Low Plasma Lipids Are Associated with Relapsing and Lethal Visceral Leishmaniasis in HIV-Infected Patients
Abstract
1. Introduction
2. Methods
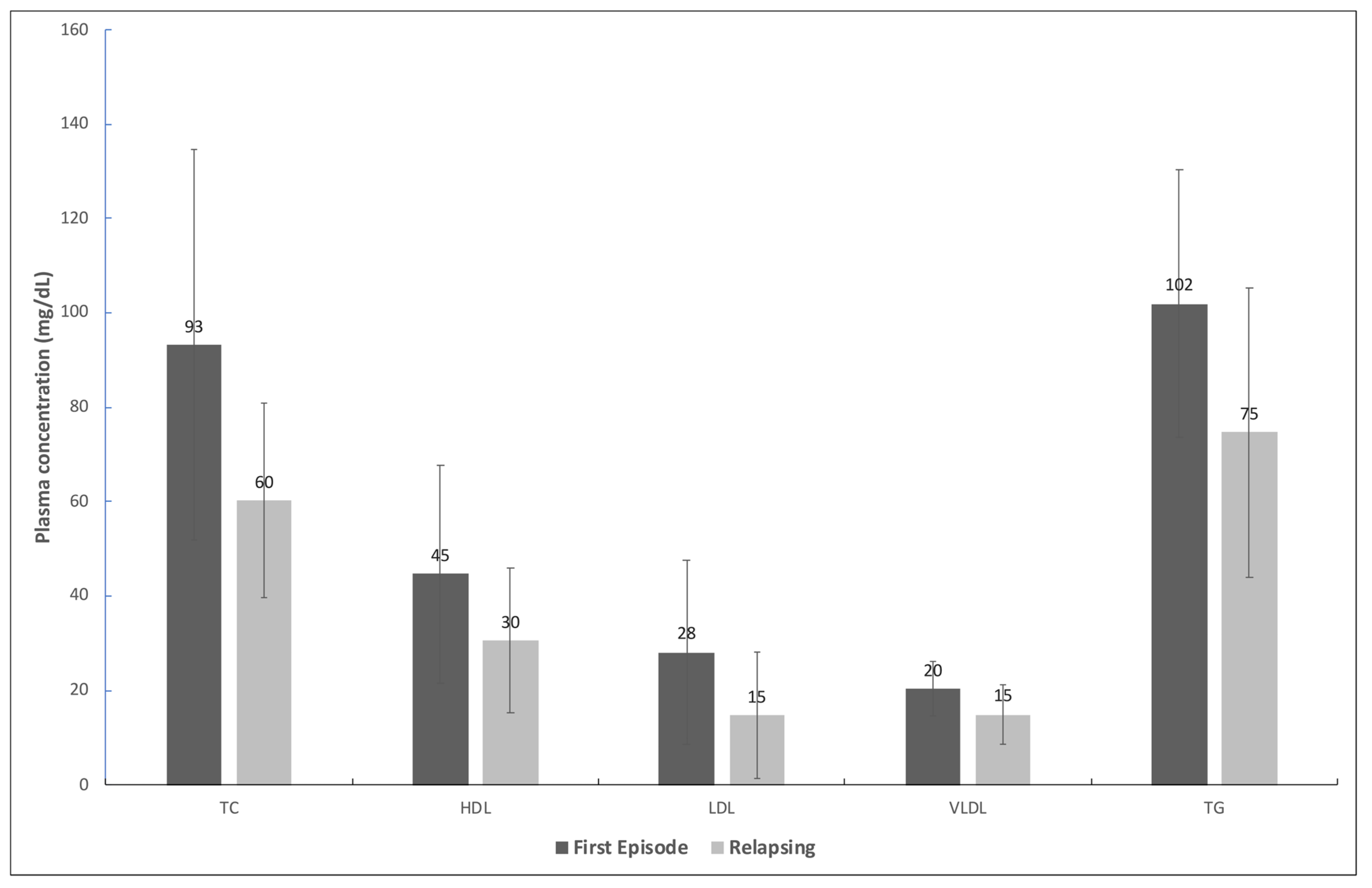
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burza, S.; Croft, S.L.; Boelaert, M. Leishmaniasis. Lancet 2018, 392, 951–970. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.L.; Rocha, R.L.; Carvalho, R.M.A.; Lima-Neto, A.S.; O Harhay, M.; Costa, C.H.N.; Barral-Neto, M.; Barral, A.P. Serum cytokines associated with severity and complications of kala-azar. Pathog. Glob. Health 2013, 107, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Wasunna, K.M.; Raynes, J.G.; Were, J.B.O.; Muigai, R.; Sherwood, J.; Gachihi, G.; Carpenter, L.; McAdam, K.P.W.J. Acute phase protein concentrations predict parasite clearance rate during therapy for visceral leishmaniasis. Trans. R. Soc. Trop. Med. Hyg. 1995, 89, 678–681. [Google Scholar] [CrossRef] [PubMed]
- Alvar, J.; Vélez, I.D.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; Jannin, J.; den Boer, M. Who Leishmaniasis Control the WHO Leishmaniasis Control Team Leishmaniasis Worldwide and Global Estimates of Its Incidence. PLoS ONE 2012, 7, e35671. [Google Scholar] [CrossRef] [PubMed]
- Reinaldo, L.G.C.; Araújo-Júnior, R.J.C.; Diniz, T.M.; Moura, R.D.; Meneses-Filho, A.J.; Furtado, C.V.V.M.; Santos, W.L.C.; Costa, D.L.; Eulálio, K.D.; Ferreira, G.R.; et al. Splenectomy in Patients with Visceral Leishmaniasis Resistant to Conventional Therapy and Secondary Prophylaxis: A Retrospective Cohort. Am. J. Trop. Med. Hyg. 2022, 107, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Cota, G.F.; de Sousa, M.R.; Rabello, A. Predictors of Visceral Leishmaniasis Relapse in HIV-Infected Patients: A Systematic Review. PLoS Neglected Trop. Dis. 2011, 5, e1153. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization & Cochrane Response. WHO Guideline for the Treatment of Visceral Leishmaniasis in HIV Coinfected Patients in East Africa and South-East Asia: Web Annex A: A Systematic Review on the Treatment of Visceral Leishmaniasis in HIV-Leishmania Coinfected Persons in East Africa and So. Licence: CC BY-NC-SA 3.0 IGO. 2022. Available online: https://apps.who.int/iris/handle/10665/354546 (accessed on 22 May 2024).
- Doitsh, G.; Greene, W.C. Dissecting How CD4 T Cells Are Lost During HIV Infection. Cell Host Microbe 2016, 19, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, P.; Prajapati, V.K.; Rai, M.; Sundar, S. Unusual Case of Resistance to Amphotericin B in Visceral Leishmaniasis in a Region in India Where Leishmaniasis Is Not Endemic. J. Clin. Microbiol. 2011, 49, 3088–3091. [Google Scholar] [CrossRef] [PubMed]
