Global Distribution of Canine Visceral Leishmaniasis and the Role of the Dog in the Epidemiology of the Disease
Abstract
1. Introduction
2. Distribution of Canine Visceral Leishmaniasis in the World
2.1. Africa
2.2. The Americas
2.3. Asia
2.4. Europe
| Continent | Country | Location | Parasite Species | % of Dogs Positive for Leishmania (Diagnostic Test Used) | References |
|---|---|---|---|---|---|
| Africa | Ethiopia | Amhara region | L. donovani Complex * | 3.8% (RIFI and ELISA); 2.8% (PCR) | [145] |
| Kafta Humera district | L. donovani | 27.7% (DAT); 14.8% (KDRT) | [146] | ||
| Addis Zemen, Humera, and Sheraro | L. donovani Complex * | 5.9% (PCR) | [147] | ||
| Benishangul-Gumuz region | NA | 13.9% (rK39 ICT); 5.6 (DAT) | [148] | ||
| Burkina-Faso | Bobo-Dioulasso | L. infantum | 5.88% (Serological test) | [39] | |
| Côte d’Ivoire | Abidjan and Yamoussoukro cities | L. infantum | 8.9% (PCR or IFAT) | [40] | |
| Nigeria | Oyo, Ogun, and Kwara | NA | 4.4% (ELISA) | [41] | |
| Zambia | Southern Province | L. infantum | NA (Serological tests and PCR) | [42] | |
| Asia | Iran | Meshkin-Shahr district | L. infantum | 15.8% (DAT) | [149] |
| Boyer Ahmad district | L. infantum | 10% (DAT) | [150] | ||
| Kouhsar district | L. infantum | 3.6% (DAT) | [151] | ||
| Khorasan Razavi province | L. infantum | 7.6% (IFAT) | [152] | ||
| Kerman province | L. infantum | 11.25% (DAT) | [153] | ||
| Kerman and Sistan-Baluchestan provinces | L. infantum | 15.4% (ELISA) | [154] | ||
| Tehran and Alborz provinces | L. infantum | 4.9% (DAT) | [155] | ||
| Meshkin-Shahr district | L. infantum | 23.4% (DAT) | [156] | ||
| Hamedan province | L. infantum | 3.95% (ELISA) | [157] | ||
| Jiroft district | L. infantum | 7.9% (DAT) | [158] | ||
| Ardabil, Alborz, and East-Azerbaijan provinces | L. infantum | 100% (DAT, rK39, and PCR) | [84] | ||
| Alborz province | L. infantum | 2.97% (DAT) | [159] | ||
| Golestan province | L. infantum | 18% (PCR | [160] | ||
| Nepal | Kathmandu | L. donovani Complex * | 18.57% (PCR) | [93] | |
| Pakistan | Chilas, Abbotabad, Bagh, Poonch, and Muzafarabad districts | L. donovani Complex * | 18% (DAT); 26.6% (ELISA) | [94] | |
| Philippines | NA | L. infantum | NA | [98] | |
| Vietnam | NA | L. infantum | NA | [98] | |
| America | Brazil | Belo Horizonte | L. infantum | 64.6% (IFAT) | [63] |
| L. infantum | 56.7% (PCR) | [64] | |||
| Boa Vista | L. infantum | 10.3% (RIFI) | [65] | ||
| Baixada Cuiabana | L. infantum | 16.58% (PCR) | [66] | ||
| Cuiabá | L. infantum | 1.14% (DPP and ELISA) | [67] | ||
| Florianópolis | L. infantum | 1.37% (ELISA and RIFI) | [68] | ||
| Niterói | NA | 15.5% (ELISA and IFAT) | [69] | ||
| Porto Alegre metropolitan area | NA | 4% (PCR) | [70] | ||
| Vitória | NA | 13% (ELISA); 6% (RIFI) | [71] | ||
| Piacatu | L. infantum | 16.08% (ELISA) | [72] | ||
| Divinópolis | NA | 4.63% (ELISA and IFAT) | [73] | ||
| Governador Valadares | NA | 30.2% (IFAT) | [74] | ||
| NA | 29% (ELISA and RIFI) | [75] | |||
| Campina Grande | NA | 8.4% (IFAT) and 4.3% (ELISA) | [76] | ||
| Santarém | L. infantum | 23.3% (ELISA and rK39) | [77] | ||
| Europe | Bulgaria | Petrich | L. infantum | NA (IFAT and PCR) | [133] |
| Georgia | Tbilisi and Kutaisi | L. donovani Complex * | 20% (rK39) | [134] | |
| Kvareli and Sagarejo districts | 19.5% and 11.4% (rK39) | [135] | |||
| Germany | NA | NA | 11.8% (ELISA) | [136] | |
| Hungary | Tolna province | L. infantum | 30% (IFAT and PCR) | [137] | |
| Kosovo | Prizren, Gjakova, Rahovec, and Deçan | L. infantum | 18.49% (ELISA) | [138] | |
| The Netherlands | NA | NA | 32.4% (ELISA) | [136] | |
| North Macedonia | Skopje and Prilep | L. infantum | 2.5% (PCR) | [139] | |
| Romania | Vâlcea County | L. infantum | NA (FASTest®LEISH) | [140] | |
| Ramnicu Vâlcea | L. infantum | 8.75% (ELISA); 10% (PCR) | [161] | ||
| Galați | NA | 8.33% (ELISA) | [141] | ||
| Argeș County | L. infantum | 20.1% (PCR) | [142] | ||
| Slovenia | Kostelo | L. infantum | NA (IFAT) | [143] | |
| Switzerland | NA | NA | 12.2% (ELISA) | [136] | |
| United Kingdom | NA | L. infantum | NA (ELISA and PCR) | [144] |
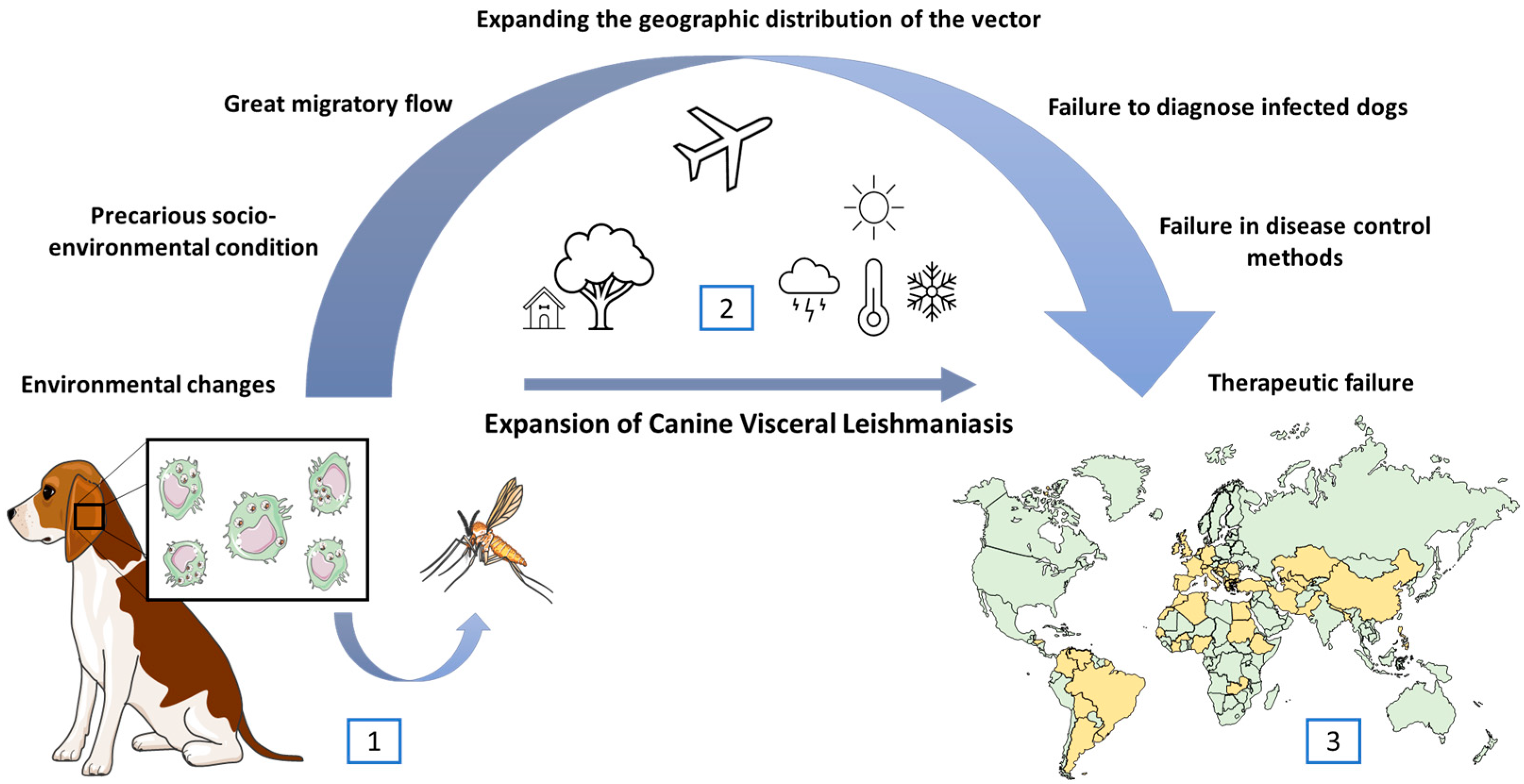
3. Factors Influencing the Territorial Expansion and Transmission Maintenance of Visceral Leishmaniasis
4. The Dog’s Role in the Visceral Leishmaniasis Transmission Cycle
5. The Euthanasia Challenges for Controlling the Spread of Canine Visceral Leishmaniasis
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alves, F.; Bilbe, G.; Blesson, S.; Goyal, V.; Monnerat, S.; Mowbray, C.; Muthoni Ouattara, G.; Pécoul, B.; Rijal, S.; Rode, J.; et al. Recent Development of Visceral Leishmaniasis Treatments: Successes, Pitfalls, and Perspectives. Clin. Microbiol. Rev. 2018, 31, e00048-18. [Google Scholar] [CrossRef]
- Alvar, J.; Vélez, I.D.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; Jannin, J.; den Boer, M. Leishmaniasis Worldwide and Global Estimates of Its Incidence. PLoS ONE 2012, 7, e35671. [Google Scholar] [CrossRef]
- Alemayehu, B.; Alemayehu, M. Leishmaniasis: A Review on Parasite, Vector and Reservoir Host. Health Sci. J. 2017, 11, 1. [Google Scholar] [CrossRef]
- Rossi, M.; Fasel, N. How to Master the Host Immune System? Leishmania Parasites Have the Solutions! Int. Immunol. 2018, 30, 103–111. [Google Scholar] [CrossRef]
- World Health Organization. Leishmaniasis. Available online: https://www.who.int/news-room/fact-sheets/detail/Leishmaniasis (accessed on 25 March 2024).
- McGwire, B.S.; Satoskar, A.R. Leishmaniasis: Clinical Syndromes and Treatment. QJM 2014, 107, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Organização Panamericana da Saúde. Leishmanioses: Informe Epidemiológico das Américas, No. 10 (Dezembro 2021). Available online: https://iris.paho.org/handle/10665.2/55386 (accessed on 20 February 2023).
- Maurìcio, I.L.; Stothard, J.R.; Miles, M.A. The Strange Case of Leishmania chagasi. Parasitol. Today 2000, 16, 188–189. [Google Scholar] [CrossRef]
- Lukeš, J.; Mauricio, I.L.; Schönian, G.; Dujardin, J.-C.; Soteriadou, K.; Dedet, J.-P.; Kuhls, K.; Tintaya, K.W.Q.; Jirků, M.; Chocholová, E.; et al. Evolutionary and Geographical History of the Leishmania donovani Complex with a Revision of Current Taxonomy. Proc. Natl. Acad. Sci. USA 2007, 104, 9375–9380. [Google Scholar] [CrossRef] [PubMed]
- Roque, A.L.R.; Jansen, A.M. Wild and Synanthropic Reservoirs of Leishmania Species in the Americas. Int. J. Parasitol. Parasites Wildl. 2014, 3, 251–262. [Google Scholar] [CrossRef]
- Roatt, B.M.; Aguiar-Soares, R.D.D.O.; Coura-Vital, W.; Ker, H.G.; Moreira, N.D.D.; Vitoriano-Souza, J.; Giunchetti, R.C.; Carneiro, C.M.; Reis, A.B. Immunotherapy and Immunochemotherapy in Visceral Leishmaniasis: Promising Treatments for This Neglected Disease. Front. Immunol. 2014, 5, 272. [Google Scholar] [CrossRef]
- Burza, S.; Croft, S.L.; Boelaert, M. Leishmaniasis. Lancet 2018, 392, 951–970. [Google Scholar] [CrossRef]
- Moreno, J.; Alvar, J. Canine Leishmaniasis: Epidemiological risk and the experimental model. Trends Parasitol. 2002, 18, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Solano-Gallego, L.; Koutinas, A.; Miró, G.; Cardoso, L.; Pennisi, M.G.; Ferrer, L.; Bourdeau, P.; Oliva, G.; Baneth, G. Directions for the Diagnosis, Clinical Staging, Treatment and Prevention of Canine Leishmaniosis. Vet. Parasitol. 2009, 165, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Dantas-Torres, F.; Solano-Gallego, L.; Baneth, G.; Ribeiro, V.M.; de Paiva-Cavalcanti, M.; Otranto, D. Canine Leishmaniosis in the Old and New Worlds: Unveiled Similarities and Differences. Trends Parasitol. 2012, 28, 531–538. [Google Scholar] [CrossRef]
- Ulchar, I.; Celeska, I.; Stefanovska, J.; Jakimovska, A. Hematological and Biochemical Parameters in Symptomatic and Asymptomatic Leishmania-Seropositive Dogs. Maced. Vet. Rev. 2015, 38, 175–182. [Google Scholar] [CrossRef]
- Gonçalves, A.A.M.; Leite, J.C.; Resende, L.A.; Mariano, R.M.D.S.; Silveira, P.; Melo-Júnior, O.A.D.O.; Ribeiro, H.S.; de Oliveira, D.S.; Soares, D.F.; Santos, T.A.P.; et al. An Overview of Immunotherapeutic Approaches against Canine Visceral Leishmaniasis: What Has Been Tested on Dogs and a New Perspective on Improving Treatment Efficacy. Front. Cell. Infect. Microbiol. 2019, 9, 427. [Google Scholar] [CrossRef]
- Ready, P. Epidemiology of Visceral Leishmaniasis. Clin. Epidemiol. 2014, 6, 147. [Google Scholar] [CrossRef] [PubMed]
- Kabbout, N.; Merzoug, D.; Chenchouni, H. Ecological Status of Phlebotomine Sandflies (Diptera: Psychodidae) in Rural Communities of Northeastern Algeria. J. Arthropod Borne Dis. 2016, 10, 24–38. [Google Scholar] [PubMed]
- Harrat, Z.; Belkaid, M. Les Leishmanioses Dans l’Algérois. Données Épidémiologiques. In Proceedings of the 6ème Congrès International Francophone de Médecine Tropicale “Santé et Urbanisation en Afrique; Available online: https://pathexo.societe-mtsi.fr/documents/articles-bull/T96-3-DK42.pdf (accessed on 10 July 2023).
