Characterising the Metabolomic Diversity and Biological Potentials of Extracts from Different Parts of Two Cistus Species Using UHPLC-MS/MS and In Vitro Techniques
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Collection
2.2. Plant Extract Preparation
2.3. Assay for Total Phenolic and Flavonoid Contents
2.4. UHPLC-MS/MS Analysis
2.5. Assays for In Vitro Antioxidant Capacity
2.6. Inhibitory Effects against Some Key Enzymes
2.7. Antimicrobial Activity
2.8. Antibacterial/Antifungal Susceptibility Testing
2.9. Cell Culture
2.10. Determination of Cellular Viability
2.11. Statistical Analysis
3. Results
3.1. Chemical Composition
3.2. Total Phenolic and Flavonoid Contents
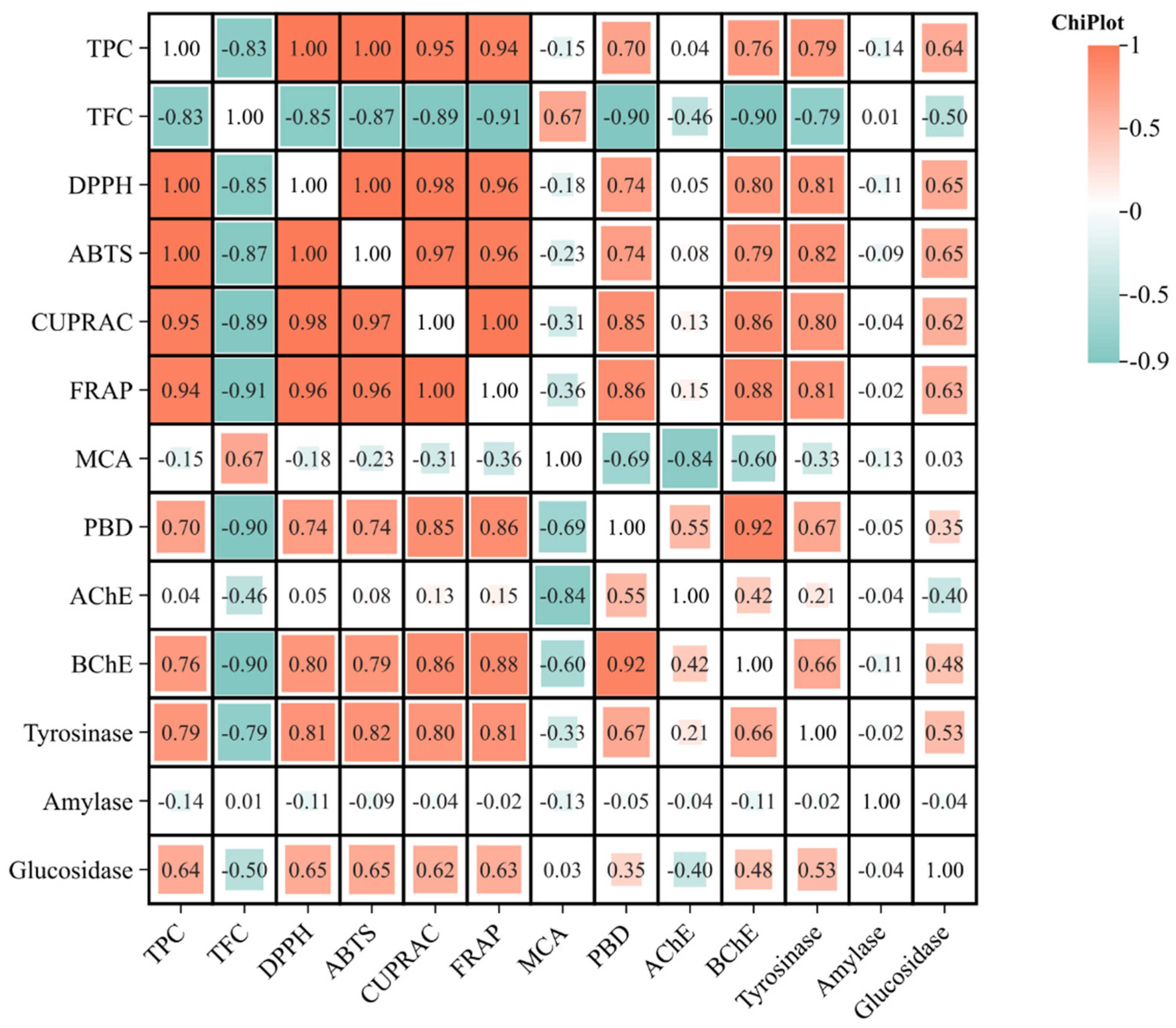
3.3. Antioxidant Activities
3.4. Enzyme Inhibitory Activities
3.5. Antimicrobial Activity
3.6. Cytotoxic Effects
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nilofar, N.; Ahmed, S.; Zengin, G.; Di Simone, S.C.; Acquaviva, A.; Libero, M.L.; Chiavaroli, A.; Orlando, G.; Tacchini, M.; Di Vito, M. Combining the pharmaceutical and toxicological properties of select essential oils with their chemical components by GC-MS analysis. Chem. Biodivers. 2024, 21, e202400738. [Google Scholar] [CrossRef] [PubMed]
- Dimcheva, V.; Karsheva, M. Cistus incanus from Strandja mountain as a source of bioactive antioxidants. Plants 2018, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Nilofar, N.; Zengin, G.; Acar, M.; Bouyayha, A.; Youssra, A.; Eldahshan, O.; Fayez, S.; Fahmy, N. Assessing the chemical composition, antioxidant and enzyme inhibitory effects of Pentapleura subulifera and Cyclotrichium glabrescens extracts. Chem. Biodivers. 2024, 21, e202301651. [Google Scholar] [CrossRef] [PubMed]
- Nilofar; Eyupoglu, O.E.; Nazzaro, F.; Fratianni, F.; Ahmed, S.; Ferrante, C.; Senkardes, I.; Zengin, G. An analytical framework combining online high-performance liquid chromatography methodologies and biological properties of different extracts of Leonurus cardiaca. J. Sep. Sci. 2024, 47, 2300695. [Google Scholar] [CrossRef]
- Guzmán, B.; Vargas, P. Historical biogeography and character evolution of Cistaceae (Malvales) based on analysis of plastid rbcL and trnL-trnF sequences. Org. Divers. Evol. 2009, 9, 83–99. [Google Scholar] [CrossRef]
- Nur Onal, F.; Ozturk, I.; Aydin Kose, F.; Der, G.; Kilinc, E.; Baykan, S. Comparative evaluation of polyphenol contents and biological activities of five Cistus L. species native to Turkey. Chem. Biodivers. 2023, 20, e202200915. [Google Scholar] [CrossRef]
- Papaefthimiou, D.; Papanikolaou, A.; Falara, V.; Givanoudi, S.; Kostas, S.; Kanellis, A.K. Genus Cistus: A model for exploring labdane-type diterpenes’ biosynthesis and a natural source of high value products with biological, aromatic, and pharmacological properties. Front. Chem. 2014, 2, 35. [Google Scholar] [CrossRef]
- Nefzi, K.; Charfi, K.; Maaroufi, A.; Hosni, K.; Msaada, K.; Baraket, M.; Nasr, Z. Biological activities and determination of the mode of action of Tunisian Globularia alypum and Cistus monspeliensis ethanolic extracts. Int. J. Environ. Health Res. 2024, 34, 127–137. [Google Scholar] [CrossRef]
- Tomou, E.-M.; Lytra, K.; Rallis, S.; Tzakos, A.G.; Skaltsa, H. An updated review of genus Cistus L. since 2014: Traditional uses, phytochemistry, and pharmacological properties. Phytochem. Rev. 2022, 21, 2049–2087. [Google Scholar] [CrossRef]
- Şen Gökmen, F.; Özbek, F.; Duman, H. Morphological features of pollen, fruits, and seeds of Turkish Cistus species (Cistaceae). Plant Biosyst. 2024, 158, 650–667. [Google Scholar] [CrossRef]
- Yeşilada, E.; Honda, G.; Sezik, E.; Tabata, M.; Fujita, T.; Tanaka, T.; Takeda, Y.; Takaishi, Y. Traditional medicine in Turkey. V. Folk medicine in the inner Taurus Mountains. J. Ethnopharmacol. 1995, 46, 133–152. [Google Scholar] [CrossRef] [PubMed]
- Tuzlacı, E.; İşbilen, D.A.; Bulut, G. Turkish folk medicinal plants, VIII: Lalapaşa (Edirne). Marmara Pharm. J. 2010, 14, 47–52. [Google Scholar] [CrossRef]
- Zalegh, I.; Akssira, M.; Bourhia, M.; Mellouki, F.; Rhallabi, N.; Salamatullah, A.M.; Alkaltham, M.S.; Khalil Alyahya, H.; Mhand, R.A. A review on Cistus sp.: Phytochemical and antimicrobial activities. Plants 2021, 10, 1214. [Google Scholar] [CrossRef] [PubMed]
- Gülz, P.-G.; Herrmann, T.; Hangst, K. Leaf trichomes in the genus Cistus. Flora 1996, 191, 85–104. [Google Scholar] [CrossRef]