- E Kip, A.; Blesson, S.; Alves, F.; Wasunna, M.; Kimutai, R.; Menza, P.; Mengesha, B.; Beijnen, J.H.; Hailu, A.; Diro, E.; et al. Low antileishmanial drug exposure in HIV-positive visceral leishmaniasis patients on antiretrovirals: An Ethiopian cohort study. J. Antimicrob. Chemother. 2021, 76, 1258–1268. [Google Scholar] [CrossRef]
- Wasan, K.M.; Kennedy, A.L.; Cassidy, S.M.; Ramaswamy, M.; Holtorf, L.; Chou, J.W.-L.; Pritchard, P.H. Pharmacokinetics, Distribution in Serum Lipoproteins and Tissues, and Renal Toxicities of Amphotericin B and Amphotericin B Lipid Complex in a Hypercholesterolemic Rabbit Model: Single-Dose Studies. Antimicrob. Agents Chemother. 1998, 42, 3146–3152. [Google Scholar] [CrossRef]
- Zauli-Nascimento, R.C.; Miguel, D.C.; Yokoyama-Yasunaka, J.K.U.; Pereira, L.I.A.; De Oliveira, M.A.P.; Ribeiro-Dias, F.; Dorta, M.L.; Uliana, S.R.B. In vitro sensitivity of Leishmania (Viannia) braziliensis and Leishmania (Leishmania) amazonensis Brazilian isolates to meglumine antimoniate and amphotericin B. Trop. Med. Int. Health 2010, 15, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Rastrojo, A.; Garcia-Hernandez, R.; Vargas, P.; Camacho, E.; Corvo, L.; Imamura, H.; Dujardin, J.-C.; Castanys, S.; Aguado, B.; Gamarro, F.; et al. Genomic and transcriptomic alterations in Leishmania donovani lines experimentally resistant to antileishmanial drugs. Int. J. Parasitol. Drugs Drug Resist. 2018, 8, 246–264. [Google Scholar] [CrossRef] [PubMed]
- Morizot, G.; Jouffroy, R.; Faye, A.; Chabert, P.; Belhouari, K.; Calin, R.; Charlier, C.; Miailhes, P.; Siriez, J.-Y.; Mouri, O.; et al. Antimony to Cure Visceral Leishmaniasis Unresponsive to Liposomal Amphotericin B. PLoS Neglected Trop. Dis. 2016, 10, e0004304. [Google Scholar] [CrossRef] [PubMed]
- Ponte-Sucre, A.; Gamarro, F.; Dujardin, J.-C.; Barrett, M.P.; López-Vélez, R.; García-Hernández, R.; Pountain, A.W.; Mwenechanya, R.; Papadopoulou, B. Drug resistance and treatment failure in leishmaniasis: A 21st century challenge. PLoS Neglected Trop. Dis. 2017, 11, e0006052. [Google Scholar] [CrossRef] [PubMed]
- Lachaud, L.; Bourgeois, N.; Plourde, M.; Leprohon, P.; Bastien, P.; Ouellette, M. Parasite Susceptibility to Amphotericin B in Failures of Treatment for Visceral Leishmaniasis in Patients Coinfected with HIV Type 1 and Leishmania infantum. Clin. Infect. Dis. 2009, 48, e16–e22. [Google Scholar] [CrossRef] [PubMed]
- Durand, R.; Paul, M.; Pratlong, F.; Rivollet, D.; Dubreuil-Lemaire, M.-L.; Houin, R.; Astier, A.; Deniau, M. Leishmania infantum: Lack of Parasite Resistance to Amphotericin B in a Clinically Resistant Visceral Leishmaniasis. Antimicrob. Agents Chemother. 1998, 42, 2141–2143. [Google Scholar] [CrossRef]
- Cota, G.F.; de Sousa, M.R.; de Assis, T.S.M.; Pinto, B.F.; Rabello, A. Exploring prognosis in chronic relapsing visceral leishmaniasis among HIV-infected patients: Circulating Leishmania DNA. Acta Trop. 2017, 172, 186–191. [Google Scholar] [CrossRef]