- Adel, A.; Saegerman, C.; Speybroeck, N.; Praet, N.; Victor, B.; De Deken, R.; Soukehal, A.; Berkvens, D. Canine Leishmaniasis in Algeria: True Prevalence and Diagnostic Test Characteristics in Groups of Dogs of Different Functional Type. Vet. Parasitol. 2010, 172, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Adel, A.; Abatih, E.; Speybroeck, N.; Soukehal, A.; Bouguedour, R.; Boughalem, K.; Bouhbal, A.; Djerbal, M.; Saegerman, C.; Berkvens, D. Estimation of Canine Leishmania Infection Prevalence in Six Cities of the Algerian Littoral Zone Using a Bayesian Approach. PLoS ONE 2015, 10, e0117313. [Google Scholar] [CrossRef]
- Medkour, H.; Laidoudi, Y.; Lafri, I.; Davoust, B.; Mekroud, A.; Bitam, I.; Mediannikov, O. Canine Vector-Borne Protozoa: Molecular and Serological Investigation for Leishmania spp., Trypanosoma spp., Babesia spp., and Hepatozoon spp. in Dogs from Northern Algeria. Vet. Parasitol. Reg. Stud. Rep. 2020, 19, 100353. [Google Scholar] [CrossRef]
- Bia, T.; Sanchez, C.; Zait, H.; Kouidri, M.; Mabrouk, S.K.; Nieto, J.; Ammar, S.S.M.; Moreno, J.; Ahlem, B.N. Diagnosis and Prevalence of Canine Leishmaniasis in the Atlas Shepherd Dog. Vet. Parasitol. Reg. Stud. Rep. 2022, 36, 100787. [Google Scholar] [CrossRef] [PubMed]
- Jeaume, G. Un Cas de Leishmaniose Naturelle Généralisée Chez Le Chien Au Maroc. Bulletiin Société Pathol. Exot. 1932, 25, 225–227. [Google Scholar]
- Kahime, K.; Boussaa, S.; Nhammi, H.; Boumezzough, A. Urbanization of Human Visceral Leishmaniasis in Morocco. Parasite Epidemiol. Control 2017, 2, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Idrissi, H.; Hakkour, M.; Duchateau, L.; Zanatta, R.; Kachani, M.; Azrib, R.; Daminet, S.; Kichou, F.; El Asatey, S.; Tazi, N.; et al. Canine Leishmaniasis in Morocco: A Descriptive Prospective Clinical Study. Vet. Med. Int. 2021, 2021, 6304127. [Google Scholar] [CrossRef] [PubMed]
- Nicolle, C.; Comte, C. Origine Canine Du Kala-Azar. Bull. Soc. Pathol. Exot. 1908, 1, 299–301. [Google Scholar]
- Diouani, M.F.; Ben Alaya Bouafif, N.; Bettaib, J.; Louzir, H.; Jedidi, S.; Ftaiti, A.; Zaatour, A.; Jomaa, I.; Dellagi, K.; Ben Ismail, R.; et al. Dogs L. infantum Infection from an Endemic Region of the North of Tunisia: A Prospective Study. Arch. Inst. Pasteur Tunis. 2008, 85, 55–61. [Google Scholar] [PubMed]
- Chargui, N.; Haouas, N.; Gorcii, M.; Akrout Messaidi, F.; Zribi, M.; Babba, H. Increase of Canine Leishmaniasis in a Previously Low-Endemicity Area in Tunisia. Parasite 2007, 14, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Chargui, N.; Haouas, N.; Gorcii, M.; Lahmar, S.; Guesmi, M.; Ben Abdelhafidh, A.; Mezhoud, H.; Babba, H. Use of PCR, IFAT and in Vitro Culture in the Detection of Leishmania infantum Infection in Dogs and Evaluation of the Prevalence of Canine Leishmaniasis in a Low Endemic Area in Tunisia. Parasite 2009, 16, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Zoghlami, Z.; Chouihi, E.; Barhoumi, W.; Dachraoui, K.; Massoudi, N.; Helel, K.B.; Habboul, Z.; Hadhri, M.H.; Limam, S.; Mhadhbi, M.; et al. Interaction between Canine and Human Visceral Leishmaniases in a Holoendemic Focus of Central Tunisia. Acta Trop. 2014, 139, 32–38. [Google Scholar] [CrossRef]
- Bouattour, A.; Amri, A.; Belkhiria, J.A.; Rhim, A.; Fezaa, O.; Gantier, J.-C.; M’ghirbi, Y. Canine Leishmaniosis in Tunisia: Growing Prevalence, Larger Zones of Infection. PLoS Negl. Trop. Dis. 2021, 15, e0009990. [Google Scholar] [CrossRef]
- Selim, A.; Shoulah, S.; Abdelhady, A.; Alouffi, A.; Alraey, Y.; Al-Salem, W. Seroprevalence and Risk Factors Associated with Canine Leishmaniasis in Egypt. Vet. Sci. 2021, 8, 236. [Google Scholar] [CrossRef] [PubMed]
- Leta, S.; Dao, T.H.T.; Mesele, F.; Alemayehu, G. Visceral Leishmaniasis in Ethiopia: An Evolving Disease. PLoS Negl. Trop. Dis. 2014, 8, e3131. [Google Scholar] [CrossRef] [PubMed]
- Dereure, J.; Boni, M.; Pratlong, F.; el Hadi Osman, M.; Bucheton, B.; el-Safi, S.; Feugier, E.; Musa, M.K.; Davoust, B.; Dessein, A.; et al. Visceral Leishmaniasis in Sudan: First Identifications of Leishmania from Dogs. Trans. R. Soc. Trop. Med. Hyg. 2000, 94, 154–155. [Google Scholar] [CrossRef] [PubMed]
- Dereure, J.; El-Safi, S.H.; Bucheton, B.; Boni, M.; Kheir, M.M.; Davoust, B.; Pratlong, F.; Feugier, E.; Lambert, M.; Dessein, A.; et al. Visceral Leishmaniasis in Eastern Sudan: Parasite Identification in Humans and Dogs; Host-Parasite Relationships. Microbes Infect. 2003, 5, 1103–1108. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.M.; Osman, O.F.; El-Raba’a, F.M.; Schallig, H.D.; Elnaiem, D.-E.A. Role of the Domestic Dog as a Reservoir Host of Leishmania donovani in Eastern Sudan. Parasit. Vectors 2009, 2, 26. [Google Scholar] [CrossRef] [PubMed]
- Sangare, I.; Djibougou, A.; Koudraogo, B.; Drabo, F.; Diabate, A.; Laure Banu, A.; Fournet, F.; Price, H.; Tinga Guig, R.; Kounbobr, D.R. First Detection of Leishmania infantum in Domestic Dogs from Burkina Faso (West Africa). Res. J. Parasitol. 2016, 12, 27–32. [Google Scholar] [CrossRef]
- Medkour, H.; Laidoudi, Y.; Athias, E.; Bouam, A.; Dizoé, S.; Davoust, B.; Mediannikov, O. Molecular and Serological Detection of Animal and Human Vector-Borne Pathogens in the Blood of Dogs from Côte d’Ivoire. Comp. Immunol. Microbiol. Infect. Dis. 2020, 69, 101412. [Google Scholar] [CrossRef] [PubMed]
- Adediran, O.A.; Kolapo, T.U.; Uwalaka, E.C. Seroprevalence of Canine Leishmaniasis in Kwara, Oyo and Ogun States of Nigeria. J. Parasit. Dis. 2016, 40, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Squarre, D.; Chambaro, H.M.; Hayashida, K.; Moonga, L.C.; Qiu, Y.; Goto, Y.; Oparaocha, E.; Mumba, C.; Muleya, W.; Bwalya, P.; et al. Autochthonous Leishmania infantum in Dogs, Zambia, 2021. Emerg. Infect. Dis. 2022, 28, 888–890. [Google Scholar] [CrossRef]
- Kuhls, K.; Alam, M.Z.; Cupolillo, E.; Ferreira, G.E.M.; Mauricio, I.L.; Oddone, R.; Feliciangeli, M.D.; Wirth, T.; Miles, M.A.; Schönian, G. Comparative Microsatellite Typing of New World Leishmania infantum Reveals Low Heterogeneity among Populations and Its Recent Old World Origin. PLoS Negl. Trop. Dis. 2011, 5, e1155. [Google Scholar] [CrossRef]
- Organização Pan-Americada da Saúde. Leishmanioses: Informe Epidemiológico das Américas, dezembro 2020. Available online: https://iris.paho.org/handle/10665.2/53091 (accessed on 12 October 2022).