- Benali, T.; Bouyahya, A.; Habbadi, K.; Zengin, G.; Khabbach, A.; Hammani, K. Chemical composition and antibacterial activity of the essential oil and extracts of Cistus ladaniferus subsp. ladanifer and Mentha suaveolens against phytopathogenic bacteria and their ecofriendly management of phytopathogenic bacteria. Biocatal. Agric. Biotechnol. 2020, 28, 101696. [Google Scholar] [CrossRef]
- Ammendola, M.; Haponska, M.; Balik, K.; Modrakowska, P.; Matulewicz, K.; Kazmierski, L.; Lis, A.; Kozlowska, J.; Garcia-Valls, R.; Giamberini, M. Stability and anti-proliferative properties of biologically active compounds extracted from Cistus L. after sterilization treatments. Sci. Rep. 2020, 10, 6521. [Google Scholar] [CrossRef]
- Bouothmany, K.; Bourhia, M.; Aoussar, N.; Attaleb, M.; Salamatullah, A.M.; Nafidi, H.-A.; Mellouki, F.; El Mzibri, M.; Aboul-Soud, M.A.; Benbacer, L. Leaf extracts of Cistus ladanifer exhibit potent antioxidant and antiproliferative activities against liver, prostate and breast cancer cells. Appl. Sci. 2022, 12, 8603. [Google Scholar] [CrossRef]
- Haida, S.; Bakkouche, K.; Kribii, A.R.; Kribii, A. Chemical composition of essential oil, phenolic compounds content, and antioxidant activity of cistus monspeliensis from Northern Morocco. Biochem. Res. Int. 2021, 2021, 6669877. [Google Scholar] [CrossRef]
- Kubica, P.; Ekiert, H.; Ekiert, R.J.; Szopa, A. Species of the genus Cistus sp.–taxonomy, distribution, chemical composition, therapeutic applications and biotechnological studies. Postępy Fitoter. 2016, 3, 179–188. [Google Scholar]
- Gori, A.; Ferrini, F.; Marzano, M.C.; Tattini, M.; Centritto, M.; Baratto, M.C.; Pogni, R.; Brunetti, C. Characterisation and antioxidant activity of crude extract and polyphenolic rich fractions from C. incanus leaves. Int. J. Mol. Sci. 2016, 17, 1344. [Google Scholar] [CrossRef]
- Gaweł-Bęben, K.; Kukula-Koch, W.; Hoian, U.; Czop, M.; Strzępek-Gomółka, M.; Antosiewicz, B. Characterization of Cistus× incanus L. and Cistus ladanifer L. extracts as potential multifunctional antioxidant ingredients for skin protecting cosmetics. Antioxidants 2020, 9, 202. [Google Scholar] [CrossRef] [PubMed]
- Carev, I.; Maravić, A.; Ilić, N.; Čikeš Čulić, V.; Politeo, O.; Zorić, Z.; Radan, M. UPLC-MS/MS phytochemical analysis of two Croatian Cistus species and their biological activity. Life 2020, 10, 112. [Google Scholar] [CrossRef] [PubMed]
- Barrajón-Catalán, E.; Fernández-Arroyo, S.; Roldán, C.; Guillén, E.; Saura, D.; Segura-Carretero, A.; Micol, V. A systematic study of the polyphenolic composition of aqueous extracts deriving from several Cistus genus species: Evolutionary relationship. Phytochem. Anal. 2011, 22, 303–312. [Google Scholar] [CrossRef] [PubMed]
- de Dato, G.D.; Micali, M.; Abou Jaoudé, R.; Liberati, D.; De Angelis, P. Earlier summer drought affects leaf functioning of the Mediterranean species Cistus monspeliensis L. Environ. Exp. Bot. 2013, 93, 13–19. [Google Scholar] [CrossRef]
- Farley, R.A.; McNeilly, T. Diversity and divergence in Cistus salvifolius (L.) populations from contrasting habitats. Hereditas 2000, 132, 183–192. [Google Scholar] [CrossRef]
- Hegnauer, R.; Hegnauer, M. Chemotaxonomie der Pflanzen: Eine Übersicht über die Verbreitung und die systematische Bedeutung der Pflanzenstoffe. 2. Monocotyledoneae; Birkhäuser: Basel, Switzerland, 1963. [Google Scholar]
- Slinkard, K.; Singleton, V.L. Total phenol analysis: Automation and comparison with manual methods. Am. J. Enol. Vitic. 1977, 28, 49–55. [Google Scholar] [CrossRef]
- Grochowski, D.M.; Uysal, S.; Aktumsek, A.; Granica, S.; Zengin, G.; Ceylan, R.; Locatelli, M.; Tomczyk, M. In vitro enzyme inhibitory properties, antioxidant activities, and phytochemical profile of Potentilla thuringiaca. Phytochem. Lett. 2017, 20, 365–372. [Google Scholar] [CrossRef]
- Pagiotti, R.; Angelini, P.; Rubini, A.; Tirillini, B.; Granetti, B.; Venanzoni, R. Identification and characterisation of human pathogenic filamentous fungi and susceptibility to Thymus schimperi essential oil. Mycoses 2011, 54, e364–e376. [Google Scholar] [CrossRef]
- Angelini, P.; Pellegrino, R.M.; Tirillini, B.; Flores, G.A.; Alabed, H.B.; Ianni, F.; Blasi, F.; Cossignani, L.; Venanzoni, R.; Orlando, G. Metabolomic profiling and biological activities of Pleurotus columbinus Quél. Cultivated on different agri-food byproducts. Antibiotics 2021, 10, 1245. [Google Scholar] [CrossRef]
- M27-A3; Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts, Approved Standard-Third Edition. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008.