- Costa, D.L.; Rocha, R.L.; Chaves, E.D.B.F.; Batista, V.G.D.V.; Costa, H.L.; Costa, C.H.N. Predicting death from kala-azar: Construction, development, and validation of a score set and accompanying software. Rev. Soc. Bras. Med. Trop. 2016, 49, 728–740. [Google Scholar] [CrossRef] [PubMed]
- Takele, Y.; Mulaw, T.; Adem, E.; Shaw, C.J.; Franssen, S.U.; Womersley, R.; Kaforou, M.; Taylor, G.P.; Levin, M.; Müller, I.; et al. Immunological factors, but not clinical features, predict visceral leishmaniasis relapse in patients co-infected with HIV. Cell Rep. Med. 2022, 3, 100487. [Google Scholar] [CrossRef]
- Silva-Freitas, M.L.; Cota, G.F.; Machado-De-Assis, T.S.; Giacoia-Gripp, C.; Rabello, A.; Da-Cruz, A.M.; Santos-Oliveira, J.R. Immune Activation and Bacterial Translocation: A Link between Impaired Immune Recovery and Frequent Visceral Leishmaniasis Relapses in HIV-Infected Patients. PLoS ONE 2016, 11, e0167512. [Google Scholar] [CrossRef]
- Gautam, S.; Kumar, R.; Singh, N.; Singh, A.K.; Rai, M.; Sacks, D.; Sundar, S.; Nylén, S. CD8 T Cell Exhaustion in Human Visceral Leishmaniasis. J. Infect. Dis. 2014, 209, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Silva-Freitas, M.L.; Corrêa-Castro, G.; Cota, G.F.; Giacoia-Gripp, C.; Rabello, A.; Dutra, J.T.; De Vasconcelos, Z.F.M.; Savino, W.; Da-Cruz, A.M.; Santos-Oliveira, J.R. Impaired Thymic Output Can Be Related to the Low Immune Reconstitution and T Cell Repertoire Disturbances in Relapsing Visceral Leishmaniasis Associated HIV/AIDS Patients. Front. Immunol. 2020. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Francois, B.; Jeannet, R.; Daix, T.; Walton, A.H.; Shotwell, M.S.; Unsinger, J.; Monneret, G.; Rimmele, T.; Blood, T.; Morre, M.; et al. Interleukin-7 restores lymphocytes in septic shock: The IRIS-7 randomized clinical trial. JCI Insight. 2018. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Maksoud, S.; El Hokayem, J. The cytokine/chemokine response in Leishmania/HIV infection and co-infection. Heliyon 2023, 9, e15055. [Google Scholar] [CrossRef] [PubMed]
- Szwarcwald, C.L.; Malta, D.C.; Pereira, C.A.; Figueiredo, A.W.; Almeida, W.d.S.d.; Machado, I.E.; Bacal, N.S.; da Silva, A.G.; Júnior, J.B.d.S.; Rosenfeld, L.G. Valores de referência para exames laboratoriais de colesterol, hemoglobina glicosilada e creatinina da população adulta brasileira. Rev. Bras. Epidemiol. 2019. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Lal, C.S.; Kumar, A.; Kumar, S.; Pandey, K.; Kumar, N.; Bimal, S.; Sinha, P.K.; Das, P. Hypocholesterolemia and increased triglyceride in pediatric visceral leishmaniasis. Clin. Chim. Acta 2007, 382, 151–153. [Google Scholar] [CrossRef] [PubMed]
