant reduction in VL incidence among LST-converted individuals. | [23,24,25,26] |
| Leishmune | Second generation | FML | Saponin | Dogs | III | Between 92 and 95% of vaccinated dogs were protected against canine VL. | [27] |
| CaniLeish | Second generation | LiESP | Saponin | Dogs | III | Vaccinated dogs developed a Th1 immune response within three weeks, and the vaccine exhibited a protection against infection rate of 99.4%. | [28,29] |
| Leish-Tec | Second generation | L. donovani A2 protein | Saponin | Dogs | III | Vaccination of infected healthy animals significantly reduced clinical progression and decreased mortality. | [30] |
| LetiFend | Second generation | L. infantum proteins (H2A, LiP2a, LiP2b, and LiP0) | None | Dogs | III | Overall efficacy in the prevention of confirmed cases of canine leishmaniasis in endemic areas with high disease pressure was shown to be 72%. | [18] |
| Leish-F1 | Second generation | TSA, LmSTI1, and LeIF | MPL-SE | Humans | I | The vaccine was safe and well tolerated by participants and induced T cell production of IFN-γ and other cytokines in response to stimulation with the antigen. | [31] |
| Leish-F2 | Second generation | TSA, LmSTI1, and LeIF | MPL-SE | Humans | II | Showed potential therapeutic effects on CL patients when combined with the adjuvant. | [32] |
| Leish-F3 | Second generation | NH36 and SMT | MPL-SE and GLA-SE | Humans | I | Subjects vaccinated with Leish-F3 and GLA-SE had significant levels of antigen-specific IgG antibodies in their serum, along with IFN-γ, TNF, and IL-2 secretion in response to the antigen. | [33] |
| ChAd63-KH | Third generation | KMP-11 and HASPB | None | Humans | II | It elicited a variety of CD8+ T cells specific to Leishmania antigens in PKDL patients. Vaccination was safe and effectively stimulated the production of IFN-γ and the activation of dendritic cells. | [34] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayala, A.; Llanes, A.; Lleonart, R.; Restrepo, C.M. Advances in Leishmania Vaccines: Current Development and Future Prospects. Pathogens 2024, 13, 812. https://doi.org/10.3390/pathogens13090812
Ayala A, Llanes A, Lleonart R, Restrepo CM. Advances in Leishmania Vaccines: Current Development and Future Prospects. Pathogens. 2024; 13(9):812. https://doi.org/10.3390/pathogens13090812
Chicago/Turabian StyleAyala, Andreina, Alejandro Llanes, Ricardo Lleonart, and Carlos M. Restrepo. 2024. "Advances in Leishmania Vaccines: Current Development and Future Prospects" Pathogens 13, no. 9: 812. https://doi.org/10.3390/pathogens13090812
APA StyleAyala, A., Llanes, A., Lleonart, R., & Restrepo, C. M. (2024). Advances in Leishmania Vaccines: Current Development and Future Prospects. Pathogens, 13(9), 812. https://doi.org/10.3390/pathogens13090812