- M27-S4; Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; 4th Informational Supplement. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012.
- M07-A9; Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, Approved Standard, 9th ed. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012.
- M38-A2; Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi; Approved Standard-Second Edition. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008.
- Mahomoodally, M.F.; Sinan, K.I.; Bene, K.; Zengin, G.; Orlando, G.; Menghini, L.; Veschi, S.; Chiavaroli, A.; Recinella, L.; Brunetti, L. Bridelia speciosa Müll. Arg. stem bark extracts as a potential biomedicine: From tropical western Africa to the pharmacy shelf. Antioxidants 2020, 9, 128. [Google Scholar] [CrossRef]
- Velioglu, Y.S. 10 Food Acids: Organic Acids, Volatile Organic Acids, and Phenolic Acids. In Advances in Food Biochemistry; CRC Press: Boca Raton, FL, USA, 2009; p. 313. [Google Scholar]
- da Silva, S.M.; Koehnlein, E.A.; Bracht, A.; Castoldi, R.; de Morais, G.R.; Baesso, M.L.; Peralta, R.A.; de Souza, C.G.M.; de Sá-Nakanishi, A.B.; Peralta, R.M. Inhibition of salivary and pancreatic α-amylases by a pinhão coat (Araucaria angustifolia) extract rich in condensed tannin. Food Res. Int. 2014, 56, 1–8. [Google Scholar] [CrossRef]
- Sagra, M.E.T. Optimization of the Extraction of Procyanidin b-2 Rich Extract from Unfermented Cocoa Using Response Surface Methodology and Interaction of Procyanidin b-2 Rich Cocoa Extract with Collagenase and Elastase as Biomarkers of Skin Aging. Master’s Thesis, Louisiana State University and Agricultural & Mechanical College, Baton Rouge, LA, USA, 2017. [Google Scholar]
- Gnonlonfin, G.B.; Sanni, A.; Brimer, L. Review scopoletin—A coumarin phytoalexin with medicinal properties. Crit. Rev. Plant Sci. 2012, 31, 47–56. [Google Scholar] [CrossRef]
- Spínola, V.; Castilho, P.C. Phytochemical profile, chemotaxonomic studies, and in vitro antioxidant activities of two endemisms from Madeira Archipelago: Melanoselinum decipiens and Monizia edulis (Apiaceae). Chem. Biodivers. 2016, 13, 1290–1306. [Google Scholar] [CrossRef] [PubMed]
- Kiokias, S.; Proestos, C.; Oreopoulou, V. Phenolic acids of plant origin—A review on their antioxidant activity in vitro (o/w emulsion systems) along with their in vivo health biochemical properties. Foods 2020, 9, 534. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Tuo, X.; Wang, L.; Tundis, R.; Portillo, M.P.; Simal-Gandara, J.; Yu, Y.; Zou, L.; Xiao, J.; Deng, J. Bioactive procyanidins from dietary sources: The relationship between bioactivity and polymerization degree. Trends Food Sci. Technol. 2021, 111, 114–127. [Google Scholar] [CrossRef]
- Guo, J.-M.; Lu, Y.-W.; Shang, E.-X.; Li, T.; Liu, Y.; Duan, J.-A.; Qian, D.-W.; Tang, Y.-P. Metabolite identification strategy of non-targeted metabolomics and its application for the identification of components in Chinese multicomponent medicine Abelmoschus manihot L. Phytomedicine 2015, 22, 579–587. [Google Scholar] [CrossRef]
- Walker, R.P.; Famiani, F. Organic acids in fruits: Metabolism, functions and contents. Hortic. Rev. 2018, 45, 371–430. [Google Scholar]
- Dhanaraj, S. A critical review on quercetin bioflavonoid and its derivatives: Scope, synthesis, and biological applications with future prospects. Arab. J. Chem. 2023, 16, 104881. [Google Scholar]
- Saleem, A.; Akhtar, M.F.; Sharif, A.; Akhtar, B.; Siddique, R.; Ashraf, G.M.; Alghamdi, B.S.; Alharthy, S.A. Anticancer, cardio-protective and anti-inflammatory potential of natural-sources-derived phenolic acids. Molecules 2022, 27, 7286. [Google Scholar] [CrossRef]
- Sehrawat, R.; Rathee, P.; Akkol, E.K.; Khatkar, S.; Lather, A.; Redhu, N.; Khatkar, A. Phenolic acids-versatile natural moiety with numerous biological applications. Curr. Top. Med. Chem. 2022, 22, 1472–1484. [Google Scholar] [CrossRef]
- Cardullo, N.; Muccilli, V.; Cunsolo, V.; Tringali, C. Mass spectrometry and 1H-NMR study of Schinopsis lorentzii (Quebracho) tannins as a source of hypoglycemic and antioxidant principles. Molecules 2020, 25, 3257. [Google Scholar] [CrossRef] [PubMed]
- Laddha, A.P.; Kulkarni, Y.A. Tannins and vascular complications of Diabetes: An update. Phytomedicine 2019, 56, 229–245. [Google Scholar] [CrossRef] [PubMed]
- Kaurinovic, B.; Vastag, D. Flavonoids and Phenolic Acids as Potential Natural Antioxidants; IntechOpen: London, UK, 2019. [Google Scholar]
- Allegra, M. Antioxidant and anti-inflammatory properties of plants extract. Antioxidants 2019, 8, 549. [Google Scholar] [CrossRef] [PubMed]
- Laganà, P.; Anastasi, G.; Marano, F.; Piccione, S.; Singla, R.K.; Dubey, A.K.; Delia, S.; Coniglio, M.A.; Facciolà, A.; Di Pietro, A. Phenolic substances in foods: Health effects as anti-inflammatory and antimicrobial agents. J. AOAC Int. 2019, 102, 1378–1387. [Google Scholar] [CrossRef]
- Pérez-Cano, F.J.; Castell, M. Flavonoids, inflammation and immune system. Nutrients 2016, 8, 659. [Google Scholar] [CrossRef]
- Tawaha, K.; Alali, F.Q.; Gharaibeh, M.; Mohammad, M.; El-Elimat, T. Antioxidant activity and total phenolic content of selected Jordanian plant species. Food Chem. 2007, 104, 1372–1378. [Google Scholar] [CrossRef]
- Bouyahya, A.; Abrini, J.; Khay, E.-O.; Charfi, S.; Boujida, N.; EL-Harsal, A.; Talbaoui, A.; ET-Touys, A.; Bakri, Y.; Dakka, N. In vitro antibacterial activity of organic extracts from north-west Moroccan medicinal plant Myrtus communis (L.). Biotechnol. J. Int. 2016, 16, 1–9. [Google Scholar] [CrossRef]
