New BB0108, BB0126, BB0298, BB0323, and BB0689 Chromosomally Encoded Recombinant Proteins of Borrelia burgdorferi sensu lato for Serodiagnosis of Lyme Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Serum Samples
2.2. Construction of Recombinant Plasmids
2.3. Expression and Purification of Recombinant Proteins
2.4. Western Blot
2.5. ELISA
2.6. Statistical Analysis
3. Results
3.1. Construction of Recombinant Plasmids
3.2. Expression and Purification of Recombinant Proteins
3.3. Western Blot
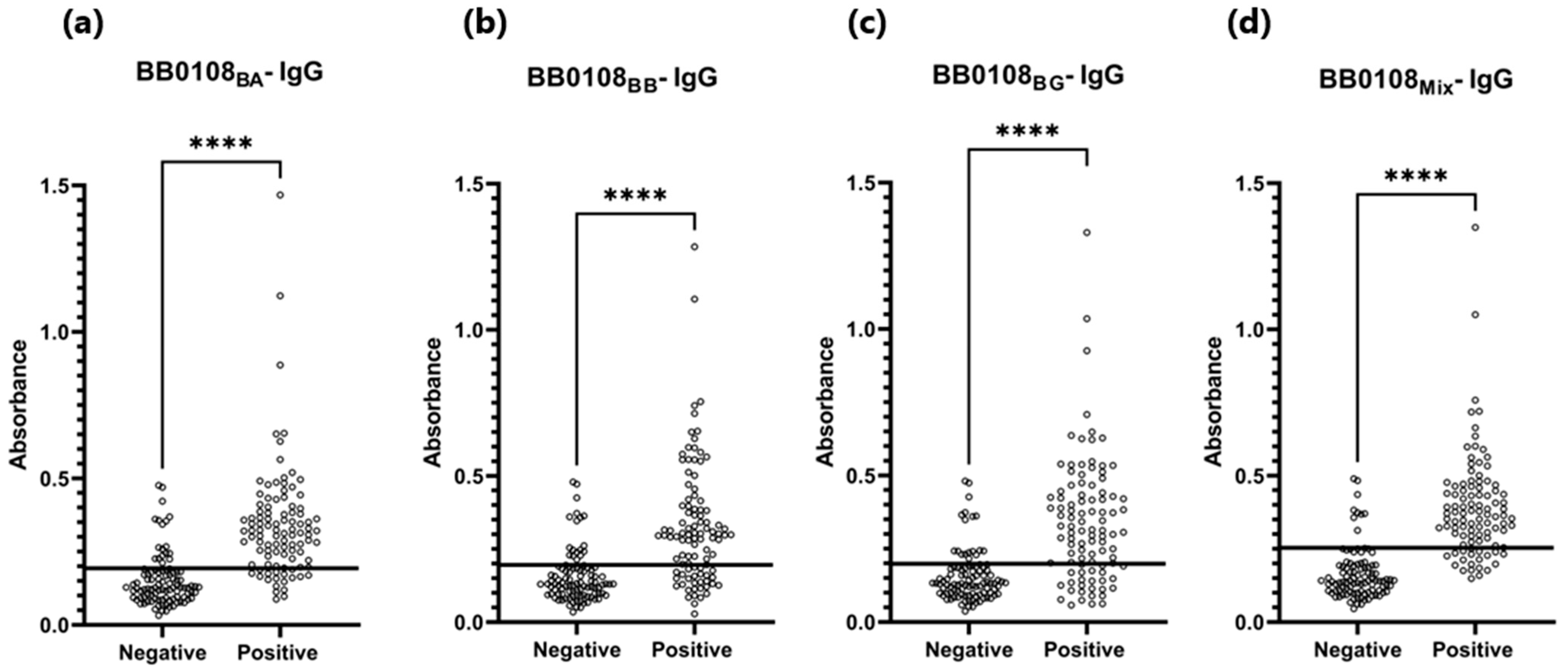
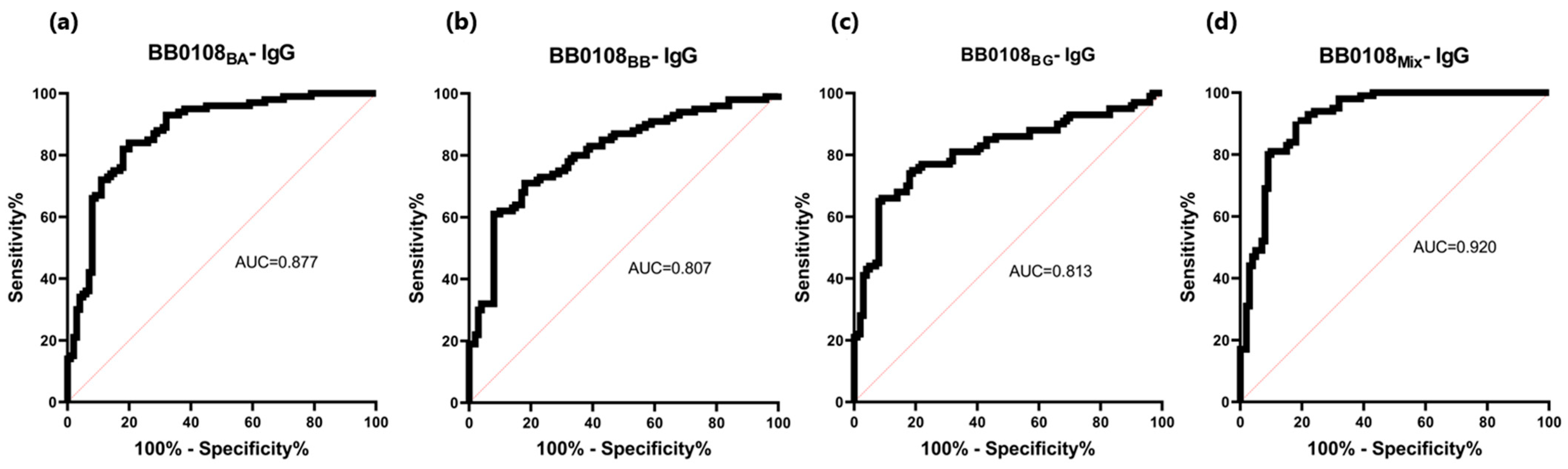
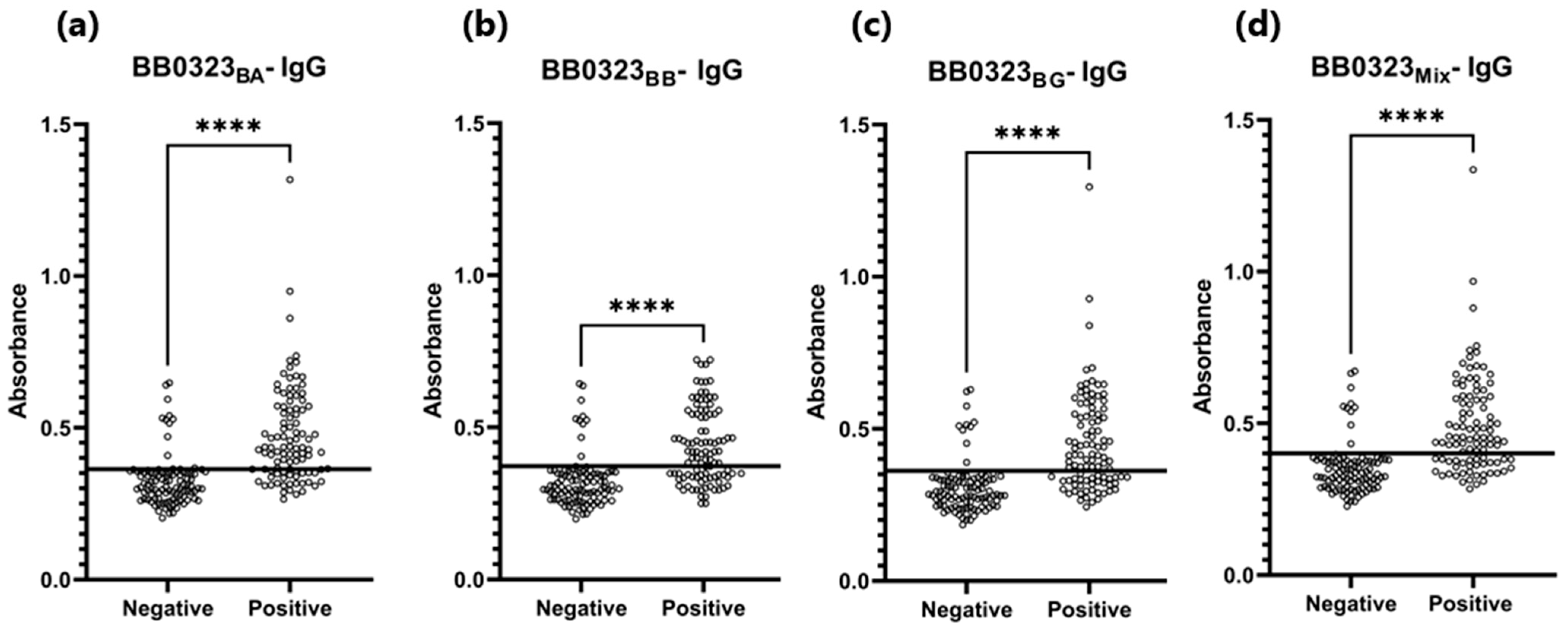
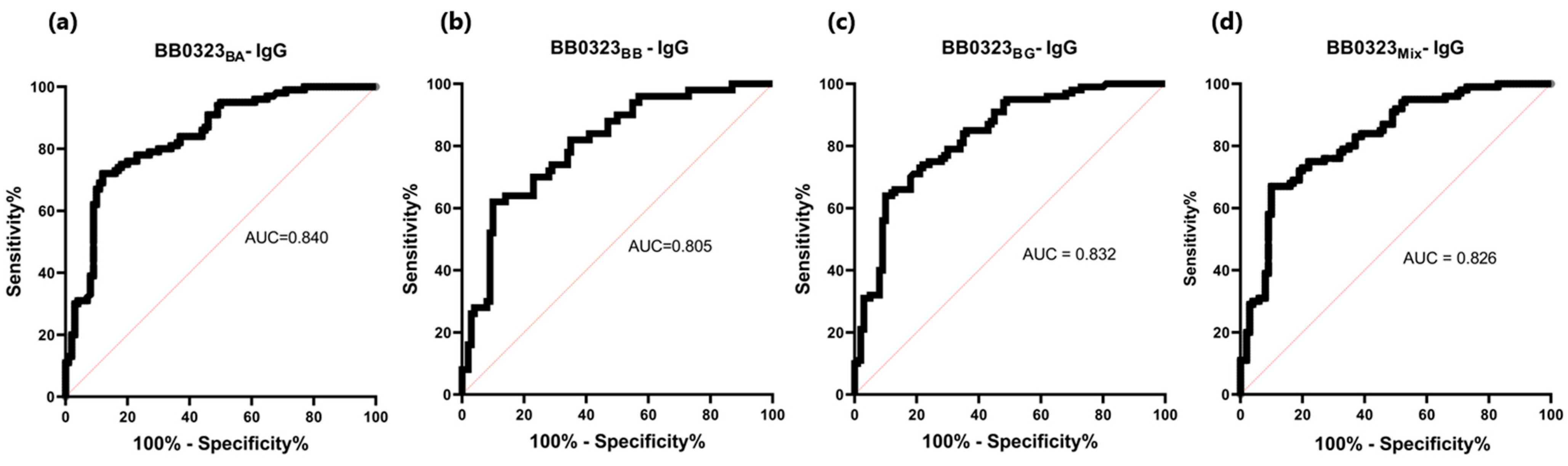
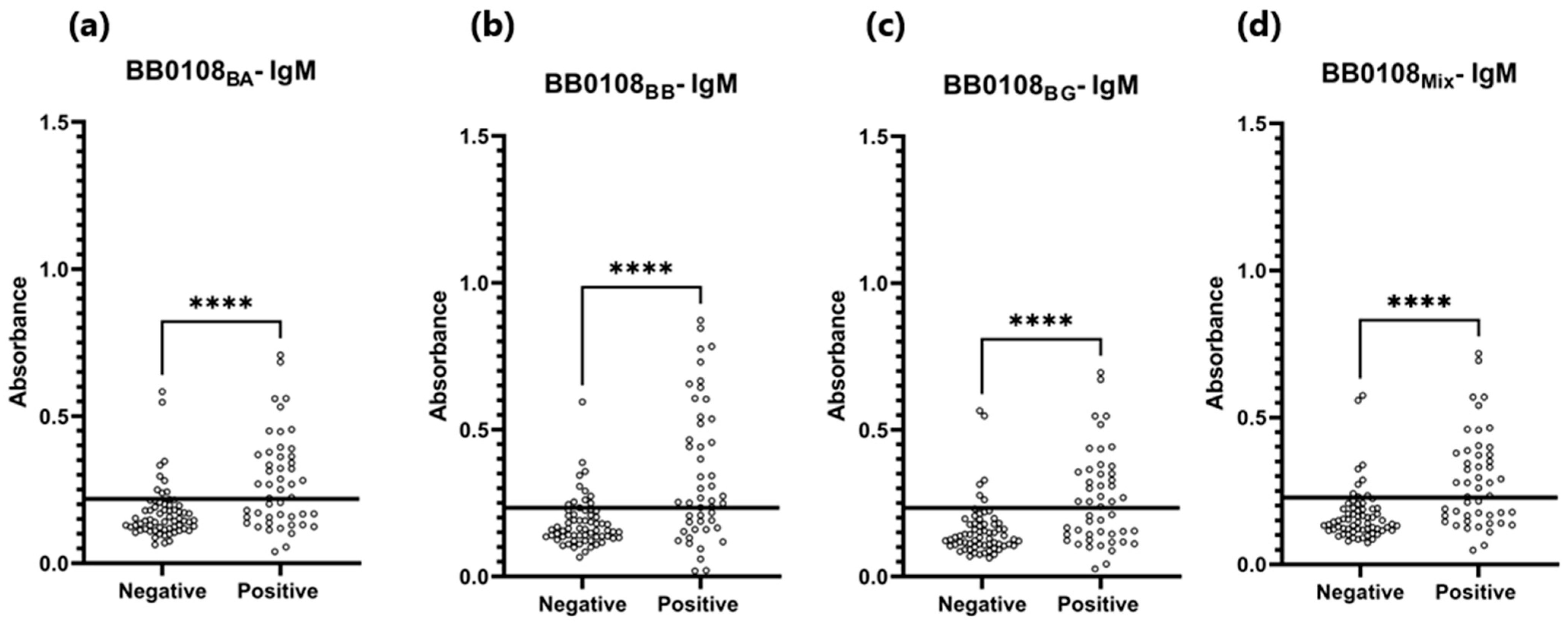
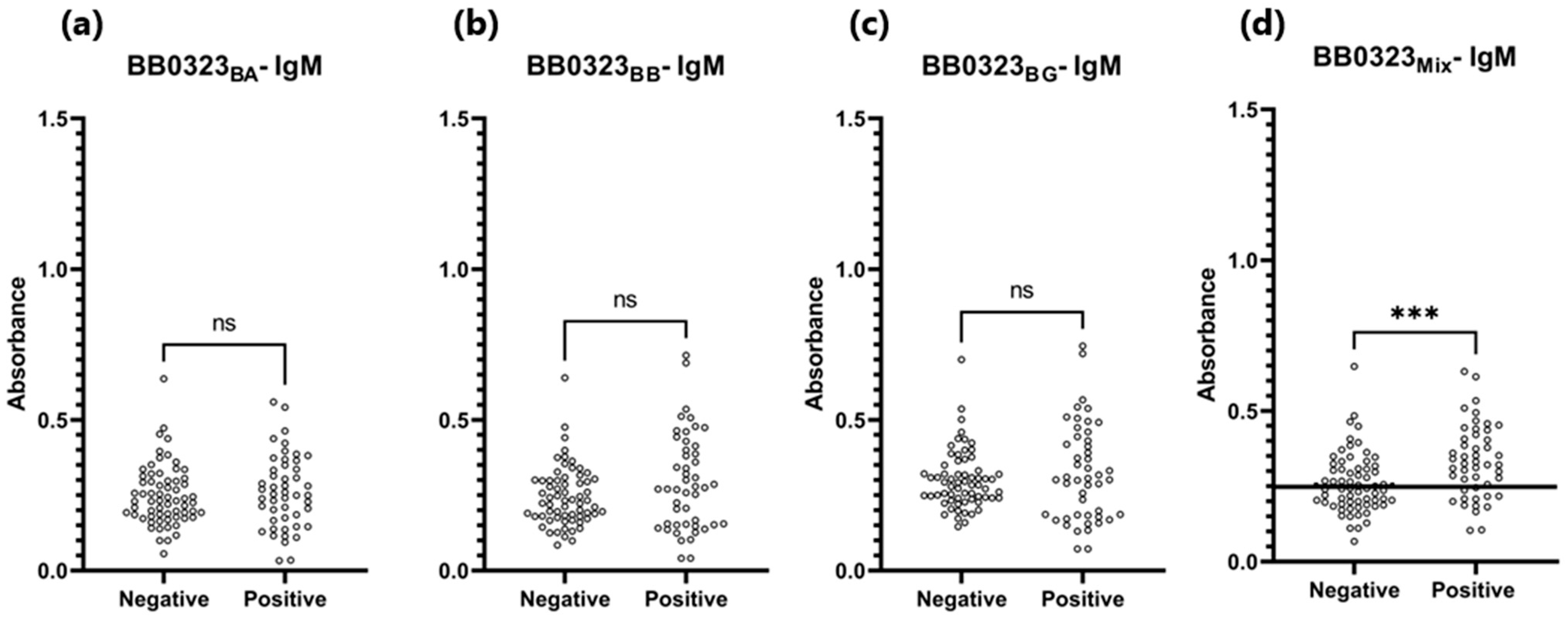
3.4. ELISA
| Recombinant Protein | Optimal Cut-Off | Sensitivity [%] | Specificity [%] | AUC | Mean Absorbance | Median Absorbance |
|---|---|---|---|---|---|---|
| BB0108BA | 0.193 | 82% (82/100) * | 82% (18/100) * | 0.877 | P 1: 0.340 N 2: 0.153 | P 1: 0.317 N 2: 0.128 |
| BB0108BB | 0.196 | 71% (71/100) * | 82% (18/100) * | 0.807 | P 1: 0.323 N 2: 0.155 | P 1: 0.297 N 2: 0.130 |
| BB0108BG | 0.199 | 74% (74/100) * | 82% (18/100) * | 0.813 | P 1: 0.355 N 2: 0.157 | P 1: 0.314 N 2: 0.132 |
| BB0108Mix | 0.254 | 80% (80/100) * | 91% (9/100) * | 0.920 | P 1: 0.391 N 2: 0.166 | P 1: 0.355 N 2: 0.141 |
| BB0323BA | 0.363 | 72% (72/100) * | 88% (12/100) * | 0.840 | P 1: 0.473 N 2: 0.326 | P 1: 0.433 N 2: 0.309 |
| BB0323BB | 0.372 | 62% (62/100) * | 90% (10/100) * | 0.805 | P 1: 0.436 N 2: 0.321 | P 1: 0.416 N 2: 0.299 |
| BB0323BG | 0.362 | 64% (64/100) * | 90% (10/100) * | 0.832 | P 1: 0.450 N 2: 0.307 | P 1: 0.413 N 2: 0.285 |
| BB0323Mix | 0.401 | 67% (67/100) * | 90% (10/100) * | 0.826 | P 1: 0.492 N 2: 0.350 | P 1: 0.452 N 2: 0.331 |
| Recombinant Protein | Optimal Cut-Off | Sensitivity [%] | Specificity [%] | AUC | Mean Absorbance | Median Absorbance |
|---|---|---|---|---|---|---|
| BB0108BA | 0.219 | 56% (27/48) * | 86% (9/65) * | 0.724 | P 1: 0.277 N 2: 0.171 | P 1: 0.259 N 2: 0.145 |
| BB0108BB | 0.234 | 63% (30/48) * | 83% (12/65) * | 0.736 | P 1: 0.259 N 2: 0.183 | P 1: 0.263 N 2: 0.159 |
| BB0108BG | 0.234 | 52% (25/48) * | 91% (6/65) * | 0.729 | P 1: 0.264 N 2: 0.157 | P 1: 0.269 N 2: 0.144 |
| BB0108Mix | 0.228 | 56% (27/48) * | 86% (9/65) * | 0.765 | P 1: 0.287 N 2: 0.168 | P 1: 0.246 N 2: 0.133 |
| BB0323BA | No statistical difference in absorbance for positive and negative sera (p = 0.575) | 0.533 | P 1: 0.262 N 2: 0.250 | P 1: 0.252 N 2: 0.232 | ||
| BB0323BB | No statistical difference in absorbance for positive and negative sera (p = 0.052) | 0.572 | P 1: 0.294 N 2: 0.246 | P 1: 0.273 N 2: 0.233 | ||
| BB0323BG | No statistical difference in absorbance for positive and negative sera (p = 0.319) | 0.526 | P 1: 0.325 N 2: 0.301 | P 1: 0.304 N 2: 0.284 | ||
| BB0323Mix | 0.248 | 60% (29/48) * | 71% (29/65) * | 0.686 | P 1: 0.333 N 2: 0.261 | P 1: 0.323 N 2: 0.243 |


4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Trevisan, G.; Cinco, M.; Trevisini, S.; Di Meo, N.; Chersi, K.; Ruscio, M.; Forgione, P.; Bonin, S. Borreliae Part 1: Borrelia Lyme group and echidna-reptile group. Biology 2021, 10, 1036. [Google Scholar] [CrossRef] [PubMed]