- Salomon, O.; Sinagra, A.; Nevot, M.; Barberian, G.; Paulin, P.; Estevez, J.; Riarte, A.; Estevez, J. First Visceral Leishmaniasis Focus in Argentina. Mem. Inst. Oswaldo Cruz 2008, 103, 109–111. [Google Scholar] [CrossRef] [PubMed]
- Acardi, S.A.; Liotta, D.J.; Santini, M.S.; Romagosa, C.M.; Salomón, O.D. Detection of Leishmania infantum in Naturally Infected Lutzomyia longipalpis (Diptera: Psychodidae: Phlebotominae) and Canis Familiaris in Misiones, Argentina: The First Report of a PCR-RFLP and Sequencing-Based Confirmation Assay. Mem. Inst. Oswaldo Cruz 2010, 105, 796–799. [Google Scholar] [CrossRef] [PubMed]
- Cruz, I.; Acosta, L.; Gutiérrez, M.N.; Nieto, J.; Cañavate, C.; Deschutter, J.; Bornay-Llinares, F.J. A Canine Leishmaniasis Pilot Survey in an Emerging Focus of Visceral Leishmaniasis: Posadas (Misiones, Argentina). BMC Infect. Dis. 2010, 10, 342. [Google Scholar] [CrossRef] [PubMed]
- Acosta, L.; Díaz, R.; Torres, P.; Silva, G.; Ramos, M.; Fattore, G.; Deschutter, E.J.; Bornay-Llinares, F.J. Identification of Leishmania infantum in puerto iguazú, misiones, argentina. Rev. Inst. Med. Trop. Sao Paulo 2015, 57, 175–176. [Google Scholar] [CrossRef]
- Fujisawa, K.; Silcott-Niles, C.; Simonson, P.; Lamattina, D.; Humeres, C.A.; Bhattacharyya, T.; Mertens, P.; Thunissen, C.; O’Rourke, V.; Pańczuk, M.; et al. Emergent Canine Visceral Leishmaniasis in Argentina: Comparative Diagnostics and Relevance to Proliferation of Human Disease. PLoS Negl. Trop. Dis. 2021, 15, e0009552. [Google Scholar] [CrossRef] [PubMed]
- Lamattina, D.; Berrozpe, P.E.; Casas, N.; Moya, S.L.; Giuliani, M.G.; Costa, S.A.; Arrabal, J.P.; Martínez, M.F.; Rivero, M.R.; Salas, M.; et al. Twice upon a Time: The Progression of Canine Visceral Leishmaniasis in an Argentinean City. PLoS ONE 2019, 14, e0219395. [Google Scholar] [CrossRef]
- Barroso, P.A.; Marco, J.D.; Locatelli, F.M.; Cardozo, R.M.; Hoyos, C.L.; Mora, M.C.; García Bustos, M.F.; López-Quiroga, I.; Mimori, T.; Gentile, A.G.; et al. Visceral Leishmaniasis Caused by Leishmania infantum in Salta, Argentina: Possible Reservoirs and Vectors. Am. J. Trop. Med. Hyg. 2015, 93, 334–339. [Google Scholar] [CrossRef]
- Satragno, D.; Faral-Tello, P.; Canneva, B.; Verger, L.; Lozano, A.; Vitale, E.; Greif, G.; Soto, C.; Robello, C.; Basmadjián, Y. Autochthonous Outbreak and Expansion of Canine Visceral Leishmaniasis, Uruguay. Emerg. Infect. Dis. 2017, 23, 536–538. [Google Scholar] [CrossRef]
- Le Pont, F.; Mollinedo, S.; Mouchet, J.; Desjeux, P. Leishmaniose en Bolivie. IV. Le Chien Dans les Cycles des Leishmanioses en Bolivie. Mem. Inst. Oswaldo Cruz 1989, 84, 417–421. [Google Scholar] [CrossRef]
- Zambrano-Hernandez, P.C.; Ayala Sotelo, M.S.; Fuya Oviedo, O.P.; Montenegro Puentes, C.A.; Aya Vanegas, N.M.; Rodriguez Toro, J.G.; Becerra Osorio, S.L.; Aguilera Jaramillo, G.; Lozano Polanco, C.A.; Rojas Garcia, M.C.; et al. Brote Urbano de Leishmaniasis Visceral En Neiva (Huila), 2012. Rev. Salud Pública 2015, 17, 514–527. [Google Scholar] [CrossRef]
- Picón, Y.; Almario, G.; Rodríguez, V.; Garcia, N.V. Seroprevalence, Clinical, and Pathological Characteristics of Canine Leishmaniasis in a Central Region of Colombia. J. Vet. Res. 2020, 64, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Arbeláez, N.; Moreno, J.; Murillo, J.; Montoya, A.; Robledo, S.M.; Vélez, A.; Vélez, I.D. First Report of an Urban Case of Canine Visceral Leishmaniasis in the Municipality of Cali, Colombia. Am. J. Trop. Med. Hyg. 2020, 102, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Rueda-Concha, K.L.; Payares-Mercado, A.; Guerra-Castillo, J.; Melendrez, J.; Arroyo-Munive, Y.; Martínez-Abad, L.; Cochero, S.; Bejarano, E.E.; Paternina, L.E. Circulación de Leishmania infantum y Trypanosoma Cruzi En Perros Domésticos de Áreas Urbanas de Sincelejo, Región Caribe de Colombia. Biomédica 2022, 42, 633–649. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Ramírez, R.D.; Lugo-Vargas, R.; Petano-Duque, J.M.; Cruz-Méndez, J.S.; Rondón-Barragán, I.S. First Study on Microscopic and Molecular Detection of Acanthocheilonema Reconditum and Leishmania infantum Coinfection in Dogs in Southwest Colombia. Vet. World 2023, 16, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Rivas, A.K.; Alcover, M.M.; Martínez-Orellana, P.; Montserrat-Sangrà, S.; Nachum-Biala, Y.; Fisa, R.; Riera, C.; Baneth, G.; Solano-Gallego, L. Serological and Molecular Survey of Leishmania Infection in Dogs from Venezuela. Vet. Parasitol. Reg. Stud. Rep. 2020, 21, 100420. [Google Scholar] [CrossRef] [PubMed]
- Segura, G.B.R.; Ochoa, W.H.S.; da Matta, V.L.R.; Martínez, M.; Tercero, C.R.; Gonzalez, R.R.; Pacheco, C.M.S.; Flores, G.V.A.; Silveira, F.T.; Henriquez, M.M.R.; et al. Can Domestic Dogs Be Considered a Good Reservoir of Leishmania (L.) infantum chagasi in an Endemic Area of Nonulcerated Cutaneous Leishmaniasis in Southern Honduras? Rev. Inst. Med. Trop. Sao Paulo 2023, 65, e24. [Google Scholar] [CrossRef] [PubMed]
- Maia-Elkhoury, A.N.S.; Alves, W.A.; de Sousa-Gomes, M.L.; de Sena, J.M.; Luna, E.A. Visceral Leishmaniasis in Brazil: Trends and Challenges. Cad. Saude Publica 2008, 24, 2941–2947. [Google Scholar] [CrossRef] [PubMed]
- Harhay, M.O.; Olliaro, P.L.; Costa, D.L.; Costa, C.H.N. Urban Parasitology: Visceral Leishmaniasis in Brazil. Trends Parasitol. 2011, 27, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.S.; Gontijo, C.M.; Pacheco, R.S.; Fiuza, V.O.; Brazil, R.P. Visceral Leishmaniasis in the Metropolitan Region of Belo Horizonte, State of Minas Gerais, Brazil. Mem. Inst. Oswaldo Cruz 2001, 96, 285–291. [Google Scholar] [CrossRef]
- Alves Souza, N.; Souza Leite, R.; de Oliveira Silva, S.; Groenner Penna, M.; Figueiredo Felicori Vilela, L.; Melo, M.N.; de Andrade, A.S.R. Detection of Mixed Leishmania Infections in Dogs from an Endemic Area in Southeastern Brazil. Acta Trop. 2019, 193, 12–17. [Google Scholar] [CrossRef]
- Guerra, J.A.O.; Barros, M.L.B.; Fé, N.F.; Guerra, M.V.F.; Castellon, E.; Paes, M.G.; Sherlock, Í.A. Leishmaniose Visceral Entre Índios No Estado de Roraima, Brasil: Aspectos Clínicoepidemiológicos de Casos Observados No Período de 1989 a 1993. Rev. Soc. Bras. Med. Trop. 2004, 37, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Dias, A.F.L.R.; Almeida, A.B.P.F.; Rodrigues, J.Y.; Nakazato, L.; Fujimori, M.; Sousa, V.R.F. Cytological and Molecular Detection of Leishmania spp. in Different Biological Tissues of Dogs in Areas Endemic for Visceral Leishmaniasis. Arq. Bras. Med. Vet. Zootec. 2019, 71, 2103–2106. [Google Scholar] [CrossRef]
- Menegatti, J.A.; Oliveira Júnior, G.J.; Silva, L.C.F.; Oliveira, A.; Bica, D.L.C.; Santos, P.V.B.A.; Cunha Filho, L.F.C.; Lunardi, M. Fauna Flebotomínica e Soroprevalência Para Leishmaniose Visceral Canina em Área Urbana Na Região Centro-Oeste do Brasil. Arq. Bras. Med. Vet. Zootec. 2020, 72, 1197–1205. [Google Scholar] [CrossRef]
- Steindel, M.; Menin, Á.; Evangelista, T.; Stoco, P.H.; Marlow, M.A.; Fleith, R.C.; Pilati, C.; Grisard, E.C. Outbreak of Autochthonous Canine Visceral Leishmaniasis in Santa Catarina, Brazil. Pesqui. Veterinária Bras. 2013, 33, 490–496. [Google Scholar] [CrossRef]
- De Oliveira, A.C.; Figueiredo, F.B.; Silva, V.L.; Santos, F.N.; de Souza, M.B.; Madeira, M.D.F.; Abrantes, T.R.; Périssé, A.R.S. Canine visceral leishmaniasis case investigation in the jacare region of niteroi, rio de janeiro, brazil. Rev. Inst. Med. Trop. Sao Paulo 2015, 57, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Riboldi, E.; Carvalho, F.; Romão, P.R.T.; Barcellos, R.B.; Bello, G.L.; Ramos, R.R.; de Oliveira, R.T.; Júnior, J.P.A.; Rossetti, M.L.; Dallegrave, E. Molecular Method Confirms Canine Leishmania Infection Detected by Serological Methods in Non-Endemic Area of Brazil. Korean J. Parasitol. 2018, 56, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Tonini, M.A.L.; Lemos, E.M.; Reis, A.B.; Vital, W.C.; Dias, E.S.; Dietze, R. First Description of Autochthonous Canine Visceral Leishmaniasis in the Metropolitan Region of Vitória, State of Espírito Santo, Brazil. Rev. Soc. Bras. Med. Trop. 2012, 45, 754–756. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, T.F.; Benitez, A.D.N.; Sevá, A.D.P.; Okamura, L.H.; Galvão, A.B.; Gomes, J.F.; Bresciani, K.D.S.; Cardoso, T.C. Spatial and Seroepidemiology of Canine Visceral Leishmaniasis in an Endemic Southeast Brazilian Area. Rev. Soc. Bras. Med. Trop. 2020, 53, e20190525. [Google Scholar] [CrossRef] [PubMed]
- Teixeira-Neto, R.G.; da Silva, E.S.; Nascimento, R.A.; Belo, V.S.; de Oliveira, C.D.L.; Pinheiro, L.C.; Gontijo, C.M.F. Canine Visceral Leishmaniasis in an Urban Setting of Southeastern Brazil: An Ecological Study Involving Spatial Analysis. Parasit. Vectors 2014, 7, 485. [Google Scholar] [CrossRef]
- Barata, R.A.; Peixoto, J.C.; Tanure, A.; Gomes, M.E.; Apolinário, E.C.; Bodevan, E.C.; de Araújo, H.S.; Dias, E.S.; Pinheiro, A.D.C. Epidemiology of Visceral Leishmaniasis in a Reemerging Focus of Intense Transmission in Minas Gerais State, Brazil. Biomed. Res. Int. 2013, 2013, 1–6. [Google Scholar] [CrossRef]
- Pinheiro, A.D.C.; da Costa, A.S.V.; de Oliveira, R.S.; Reis, M.L.C. Epidemiological Aspects and Spatial Distribution of Visceral Leishmaniasis in Governador Valadares, Brazil, between 2008 and 2012. Rev. Soc. Bras. Med. Trop. 2020, 53, e20190216. [Google Scholar] [CrossRef] [PubMed]
- Brito, F.G.; Langoni, H.; da Silva, R.C.; Rotondano, T.E.D.F.; de Melo, M.A.; da Paz, G.S. Canine Visceral Leishmaniasis in the Northeast Region of Brazil. J. Venom. Anim. Toxins Incl. Trop. Dis. 2016, 22, 15. [Google Scholar] [CrossRef] [PubMed]
- Valadas, S.; Minervino, A.H.H.; Lima, V.M.F.; Soares, R.M.; Ortolani, E.L.; Gennari, S.M. Occurrence of Antibodies Anti-Neospora Caninum, Anti-Toxoplasma Gondii, and Anti-Leishmania chagasi in Serum of Dogs from Pará State, Amazon, Brazil. Parasitol. Res. 2010, 107, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.N.C.C.; Blangiardo, M.; Rodas, L.A.C.; Nunes, C.M.; Hiramoto, R.M.; Tolezano, J.E.; Bonfietti, L.X.; Bermudi, P.M.M.; Cipriano, R.S.; Cardoso, G.C.D.; et al. Canine Visceral Leishmaniasis in Araçatuba, State of São Paulo, Brazil, and Its Relationship with Characteristics of Dogs and Their Owners: A Cross-Sectional and Spatial Analysis Using a Geostatistical Approach. BMC Vet. Res. 2018, 14, 229. [Google Scholar] [CrossRef] [PubMed]