- Rice-Evans, C. Flavonoid antioxidants. Curr. Med. Chem. 2001, 8, 797–807. [Google Scholar] [CrossRef]
- Shahidi, F.; Naczk, M. 11 Analysis of Polyphenols. In Methods of Analysis of Food Components and Additives; CRC Press: Boca Raton, FL, USA, 2011; p. 253. [Google Scholar]
- Saleem, H.; Khurshid, U.; Sarfraz, M.; Tousif, M.I.; Alamri, A.; Anwar, S.; Alamri, A.; Ahmad, I.; Abdallah, H.H.; Mahomoodally, F.M. A comprehensive phytochemical, biological, toxicological and molecular docking evaluation of Suaeda fruticosa (L.) Forssk.: An edible halophyte medicinal plant. Food Chem. Toxicol. 2021, 154, 112348. [Google Scholar] [CrossRef]
- Li, Q.; Wang, X.; Chen, J.; Liu, C.; Li, T.; McClements, D.J.; Dai, T.; Liu, J. Antioxidant activity of proanthocyanidins-rich fractions from Choerospondias axillaris peels using a combination of chemical-based methods and cellular-based assay. Food Chem. 2016, 208, 309–317. [Google Scholar] [CrossRef]
- Wilkinson, D. Pharmacotherapy of Alzheimer’s disease. Psychiatry 2008, 7, 9–14. [Google Scholar] [CrossRef]
- Altamura, S.; Muckenthaler, M.U. Iron toxicity in diseases of aging: Alzheimer’s disease, Parkinson’s disease and atherosclerosis. J. Alzheimer’s Dis. 2009, 16, 879–895. [Google Scholar] [CrossRef] [PubMed]
- Akkol, E.K.; Orhan, I.E.; Yeşilada, E. Anticholinesterase and antioxidant effects of the ethanol extract, ethanol fractions and isolated flavonoids from Cistus laurifolius L. leaves. Food Chem. 2012, 131, 626–631. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Jemia, M.B.; Senatore, F.; Bruno, M.; Menichini, F.; Tundis, R. Chemistry and functional properties in prevention of neurodegenerative disorders of five Cistus species essential oils. Food Chem. Toxicol. 2013, 59, 586–594. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2017, 32, 403–425. [Google Scholar] [CrossRef]
- Leyden, J.; Shergill, B.; Micali, G.; Downie, J.; Wallo, W. Natural options for the management of hyperpigmentation. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 1140–1145. [Google Scholar] [CrossRef]
- Bailes, B.K. Diabetes mellitus and its chronic complications. AORN J. 2002, 76, 265–282. [Google Scholar] [CrossRef]
- Williamson, G. Possible effects of dietary polyphenols on sugar absorption and digestion. Mol. Nutr. Food Res. 2013, 57, 48–57. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Marrelli, M.; Pugliese, A.; Conforti, F.; Nadjafi, F.; Menichini, F.; Tundis, R. Crocus cancellatus subsp. damascenus stigmas: Chemical profile, and inhibition of α-amylase, α-glucosidase and lipase, key enzymes related to type 2 diabetes and obesity. J. Enzym. Inhib. Med. Chem. 2016, 31, 212–218. [Google Scholar] [CrossRef]
- Sayah, K.; Marmouzi, I.; Naceiri Mrabti, H.; Cherrah, Y.; Faouzi, M.E.A. Antioxidant activity and inhibitory potential of Cistus salviifolius (L.) and Cistus monspeliensis (L.) aerial parts extracts against key enzymes linked to hyperglycemia. BioMed Res. Int. 2017, 2017, 2789482. [Google Scholar] [CrossRef]
- Frazão, D.F.; Martins-Gomes, C.; Díaz, T.S.; Delgado, F.; Gonçalves, J.C.; Silva, A.M. Labdanum resin from Cistus ladanifer L. as a source of compounds with anti-diabetic, neuroprotective and anti-proliferative activity. Molecules 2024, 29, 2222. [Google Scholar] [CrossRef]
- İnan, Y.; Akyüz, S.; Kurt-Celep, I.; Celep, E.; Yesilada, E. Influence of in vitro human digestion simulation on the phenolics contents and biological activities of the aqueous extracts from Turkish Cistus Species. Molecules 2021, 26, 5322. [Google Scholar] [CrossRef] [PubMed]
- Khachebaa, I.; Boussoussaa, H.; Djeridane, A.; Bekhaoua, A.; Bensayah, N.; Yousfi, M. α-Glucosidase inhibitory effect and antioxidant activity of the extracts of eighteen plant traditionally used in Algeria for diabetes. Curr. Enzym. Inhib. 2017, 13, 1–12. [Google Scholar] [CrossRef]
- Delcour, A.H. Outer membrane permeability and antibiotic resistance. Biochim. Et Biophys. Acta (BBA)-Proteins Proteom. 2009, 1794, 808–816. [Google Scholar] [CrossRef] [PubMed]
- Covino, S.; D’Ellena, E.; Tirillini, B.; Angeles, G.; Arcangeli, A.; Bistocchi, G.; Venanzoni, R.; Angelini, P. Characterization of biological activities of methanol extract of Fuscoporia torulosa (Basidiomycetes) from Italy. Int. J. Med. Mushrooms 2019, 21, 1051–1063. [Google Scholar] [CrossRef]
- Flores, G.A.; Girometta, C.E.; Cusumano, G.; Angelini, P.; Tirillini, B.; Ianni, F.; Blasi, F.; Cossignani, L.; Pellegrino, R.M.; Emiliani, C. Untargeted metabolomics used to describe the chemical composition, antioxidant and antimicrobial effects of extracts from Pleurotus spp. mycelium grown in different culture media. Antibiotics 2022, 11, 1468. [Google Scholar] [CrossRef]
- Flores, G.A.; Girometta, C.E.; Cusumano, G.; Pellegrino, R.M.; Silviani, S.; Bistocchi, G.; Arcangeli, A.; Ianni, F.; Blasi, F.; Cossignani, L. Diversity of Pleurotus spp.(Agaricomycetes) and their metabolites of Nutraceutical and Therapeutic Importance. Int. J. Med. Mushrooms 2023, 25, 1–20. [Google Scholar] [CrossRef]
- Seil, J.T.; Webster, T.J. Antimicrobial applications of nanotechnology: Methods and literature. Int. J. Nanomed. 2012, 7, 2767–2781. [Google Scholar]
- Dresch, P.; D’ Aguanno, M.N.; Rosam, K.; Grienke, U.; Rollinger, J.M.; Peintner, U. Fungal strain matters: Colony growth and bioactivity of the European medicinal polypores Fomes fomentarius, Fomitopsis pinicola. Amb Express 2015, 5, 4. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices. Part 5: Tests for In Vitro Cytotoxicity. ISO: Geneva, Switzerland, 2009; pp. 1–34.