- Cirkovic, V.; Veinovic, G.; Stankovic, D.; Mihaljica, D.; Sukara, R.; Tomanovic, S. Evolutionary dynamics and geographical dispersal of Borrelia lusitaniae. Front. Microbiol. 2024, 15, 1330914. [Google Scholar] [CrossRef] [PubMed]
- Steinbrink, A.; Brugger, K.; Margos, G.; Kraiczy, P.; Klimpel, S. The evolving story of Borrelia burgdorferi sensu lato transmission in Europe. Parasitol. Res. 2022, 121, 781–803. [Google Scholar] [CrossRef] [PubMed]
- Pritt, B.S.; Respicio-Kingry, L.B.; Sloan, L.M.; Schriefer, M.E.; Replogle, A.J.; Bjork, J.; Liu, G.; Kingry, L.C.; Mead, P.S.; Neitzel, D.F.; et al. Borrelia mayonii Sp. Nov., a Member of the Borrelia burgdorferi sensu lato complex, detected in patients and ticks in the Upper Midwestern United States. Int. J. Syst. Evol. Microbiol. 2016, 123, 4757–4763. [Google Scholar] [CrossRef]
- Wang, G.; van Dam, A.P.; Schwartz, I.; Dankert, J. Molecular typing of Borrelia burgdorferi sensu lato: Taxonomic, epidemiological, and clinical implications. Clin. Microbiol. Rev. 1999, 12, 633–653. [Google Scholar] [CrossRef]
- Hofmann, H.; Fingerle, V.; Hunfeld, K.P.; Huppertz, H.I.; Krause, A.; Rauer, S.; Ruf, B. Cutaneous Lyme borreliosis: Guideline of the german dermatology society. Ger. Med. Sci. 2017, 15, 1–31. [Google Scholar] [CrossRef]
- Ružić-Sabljić, E.; Lotrič-Furlan, S.; Maraspin, V.; Cimperman, J.; Logar, M.; Jurca, T.; Strle, F. Comparison of isolation rate of Borrelia burgdorferi sensu lato in MKP and BSK-II Medium. Int. J. Med Microbiol. 2006, 296, 267–273. [Google Scholar] [CrossRef]
- Eldin, C.; Raffetin, A.; Bouiller, K.; Hansmann, Y.; Roblot, F.; Raoult, D.; Parola, P. Review of European and American guidelines for the diagnosis of Lyme borreliosis. Med. Mal. Infect. 2019, 49, 121–132. [Google Scholar] [CrossRef]
- Unlu, A.M.; Andersen, N.S.; Larsen, S.L.; Skarphedinsson, S.; Chrysidis, S.; Knudtzen, F.C.; Lage-Hansen, P.R. Differentiating Lyme arthritis: A case-based review. Rheumatol. Int. 2024. Online ahead of print. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Recommendations for test performance and interpretation from the second national conference on serologic diagnosis of Lyme disease. JAMA 1995, 274, 937. [Google Scholar] [CrossRef]
- Brisson, D.; Drecktrah, D.; Eggers, C.H.; Samuels, D.S. Genetics of Borrelia burgdorferi. Annu. Rev. Genet. 2012, 46, 515–536. [Google Scholar] [CrossRef]
- Ohnishi, J.; Piesman, J.; de Silva, A.M. Antigenic and genetic heterogeneity of Borrelia burgdorferi populations transmitted by ticks. Proc. Natl. Acad. Sci. USA 2001, 98, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Casjens, S.R.; Gilcrease, E.B.; Vujadinovic, M.; Mongodin, E.F.; Luft, B.J.; Schutzer, S.E.; Fraser, C.M.; Qiu, W.G. Plasmid diversity and phylogenetic consistency in the Lyme disease agent Borrelia burgdorferi. BMC Genom. 2017, 18, 165. [Google Scholar] [CrossRef] [PubMed]
- Ojaimi, C.; Brooks, C.; Casjens, S.; Rosa, P.; Elias, A.; Barbour, A.; Jasinskas, A.; Benach, J.; Katona, L.; Radolf, J.; et al. Profiling of temperature-induced changes in Borrelia burgdorferi gene expression by using whole genome arrays. Infect. Immun. 2003, 71, 1689–1705. [Google Scholar] [CrossRef]
- Schwartz, I.; Margos, G.; Casjens, S.R.; Qiu, W.G.; Eggers, C.H. Multipartite genome of Lyme disease Borrelia: Structure, variation and prophages. Curr. Issues Mol. Biol. 2021, 42, 409–454. [Google Scholar] [CrossRef] [PubMed]