- Medkour, H.; Davoust, B.; Dulieu, F.; Maurizi, L.; Lamour, T.; Marié, J.-L.; Mediannikov, O. Potential Animal Reservoirs (Dogs and Bats) of Human Visceral Leishmaniasis Due to Leishmania infantum in French Guiana. PLoS Negl. Trop. Dis. 2019, 13, e0007456. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.Z.; Kovalenko, D.A.; Kuhls, K.; Nasyrova, R.M.; Ponomareva, V.I.; Fatullaeva, A.A.; Razakov, S.A.; Schnur, L.F.; Schönian, G. Identification of the Agent Causing Visceral Leishmaniasis in Uzbeki and Tajiki Foci by Analysing Parasite DNA Extracted from Patients’ Giemsa-Stained Tissue Preparations. Parasitology 2009, 136, 981–986. [Google Scholar] [CrossRef] [PubMed]
- Akter, S.; Alam, M.Z.; Nakao, R.; Yasin, G.; Kato, H.; Katakura, K. Molecular and Serological Evidence of Leishmania Infection in Stray Dogs from Visceral Leishmaniasis–Endemic Areas of Bangladesh. Am. J. Trop. Med. Hyg. 2016, 95, 795–799. [Google Scholar] [CrossRef] [PubMed]
- Karunaweera, N.D.; Ferreira, M.U. Leishmaniasis: Current Challenges and Prospects for Elimination with Special Focus on the South Asian Region. Parasitology 2018, 145, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, I.; Aflatoonian, M.R.; Daei Parizi, M.H.; Hosseininasab, A.; Mostafavi, M.; Bamorovat, M.; Aghaei Afshar, A.; Mohebali, M.; Keshavarz, H.; Daneshvar, H.; et al. Visceral Leishmaniasis in Southeastern Iran: A Narrative Review. Iran. J. Parasitol. 2017, 12, 1–11. [Google Scholar]
- Dalimi, A.; Mohammadiha, A.; Mohebali, M.; Mirzaei, A.; Mahmoudi, M. Molecular Identification and Intra-Species Variations among Leishmania infantum Isolated from Human and Canine Visceral Leishmaniasis in Iran. Iran. J. Parasitol. 2018, 13, 567–576. [Google Scholar]
- Baneth, G.; Dank, G.; Keren-Kornblatt, E.; Sekeles, E.; Adini, I.; Eisenberger, C.L.; Schnur, L.F.; King, R.; Jaffe, C.L. Emergence of Visceral Leishmaniasis in Central Israel. Am. J. Trop. Med. Hyg. 1998, 59, 722–725. [Google Scholar] [CrossRef] [PubMed]
- Nasereddin, A.; Baneth, G.; Schönian, G.; Kanaan, M.; Jaffe, C.L. Molecular Fingerprinting of Leishmania infantum Strains Following an Outbreak of Visceral Leishmaniasis in Central Israel. J. Clin. Microbiol. 2005, 43, 6054–6059. [Google Scholar] [CrossRef] [PubMed]
- Hamarsheh, O.; Nasereddin, A.; Damaj, S.; Sawalha, S.; Al-Jawabreh, H.; Azmi, K.; Amro, A.; Ereqat, S.; Abdeen, Z.; Al-Jawabreh, A. Serological and Molecular Survey of Leishmania Parasites in Apparently Healthy Dogs in the West Bank, Palestine. Parasit. Vectors 2012, 5, 183. [Google Scholar] [CrossRef] [PubMed]
- Baneth, G.; Nachum-Biala, Y.; Adamsky, O.; Gunther, I. Leishmania Tropica and Leishmania infantum Infection in Dogs and Cats in Central Israel. Parasit. Vectors 2022, 15, 147. [Google Scholar] [CrossRef] [PubMed]
- Ozbel, Y.; Oskam, L.; Ozensoy, S.; Turgay, N.; Alkan, M.Z.; Jaffe, C.L.; Ozcel, M.A. A Survey on Canine Leishmaniasis in Western Turkey by Parasite, DNA and Antibody Detection Assays. Acta Trop. 2000, 74, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Toz, S.O.; Culha, G.; Zeyrek, F.Y.; Ertabaklar, H.; Alkan, M.Z.; Vardarlı, A.T.; Gunduz, C.; Ozbel, Y. A Real-Time ITS1-PCR Based Method in the Diagnosis and Species Identification of Leishmania Parasite from Human and Dog Clinical Samples in Turkey. PLoS Negl. Trop. Dis. 2013, 7, e2205. [Google Scholar] [CrossRef] [PubMed]
- Koenhemsi, L.; Fabrizio, V.; Mariella, P.; Antonella, M.; Or, E. Seroprevalence of Leishmaniosis Among Healthy Dogs in Istanbul. Isr. J. Vet. Med. 2020, 75, 31–34. [Google Scholar]
- Strelkova, M.V.; Ponirovsky, E.N.; Morozov, E.N.; Zhirenkina, E.N.; Razakov, S.A.; Kovalenko, D.A.; Schnur, L.F.; Schönian, G. A Narrative Review of Visceral Leishmaniasis in Armenia, Azerbaijan, Georgia, Kazakhstan, Kyrgyzstan, Tajikistan, Turkmenistan, Uzbekistan, the Crimean Peninsula and Southern Russia. Parasit. Vectors 2015, 8, 330. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Regañón, D.; Agulla, B.; Piya, B.; Fernández-Ruiz, N.; Villaescusa, A.; García-Sancho, M.; Rodríguez-Franco, F.; Sainz, Á. Stray Dogs in Nepal Have High Prevalence of Vector-Borne Pathogens: A Molecular Survey. Parasit. Vectors 2020, 13, 174. [Google Scholar] [CrossRef]
- Rab, M.A.; Frame, I.A.; Evans, D.A. The Role of Dogs in the Epidemiology of Human Visceral Leishmaniasis in Northern Pakistan. Trans. R. Soc. Trop. Med. Hyg. 1995, 89, 612–615. [Google Scholar] [CrossRef]
- Shang, L.; Peng, W.; Jin, H.; Xu, D.; Zhong, N.; Wang, W.; Wu, Y.; Liu, Q. The Prevalence of Canine Leishmania infantum Infection in Sichuan Province, Southwestern China Detected by Real Time PCR. Parasit. Vectors 2011, 4, 173. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-Y.; Ha, Y.; Gao, C.-H.; Wang, Y.; Yang, Y.-T.; Chen, H.-T. The Prevalence of Canine Leishmania infantum Infection in Western China Detected by PCR and Serological Tests. Parasit. Vectors 2011, 4, 69. [Google Scholar] [CrossRef] [PubMed]
- Sandy, J.; Matthews, A.; Nachum-Biala, Y.; Baneth, G. First Report of Autochthonous Canine Leishmaniasis in Hong Kong. Microorganisms 2022, 10, 1873. [Google Scholar] [CrossRef] [PubMed]
- Colella, V.; Nguyen, V.L.; Tan, D.Y.; Lu, N.; Fang, F.; Zhijuan, Y.; Wang, J.; Liu, X.; Chen, X.; Dong, J.; et al. Zoonotic Vectorborne Pathogens and Ectoparasites of Dogs and Cats in Eastern and Southeast Asia. Emerg. Infect. Dis. 2020, 26, 1221–1233. [Google Scholar] [CrossRef] [PubMed]
- Dujardin, J.-C.; Campino, L.; Cañavate, C.; Dedet, J.-P.; Gradoni, L.; Soteriadou, K.; Mazeris, A.; Ozbel, Y.; Boelaert, M. Spread of Vector-Borne Diseases and Neglect of Leishmaniasis, Europe. Emerg. Infect. Dis. 2008, 14, 1013–1018. [Google Scholar] [CrossRef] [PubMed]
- Velo, E.; Bongiorno, G.; Kadriaj, P.; Myrseli, T.; Crilly, J.; Lika, A.; Mersini, K.; Di Muccio, T.; Bino, S.; Gramiccia, M.; et al. The Current Status of Phlebotomine Sand Flies in Albania and Incrimination of Phlebotomus neglectus (Diptera, Psychodidae) as the Main Vector of Leishmania infantum. PLoS ONE 2017, 12, e0179118. [Google Scholar] [CrossRef]
- Colella, V.; Hodžić, A.; Iatta, R.; Baneth, G.; Alić, A.; Otranto, D. Zoonotic Leishmaniasis, Bosnia and Herzegovina. Emerg. Infect. Dis. 2019, 25, 385–386. [Google Scholar] [CrossRef] [PubMed]
- Živičnjak, T.; Martinković, F.; Marinculić, A.; Mrljak, V.; Kučer, N.; Matijatko, V.; Mihaljević, Ž.; Barić-Rafaj, R. A Seroepidemiologic Survey of Canine Visceral Leishmaniosis among Apparently Healthy Dogs in Croatia. Vet. Parasitol. 2005, 131, 35–43. [Google Scholar] [CrossRef]
- Živičnjak, T.; Martinković, F.; Khoury, C.; Bongiorno, G.; Bosnić, S.; Lukačević, D.; Maroli, M. Serological and Entomological Studies of Canine Leishmaniosis in Croatia. Vet. Arh. 2011, 81, 99–110. [Google Scholar]
- Deplazes, P.; Grimm, F.; Papaprodromou, M.; Cavaliero, T.; Gramiccia, M.; Christofi, G.; Christofi, N.; Economides, P.; Eckert, J. Canine Leishmaniosis in Cyprus Due to Leishmania infantum MON 1. Acta Trop. 1998, 71, 169–178. [Google Scholar] [CrossRef]
- Mazeris, A.; Soteriadou, K.; Dedet, J.P.; Haralambous, C.; Tsatsaris, A.; Moschandreas, J.; Messaritakis, I.; Christodoulou, V.; Papadopoulos, B.; Ivović, V.; et al. Leishmaniases and the Cyprus Paradox. Am. J. Trop. Med. Hyg. 2010, 82, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Ruh, E.; Taylan Ozkan, A. Leishmaniasis in Northern Cyprus. Eur. J. Ther. 2019, 25, 1–5. [Google Scholar] [CrossRef]
- Chamaillé, L.; Tran, A.; Meunier, A.; Bourdoiseau, G.; Ready, P.; Dedet, J.-P. Environmental Risk Mapping of Canine Leishmaniasis in France. Parasit. Vectors 2010, 3, 31. [Google Scholar] [CrossRef]
- Pomares, C.; Marty, P.; Bañuls, A.L.; Lemichez, E.; Pratlong, F.; Faucher, B.; Jeddi, F.; Moore, S.; Michel, G.; Aluru, S.; et al. Genetic Diversity and Population Structure of Leishmania infantum from Southeastern France: Evaluation Using Multi-Locus Microsatellite Typing. PLoS Negl. Trop. Dis. 2016, 10, e0004303. [Google Scholar] [CrossRef] [PubMed]
- Lachaud, L.; Chabbert, E.; Dubessay, P.; Dereure, J.; Lamothe, J.; Dedet, J.-P.; Bastien, P. Value of Two PCR Methods for the Diagnosis of Canine Visceral Leishmaniasis and the Detection of Asymptomatic Carriers. Parasitology 2002, 125, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Aoun, O.; Mary, C.; Roqueplo, C.; Marié, J.-L.; Terrier, O.; Levieuge, A.; Davoust, B. Canine Leishmaniasis in South-East of France: Screening of Leishmania infantum Antibodies (Western Blotting, ELISA) and Parasitaemia Levels by PCR Quantification. Vet. Parasitol. 2009, 166, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Le Rutte, E.A.; van der Wilt, L.S.; Bulstra, C.A.; Nieboer, D.; Kontoroupis, P.; de Vlas, S.J.; Richardus, J.H. Incidence and Geographical Distribution of Canine Leishmaniosis in 2016–2017 in Spain and France. Vet. Parasitol. Reg. Stud. Rep. 2021, 25, 100613. [Google Scholar] [CrossRef] [PubMed]
- Ntais, P.; Sifaki-Pistola, D.; Christodoulou, V.; Messaritakis, I.; Pratlong, F.; Poupalos, G.; Antoniou, M. Leishmaniases in Greece. Am. J. Trop. Med. Hyg. 2013, 89, 906–915. [Google Scholar] [CrossRef] [PubMed]
- Symeonidou, I.; Angelou, A.; Theodoridis, A.; Sioutas, G.; Papadopoulos, E. Canine Leishmaniosis in Greece: An Updated Countrywide Serological Study and Associated Risk Factors. Pathogens 2021, 10, 1129. [Google Scholar] [CrossRef]
- Tamponi, C.; Scarpa, F.; Carta, S.; Knoll, S.; Sanna, D.; Gai, C.; Pipia, A.P.; Dessì, G.; Casu, M.; Varcasia, A.; et al. Seroprevalence and Risk Factors Associated with Leishmania infantum in Dogs in Sardinia (Italy), an Endemic Island for Leishmaniasis. Parasitol. Res. 2021, 120, 289–300. [Google Scholar] [CrossRef]
- Ferroglio, E.; Maroli, M.; Gastaldo, S.; Mignone, W.; Rossi, L. Canine Leishmaniasis, Italy. Emerg. Infect. Dis. 2005, 11, 1618–1620. [Google Scholar] [CrossRef] [PubMed]
- Baldelli, R.; Piva, S.; Salvatore, D.; Parigi, M.; Melloni, O.; Tamba, M.; Bellini, R.; Poglayen, G. Canine Leishmaniasis Surveillance in a Northern Italy Kennel. Vet. Parasitol. 2011, 179, 57–61. [Google Scholar] [CrossRef]
- Santi, A.; Renzi, M.; Baldelli, R.; Calzolari, M.; Caminiti, A.; Dell’Anna, S.; Galletti, G.; Lombardini, A.; Paternoster, G.; Tamba, M. A Surveillance Program on Canine Leishmaniasis in the Public Kennels of Emilia-Romagna Region, Northern Italy. Vector-Borne Zoonotic Dis. 2014, 14, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Roldan, J.; Benelli, G.; Panarese, R.; Iatta, R.; Furlanello, T.; Beugnet, F.; Zatelli, A.; Otranto, D. Leishmania infantum and Dirofilaria Immitis Infections in Italy, 2009–2019: Changing Distribution Patterns. Parasit Vectors 2020, 13, 193. [Google Scholar] [CrossRef] [PubMed]