- Heydari, M.; Rauf, A.; Thiruvengadam, M.; Chen, X.; Hashempur, M.H. Clinical safety of natural products, an evidence-based approach. Front. Pharmacol. 2022, 13, 960556. [Google Scholar] [CrossRef]
- Vitali, F.; Pennisi, G.; Attaguile, G.; Savoca, F.; Tita, B. Antiproliferative and cytotoxic activity of extracts from Cistus incanus L. and Cistus monspeliensis L. on human prostate cell lines. Nat. Prod. Res. 2011, 25, 188–202. [Google Scholar] [CrossRef] [PubMed]
- Ben Jemia, M.; Kchouk, M.E.; Senatore, F.; Autore, G.; Marzocco, S.; De Feo, V.; Bruno, M. Antiproliferative activity of hexane extract from Tunisian Cistus libanotis, Cistus monspeliensis and Cistus villosus. Chem. Cent. J. 2013, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Moreira, H.; Slezak, A.; Szyjka, A.; Oszmianski, J.; Gasiorowski, K. Antioxidant and cancer chemopreventive activities of Cistus and pomegranate polyphenols. Acta Poloniae Pharm. 2017, 74, 688–698. [Google Scholar] [PubMed]
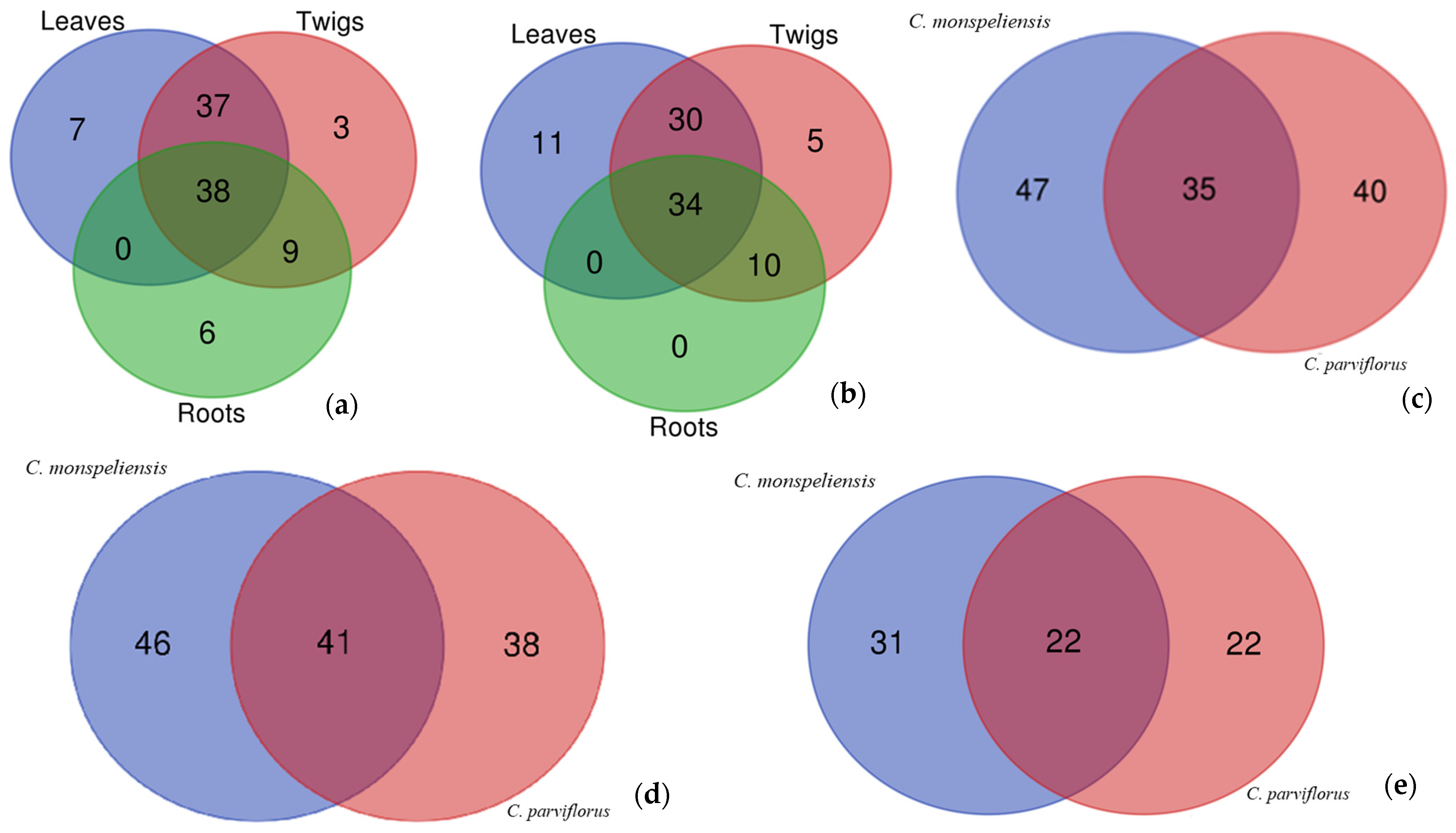
| Compounds | Leaves | Twigs | Roots |
|---|---|---|---|
| Quinic acid | + | + | + |
| Citric acid | + | + | + |
| Arbutin | + | + | + |
| Prodelphinidin B isomer 1 | - | + | + |
| Gallic acid (3,4,5-Trihydroxybenzoic acid) | + | + | + |
| Prodelphinidin B isomer 2 | - | + | + |
| Protocatechuic acid (3,4-Dihydroxybenzoic acid) | + | + | + |
| Prodelphinidin B isomer 3 | - | + | + |
| Gallocatechin | + | + | + |
| Prodelphinidin B isomer 4 | - | + | + |
| Procyanidin B isomer 1 | - | + | + |
| Punicalagin isomer | + | - | - |
| Flavogallonic acid dilactone or isomer | + | + | - |
| Punicalagin | + | - | - |
| Prodelphinidin B isomer 5 | - | + | + |
| Procyanidin B isomer 2 | - | + | + |
| Esculin (Esculetin-6-O-glucoside) | + | + | + |
| Unidentified hydroxybenzoic acid derivative 1 | + | + | + |
| Catechin | + | + | + |
| Epigallocatechin | + | + | + |
| Magnolioside (Isoscopoletin-6-O-glucoside) | - | + | + |
| Scopolin (Scopoletin-7-O-glucoside) | - | + | + |
| Caffeic acid-O-glucoside | + | + | - |
| Caffeic acid | + | + | + |
| Unidentified hydroxybenzoic acid derivative 2 | + | + | + |
| Naringenin-6,8-di-C-glucoside | + | + | - |
| Epigallocatechin-3-O-gallate (Teatannin II) | + | + | + |
| Epicatechin | + | + | + |
| Isoscopoletin (6-Hydroxy-7-methoxycoumarin) | + | + | + |
| p-Coumaric acid | + | + | + |
| Vicenin-2 (Apigenin-6,8-di-C-glucoside) | + | + | - |
| Scopoletin (7-Hydroxy-6-methoxycoumarin) | + | + | + |
| Methyl flavogallonate | + | + | - |
| Ellagic acid-4-O-glucoside | + | + | - |
| Quercetin-O-dirhamnosylhexoside | + | + | - |
| Myricetin-3-O-glucoside (Isomyricitrin) | + | + | + |
| Quercetin-O-galloylhexoside | + | - | - |
| Kaempferol-O-dirhamnosylhexoside | + | + | - |
| Myricetin-3-O-pentoside | + | + | - |
| 3-O-Methylellagic acid-4′-O-glucoside | - | + | - |
| Myricitrin (Myricetin-3-O-rhamnoside) | + | + | + |
| Kaempferol-3-O-neohesperidoside | + | + | - |
| Myricetin-O-malonylhexoside | + | - | - |
| Hyperoside (Quercetin-3-O-galactoside) | + | + | + |
| Ellagic acid-O-pentoside | + | + | + |
| Isoquercitrin (Quercetin-3-O-glucoside) | + | + | - |
| Rutin (Quercetin-3-O-rutinoside) | + | + | - |
| Eschweilenol C (Ellagic acid-4-O-rhamnoside) | - | + | - |
| Avicularin (Quercetin-3-O-arabinofuranoside) | + | + | - |
| Ellagic acid | + | + | + |
| Kaempferol-7-O-glucoside | + | + | - |
| Quercetin-O-malonylhexoside | + | - | - |
| Guaijaverin (Quercetin-3-O-arabinopyranoside) | + | - | - |
| Dimethoxy-trihydroxyflavone-O-hexoside | + | + | + |
| Astragalin (Kaempferol-3-O-glucoside) | + | + | + |
| Kaempferol-3-O-rutinoside (Nicotiflorin) | + | + | - |
| Ducheside A (3-O-Methylellagic acid-4′-O-xyloside) | - | + | - |
| 3-O-Methylellagic acid-4′-O-rhamnoside | - | + | + |
| 3-O-Methylellagic acid | - | + | - |
| Isorhamnetin-3-O-rutinoside (Narcissin) | + | - | - |
| Kaempferol-O-malonylhexoside | + | - | - |
| Pinobanksin (3,5,7-Trihydroxyflavanone) | + | + | - |
| Naringenin (4′,5,7-Trihydroxyflavanone) | + | + | + |
| Quercetin (3,3′,4′,5,7-Pentahydroxyflavone) | + | + | + |
| Kaempferol-3-O-[rhamnosyl-(1→2)-(6″-O-trans-p-coumaroyl)]glucoside | + | + | - |
| Di-O-methylellagic acid | - | + | - |
| Tiliroside (6″-O-trans-p-Coumaroylastragalin) | + | + | + |
| Quercetin-3-O-methyl ether | + | + | - |