- Roessler, D.; Hauser, U.; Wilske, B. Heterogeneity of BmpA (P39) among European isolates of Borrelia burgdorferi sensu lato and influence of interspecies variability on serodiagnosis. J. Clin. Microbiol. 1997, 35, 2752–2758. [Google Scholar] [CrossRef] [PubMed]
- Heikkilä, T.; Seppälä, I.; Saxen, H.; Panelius, J.; Yrjänäinen, H. Species-specific serodiagnosis of Lyme arthritis and neuroborreliosis due to Borrelia burgdorferi sensu stricto, B. afzelii, and B. garinii by using decorin binding protein A. J. Clin. Microbiol. 2002, 40, 453–460. [Google Scholar] [CrossRef]
- Goettner, G.; Schulte-Spechtel, U.; Hillermann, R.; Liegl, G.; Wilske, B.; Fingerle, V. Improvement of Lyme borreliosis serodiagnosis by a newly developed recombinant immunoglobulin G (IgG) and IgM line immunoblot assay and addition of VlsE and DbpA homologues. J. Clin. Microbiol. 2005, 43, 3602–3609. [Google Scholar] [CrossRef]
- Fraser, C.M.; Casjens, S.; Huang, W.M.; Sutton, G.G.; Clayton, R.; Lathigra, R.; White, O.; Ketchum, K.A.; Dodson, R.; Hickey, E.K.; et al. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 1997, 390, 580–586. [Google Scholar] [CrossRef]
- Magnarelli, L.A.; Lawrenz, M.; Fikrig, E.; Norris, S.J. Comparative reactivity of human sera to recombinant VlsE and other Borrelia burgdorferi antigens in class-specific enzyme-linked immunosorbent assays for Lyme borreliosis. J. Med. Microbiol. 2002, 51, 649–655. [Google Scholar] [CrossRef]
- Barbour, A.G.; Jasinskas, A.; Kayala, M.A.; Davies, D.H.; Steere, A.C.; Baldi, P.; Felgner, P.L. A Genome-wide proteome array reveals a limited set of immunogens in natural infections of humans and white-footed mice with Borrelia burgdorferi. Infect. Immun. 2008, 76, 3374–3389. [Google Scholar] [CrossRef] [PubMed]
- Grąźlewska, W.; Sołowińska, K.; Holec-Gąsior, L. In silico epitope prediction of Borrelia burgdorferi sensu lato antigens for the detection of specific antibodies. J. Immunol. Methods 2024, 524, 113596. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowski, S.; Kur, J. Cloning, overexpression, and purification of the recombinant His-Tagged SSB protein of Escherichia coli and use in polymerase chain reaction amplification. Protein Expr. Purif. 1999, 16, 96–102. [Google Scholar] [CrossRef]
- Perkins, N.J.; Schisterman, E.F. The inconsistency of “optimal” cutpoints obtained using two criteria based on the receiver operating characteristic curve. Am. J. Epidemiol. 2006, 163, 670–675. [Google Scholar] [CrossRef]
- Lohr, B.; Fingerle, V.; Norris, D.E.; Hunfeld, K.-P. Laboratory diagnosis of Lyme borreliosis: Current state of the art and future perspectives. Crit. Rev. Clin. Lab. Sci. 2018, 55, 219–245. [Google Scholar] [CrossRef] [PubMed]
- Golkocheva-Markova, E.; Nenova, R.; Stoilov, R.; Christova, I.; Najdenski, H. Cross-reactivity between Yersinia outer membrane proteins and anti-Francisella and anti-Borrelia antibodies in serodiagnosis of Yersinia-triggered reactive arthritis. Comptes Rendus L’Acad. Bulg. Sci. 2011, 64, 61–66. [Google Scholar]
- Wojciechowska-Koszko, I.; Kwiatkowski, P.; Sienkiewicz, M.; Kowalczyk, M.; Kowalczyk, E.; Dołęgowska, B. Cross-reactive results in serological tests for borreliosis in patients with active viral infections. Pathogens 2022, 11, 203. [Google Scholar] [CrossRef]