- Moirano, G.; Zanet, S.; Giorgi, E.; Battisti, E.; Falzoi, S.; Acquaotta, F.; Fratianni, S.; Richiardi, L.; Ferroglio, E.; Maule, M. Integrating Environmental, Entomological, Animal, and Human Data to Model the Leishmania infantum Transmission Risk in a Newly Endemic Area in Northern Italy. One Health 2020, 10, 100159. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, D.; Di Francesco, A.; Parigi, M.; Poglayen, G.; Battistini, M.; Baldelli, R. Programma di Sorveglianza della Leishmaniosi Canina in un Canile della Repubblica di San Marino. Vet. Ital. 2013, 49, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Headington, C.E.; Barbara, C.H.; Lambson, B.E.; Hart, D.T.; Barker, D.C. Diagnosis of Leishmaniasis in Maltese Dogs with the Aid of the Polymerase Chain Reaction. Trans. R. Soc. Trop. Med. Hyg. 2002, 96, S195–S197. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, L.; Mendão, C.; Madeira de Carvalho, L. Prevalence of Dirofilaria immitis, Ehrlichia canis, Borrelia burgdorferi Sensu Lato, Anaplasma spp. and Leishmania infantum in Apparently Healthy and CVBD-Suspect Dogs in Portugal—A National Serological Study. Parasit. Vectors 2012, 5, 62. [Google Scholar] [CrossRef] [PubMed]
- Maia, C.; Dionisio, L.; Afonso, M.O.; Neto, L.; Cristovao, J.M.; Campino, L. Leishmania Infection and Host-Blood Feeding Preferences of Phlebotomine Sandflies and Canine Leishmaniasis in an Endemic European Area, the Algarve Region in Portugal. Mem. Inst. Oswaldo Cruz 2013, 108, 481–487. [Google Scholar] [CrossRef]
- Maia, C.; Coimbra, M.; Ramos, C.; Cristóvão, J.; Cardoso, L.; Campino, L. Serological Investigation of Leishmania infantum, Dirofilaria Immitis and Angiostrongylus Vasorum in Dogs from Southern Portugal. Parasit. Vectors 2015, 8, 152. [Google Scholar] [CrossRef]
- Schallig, H.D.; Cardoso, L.; Semião-Santos, S.J. Seroepidemiology of Canine Leishmaniosis in Évora (Southern Portugal): 20-Year Trends. Parasit. Vectors 2013, 6, 100. [Google Scholar] [CrossRef] [PubMed]
- Maia, C.; Altet, L.; Serrano, L.; Cristóvão, J.M.; Tabar, M.D.; Francino, O.; Cardoso, L.; Campino, L.; Roura, X. Molecular Detection of Leishmania infantum, Filariae and Wolbachia Spp. in Dogs from Southern Portugal. Parasit. Vectors 2016, 9, 170. [Google Scholar] [CrossRef] [PubMed]
- Maia, C.; Alwassouf, S.; Cristóvão, J.M.; Ayhan, N.; Pereira, A.; Charrel, R.N.; Campino, L. Serological Association between Leishmania infantum and Sand Fly Fever Sicilian (but Not Toscana) Virus in Sheltered Dogs from Southern Portugal. Parasit. Vectors 2017, 10, 92. [Google Scholar] [CrossRef]
- Almeida, M.; Maia, C.; Cristóvão, J.M.; Morgado, C.; Barbosa, I.; Ibars, R.F.; Campino, L.; Gonçalves, L.; Cortes, S. Seroprevalence and Risk Factors Associated with Leishmania Infection in Dogs from Portugal. Microorganisms 2022, 10, 2262. [Google Scholar] [CrossRef] [PubMed]
- Montoya-Alonso, J.A.; Morchón, R.; Costa-Rodríguez, N.; Matos, J.I.; Falcón-Cordón, Y.; Carretón, E. Current Distribution of Selected Vector-Borne Diseases in Dogs in Spain. Front. Vet. Sci. 2020, 7. [Google Scholar] [CrossRef]
- Gálvez, R.; Montoya, A.; Cruz, I.; Fernández, C.; Martín, O.; Checa, R.; Chicharro, C.; Migueláñez, S.; Marino, V.; Miró, G. Latest Trends in Leishmania infantum Infection in Dogs in Spain, Part I: Mapped Seroprevalence and Sand Fly Distributions. Parasit. Vectors 2020, 13, 204. [Google Scholar] [CrossRef] [PubMed]
- Montoya, A.; Gálvez, R.; Checa, R.; Sarquis, J.; Plaza, A.; Barrera, J.P.; Marino, V.; Miró, G. Latest Trends in L. infantum Infection in Dogs in Spain, Part II: Current Clinical Management and Control According to a National Survey of Veterinary Practitioners. Parasit. Vectors 2020, 13, 205. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Regañón, D.; Roura, X.; Suárez, M.L.; León, M.; Sainz, Á. Serological Evaluation of Selected Vector-Borne Pathogens in Owned Dogs from Northern Spain Based on a Multicenter Study Using a Commercial Test. Parasit. Vectors 2020, 13, 301. [Google Scholar] [CrossRef] [PubMed]
- Tsatchev, I.; Kyriazis, I.D.; Boutsini, S.; Karagouni, E.; Dotsika, E. First Report of Canine Visceral Leishmaniasis in Bulgaria. Turk. J. Vet. Anim. Sci. 2010, 34, 465–469. [Google Scholar] [CrossRef]
- Babuadze, G.; Alvar, J.; Argaw, D.; de Koning, H.P.; Iosava, M.; Kekelidze, M.; Tsertsvadze, N.; Tsereteli, D.; Chakhunashvili, G.; Mamatsashvili, T.; et al. Epidemiology of Visceral Leishmaniasis in Georgia. PLoS Negl. Trop. Dis. 2014, 8, e2725. [Google Scholar] [CrossRef]
- Babuadze, G.; Farlow, J.; de Koning, H.P.; Carrillo, E.; Chakhunashvili, G.; Murskvaladze, M.; Kekelidze, M.; Karseladze, I.; Kokaia, N.; Kalandadze, I.; et al. Seroepidemiology and Molecular Diversity of Leishmania donovani Complex in Georgia. Parasit. Vectors 2016, 9, 279. [Google Scholar] [CrossRef] [PubMed]
- Miró, G.; Wright, I.; Michael, H.; Burton, W.; Hegarty, E.; Rodón, J.; Buch, J.; Pantchev, N.; von Samson-Himmelstjerna, G. Seropositivity of Main Vector-Borne Pathogens in Dogs across Europe. Parasit. Vectors 2022, 15, 189. [Google Scholar] [CrossRef] [PubMed]
- Tánczos, B.; Balogh, N.; Király, L.; Biksi, I.; Szeredi, L.; Gyurkovsky, M.; Scalone, A.; Fiorentino, E.; Gramiccia, M.; Farkas, R. First Record of Autochthonous Canine Leishmaniasis in Hungary. Vector-Borne Zoonotic Dis. 2012, 12, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Xhekaj, B.; Alishani, M.; Rexhepi, A.; Jakupi, X.; Sherifi, K. Serological Survey of Canine Leishmaniasis in Southwestern Region of Kosovo. Vet. Ital. 2020, 56, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Tasić-Otašević, S.; Savić, S.; Jurhar-Pavlova, M.; Stefanovska, J.; Stalević, M.; Ignjatović, A.; Ranđelović, M.; Gajić, B.; Cvetkovikj, A.; Gabrielli, S. Molecular Survey of Dirofilaria and Leishmania Species in Dogs from Central Balkan. Animals 2022, 12, 911. [Google Scholar] [CrossRef] [PubMed]
- Mircean, V.; Dumitrache, M.O.; Mircean, M.; Bolfa, P.; Györke, A.; Mihalca, A.D. Autochthonous Canine Leishmaniasis in Romania: Neglected or (Re)Emerging? Parasit. Vectors 2014, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- Cîmpan, A.A.; Diakou, A.; Papadopoulos, E.; Miron, L.D. Serological study of exposure to Leishmania in dogs living in shelters, in south-east romania. Rev. Rom. Med. Vet. 2019, 29, 54–58. [Google Scholar]
- Cazan, C.D.; Ionică, A.M.; Matei, I.A.; D’Amico, G.; Muñoz, C.; Berriatua, E.; Dumitrache, M.O. Detection of Leishmania infantum DNA and Antibodies against Anaplasma spp., Borrelia burgdorferi s.l. and Ehrlichia canis in a Dog Kennel in South-Central Romania. Acta Vet. Scand. 2020, 62, 42. [Google Scholar] [CrossRef] [PubMed]
- Kotnik, T. Dog Leishmaniasis in Slovenia: A Probable Creation of the First Enzootic Focus—A Case Report. Vet. Arh. 2020, 90, 317–322. [Google Scholar] [CrossRef]
- McKenna, M.; Attipa, C.; Tasker, S.; Augusto, M. Leishmaniosis in a Dog with No Travel History outside of the UK. Vet. Rec. 2019, 184, 441. [Google Scholar] [CrossRef]
- Bashaye, S.; Nombela, N.; Argaw, D.; Mulugeta, A.; Herrero, M.; Nieto, J.; Chicharro, C.; Cañavate, C.; Aparicio, P.; Vélez, I.D.; et al. Risk Factors for Visceral Leishmaniasis in a New Epidemic Site in Amhara Region, Ethiopia. Am. J. Trop. Med. Hyg. 2009, 81, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Kalayou, S.; Tadelle, H.; Bsrat, A.; Abebe, N.; Haileselassie, M.; Schallig, H.D.F.H. Serological Evidence of Leishmania donovani Infection in Apparently Healthy Dogs Using Direct Agglutination Test (DAT) and Rk39 Dipstick Tests in Kafta Humera, North-West Ethiopia. Transbound. Emerg. Dis. 2011, 58, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Rohousova, I.; Talmi-Frank, D.; Kostalova, T.; Polanska, N.; Lestinova, T.; Kassahun, A.; Yasur-Landau, D.; Maia, C.; King, R.; Votypka, J.; et al. Exposure to Leishmania spp. and Sand Flies in Domestic Animals in Northwestern Ethiopia. Parasit. Vectors 2015, 8, 360. [Google Scholar] [CrossRef] [PubMed]
- Bejano, S.; Shumie, G.; Kumar, A.; Asemahagn, E.; Damte, D.; Woldie, S.; Mulugeta, A.; Manaye, N.; Genetu, A.; Gadisa, E.; et al. Prevalence of Asymptomatic Visceral Leishmaniasis in Human and Dog, Benishangul Gumuz Regional State, Western Ethiopia. Parasit. Vectors 2021, 14, 39. [Google Scholar] [CrossRef] [PubMed]
- Sharifdini, M.; Mohebali, M.; Keshavarz, H.; Hosseininejad, M.; Hajjaran, H.; Akhoundi, B.; Foroushani, A.R.; Zarei, Z.; Charehdar, S. Neospora Caninum and Leishmania infantum Co-Infection in Domestic Dogs (Canis Familiaris) in Meshkin-Shahr District, Northwestern Iran. Iran. J. Arthropod Borne Dis. 2011, 1, 60–68. [Google Scholar]
- Moshfe, A.; Mohebali, M.; Afshoun, E.; Mousavizadeh, A.; Zarei, Z.; Abidi, H.; Akhoundi, B.; Barati, V.; Joukar, S. Canine Visceral Leishmaniasis in Boyer Ahmad District, Kohgiluyeh & Boyer Ahmad Province, Southwest of Iran. Iran. J. Parasitol. 2012, 7, 75–81. [Google Scholar] [PubMed]
- Haddadzade, H.; Fattahi, R.; Mohebali, M.; Akhoundi, B.; Ebrahimzade, E. Seroepidemiologcal Investigation of Visceral Leishmaniasis in Stray and Owned Dogs in Alborz Province, Central Iran Using Direct Agglutination Test. Iran. J. Parasitol. 2013, 8, 152–157. [Google Scholar]
- Sabzevari, S.; Razmi, G.R.; Naghibi, A.; Khoshnegah, J. A Serological Study of Leishmania infantum in Dogs of Khorasan Razavi Province, Iran. J. Parasit. Dis. 2013, 37, 189–191. [Google Scholar] [CrossRef]
- Bamorovat, M.; Sharifi, I.; Mohammadi, M.A.; Fasihi Harandi, M.; Mohebali, M.; Malekpour Afshar, R.; Babaei, Z.; Ziaali, N.; Aflatoonian, M.R. Canine Visceral Leishmaniasis in Kerman, Southeast of Iran: A Seroepidemiological, Histopathological and Molecular Study. Iran. J. Parasitol. 2014, 9, 342–349. [Google Scholar]
- Mahshid, M.; Baharak, A.; Iraj, S.; Sina, K.; Javad, K.; Mehdi, B. Seroprevalence of Canine Visceral Leishmaniasis in Southeast of Iran. J. Parasit. Dis. 2014, 38, 218–222. [Google Scholar] [CrossRef]
- Malmasi, A.; Janitabar, S.; Mohebali, M.; Akhoundi, B.; Maazi, N.; Aramoon, M.; Khorrami, N.; Seifi, H.A. Seroepidemiologic Survey of Canine Visceral Leishmaniasis in Tehran and Alborz Provinces of Iran. J. Arthropod Borne Dis. 2014, 8, 132–138. [Google Scholar]
- Barati, M.; Mohebali, M.; Alimohammadian, M.H.; Khamesipour, A.; Akhoundi, B.; Zarei, Z. Canine Visceral Leishmaniasis: Seroprevalence Survey of Asymptomatic Dogs in an Endemic Area of Northwestern Iran. J. Parasit. Dis. 2015, 39, 221–224. [Google Scholar] [CrossRef]