| 3″-O-trans-p-Coumaroylastragalin | + | + | + |
| Axillarin (3,6-Dimethoxy-3′,4′,5,7-tetrahydroxyflavone) | + | + | - |
| Kaempferol (3,4′,5,7-Tetrahydroxyflavone) | + | + | - |
| Isorhamnetin (3′-Methoxy-3,4′,5,7-tetrahydroxyflavone) | + | + | - |
| Apigenin (4′,5,7-Trihydroxyflavone) | + | + | - |
| Chrysoeriol (3′-Methoxy-4′,5,7-trihydroxyflavone) | + | + | - |
| Isokaempferide (3-Methoxy-4′,5,7-trihydroxyflavone) | + | + | - |
| 3,8-Dimethoxy-4′,5,7-trihydroxyflavone | + | + | + |
| Rhamnetin (7-Methoxy-3,3′,4′,5-tetrahydroxyflavone) | + | + | - |
| Jaceidin (4′,5,7-Trihydroxy-3,3′,6-trimethoxyflavone) | + | - | - |
| Pinocembrin (5,7-Dihydroxyflavanone) | + | + | + |
| 3,6-Dimethoxy-4′,5,7-trihydroxyflavone | + | + | + |
| 4′,5,7-Trihydroxy-3,3′,8-trimethoxyflavone (Gossypetin-3,3′,8-trimethyl ether) | + | + | - |
| Kaempferol-3-O-(3,6-di-p-coumaroylglucoside) | + | + | - |
| Dihydroxy-trimethoxy(iso)flavone isomer 1 | + | - | - |
| Dihydroxy-trimethoxy(iso)flavone isomer 2 | + | + | - |
| Dihydroxy-tetramethoxy(iso)flavone | + | + | - |
| 5,7-Dihydroxy-3,4′,8-trimethoxyflavone (Herbacetin-3,4′,8-trimethyl ether) | + | + | + |
| 5,7-Dihydroxy-3,3′,4′,8-tetramethoxyflavone (Gossypetin-3,3′,4′,8-tetramethyl ether) | + | + | + |
| Flindulatin (5-Hydroxy-3,4′,7,8-tetramethoxyflavone) | + | + | + |
| Kaempferol-3,4′,7-trimethyl ether (5-Hydroxy-3,4′,7-trimethoxyflavone) | + | + | - |
| Pheophytin A | + | - | - |
| Compounds | Leaves | Twigs | Roots |
|---|---|---|---|
| Quinic acid | + | + | + |
| Malic acid | + | + | + |
| Citric acid | + | + | + |
| Gallic acid (3,4,5-Trihydroxybenzoic acid) | + | + | + |
| Gentisic acid (2,5-Dihydroxybenzoic acid) | + | + | + |
| Gallocatechin | + | + | + |
| Gentisic acid-O-glucoside | + | + | + |
| Procyanidin B isomer 1 | - | + | + |
| Flavogallonic acid dilactone or isomer | + | + | - |
| Uralenneoside | + | + | + |
| Procyanidin B isomer 2 | - | + | + |
| Esculin (Esculetin-6-O-glucoside) | + | + | - |
| Unidentified hydroxybenzoic acid derivative 1 | - | - | + |
| Catechin | + | + | + |
| Epigallocatechin | + | + | + |
| Procyanidin B isomer 3 | - | + | + |
| Magnolioside (Isoscopoletin-6-O-glucoside) | - | + | + |
| Scopolin (Scopoletin-7-O-glucoside) | + | + | + |
| Esculetin (6,7-Dihydroxycoumarin) | + | + | |
| Procyanidin B isomer 4 | - | + | + |
| Fraxetin-O-glucoside | - | + | - |
| Monspelioside (1-(3,5-Dihydroxy-2-methylphenyl)ethanone-5-O-glucoside) | + | + | + |
| Unidentified hydroxybenzoic acid derivative 2 | - | - | + |
| Naringenin-6,8-di-C-glucoside | - | + | + |
| Epicatechin | + | + | + |
| Fraxetin (7,8-Dihydroxy-6-methoxycoumarin) | + | + | - |
| Isoscopoletin (6-Hydroxy-7-methoxycoumarin) | - | + | + |
| p-Coumaric acid | + | + | + |
| Vicenin-2 (Apigenin-6,8-di-C-glucoside) | + | + | + |
| Scopoletin (7-Hydroxy-6-methoxycoumarin) | + | + | + |
| Taxifolin (Dihydroquercetin) | + | + | - |
| Ellagic acid-4-O-glucoside | + | + | - |
| Sinapic acid | + | - | - |
| Dimethoxy-hydroxycoumarin | + | + | + |
| Myricetin-3-O-glucoside (Isomyricitrin) | + | + | + |
| Scoparone (6,7-Dimethoxycoumarin) | + | - | - |
| Myricetin-O-pentoside isomer 1 | + | + | - |
| Myricetin-O-pentoside isomer 2 | + | + | - |
| Dihydrokaempferol (3,4′,5,7-Tetrahydroxyflavanone) | + | + | - |
| Myricitrin (Myricetin-3-O-rhamnoside) | + | + | - |
| Quercetin-O-pentosylhexoside | + | - | - |
| Myricetin-O-pentoside isomer 3 | + | + | - |
| Hyperoside (Quercetin-3-O-galactoside) | + | + | + |
| Isoquercitrin (Quercetin-3-O-glucoside) | - | + | + |
| Trimethoxycoumarin | + | - | - |
| Ellagic acid-O-pentoside | + | + | - |
| Rutin (Quercetin-3-O-rutinoside) | + | - | - |
| Eschweilenol A or isomer | + | - | - |
| Ellagic acid | + | + | + |
| Kaempferol-7-O-glucoside | + | + | - |
| Myricetin (3,3′,4′,5,5′,7-Hexahydroxyflavone) | + | + | - |
| Quercitrin (Quercetin-3-O-rhamnoside) | + | + | + |
| 3-O-Methylellagic acid-4′-O-rhamnoside | - | + | + |
| Pinobanksin (3,5,7-Trihydroxyflavanone) | + | + | - |
| Quercetin-O-coumaroylhexoside | + | + | - |
| Naringenin (4′,5,7-Trihydroxyflavanone) | - | + | - |
| Quercetin (3,3′,4′,5,7-Pentahydroxyflavone) | + | + | + |
| Trihydroxy-trimethoxy(iso)flavone-O-hexoside | + | + | - |
| 3,4′-Di-O-methylellagic acid | - | + | - |
| Luteolin (3′,4′,5,7-Tetrahydroxyflavone) | + | + | - |