- Bruckbauer, H.R.; Preac-Mursic, V.; Fuchs, R.; Wilske, B. Cross-reactive proteins of Borrelia burgdorferi. Eur. J. Clin. Microbiol. 1992, 11, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Smit, P.W.; Kurkela, S.; Kuusi, M.; Vapalahti, O. Evaluation of two commercially available rapid diagnostic tests for Lyme borreliosis. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 34, 109–113. [Google Scholar] [CrossRef]
- Heikkilä, T.; Huppertz, H.I.; Seppälä, I.; Sillanpää, H.; Saxen, H.; Lahdenne, P. Recombinant or peptide antigens in the serology of Lyme arthritis in children. J. Infect. Dis. 2003, 187, 1888–1894. [Google Scholar] [CrossRef]
- Panelius, J.; Lahdenne, P.; Saxén, H.; Carlsson, S.A.; Heikkilä, T.; Peltomaa, M.; Lauhio, A.; Seppälä, I. Diagnosis of Lyme neuroborreliosis with antibodies to recombinant proteins DbpA, BBK32, and OspC, and VIsE IR6peptide. J. Neurol. 2003, 250, 1318–1327. [Google Scholar] [CrossRef] [PubMed]
- Heikkilä, T.; Seppälä, I.; Saxén, H.; Panelius, J.; Peltomaa, M.; Julin, T.; Carlsson, S.A.; Lahdenne, P. Recombinant BBK32 protein in serodiagnosis of early and late Lyme borreliosis. J. Clin. Microbiol. 2002, 40, 1174–1180. [Google Scholar] [CrossRef]
- Glatz, M.; Fingerle, V.; Wilske, B.; Ambros-Rudolph, C.; Kerl, H.; Müllegger, R.R. Immunoblot analysis of the seroreactivity to recombinant Borrelia burgdorferi sensu lato antigens, including VlsE, in the long-term course of treated patients with erythema migrans. Dermatology 2008, 216, 93–103. [Google Scholar] [CrossRef]
- Hsieh, Y.F.; Liu, H.W.; Hsu, T.C.; Wei, J.C.C.; Shih, C.M.; Krause, P.J.; Tsay, G.J. Serum reactivity against Borrelia burgdorferi OspA in patients with rheumatoid arthritis. Clin. Vaccine Immunol. 2007, 14, 1437–1441. [Google Scholar] [CrossRef] [PubMed]
- Magnarelli, L.; Ijdo, J.W.; Padula, S.J.; Flavell, R.; Fikrig, E. Serologic diagnosis of Lyme borreliosis by using enzyme-linked immunosorbent assays with recombinant antigens. J. Clin. Microbiol. 2000, 38, 1735–1739. [Google Scholar] [CrossRef]
- Cornero, R.; Irfan, S.S.; Cachaco, S.; Zhou, W.; Byne, A.; Howard, M.; McIntyre, H.; Birkaya, B.; Liotta, L.; Luchini, A. Identification of unambiguous Borrelia peptides in human urine using affinity capture and mass spectrometry. Methods Mol. Biol. 2024, 2742, 105–122. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, X.; Kumar, M.; Pal, U. BB0323 function is essential for Borrelia burgdorferi virulence and persistence through tick-rodent transmission cycle. J. Infect. Dis. 2009, 200, 1318–1330. [Google Scholar] [CrossRef]
- Xu, Y.; Bruno, J.F.; Luft, B.J. Profiling the humoral immune response to Borrelia burgdorferi infection with protein microarrays. Microb. Pathog. 2008, 45, 403–407. [Google Scholar] [CrossRef]
- Brangulis, K.; Jaudzems, K.; Petrovskis, I.; Akopjana, I.; Kazaks, A.; Tars, K. Structural and functional analysis of BB0689 from Borrelia burgdorferi, a member of the bacterial CAP superfamily. J. Struct. Biol. 2015, 192, 320–330. [Google Scholar] [CrossRef]
- Brooks, C.S.; Vuppala, S.R.; Jett, A.M.; Akins, D.R. Identification of Borrelia burgdorferi outer surface proteins. Infect. Immun. 2006, 74, 296–304. [Google Scholar] [CrossRef]