- Gharekhani, J.; Heidari, H.; Hajian-Bidar, H.; Abbasi-Doulatshahi, E.; Edalati-Shokat, H. Prevalence of Anti-Leishmania infantum Antibodies in Dogs from West of Iran. J. Parasit. Dis. 2016, 40, 964–967. [Google Scholar] [CrossRef] [PubMed]
- Afshar, M.J.A.; Sharifi, I.; Bamorovat, M.; Mohebali, M.; Bahreini, M.S.; Naderi, A. Canine Visceral Leishmaniasis; A Seroepidemiological Survey in Jiroft District, Southern Kerman Province, Southeastern Iran in 2015. Iran. J. Parasitol. 2018, 13, 67–71. [Google Scholar]
- Heidari, A.; Mohebali, M.; Vahed, M.; Kabir, K.; Zarei, Z.; Akhoundi, B.; Elikaee, S.; Barati, H.; Sezavar, M.; Keshavarz, H.; et al. Molecular and Seroepidemiological Survey of Visceral Leishmaniasis in Owned Dogs (Canis Familiaris) in New Foci of Rural Areas of Alborz Province, Central Part of Iran: A Cross-Sectional Study in 2017. J. Arthropod Borne Dis. 2020, 14, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Fakhar, M.; Derakhshani-nia, M.; Gohardehi, S.; Karamian, M.; Hezarjaribi, H.Z.; Mohebali, M.; Akhoundi, B.; Sharbatkhori, M. Domestic Dogs Carriers of Leishmania infantum, Leishmania tropica and Crithidia fasciculata as Potential Reservoirs for Human Visceral Leishmaniasis in Northeastern Iran. Vet. Med. Sci. 2022, 8, 2329–2336. [Google Scholar] [CrossRef]
- Dumitrache, M.O.; Nachum-Biala, Y.; Gilad, M.; Mircean, V.; Cazan, C.D.; Mihalca, A.D.; Baneth, G. The Quest for Canine Leishmaniasis in Romania: The Presence of an Autochthonous Focus with Subclinical Infections in an Area Where Disease Occurred. Parasit. Vectors 2016, 9, 297. [Google Scholar] [CrossRef] [PubMed]
- Marcondes, M.; Rossi, C.N. Leishmaniose Visceral No Brasil. Braz. J. Vet. Res. Anim. Sci. 2013, 50, 341–352. [Google Scholar] [CrossRef]
- Desjeux, P. The Increase in Risk Factors for Leishmaniasis Worldwide. Trans. R. Soc. Trop. Med. Hyg. 2001, 95, 239–243. [Google Scholar] [CrossRef]
- Barbosa, I.R.; Carlota, F.C.; Andrade-Neto, V.F. de Seroepidemiological Survey of Canine Leishmania Infections from Peripheral Areas in Natal, Northeast Brazil. Open Microbiol. J. 2015, 9, 43–47. [Google Scholar] [CrossRef]
- Ready, P.D. Leishmaniasis Emergence and Climate Change. Rev. Sci. Tech. 2008, 27, 399–412. [Google Scholar] [CrossRef] [PubMed]
- El Omari, H.; Chahlaoui, A.; Talbi, F.Z.; El Mouhdi, K.; El Ouali Lalami, A. Impact of Climatic Factors on the Seasonal Fluctuation of Leishmaniasis Vectors in Central Morocco (Meknes Prefecture). Can. J. Infect. Dis. Med. Microbiol. 2020, 2020, 6098149. [Google Scholar] [CrossRef] [PubMed]
- Gramiccia, M.; Gradoni, L. The Current Status of Zoonotic Leishmaniases and Approaches to Disease Control. Int. J. Parasitol. 2005, 35, 1169–1180. [Google Scholar] [CrossRef] [PubMed]
- Ready, P.D. Leishmaniasis Emergence in Europe. Euro Surveill. 2010, 15, 19505. [Google Scholar] [CrossRef]
- Koch, L.K.; Kochmann, J.; Klimpel, S.; Cunze, S. Modeling the Climatic Suitability of Leishmaniasis Vector Species in Europe. Sci. Rep. 2017, 7, 13325. [Google Scholar] [CrossRef]
- Abdullah, A.Y.M.; Dewan, A.; Shogib, M.R.I.; Rahman, M.M.; Hossain, M.F. Environmental Factors Associated with the Distribution of Visceral Leishmaniasis in Endemic Areas of Bangladesh: Modeling the Ecological Niche. Trop. Med. Health 2017, 45, 13. [Google Scholar] [CrossRef]
- Dos Santos, C.V.B.; Sevá, A.D.P.; Werneck, G.L.; Struchiner, C.J. Does Deforestation Drive Visceral Leishmaniasis Transmission? A Causal Analysis. Proc. R. Soc. B Biol. Sci. 2021, 288, 20211537. [Google Scholar] [CrossRef]
- El-Sayed, A.; Kamel, M. Climatic Changes and Their Role in Emergence and Re-Emergence of Diseases. Environ. Sci. Pollut. Res. 2020, 27, 22336–22352. [Google Scholar] [CrossRef]
- Al-Salem, W.; Herricks, J.R.; Hotez, P.J. A Review of Visceral Leishmaniasis during the Conflict in South Sudan and the Consequences for East African Countries. Parasit. Vectors 2016, 9, 460. [Google Scholar] [CrossRef]
- Sunyoto, T.; Potet, J.; Boelaert, M. Visceral Leishmaniasis in Somalia: A Review of Epidemiology and Access to Care. PLoS Negl. Trop. Dis. 2017, 11, e0005231. [Google Scholar] [CrossRef]
- de Carvalho, A.G.; Luz, J.G.G.; Rodrigues, L.D.; Dias, J.V.L.; Fontes, C.J.F. High Seroprevalence and Peripheral Spatial Distribution of Visceral Leishmaniasis among Domestic Dogs in an Emerging Urban Focus in Central Brazil: A Cross-Sectional Study. Pathog. Glob. Health 2018, 112, 29–36. [Google Scholar] [CrossRef]
- Carvalho, A.G.; Luz, J.G.G.; Rodrigues, L.D.; Dias, J.V.L.; Fontes, C.J.F. Factors Associated with Leishmania spp. Infection in Domestic Dogs from an Emerging Area of High Endemicity for Visceral Leishmaniasis in Central-Western Brazil. Res. Vet. Sci. 2019, 125, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Luz, J.G.G.; Carvalho, A.G.; Naves, D.B.; Dias, J.V.L.; Fontes, C.J.F. Are Backyard Characteristics Relevant Factors for the Occurrence of Human Visceral Leishmaniasis in Central-Western Brazil? Trans. R. Soc. Trop. Med. Hyg. 2020, 114, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, A.I.P.; Silva, D.M.; de Freitas, L.R.S.; Romero, G.A.S. A Cross-Sectional Approach Including Dog Owner Characteristics as Predictors of Visceral Leishmaniasis Infection in Dogs. Mem. Inst. Oswaldo Cruz 2020, 115, e190349. [Google Scholar] [CrossRef] [PubMed]
- Cortes, S.; Afonso, M.O.; Alves-Pires, C.; Campino, L. Stray Dogs and Leishmaniasis in Urban Areas, Portugal. Emerg. Infect. Dis. 2007, 13, 1431–1432. [Google Scholar] [CrossRef] [PubMed]
- Martín-Sánchez, J.; Morales-Yuste, M.; Acedo-Sánchez, C.; Barón, S.; Díaz, V.; Morillas-Márquez, F. Canine Leishmaniasis in Southeastern Spain. Emerg. Infect. Dis. 2009, 15, 795–798. [Google Scholar] [CrossRef]
- Coura-Vital, W.; Marques, M.J.; Veloso, V.M.; Roatt, B.M.; Aguiar-Soares, R.D.D.O.; Reis, L.E.S.; Braga, S.L.; Morais, M.H.F.; Reis, A.B.; Carneiro, M. Prevalence and Factors Associated with Leishmania infantum Infection of Dogs from an Urban Area of Brazil as Identified by Molecular Methods. PLoS Negl. Trop. Dis. 2011, 5, e1291. [Google Scholar] [CrossRef]
- Melo, S.N.; Teixeira-Neto, R.G.; Werneck, G.L.; Struchiner, C.J.; Ribeiro, R.A.N.; Sousa, L.R.; de Melo, M.O.G.; Carvalho, C.G., Jr.; Penaforte, K.M.; Manhani, M.N.; et al. Prevalence of Visceral Leishmaniasis in A Population of Free-Roaming Dogs as Determined by Multiple Sampling Efforts: A Longitudinal Study Analyzing the Effectiveness of Euthanasia. Prev. Vet. Med. 2018, 161, 19–24. [Google Scholar] [CrossRef]
- Werneck, G.L.; Costa, C.H.N.; Walker, A.M.; David, J.R.; Wand, M.; Maguire, J.H. Multilevel Modelling of the Incidence of Visceral Leishmaniasis in Teresina, Brazil. Epidemiol. Infect. 2007, 135, 195–201. [Google Scholar] [CrossRef]
- De Araújo, V.E.M.; Pinheiro, L.C.; Almeida, M.C.D.M.; Menezes, F.C.D.; Morais, M.H.F.; Reis, I.A.; Assunção, R.M.; Carneiro, M. Relative Risk of Visceral Leishmaniasis in Brazil: A Spatial Analysis in Urban Area. PLoS Negl. Trop. Dis. 2013, 7, e2540. [Google Scholar] [CrossRef]
- Leite, B.M.M.; Solcà, M.D.S.; Santos, L.C.S.; Coelho, L.B.; Amorim, L.D.A.F.; Donato, L.E.; Passos, S.M.D.S.; Almeida, A.O.D.; Veras, P.S.T.; Fraga, D.B.M. The Mass Use of Deltamethrin Collars to Control and Prevent Canine Visceral Leishmaniasis: A Field Effectiveness Study in a Highly Endemic Area. PLoS Negl. Trop. Dis. 2018, 12, e0006496. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.D.L.; Assunção, R.M.; Reis, I.A.; Proietti, F.A. Spatial Distribution of Human and Canine Visceral Leishmaniasis in Belo Horizonte, Minas Gerais State, Brasil, 1994–1997. Cad. Saude Publica 2001, 17, 1231–1239. [Google Scholar] [CrossRef] [PubMed]
- Gavgani, A.S.M.; Mohite, H.; Edrissian, G.H.; Mohebali, M.; Davies, C.R. Domestic Dog Ownership in Iran Is a Risk Factor for Human Infection with Leishmania infantum. Am. J. Trop. Med. Hyg. 2002, 67, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.C.M.; Melo, A.C.F.L.; Júnior, A.D.S.; Franco, S.O.; Rondon, F.C.M.; Bevilaqua, C.M.L. Epidemiologia Da Leishmaniose Visceral No Município de Fortaleza, Ceará. Pesqui. Veterinária Bras. 2017, 37, 1119–1124. [Google Scholar] [CrossRef]
- Gaskin, A.A.; Schantz, P.; Jackson, J.; Birkenheuer, A.; Tomlinson, L.; Gramiccia, M.; Levy, M.; Steurer, F.; Kollmar, E.; Hegarty, B.C.; et al. Visceral Leishmaniasis in a New York Foxhound Kennel. J. Vet. Intern. Med. 2002, 16, 34. [Google Scholar] [CrossRef] [PubMed]
- França-Silva, J.C.; da Costa, R.T.; Siqueira, A.M.; Machado-Coelho, G.L.L.; da Costa, C.A.; Mayrink, W.; Vieira, E.P.; Costa, J.S.; Genaro, O.; Nascimento, E. Epidemiology of Canine Visceral Leishmaniosis in the Endemic Area of Montes Claros Municipality, Minas Gerais State, Brazil. Vet. Parasitol. 2003, 111, 161–173. [Google Scholar] [CrossRef]
- Giorgobiani, E.; Chitadze, N.; Chanturya, G.; Grdzelidze, M.; Jochim, R.C.; Machablishvili, A.; Tushishvili, T.; Zedginidze, Y.; Manjgaladze, M.K.; Iashvili, N.; et al. Epidemiologic Aspects of an Emerging Focus of Visceral Leishmaniasis in Tbilisi, Georgia. PLoS Negl. Trop. Dis. 2011, 5, e1415. [Google Scholar] [CrossRef]
- Palatnik-de-Sousa, C.B.; Day, M.J. One Health: The Global Challenge of Epidemic and Endemic Leishmaniasis. Parasit. Vectors 2011, 4, 197. [Google Scholar] [CrossRef] [PubMed]
- Toepp, A.J.; Monteiro, G.R.G.; Coutinho, J.F.V.; Lima, A.L.; Larson, M.; Wilson, G.; Grinnage-Pulley, T.; Bennett, C.; Mahachi, K.; Anderson, B.; et al. Comorbid Infections Induce Progression of Visceral Leishmaniasis. Parasit. Vectors 2019, 12, 54. [Google Scholar] [CrossRef]
- Belo, V.S.; Werneck, G.L.; Barbosa, D.S.; Simões, T.C.; Nascimento, B.W.L.; da Silva, E.S.; Struchiner, C.J. Factors Associated with Visceral Leishmaniasis in the Americas: A Systematic Review and Meta-Analysis. PLoS Negl. Trop. Dis. 2013, 7, e2182. [Google Scholar] [CrossRef]
- Thomaz Soccol, V.; Pasquali, A.K.S.; Pozzolo, E.M.; Leandro, A.D.S.; Chiyo, L.; Baggio, R.A.; Michaliszyn, M.S.; Silva, C.; Cubas, P.H.; Peterlle, R.; et al. More than the Eyes Can See: The Worrying Scenario of Canine Leishmaniasis in the Brazilian Side of the Triple Border. PLoS ONE 2017, 12, e0189182. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, M.A.A.; Paula, A.A.; Camacho, L.A.B.; Marzochi, M.C.A.; Xavier, S.C.; da Silva, A.V.M.; Jansen, A.M. Canine Visceral Leishmaniasis in Barra de Guaratiba, Rio de Janeiro, Brazil: Assessment of Risk Factors. Rev. Inst. Med. Trop. Sao Paulo 2003, 45, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.d.B.P.F.d.; Mendonça, A.J.; Sousa, V.R.F. Prevalência e Epidemiologia Da Leishmaniose Visceral Em Cães e Humanos, Na Cidade de Cuiabá, Mato Grosso, Brasil. Ciência Rural 2010, 40, 1610–1615. [Google Scholar] [CrossRef]