| 3,3′-Di-O-methylellagic acid | - | - | + |
| 3,4′-Di-O-methylellagic acid | - | + | - |
| Tiliroside (6″-O-trans-p-Coumaroylastragalin) | + | + | - |
| Quercetin-3-O-methyl ether | + | + | + |
| Dimethoxy-tetrahydroxy(iso)flavone | + | + | - |
| Kaempferol (3,4′,5,7-Tetrahydroxyflavone) | + | + | - |
| Isorhamnetin-7-O-rhamnoside | + | - | - |
| Isorhamnetin (3′-Methoxy-3,4′,5,7-tetrahydroxyflavone) | + | + | + |
| Apigenin (4′,5,7-Trihydroxyflavone) | + | + | + |
| Chrysoeriol (3′-Methoxy-4′,5,7-trihydroxyflavone) | + | + | + |
| Isokaempferide (3-Methoxy-4′,5,7-trihydroxyflavone) | + | + | + |
| Dimethoxy-trihydroxy(iso)flavone | + | + | + |
| Rhamnetin (7-Methoxy-3,3′,4′,5-tetrahydroxyflavone) | + | + | + |
| Trihydroxy-trimethoxy(iso)flavone isomer 1 | + | + | + |
| Malyngic acid (9,12,13-Trihydroxy-10E,15Z-octadecadienoic acid) | - | - | + |
| Pinocembrin (5,7-Dihydroxyflavanone) | + | + | + |
| Luteolin-7-O-methyl ether (7-Methoxy-3′,4′,5-trihydroxyflavone) | + | + | + |
| Trihydroxy-trimethoxy(iso)flavone isomer 2 | + | + | + |
| Pinellic acid (9,12,13-Trihydroxy-10E-octadecenoic acid) | - | - | + |
| Dihydroxy-trimethoxy(iso)flavone isomer 1 | + | + | - |
| Dihydroxy(iso)flavone | + | + | - |
| Methoxy-trihydroxy(iso)flavone isomer 1 | + | + | - |
| Acacetin (5,7-Dihydroxy-4′-methoxyflavone) | + | + | - |
| Methoxy-trihydroxy(iso)flavone isomer 2 | + | + | - |
| Genkwanin (4′,5-Dihydroxy-7-methoxyflavone) | + | + | - |
| Kumatakenin (4′,5-Dihydroxy-3,7-dimethoxyflavone) | + | + | - |
| Dihydroxy-trimethoxy(iso)flavone isomer 2 | + | + | - |
| Ermanin (5,7-Dihydroxy-3,4′-dimethoxyflavone) | + | + | + |
| Dihydroxy-trimethoxy(iso)flavone isomer 3 | + | + | - |
| Myricetin-3,3′,4′,7-tetramethyl ether (5,5′-Dihydroxy-3,3′,4′,7-tetramethoxyflavone) | + | + | + |
| Vitexilactone or isomer | + | + | + |
| Emodin | - | - | + |
| Hydroxy-tetramethoxy(iso)flavone | + | + | - |
| Hydroxy-methoxy(iso)flavone | + | + | - |
| Apigenin-4′,7-dimethyl ether (4′,7-Dimethoxy-5-hydroxyflavone) | + | + | - |
| Kaempferol-3,4′,7-trimethyl ether (5-Hydroxy-3,4′,7-trimethoxyflavone) | + | + | - |
| 18-Hydroxy-cis-clerodan-3-ene-15-oic acid or 15-Hydroxy-cis-clerodan-3-ene-18-oic acid | + | + | + |
| 18-Hydroxy-cis-clerodan-3-ene-15-oic acid or 15-Hydroxy-cis-clerodan-3-ene-18-oic acid | - | - | + |
| 18-Hydroxy-cis-clerodan-3-ene-15-oic acid or 15-Hydroxy-cis-clerodan-3-ene-18-oic acid | - | - | + |
| Cistadiol (15,18-Dihydroxy-cis-clerodan-3-ene) | + | + | - |
| 18-Acetoxy-cis-clerodan-3-ene-15-oic acid or 15-Acetoxy-cis-clerodan-3-ene-18-oic acid | + | + | - |
| 8-Hydroxylabdan-15-oic acid | + | + | + |
| Pheophytin A | + | + | - |
| Species | Parts | TPC (mg GAE/g) | TFC (mg RE/g) |
|---|---|---|---|
| Cistus monspeliasis | Leaves | 52.07 ± 0.21 d | 56.98 ± 0.26 a |
| Roots | 103.35 ± 0.54 a | 2.43 ± 0.15 e | |
| Twigs | 98.59 ± 0.49 b | 9.29 ± 0.23 c | |
| Cistus parviflorus | Leaves | 94.44 ± 0.15 c | 43.19 ± 0.44 b |
| Roots | 101.13 ± 1.26 a | 1.91 ± 0.08 e | |
| Twigs | 96.99 ± 1.43 b | 5.86 ± 0.04 d |
| Species | Parts | DPPH (mg TE/g) | ABTS (mg TE/g) | CUPRAC (mg TE/g) | FRAP (mg TE/g) | Chelating (mg EDTAE/g) | PBD (mmol TE/g) |
|---|---|---|---|---|---|---|---|
| Cistus monspeliasis | Leaves | 74.76 ± 0.15 d | 85.62 ± 0.04 d | 123.23 ± 4.61 f | 89.38 ± 1.33 f | 7.23 ± 0.71 b | 2.90 ± 0.02 c |
| Roots | 651.30 ± 3.11 a | 851.53 ± 0.59 a | 843.01 ± 5.00 b | 481.89 ± 4.31 b | 4.58 ± 0.10 c | 4.79 ± 0.07 a | |
| Twigs | 564.54 ± 3.45 b | 784.89 ± 0.94 b | 684.74 ± 9.83 d | 384.99 ± 4.35 d | 5.30 ± 0.36 c | 4.13 ± 0.07 b | |
| Cistus parviflorus | Leaves | 532.07 ± 5.86 c | 681.94 ± 13.92 c | 609.68 ± 10.24 e | 339.03 ± 7.15 e | 15.01 ± 0.25 a | 3.05 ± 0.10 c |
| Roots | 648.83 ± 0.97 a | 854.90 ± 0.62 a | 938.11 ± 9.57 a | 541.41 ± 2.58 a | 4.49 ± 0.31 c | 5.02 ± 0.33 a | |
| Twigs | 566.34 ± 1.95 b | 783.65 ± 6.50 b | 710.45 ± 8.78 c | 430.68 ± 7.41 c | 5.03 ± 0.22 c | 3.92 ± 0.13 b |
| Species | Parts | AChE (mg GALAE/g) | BChE (mg GALAE/g) | Tyrosinase (mg KAE/g) | Amylase (mmol ACAE/g) | Glucosidase (mmol ACAE/g) |
|---|---|---|---|---|---|---|
| Cistus monspeliasis | Leaves | 2.54 ± 0.04 a | 3.69 ± 0.69 d | 63.09 ± 4.45 b | 0.63 ± 0.03 a | 0.94 ± 0.12 b |
| Roots | 2.58 ± 0.02 a | 11.37 ± 1.93 a | 70.87 ± 0.16 a | 0.58 ± 0.02 a | 1.03 ± 0.03 ab | |