- Strand, M.; Hönig, V.; Ružek, D.; Grubhoffer, L.; Regoa, R.O.M. Europe-wide meta-analysis of Borrelia burgdorferi sensu lato prevalence in questing Ixodes ricinus ticks. Appl. Environ. Microbiol. 2017, 83, e00609-17. [Google Scholar] [CrossRef] [PubMed]
- Kubiak, K.; Dziekońska-Rynko, J.; Szymańska, H.; Kubiak, D.; Dmitryjuk, M.; Dzika, E. Questing Ixodes ricinus ticks (acari, ixodidae) as a vector of Borrelia burgdorferi sensu lato and Borrelia miyamotoi in an urban area of North-Eastern Poland. Exp. Appl. Acarol. 2019, 78, 113–126. [Google Scholar] [CrossRef]
- Gałęziowska, E.; Rzymowska, J.; Najda, N.; Kołodziej, P.; Domżał-Drzewicka, R.; Rząca, M.; Muraczyńska, B.; Charzyńska-Gula, M.; Szadowska-Szlachetka, Z.; Ślusarska, B.; et al. Prevalence of Borrelia burgdorferi in ticks removed from skin of people and circumstances of being bitten—Research from the area of Poland, 2012–2014. Ann. Agric. Environ. Med. 2018, 25, 31–35. [Google Scholar] [CrossRef]
- Holec-Gąsior, L.; Ferra, B.; Drapała, D. MIC1-MAG1-SAG1 Chimeric protein, a most effective antigen for detection of human toxoplasmosis. J. Clin. Immunol. 2012, 19, 1977–1979. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Drapała, D.; Holec-Gasior, L.; Kur, J. New Recombinant chimeric antigens, P35-MAG1, MIC1-ROP1, and MAG1-ROP1, for the serodiagnosis of human toxoplasmosis. Diagn. Microbiol. Infect. Dis. 2015, 82, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Panelius, J.; Lahdenne, P.; Saxen, H.; Heikkila, T.; Seppala, I. Recombinant flagellin A proteins from Borrelia burgdorferi sensu stricto, B. afzelii, and B. garinii in serodiagnosis of Lyme borreliosis. J. Clin. Microbiol. 2001, 39, 4013–4019. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Xiong, E.; Hong, R.; Lu, Q.; Ohno, H.; Wang, J.Y. Role of the IgM Fc receptor in immunity and tolerance. Front. Immunol. 2019, 10, 529. [Google Scholar] [CrossRef]
- Hillerdal, H.; Henningsson, A.J. Serodiagnosis of Lyme Borreliosis—Is IgM in serum more harmful than helpful? Eur. J. Clin. Microbiol. 2021, 40, 1161–1168. [Google Scholar] [CrossRef]
- Mäkelä, O.; Rouslahti, E.; Seppälä, I.J.T. Affinity of IgM and IgG antibodies. Immunochemistry 1970, 7, 917–932. [Google Scholar] [CrossRef]
- Keyt, B.A.; Baliga, R.; Sinclair, A.M.; Carroll, S.F.; Peterson, M.S. Structure, function, and therapeutic use of IgM antibodies. Antibodies 2020, 2020, 53. [Google Scholar] [CrossRef]
- Johnson, B.J.B.; Robbins, K.E.; Bailey, R.E.; Cao, B.L.; Sviat, S.L.; Craven, R.B.; Mayer, L.W.; Dennis, D.T. Serodiagnosis of Lyme disease: Accuracy of a two-step approach using a flagella-based ELISA and immunoblotting. J. Infect. Dis. 1996, 174, 346–353. [Google Scholar] [CrossRef]
- Panelius, J.; Lahdenne, P.; Heikkila, T.; Peltomaa, M.; Oksi, J.; Seppala, I. Recombinant OspC from Borrelia burgdorferi sensu stricto B. afzelii and B. garinii in the serodiagnosis of Lyme borreliosis. J. Med. Microbiol. 2002, 51, 731–739. [Google Scholar] [CrossRef]
- Schulte-Spechtel, U.; Fingerle, V.; Goettner, G.; Rogge, S.; Wilske, B. Molecular analysis of decorin-binding protein A (DbpA) reveals five major groups among European Borrelia burgdorferi sensu lato strains with impact for the development of serological assays and indicates lateral gene transfer of the DbpA gene. Int. J. Med. Microbiol. 2006, 296, 250–266. [Google Scholar] [CrossRef]