- dos Santos, J.M.L.; Dantas-Torres, F.; Mattos, M.R.F.; Lino, F.R.L.; Andrade, L.S.S.; de Souza, R.C.A.; Brito, M.E.F.D.; de Brito, M.E.F.; Brandão-Filho, S.P.; Simões-Mattos, L. Prevalência de Anticorpos Antileishmania spp. Em Cães de Garanhuns, Agreste de Pernambuco. Rev. Soc. Bras. Med. Trop. 2010, 43, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Deane, L.M. Leishmaniose Visceral No Brasil. Estudos Sobre Reservatórios e Transmissores Realizados No Estado do Ceará. Ph.D. Thesis, Universidade de São Paulo, São Paulo, Brazil, 1956. [Google Scholar]
- Deane, L.M.; Deane, M.P. Visceral Leishmaniosis in Brazil. Geographical Distribution and Transmission. Rev. Inst. Med. Trop. 1962, 4, 198–212. [Google Scholar]
- Fraga, D.B.M.; Solcà, M.S.; Silva, V.M.G.; Borja, L.S.; Nascimento, E.G.; Oliveira, G.G.S.; Pontes-de-Carvalho, L.C.; Veras, P.S.T.; dos-Santos, W.L.C. Temporal Distribution of Positive Results of Tests for Detecting Leishmania Infection in Stray Dogs of an Endemic Area of Visceral Leishmaniasis in the Brazilian Tropics: A 13 Years Survey and Association with Human Disease. Vet. Parasitol. 2012, 190, 591–594. [Google Scholar] [CrossRef] [PubMed]
- Miró, G.; Gálvez, R.; Fraile, C.; Descalzo, M.A.; Molina, R. Infectivity to Phlebotomus perniciosus of Dogs Naturally Parasitized with Leishmania infantum after Different Treatments. Parasit. Vectors 2011, 4, 52. [Google Scholar] [CrossRef] [PubMed]
- Courtenay, O.; Carson, C.; Calvo-Bado, L.; Garcez, L.M.; Quinnell, R.J. Heterogeneities in Leishmania infantum Infection: Using Skin Parasite Burdens to Identify Highly Infectious Dogs. PLoS Negl. Trop. Dis. 2014, 8, e2583. [Google Scholar] [CrossRef] [PubMed]
- Maia, C.; Nunes, M.; Cristóvão, J.; Campino, L. Experimental Canine Leishmaniasis: Clinical, Parasitological and Serological Follow-Up. Acta Trop. 2010, 116, 193–199. [Google Scholar] [CrossRef]
- Michalsky, É.M.; Rocha, M.F.; da Rocha Lima, A.C.V.M.; França-Silva, J.C.; Pires, M.Q.; Oliveira, F.S.; Pacheco, R.S.; dos Santos, S.L.; Barata, R.A.; Romanha, Á.J.; et al. Infectivity of Seropositive Dogs, Showing Different Clinical Forms of Leishmaniasis, to Lutzomyia Longipalpis Phlebotomine Sand Flies. Vet. Parasitol. 2007, 147, 67–76. [Google Scholar] [CrossRef]
- Verçosa, B.; Lemos, C.; Mendonça, I.; Silva, S.; de Carvalho, S.; Goto, H.; Costa, F. Transmission Potential, Skin Inflammatory Response, and Parasitism of Symptomatic and Asymptomatic Dogs with Visceral Leishmaniasis. BMC Vet. Res. 2008, 4, 45. [Google Scholar] [CrossRef] [PubMed]
- Guarga, J.L.; Lucientes, J.; Peribáñez, M.A.; Molina, R.; Gracia, M.J.; Castillo, J.A. Experimental Infection of Phlebotomusperniciosus and Determination of the Natural Infection Rates of Leishmania infantum in Dogs. Acta Trop. 2000, 77, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Molina, R.; Amela, C.; Nieto, J.; San-Andrés, M.; González, F.; Castillo, J.A.; Lucientes, J.; Alvar, J. Infectivity of Dogs Naturally Infected with Leishmania infantum to Colonized Phlebotomus perniciosus. Trans. R. Soc. Trop. Med. Hyg. 1994, 88, 491–493. [Google Scholar] [CrossRef] [PubMed]
- Solano-Gallego, L.; Morell, P.; Arboix, M.; Alberola, J.; Ferrer, L. Prevalence of Leishmania infantum Infection in Dogs Living in an Area of Canine Leishmaniasis Endemicity Using PCR on Several Tissues and Serology. J. Clin. Microbiol. 2001, 39, 560–563. [Google Scholar] [CrossRef]
- Moshfe, A.; Mohebali, M.; Edrissian, G.; Zarei, Z.; Akhoundi, B.; Kazemi, B.; Jamshidi, S.; Mahmoodi, M. Canine Visceral Leishmaniasis: Asymptomatic Infected Dogs as a Source of L. infantum Infection. Acta Trop. 2009, 112, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Coura-Vital, W.; Reis, A.B.; Fausto, M.A.; Leal, G.G.D.A.; Marques, M.J.; Veloso, V.M.; Carneiro, M. Risk Factors for Seroconversion by Leishmania infantum in a Cohort of Dogs from an Endemic Area of Brazil. PLoS ONE 2013, 8, e71833. [Google Scholar] [CrossRef] [PubMed]
- Lopes, J.V.; Michalsky, É.M.; Lara Silva, F.D.O.; Lima, A.C.V.M.R.; de Avelar, D.M.; da Costa, A.A.J.; França-Silva, J.C.; Regina-Silva, S.; Fortes-Dias, C.L.; Dias, E.S. Seroprevalence and Molecular Characterization of Leishmania in Dogs from an Endemic Area of Zoonotic Visceral Leishmaniasis in Brazil. Int. J. Vet. Sci. Med. 2017, 5, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Miguel, D.C.; Guarnier, D.C. Canine and Human Leishmaniasis: Disease Progression to Brazilian Urbanized Areas. Int. J. Trop. Dis. 2019, 2, 23. [Google Scholar] [CrossRef]
- Travi, B.L.; Cordeiro-da-Silva, A.; Dantas-Torres, F.; Miró, G. Canine Visceral Leishmaniasis: Diagnosis and Management of the Reservoir Living among Us. PLoS Negl. Trop. Dis. 2018, 12, e0006082. [Google Scholar] [CrossRef]
- Dantas-Torres, F.; Miró, G.; Bowman, D.D.; Gradoni, L.; Otranto, D. Culling Dogs for Zoonotic Visceral Leishmaniasis Control: The Wind of Change. Trends Parasitol. 2019, 35, 97–101. [Google Scholar] [CrossRef]
- Nunes, C.M.; de Lima, V.M.F.; de Paula, H.B.; Perri, S.H.V.; de Andrade, A.M.; Dias, F.E.F.; Burattini, M.N. Dog Culling and Replacement in an Area Endemic for Visceral Leishmaniasis in Brazil. Vet. Parasitol. 2008, 153, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Sevá, A.P.; Ovallos, F.G.; Amaku, M.; Carrillo, E.; Moreno, J.; Galati, E.A.B.; Lopes, E.G.; Soares, R.M.; Ferreira, F. Canine-Based Strategies for Prevention and Control of Visceral Leishmaniasis in Brazil. PLoS ONE 2016, 11, e0160058. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, G.; Teva, A.; Ferreira, A.L.; dos Santos, C.B.; Pinto, I.D.S.; de-Azevedo, C.T.; Falqueto, A. Evaluation of a Novel Chromatographic Immunoassay Based on Dual-Path Platform Technology (DPP® CVL Rapid Test) for the Serodiagnosis of Canine Visceral Leishmaniasis. Trans. R. Soc. Trop. Med. Hyg. 2012, 106, 54–59. [Google Scholar] [CrossRef]
- Coura-Vital, W.; Ker, H.G.; Roatt, B.M.; Aguiar-Soares, R.D.O.; Leal, G.G.D.A.; Moreira, N.D.D.; Oliveira, L.A.M.; de Menezes Machado, E.M.; Morais, M.H.F.; Corrêa-Oliveira, R.; et al. Evaluation of Change in Canine Diagnosis Protocol Adopted by the Visceral Leishmaniasis Control Program in Brazil and a New Proposal for Diagnosis. PLoS ONE 2014, 9, e91009. [Google Scholar] [CrossRef] [PubMed]
- de Sousa-Paula, L.C.; da Silva, L.G.; Sales, K.G.D.S.; Dantas-Torres, F. Failure of the Dog Culling Strategy in Controlling Human Visceral Leishmaniasis in Brazil: A Screening Coverage Issue? PLoS Negl. Trop. Dis. 2019, 13, e0007553. [Google Scholar] [CrossRef] [PubMed]
- Courtenay, O.; Quinnell, R.J.; Garcez, L.M.; Shaw, J.J.; Dye, C. Infectiousness in a Cohort of Brazilian Dogs: Why Culling Fails to Control Visceral Leishmaniasis in Areas of High Transmission. J. Infect. Dis. 2002, 186, 1314–1320. [Google Scholar] [CrossRef] [PubMed]
- Moreira, E.D.; Mendes de Souza, V.M.; Sreenivasan, M.; Nascimento, E.G.; Pontes de Carvalho, L. Assessment of an Optimized Dog-Culling Program in the Dynamics of Canine Leishmania Transmission. Vet. Parasitol. 2004, 122, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Ashford, D.A.; David, J.R.; Freire, M.; David, R.; Sherlock, I.; Eulálio, M.D.C.; Sampaio, D.P.; Badaro, R. Studies on Control of Visceral Leishmaniasis: Impact of Dog Control on Canine and Human Visceral Leishmaniasis in Jacobina, Bahia, Brazil. Am. J. Trop. Med. Hyg. 1998, 59, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.H.N.; Tapety, C.M.M.; Werneck, G.L. Controle Da Leishmaniose Visceral Em Meio Urbano: Estudo de Intervenção Randomizado Fatorial. Rev. Soc. Bras. Med. Trop. 2007, 40, 415–419. [Google Scholar] [CrossRef]
- Nunes, C.M.; Pires, M.M.; da Silva, K.M.; Assis, F.D.; Filho, J.G.; Perri, S.H.V. Relationship between Dog Culling and Incidence of Human Visceral Leishmaniasis in an Endemic Area. Vet. Parasitol. 2010, 170, 131–133. [Google Scholar] [CrossRef]
- Costa, D.N.C.C.; Bermudi, P.M.M.B.; Rodas, L.A.C.; Nunes, C.M.; Hiramoto, R.M.; Tolezano, J.E.; Cipriano, R.S.; Cardoso, G.C.D.; Codeço, C.T.; Chiaravalloti-Neto, F. Human Visceral Leishmaniasis and Relationship with Vector and Canine Control Measures. Rev. Saude Publica 2018, 52, 92. [Google Scholar] [CrossRef] [PubMed]
- Bermudi, P.M.M.; Costa, D.N.C.C.; Nunes, C.M.; Tolezano, J.E.; Hiramoto, R.M.; Rodas, L.A.C.; Cipriano, R.S.; Blangiardo, M.; Chiaravalloti-Neto, F. Canine Serological Survey and Dog Culling and Its Relationship with Human Visceral Leishmaniasis in an Endemic Urban Area. BMC Infect. Dis. 2020, 20, 401. [Google Scholar] [CrossRef] [PubMed]
- Dietze, R.; Barros, G.B.; Teixeira, L.; Harris, J.; Michelson, K.; Falqueto, A.; Corey, R. Effect of Eliminating Seropositive Canines on the Transmission of Visceral Leishmaniasis in Brazil. Clin. Infect. Dis. 1997, 25, 1240–1242. [Google Scholar] [CrossRef] [PubMed]
- Paranhos-Silva, M.; Nascimento, E.G.; Melro, M.C.B.F.; Oliveira, G.G.S.; dos Santos, W.L.C.; Pontes-de-Carvalho, L.C.; Oliveira-dos-Santos, A.J. Cohort Study on Canine Emigration and Leishmania Infection in an Endemic Area for American Visceral Leishmaniasis. Implications for the Disease Control. Acta Trop. 1998, 69, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Vaz, T.P.; Gama-Melo, M.O.; Quaresma, P.F.; Gontijo, C.M.F.; Santos, G.; Barbosa, F.S.; Fontes, G. Evaluation of the Euthanasia of Seropositive Dogs for Canine Visceral Leishmaniasis as the Only Method of Controling the Disease in the Enzootic Area in the Midwestern Minas Gerais. Pesqui. Veterinária Bras. 2020, 40, 107–112. [Google Scholar] [CrossRef]
- Costa, D.N.C.C.; Codeço, C.T.; Silva, M.A.; Werneck, G.L. Culling Dogs in Scenarios of Imperfect Control: Realistic Impact on the Prevalence of Canine Visceral Leishmaniasis. PLoS Negl. Trop. Dis. 2013, 7, e2355. [Google Scholar] [CrossRef] [PubMed]
- França-Silva, J.C.; Giunchetti, R.C.; Mariano, R.M.D.S.; Machado-Coelho, G.L.L.; Teixeira, L.D.A.S.; Barata, R.A.; Michalsky, É.M.; Rocha, M.F.; Fortes-Dias, C.L.; Dias, E.S. The Program for the Control of Visceral Leishmaniasis in Brazil: The Effect of the Systematic Euthanasia of Seropositive Dogs as a Single Control Action in Porteirinha, a Brazilian City with an Intense Transmission of Visceral Leishmaniasis. Pathogens 2023, 12, 1060. [Google Scholar] [CrossRef] [PubMed]