| Twigs | 2.56 ± 0.01 a | 8.28 ± 0.92 bc | 69.99 ± 2.21 a | 0.62 ± 0.01 a | 1.03 ± 0.04 ab | |
| Cistus parviflorus | Leaves | 2.44 ± 0.03 b | 5.38 ± 0.87 cd | 68.03 ± 1.61 ab | 0.61 ± 0.02 a | 1.07 ± 0.03 ab |
| Roots | 2.53 ± 0.01 a | 10.90 ± 0.62 ab | 71.19 ± 1.34 a | 0.65 ± 0.04 a | 1.08 ± 0.01 ab | |
| Twigs | 2.52 ± 0.02 a | 8.63 ± 1.11 ab | 71.46 ± 1.38 a | 0.64 ± 0.02 a | 1.09 ± 0.03 a |
| MIC (µg/mL) | |||||
|---|---|---|---|---|---|
| Cistus Species | Parts | Escherichia coli (ATCC 10536) | Pseudomonas aeruginosa (ATCC 15442) | Bacillus subtilis (PeruMycA 6) | Salmonella typhi (PeruMycA 7) |
| Cistus monspeliensis | Leaves | 7.87 | 15.75 | 31.50 | 39.68 |
| Roots | 15.75 | 15.75 | 62.99 | 62.99 | |
| Twigs | 3.96 | 7.77 | 79.37 | 125.99 | |
| Cistus parviflorus | Leaves | 7.87 | 9.92 | 31.50 | 79.37 |
| Roots | 7.87 | 15.75 | 39.68 | 158 | |
| Twigs | 15.75 | 9.92 | 158 | 125.99 | |
| Standard | Ciprofloxacin (µg/mL) | 31.49 | 125.99 | 125.99 | 79.37 |
| MIC (μg/mL) | ||||
|---|---|---|---|---|
| Cistus Species | Parts | Candida tropicalis (YEPGA 6184) | Candida albicans (YEPGA 6379) | Candida parapsilosis (YEPGA 6551) |
| Cistus monspeliensis | Leaves | 125.99 | 31.50 | 125.99 |
| Roots | 62.99 | 158.74 | 158.74 | |
| Twigs | 62.99 | 79.37 | 62.99 | |
| Cistus parviflorus | Leaves | 79.37 | 39.68 | 31.50 |
| Roots | 39.68 | 31.50 | 62.99 | |
| Twigs | 15.75 | 39.68 | 31.50 | |
| Standard | Fluconazole (μg/mL) | 2 | 1 | 4 |
| MIC (µg/mL) | |||||||
|---|---|---|---|---|---|---|---|
| Cistus Species | Extracts | Trichophyton mentagrophytes (CCF 4823) | Trichophyton tonsurans (CCF 4834) | Arthroderma quadrifidum | Arthroderma insingulare (CCF 5417) | Trichophyton mentagrophytes (CCF 5930) | Auxarthron ostraviense DB7 |
| Cistus monspeliensis | Leaves | 125.99 | 31.49 | 79.37 | 62.99 | 125.99 | 125.99 |
| Roots | >200 | 62.99 | 125.99 | 125.99 | 158.74 | 79.37 | |
| Twigs | >200 | 158.74 | >200 | >200 | >200 | 79.37 | |
| Cistus parviflorus | Leaves | 62.99 | 62.99 | 39.68 | 31.49 | 62.99 | 125.99 |
| Roots | >200 | >200 | 125.99 | 79.37 | 158.74 | 125.99 | |
| Twigs | >200 | 125.99 | 125.99 | >200 | 158.74 | 158.74 | |
| Standard | Griseofulvin (µg/mL) | 2.52 | 0.198 | >8 | >8 | 3.174 | 3.17 |
| Cistus Species | Extracts | RAW | HepG2 | S17 |
|---|---|---|---|---|
| 0.5% DMSO | 87.7 ± 5.5 | 99.7 ± 5.7 | 99.3 ± 7.2 | |
| Cistus monspeliasis | Leaves | 60.1 ± 3.8 | 48.8 ± 1.2 | 98.3 ± 8.9 |
| Roots | 70.9 ± 4.7 | 105 ± 7 | 109 ± 8 | |
| Twigs | 73.5 ± 5.1 | 97 ± 6.6 | 105 ± 8 | |
| Cistus parviflorus | Leaves | 58.1 ± 4.8 | 82.6 ± 4.4 | 102 ± 9 |
| Roots | 71.4 ± 4.9 | 60.3 ± 2.3 | 112 ± 8 | |
| Twigs | 73.8 ± 5 | 82.6 ± 4 | 107 ± 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, S.; Zengin, G.; Selvi, S.; Ak, G.; Cziáky, Z.; Jekő, J.; Rodrigues, M.J.; Custodio, L.; Venanzoni, R.; Flores, G.A.; et al. Characterising the Metabolomic Diversity and Biological Potentials of Extracts from Different Parts of Two Cistus Species Using UHPLC-MS/MS and In Vitro Techniques. Pathogens 2024, 13, 795. https://doi.org/10.3390/pathogens13090795
Ahmed S, Zengin G, Selvi S, Ak G, Cziáky Z, Jekő J, Rodrigues MJ, Custodio L, Venanzoni R, Flores GA, et al. Characterising the Metabolomic Diversity and Biological Potentials of Extracts from Different Parts of Two Cistus Species Using UHPLC-MS/MS and In Vitro Techniques. Pathogens. 2024; 13(9):795. https://doi.org/10.3390/pathogens13090795
Chicago/Turabian StyleAhmed, Shakeel, Gokhan Zengin, Selami Selvi, Gunes Ak, Zoltán Cziáky, József Jekő, Maria J. Rodrigues, Luisa Custodio, Roberto Venanzoni, Giancarlo Angeles Flores, and et al. 2024. "Characterising the Metabolomic Diversity and Biological Potentials of Extracts from Different Parts of Two Cistus Species Using UHPLC-MS/MS and In Vitro Techniques" Pathogens 13, no. 9: 795. https://doi.org/10.3390/pathogens13090795
APA StyleAhmed, S., Zengin, G., Selvi, S., Ak, G., Cziáky, Z., Jekő, J., Rodrigues, M. J., Custodio, L., Venanzoni, R., Flores, G. A., Cusumano, G., & Angelini, P. (2024). Characterising the Metabolomic Diversity and Biological Potentials of Extracts from Different Parts of Two Cistus Species Using UHPLC-MS/MS and In Vitro Techniques. Pathogens, 13(9), 795. https://doi.org/10.3390/pathogens13090795