- Cisak, E.; Wójcik-Fatla, A.; Stojek, N.M.; Chmielewska-Badora, J.; Zwoliński, J.; Buczek, A.; Dutkiewicz, J. Prevalence of Borrelia burgdorferi genospecies in Ixodes ricinus ticks from Lublin Region (Eastern Poland). Ann. Agric. Environ. Med. 2006, 13, 301–306. [Google Scholar]
- Strzelczyk, J.K.; Gaździcka, J.; Cuber, P.; Asman, M.; Trapp, G.; Gołąbek, K.; Zalewska-Ziob, M.; Nowak-Chmura, M.; Siuda, K.; Wiczkowski, A.; et al. Prevalence of Borrelia burgdorferi sensu lato in Ixodes ricinus ticks collected from Southern Poland. Acta Parasitol. 2015, 60, 666–674. [Google Scholar] [CrossRef]
- Estrada-Peña, A.; Ortega, C.; Sánchez, N.; DeSimone, L.; Sudre, B.; Suk, J.E.; Semenza, J.C. Correlation of Borrelia burgdorferi sensu lato prevalence in questing Ixodes ricinus ticks with specific abiotic traits in the Western Palearctic. Appl. Environ. Microbiol. 2011, 77, 3838–3845. [Google Scholar] [CrossRef]
- Stanek, G.; Reiter, M. The expanding Lyme Borrelia complex-clinical significance of genomic species? Clin. Infect. Dis. 2011, 17, 487–493. [Google Scholar] [CrossRef]
- Gerber, M.A.; Shapiro, E.D.; Bell, G.L.; Sampieri, A.; Padula, S.J. Recombinant outer surface protein-C ELISA for the diagnosis of early Lyme-disease. J. Infect. Dis. 1995, 171, 724–727. [Google Scholar] [CrossRef]
- Fung, B.P.; McHugh, G.L.; Leong, J.M.; Steere, A.C. Humoral immune response to outer surface protein C of Borrelia burgdorferi in Lyme disease: Role of the immunoglobulin M response in the serodiagnosis of early infection. Infect. Immun. 1994, 62, 3213–3221. [Google Scholar] [CrossRef]
- Padula, S.J.; Dias, F.; Sampieri, A.; Craven, R.B.; Ryan, R.W. Use of Recombinant Ospc from Borrelia-burgdorferi for serodiagnosis of early Lyme-disease. J. Clin. Microbiol. 1994, 32, 1733–1738. [Google Scholar] [CrossRef]
- Liang, F.T.; Steere, A.C.; Marques, A.R.; Johnson, B.J.; Miller, J.N.; Philipp, M.T. Sensitive and specific serodiagnosis of Lyme disease by enzyme-linked immunosorbent assay with a peptide based on an immunodominant conserved region of Borrelia burgdorferi VlsE. J. Clin. Microbiol. 1999, 37, 3990–3996. [Google Scholar] [CrossRef] [PubMed]
- Talagrand-Reboul, E.; Raffetin, A.; Zachary, P.; Jaulhac, B.; Eldin, C. Immunoserological diagnosis of human borreliosis: Current knowledge and perspectives. Front. Cell. Infect. Microbiol. 2020, 10, 241. [Google Scholar] [CrossRef]
- Rawlins, M.L.; Gerstner, C.; Hill, H.R.; Litwin, C.M. Evaluation of a Western blot method for the detection of Yersinia antibodies: Evidence of serological cross-reactivity between Yersinia outer membrane proteins and Borrelia burgdorferi. Clin. Diagn. Lab. Immunol. 2005, 12, 1269–1274. [Google Scholar] [CrossRef] [PubMed]
- Goossens, H.; Nohlmans, M.K.; van den Bogaard, E. Epstein-Barr Virus and Cytomegalovirus Infections Cause False-Positive Results in IgM Two-Test Protocol for Early Lyme Borreliosis. Infection 1999, 27, 231. [Google Scholar] [CrossRef]
- Smismans, A.; Goossens, V.J.; Nulens, E.; Bruggeman, C.A. Comparison of five different immunoassays for the detection of Borrelia burgdorferi IgM and IgG antibodies. Clin. Infect. Dis. 2006, 12, 648–655. [Google Scholar] [CrossRef][Green Version]