- Latif, A.A.; Nkabinde, B.; Peba, B.; Matthee, O.; Pienaar, R.; Josemans, A.; Marumo, D.; Labuschagne, K.; Abdelatief, N.A.; Krüger, A.; et al. Risk of Establishment of Canine Leishmaniasis Infection through the Import of Dogs into South Africa. Onderstepoort J. Vet. Res. 2019, 86, e1–e11. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, F.B.; de Lima, F.E.F., Jr.; Tomio, J.E.; Indá, F.D.M.C.; Corrêa, G.L.B.; Madeira, M.D.F. Leishmaniose Visceral Canina: Dois Casos Autóctones No Município de Florianópolis, Estado de Santa Catarina. Acta Sci. Vet. 2012, 40, 1026. [Google Scholar]
- Vilas, V.J.D.R.; Maia-Elkhoury, A.N.S.; Yadon, Z.E.; Cosivi, O.; Sanchez-Vazquez, M.J. Visceral Leishmaniasis: A One Health Approach. Vet. Rec. 2014, 175, 42–44. [Google Scholar] [CrossRef]
- De Carvalho, F.L.N.; de Oliveira Riboldi, E.; Bello, G.L.; Ramos, R.R.; Barcellos, R.B.; Gehlen, M.; Halon, M.L.; Romão, P.R.T.; Dallegrave, E.; Rossetti, M.L.R. Canine Visceral Leishmaniasis Diagnosis: A Comparative Performance of Serological and Molecular Tests in Symptomatic and Asymptomatic Dogs. Epidemiol. Infect. 2018, 146, 571–576. [Google Scholar] [CrossRef]
- Pessoa-e-Silva, R.; Vaitkevicius-Antão, V.; de Andrade, T.A.S.; de Oliveira Silva, A.C.; de Oliveira, G.A.; Trajano-Silva, L.A.M.; Nakasone, E.K.N.; de Paiva-Cavalcanti, M. The Diagnosis of Canine Visceral Leishmaniasis in Brazil: Confronting Old Problems. Exp. Parasitol. 2019, 199, 9–16. [Google Scholar] [CrossRef]
- Lopes, E.G.; Sevá, A.P.; Ferreira, F.; Nunes, C.M.; Keid, L.B.; Hiramoto, R.M.; Ferreira, H.L.; Oliveira, T.M.F.S.; Bigotto, M.F.D.; Galvis-Ovallos, F.; et al. Serological and Molecular Diagnostic Tests for Canine Visceral Leishmaniasis in Brazilian Endemic Area: One out of Five Seronegative Dogs Are Infected. Epidemiol. Infect. 2017, 145, 2436–2444. [Google Scholar] [CrossRef]
- de Mendonça, I.L.; Batista, J.F.; Schallig, H.; Cruz, M.D.S.P.E.; Alonso, D.P.; Ribolla, P.E.M.; Costa, D.L.; Costa, C.H.N. The Performance of Serological Tests for Leishmania infantum Infection Screening in Dogs Depends on the Prevalence of the Disease. Rev. Inst. Med. Trop. Sao Paulo 2017, 59, e39. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ribeiro, V.M.; Miranda, J.B.; Marcelino, A.P.; de Andrade, H.M.; Reis, I.A.; Cardoso, M.S.; Gontijo, C.M.F.; Paz, G.F. Performance of Different Serological Tests in the Diagnosis of Natural Infection by Leishmania infantum in Dogs. Vet. Parasitol. 2019, 274, 108920. [Google Scholar] [CrossRef] [PubMed]
- Herrera, G.; Castillo, A.; Ayala, M.S.; Flórez, C.; Cantillo-Barraza, O.; Ramirez, J.D. Evaluation of Four Rapid Diagnostic Tests for Canine and Human Visceral Leishmaniasis in Colombia. BMC Infect. Dis. 2019, 19, 747. [Google Scholar] [CrossRef]
- Salomón, O.D.; Pérez, A.A.; Riarte, A.R.; Casas, N.; Fragueiro-Frías, V.; Negri, V.; Santini, M.S.; Liotta, D.J. Performance of Rapid Tests for Canine Visceral Leishmaniasis Diagnosis in Argentina. Medicina 2020, 80, 103–110. [Google Scholar]
- Teixeira, A.I.P.; Silva, D.M.; Vital, T.; Nitz, N.; de Carvalho, B.C.; Hecht, M.; Oliveira, D.; Oliveira, E.; Rabello, A.; Romero, G.A.S. Improving the Reference Standard for the Diagnosis of Canine Visceral Leishmaniasis: A Challenge for Current and Future Tests. Mem. Inst. Oswaldo Cruz 2019, 114, e180452. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, F.B.; de Vasconcelos, T.C.B.; Madeira, M.D.F.; Menezes, R.C.; Maia-Elkhoury, A.N.S.; Marcelino, A.P.; Werneck, G.L. Validation of the Dual-Path Platform Chromatographic Immunoassay (DPP® CVL Rapid Test) for the Serodiagnosis of Canine Visceral Leishmaniasis. Mem. Inst. Oswaldo Cruz 2018, 113, e180260. [Google Scholar] [CrossRef]
- Programa Nacional de Leishmaniasis. IV Reunión Nacional. Available online: https://www.argentina.gob.ar/sites/default/files/2021/07/pnl2009web_1_0.pdf (accessed on 23 April 2024).
- Ministério da Saúde. Manual de Vigilância e Controle da Leishmaniose Visceral. Available online: https://bvsms.saude.gov.br/bvs/publicacoes/manual_vigilancia_controle_leishmaniose_visceral_1edicao.pdf (accessed on 6 February 2022).
- Subdirección de Vigilancia y Control en Salud Pública. Instituto Nacional de Salud Guía Protocolo para la Vigilancia en Salud Pública de Leishmaisniasis; Subdirección de Vigilancia y Control en Salud Pública: Madrid, Spain.
- Ministerio de Salud Pública y Bienestar. Social Reporte de Leishmaniasis Visceral en Humanos y Caninos, Paraguay, Período 2016 al 2018; Ministerio de Salud Pública y Bienestar: Asunción, Paraguay.
- Ministerio de Salud Guía de Diagnóstico. Tratamiento y Control de la Leishmaniasis Visceral en Uruguay; Ministerio de Salud Guía de Diagnóstico: Bogotá, Colombia, 2016.
- Costa, D.N.C.C.; Codeço, C.T.; Bermudi, P.M.M.; Rodas, L.A.C.; Nunes, C.M.; Hiramoto, R.M.; Tolezano, J.E.; Chiaravalloti Neto, F. Controle Da Leishmaniose Visceral Canina Por Eutanásia: Estimativa de Efeito Baseado Em Inquérito e Modelagem Matemática. Cad. Saude Publica 2020, 36, e00221418. [Google Scholar] [CrossRef]
- Leite, J.C.; Gonçalves, A.A.M.; de Oliveira, D.S.; Resende, L.A.; Boas, D.F.V.; Ribeiro, H.S.; Pereira, D.F.S.; da Silva, A.V.; Mariano, R.M.D.S.; Reis, P.C.C.; et al. Transmission-Blocking Vaccines for Canine Visceral Leishmaniasis: New Progress and Yet New Challenges. Vaccines 2023, 11, 1565. [Google Scholar] [CrossRef] [PubMed]
- Oliva, G.; Nieto, J.; Foglia Manzillo, V.; Cappiello, S.; Fiorentino, E.; Di Muccio, T.; Scalone, A.; Moreno, J.; Chicharro, C.; Carrillo, E.; et al. A Randomised, Double-Blind, Controlled Efficacy Trial of the LiESP/QA-21 Vaccine in Naïve Dogs Exposed to Two Leishmania infantum Transmission Seasons. PLoS Negl. Trop. Dis. 2014, 8, e3213. [Google Scholar] [CrossRef] [PubMed]
- Fernández Cotrina, J.; Iniesta, V.; Monroy, I.; Baz, V.; Hugnet, C.; Marañon, F.; Fabra, M.; Gómez-Nieto, L.C.; Alonso, C. A Large-Scale Field Randomized Trial Demonstrates Safety and Efficacy of the Vaccine LetiFend® against Canine Leishmaniosis. Vaccine 2018, 36, 1972–1982. [Google Scholar] [CrossRef] [PubMed]
- Giunchetti, R.C.; Silveira, P.; Resende, L.A.; Leite, J.C.; de Oliveira Melo-Júnior, O.A.; Rodrigues- Alves, M.L.; Costa, L.M.; Lair, D.F.; Chaves, V.R.; dos Santos Soares, I.; et al. Canine Visceral Leishmaniasis Biomarkers and Their Employment in Vaccines. Vet. Parasitol. 2019, 271, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Velez, R.; Gállego, M. Commercially Approved Vaccines for Canine Leishmaniosis: A Review of Available Data on Their Safety and Efficacy. Trop. Med. Int. Health 2020, 25, 540–557. [Google Scholar] [CrossRef] [PubMed]
- De Lana, M.; Giunchetti, R.C. Dogs as a Model for Chemotherapy of Chagas Disease and Leishmaniasis. Curr. Pharm. Des. 2021, 27, 1741–1756. [Google Scholar] [CrossRef] [PubMed]
- Ribas, L.M.; Zaher, V.L.; Shimozako, H.J.; Massad, E. Estimating the Optimal Control of Zoonotic Visceral Leishmaniasis by the Use of a Mathematical Model. Sci. World J. 2013, 2013, 810380. [Google Scholar] [CrossRef] [PubMed]
- Ministério da Saúde, Brasil; Secretaria de Vigilância em Saúde. Nota Técnica Nº 5/2021-CGZV/DEIDT/SVS/MS; Ministério da Saúde: Brasília, Brasil, 2021. Available online: https://www.gov.br/saude/pt-br/assuntos/saude-de-a-a-z/l/leishmaniose-visceral/arquivos/sei_ms-nota-tecnica-n-5_leishpdf.pdf (accessed on 14 October 2022).
- Oliveira, D.S.; Zaldívar, M.F.; Gonçalves, A.A.M.; Resende, L.A.; Mariano, R.M.D.S.; Pereira, D.F.S.; Conrado, I.D.S.S.; Costa, M.A.F.; Lair, D.F.; Vilas-Boas, D.F.; et al. New Approaches to the Prevention of Visceral Leishmaniasis: A Review of Recent Patents of Potential Candidates for a Chimeric Protein Vaccine. Vaccines 2024, 12, 271. [Google Scholar] [CrossRef]
- Graciano, R.C.D.; Ribeiro, J.A.T.; Macêdo, A.K.S.; de S Lavareda, J.P.; de Oliveira, P.R.; Netto, J.B.; Nogueira, L.M.; Machado, J.M.; Camposda-Paz, M.; Giunchetti, R.C.; et al. Recent Patents Applications in Red Biotechnology: A Mini-Review. Recent. Pat. Biotechnol. 2019, 13, 170–186. [Google Scholar] [CrossRef]
| Test | Manufacturer | Sensitivity | Specificity | References |
|---|---|---|---|---|
| IFAT 1:40 | Bio-Manguinhos, Rio de Janeiro, Brazil | 96% | 18% *; 76% ** | [239] |
| IFAT 1:80 | 90% | 33% *; 93% ** | ||
| ELISA | Bio-Manguinhos, Rio de Janeiro, Brazil | 91% | 79% *; 98% ** | [239] |
| 84.20% | 95.6% | [240] | ||
| rK39 (Kalazar Detect) | InBios, Inc., Seattle, WA, USA | 88% | 74% *; 98% ** | [239] |
| 82.90% | 92.6% | [241] | ||
| 76.90% | 98.6% | [242] | ||
| 79.60% | 95.7% | [240] | ||
| FAST | Royal Tropical Institute, Amsterdam, The Netherlands | 93% | 68% *; 100% ** | [239] |
| DAT | Royal Tropical Institute, Amsterdam, The Netherlands | 96% | 33% *; 98% ** | [239] |
| TR DPP | Bio-Manguinhos, Rio de Janeiro, Brazil | 98% | 60% *; 98% ** | [239] |
| 21.74% | 92.59% | [243] | ||
| 89% | 70.2% | [244] | ||
| 85.70% | 79.6% | [241] | ||
| 93.70% | 95.9% | [242] | ||
| 97.90% | 93.6% | [240] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilas-Boas, D.F.; Nakasone, E.K.N.; Gonçalves, A.A.M.; Lair, D.F.; Oliveira, D.S.d.; Pereira, D.F.S.; Silva, G.G.; Conrado, I.d.S.S.; Resende, L.A.; Zaldívar, M.F.; et al. Global Distribution of Canine Visceral Leishmaniasis and the Role of the Dog in the Epidemiology of the Disease. Pathogens 2024, 13, 455. https://doi.org/10.3390/pathogens13060455
Vilas-Boas DF, Nakasone EKN, Gonçalves AAM, Lair DF, Oliveira DSd, Pereira DFS, Silva GG, Conrado IdSS, Resende LA, Zaldívar MF, et al. Global Distribution of Canine Visceral Leishmaniasis and the Role of the Dog in the Epidemiology of the Disease. Pathogens. 2024; 13(6):455. https://doi.org/10.3390/pathogens13060455
Chicago/Turabian StyleVilas-Boas, Diego Fernandes, Eiji Kevin Nakasone Nakasone, Ana Alice Maia Gonçalves, Daniel Ferreira Lair, Diana Souza de Oliveira, Diogo Fonseca Soares Pereira, Geralda Gabriele Silva, Ingrid dos Santos Soares Conrado, Lucilene Aparecida Resende, Maykelin Fuentes Zaldívar, and et al. 2024. "Global Distribution of Canine Visceral Leishmaniasis and the Role of the Dog in the Epidemiology of the Disease" Pathogens 13, no. 6: 455. https://doi.org/10.3390/pathogens13060455
APA StyleVilas-Boas, D. F., Nakasone, E. K. N., Gonçalves, A. A. M., Lair, D. F., Oliveira, D. S. d., Pereira, D. F. S., Silva, G. G., Conrado, I. d. S. S., Resende, L. A., Zaldívar, M. F., Mariano, R. M. d. S., Dutra, W. O., Chávez-Fumagalli, M. A., Galdino, A. S., Silveira-Lemos, D., & Giunchetti, R. C. (2024). Global Distribution of Canine Visceral Leishmaniasis and the Role of the Dog in the Epidemiology of the Disease. Pathogens, 13(6), 455. https://doi.org/10.3390/pathogens13060455