- Tuuminen, T.; Hedman, K.; Söderlund-Venermo, M.; Seppälä, I. Acute parvovirus B19 infection causes nonspecificity frequently in Borrelia and less often in Salmonella and Campylobacter serology, posing a problem in diagnosis of infectious arthropathy. Clin. Vaccine Immunol. 2011, 18, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Grąźlewska, W.; Holec-Gąsior, L.; Sołowińska, K.; Chmielewski, T.; Fiecek, B.; Contreras, M. Epitope Mapping of BmpA and BBK32 Borrelia burgdorferi sensu stricto antigens for the design of chimeric proteins with potential diagnostic value. ACS Infect. Dis. 2023, 9, 2160–2172. [Google Scholar] [CrossRef]
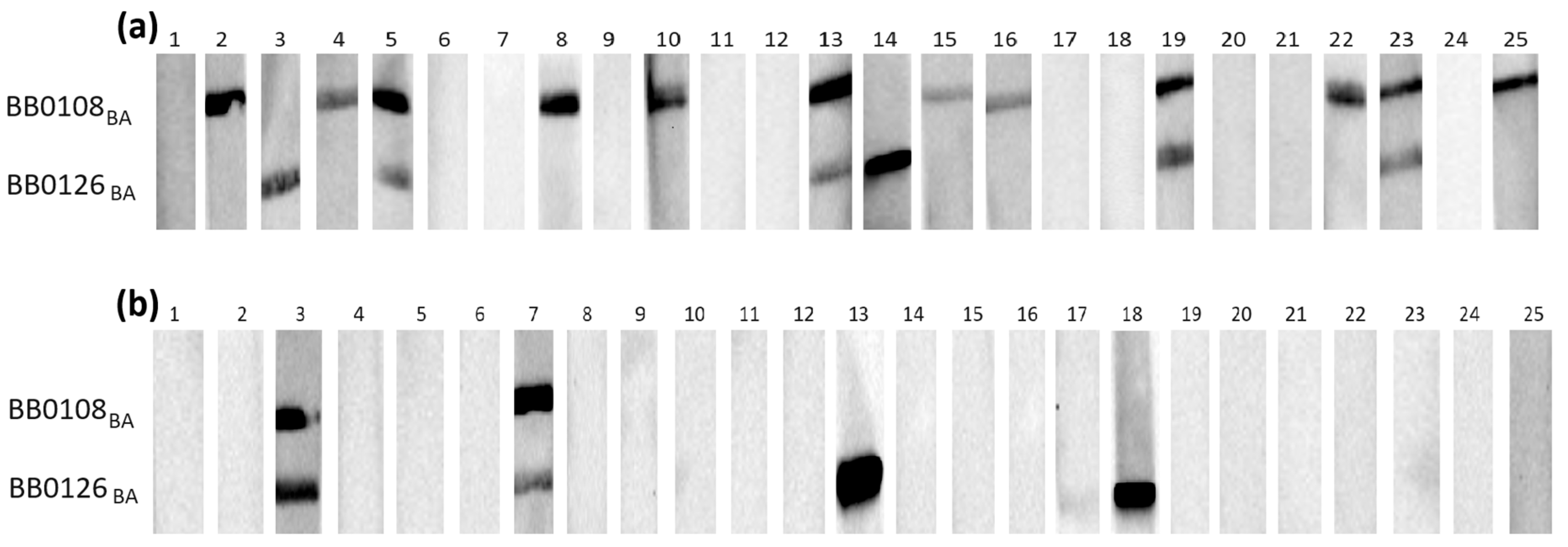
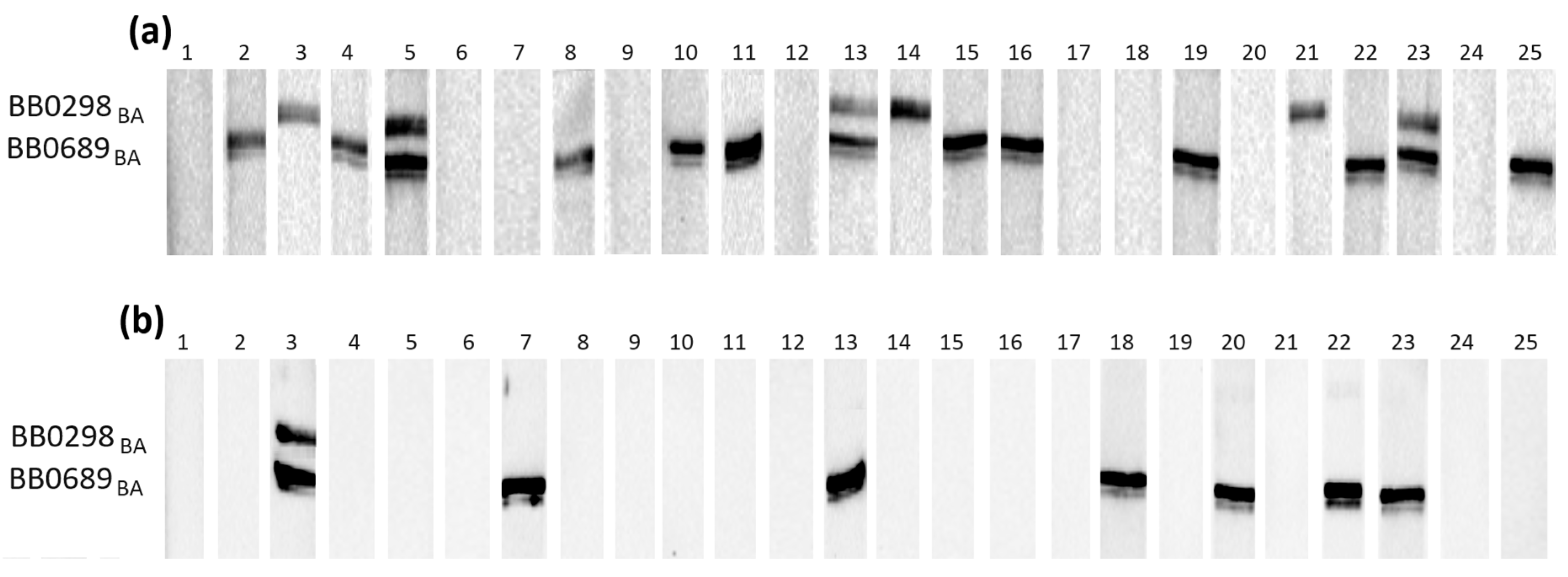

| Test Strip No. 1 | Test Strip No. 2 | Test Strip No. 3 |
|---|---|---|
| BB0108—44 kDa BB0126—30 kDa | BB0298s—32 kDa BB0698s—23 kDa | BB0323—50 kDa |
| Plasmid Name | Plasmid Length [bp] | Recombinant Protein | Protein Length [aa] | Native Protein aa Residues | Molecular Mass [kDa] | Production Efficiency [mg/L] |
|---|---|---|---|---|---|---|
| pUET1-BB0108BA | 3839 | BB0108BA | 394 | 20–336 | 44.4 | 41 |
| pUET1-BB0108BB | 3839 | BB0108BB | 394 | 20–336 | 44.3 | 76 |
| pUET1-BB0108BG | 3839 | BB0108BG | 394 | 20–336 | 44.3 | 58 |
| pUET1-BB0126BA | 3408 | BB0126BA | 259 | 22–203 | 29.5 | 47 |
| pUET1-BB0126BB | 3408 | BB0126BB | 259 | 22–203 | 29.6 | 42 |
| pUET1-BB0126BG | 3408 | BB0126BG | 259 | 22–203 | 29.5 | 55 |
| pUET1-BB0298BA | 3489 | BB0298BA | 286 | 17–225 | 32.6 | 59 |
| pUET1-BB0298BB | 3489 | BB0298BB | 286 | 17–225 | 32.5 | 61 |
| pUET1-BB0298BG | 3489 | BB0298BG | 286 | 17–225 | 32.6 | 46 |
| pUET1-BB0323BA | 3933 | BB0323BA | 434 | 23–379 | 50.5 | 43 |
| pUET1-BB0323BB | 3927 | BB0323BB | 432 | 22–376 | 50 | 39 |
| pUET1-BB0323BG | 3924 | BB0323BG | 431 | 23–376 | 50.1 | 41 |
| pUET1-BB0689BA | 3237 | BB0689BA | 202 | 28–152 | 22.9 | 73 |
| pUET1-BB0689BB | 3237 | BB0689BB | 202 | 28–152 | 22.9 | 71 |
| pUET1-BB0689BG | 3237 | BB0689BG | 202 | 28–152 | 22.9 | 74 |
| Recombinant Protein | Sensitivity (n = 25) | Specificity (n = 25) | PPV 1 | NPV 2 | Statistically Significant (p < 0.05) |
|---|---|---|---|---|---|
| BB0108BA | 48% (12/25) * | 92% (2/25) * | 86% | 64% | Yes (p = 0.0036) |
| BB0108BB | 40% (10/25) * | 96% (1/25) * | 91% | 62% | Yes (p = 0.0046) |
| BB0108BG | 44% (11/25) * | 88% (3/25) * | 79% | 61% | Yes (p = 0.0255) |
| BB0108BA+BB+BG | 48% (12/25) * | 92% (2/25) * | 86% | 64% | Yes (p = 0.0036) |
| BB0126BA | 24% (6/25) * | 84% (4/25) * | 60% | 53% | No (p = 0.7252) |
| BB0126BB | 20% (5/25) * | 88% (3/25) * | 63% | 52% | No (p = 0.7019) |
| BB0126BG | 16% (4/25) * | 100% (0/25) * | 100% | 54% | No (p = 0.1099) |
| BB0126BA+BB+BG | 24% (6/25) * | 88% (3/25) * | 67% | 54% | No (p = 0.4635) |
| BB0298BA | 24% (6/25) * | 96% (1/25) * | 86% | 56% | No (p = 0.0983) |
| BB0298BB | 20% (5/25) * | 100% (0/25) * | 100% | 56% | No (p = 0.0502) |
| BB0298BG | 24% (6/25) * | 100% (0/25) * | 100% | 57% | Yes (p = 0.0223) |
| BB0298BA+BB+BG | 24% (6/25) * | 96% (1/25) * | 86% | 56% | No (p = 0.0983) |
| BB0323BA | 44% (11/25) * | 88% (3/25) * | 79% | 61% | Yes (p = 0.0255) |
| BB0323BB | 36% (9/25) * | 92% (2/25) * | 82% | 59% | Yes (p = 0.0374) |
| BB0323BG | 44% (11/25) * | 92% (2/25) * | 85% | 62% | Yes (p = 0.0083) |
| BB0323BA+BB+BG | 44% (11/25) * | 88% (3/25) * | 79% | 61% | Yes (p = 0.0255) |
| BB0689BA | 52% (13/25) * | 72% (7/25) * | 65% | 60% | No (p = 0.1482) |
| BB0689BB | 44% (11/25) * | 76% (6/25) * | 65% | 58% | No (p = 0.2321) |
| BB0689BG | 48% (12/25) * | 72% (7/25) * | 63% | 58% | No (p = 0.2436) |
| BB0689BA+BB+BG | 60% (15/25) * | 64% (9/25) * | 63% | 62% | No (p= 0.1564) |
| Recombinant Protein | Sensitivity (n = 25) | Specificity (n = 25) | PPV 1 | NPV 2 | Statistically Significant (p < 0.05) |
|---|---|---|---|---|---|
| BB0108BA | 24% (6/25) * | 96% (1/25) * | 86% | 56% | No (p = 0.0983) |
| BB0108BB | 20% (5/25) * | 96% (1/25) * | 83% | 55% | No (p = 0.1895) |
| BB0108BG | 24% (6/25) * | 96% (1/25) * | 86% | 56% | No (p = 0.0983) |
| BB0108BA+BB+BG | 24% (6/25) * | 96% (1/25) * | 86% | 56% | No (p = 0.0983) |
| BB0126BA | 16% (4/25) * | 92% (2/25) * | 67% | 52% | No (p = 0.6671) |
| BB0126BB | 8% (2/25) * | 92% (2/25) * | 50% | 50% | No (p > 0.9999) |
| BB0126BG | 8% (2/25) * | 92% (2/25) * | 50% | 50% | No (p > 0.9999) |
| BB0126BA+BB+BG | 16% (4/25) * | 92% (2/25) * | 67% | 52% | No (p = 0.6671) |
| BB0298BA | 8% (2/25) * | 96% (1/25) * | 67% | 51% | No (p > 0.9999) |
| BB0298BB | 12% (3/25) * | 92% (2/25) * | 60% | 51% | No (p > 0.9999) |
| BB0298BG | 8% (2/25) * | 96% (1/25) * | 67% | 51% | No (p > 0.9999) |
| BB0298BA+BB+BG | 12% (3/25) * | 92% (2/25) * | 60% | 51% | No (p > 0.9999) |
| BB0323BA | 8% (2/25) * | 96% (1/25) * | 67% | 51% | No (p > 0.9999) |
| BB0323BB | 4% (1/25) * | 96% (1/25) * | 50% | 50% | No (p > 0.9999) |
| BB0323BG | 8% (2/25) * | 92% (2/25) * | 50% | 50% | No (p > 0.9999) |
| BB0323BA+BB+BG | 8% (2/25) * | 92% (2/25) * | 50% | 50% | No (p > 0.9999) |
| BB0689BA | 20% (5/25) * | 96% (1/25) * | 83% | 55% | No (p = 0.1895) |
| BB0689BB | 12% (3/25) * | 96% (1/25) * | 75% | 52% | No (p = 0.6092) |
| BB0689BG | 12% (3/25) * | 92% (2/25) * | 60% | 51% | No (p > 0.9999) |
| BB0689BA+BB+BG | 20% (5/25) * | 92% (2/25) * | 71% | 53% | No (p = 0.4174) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grąźlewska, W.; Chmielewski, T.; Fiecek, B.; Holec-Gąsior, L. New BB0108, BB0126, BB0298, BB0323, and BB0689 Chromosomally Encoded Recombinant Proteins of Borrelia burgdorferi sensu lato for Serodiagnosis of Lyme Disease. Pathogens 2024, 13, 767. https://doi.org/10.3390/pathogens13090767
Grąźlewska W, Chmielewski T, Fiecek B, Holec-Gąsior L. New BB0108, BB0126, BB0298, BB0323, and BB0689 Chromosomally Encoded Recombinant Proteins of Borrelia burgdorferi sensu lato for Serodiagnosis of Lyme Disease. Pathogens. 2024; 13(9):767. https://doi.org/10.3390/pathogens13090767
Chicago/Turabian StyleGrąźlewska, Weronika, Tomasz Chmielewski, Beata Fiecek, and Lucyna Holec-Gąsior. 2024. "New BB0108, BB0126, BB0298, BB0323, and BB0689 Chromosomally Encoded Recombinant Proteins of Borrelia burgdorferi sensu lato for Serodiagnosis of Lyme Disease" Pathogens 13, no. 9: 767. https://doi.org/10.3390/pathogens13090767
APA StyleGrąźlewska, W., Chmielewski, T., Fiecek, B., & Holec-Gąsior, L. (2024). New BB0108, BB0126, BB0298, BB0323, and BB0689 Chromosomally Encoded Recombinant Proteins of Borrelia burgdorferi sensu lato for Serodiagnosis of Lyme Disease. Pathogens, 13(9), 767. https://doi.org/10.3390/pathogens13090767