- Grunfeld, C.; Pang, M.; Doerrler, W.; Shigenaga, J.K.; Jensen, P.; Feingold, K.R. Lipids, lipoproteins, triglyceride clearance, and cytokines in human immunodeficiency virus infection and the acquired immunodeficiency syndrome. J. Clin. Endocrinol. Metab. 1992, 74, 1045–1052. [Google Scholar] [PubMed]
- Souza, S.J.; Luzia, L.A.; Santos, S.S.; Rondo, P.H.C. Lipid profile of HIV-infected patients in relation to antiretroviral therapy: A review. Rev. Assoc. Med. Bras. 2013, 59, 186–198. [Google Scholar] [CrossRef] [PubMed]
- Wallet, M.A.; Buford, T.W.; Joseph, A.-M.; Sankuratri, M.; Leeuwenburgh, C.; Pahor, M.; Manini, T.; Sleasman, J.W.; Goodenow, M.M. Increased inflammation but similar physical composition and function in older-aged, HIV-1 infected subjects. BMC Immunol. 2015, 16, 186–198. [Google Scholar] [CrossRef]
- Harhay, M.O.; Rijal, S.; Chappuis, F.; Sundar, S.; Lima, M.A.; Olliaro, P.L.; Costa, C.H.; Vaillant, M.; Costa, D.L.; Balasegaram, M.; et al. Who Is a Typical Patient with Visceral Leishmaniasis? Characterizing the Demographic and Nutritional Profile of Patients in Brazil, East Africa, and South Asia. Am. J. Trop. Med. Hyg. 2011, 84, 543–550. [Google Scholar] [CrossRef]
- Grinspoon, S.; Mulligan, K. Weight Loss and Wasting in Patients Infected with Human Immunodeficiency Virus. Clin. Infect. Dis. 2003, 36, S69–S78. [Google Scholar] [CrossRef] [PubMed]
- Carpentier, Y.A.; Scruel, O. Changes in the concentration and composition of plasma lipoproteins during the acute phase response. Curr. Opin. Clin. Nutr. Metab. Care 2002, 5, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Feingold, K.R.; Grunfeld, C. The Effect of Inflammation and Infection on Lipids and Lipoproteins. [Updated 2022 Mar 7]. In Endotext [Internet]; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Hofland, J., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. Available online: https://www.ncbi.nlm.nih.gov/books/NBK326741/ (accessed on 22 May 2024).
- Veiga, G.R.S.; Ferreira, H.S.; Sawaya, A.L.; Calado, J.; Florêncio, T.M.M.T. Dyslipidaemia and Undernutrition in Children from Impoverished Areas of Maceió, State of Alagoas, Brazil. Int. J. Environ. Res. Public Health 2010, 7, 4139–4151. [Google Scholar] [CrossRef] [PubMed]
- Verma, G.K.; Yadav, Y.S.; Yadav, R.K.; Sharma, I.K.; Bharat, K.; Yadav, K.K. Study of lipid profile levels in malnourished and healthy children: A case control study acquired pneumonia in children. Pediatr. Rev. Int. J. Pediatr. Res. 2018, 5, 156–161. [Google Scholar] [CrossRef]
- Zhang, Z.; Pereira, S.L.; Luo, M.; Matheson, E.M. Evaluation of Blood Biomarkers Associated with Risk of Malnutrition in Older Adults: A Systematic Review and Meta-Analysis. Nutrients 2017, 9, 829. [Google Scholar] [CrossRef]
- Stellaard, F. From Dietary Cholesterol to Blood Cholesterol, Physiological Lipid Fluxes, and Cholesterol Homeostasis. Nutrients 2022, 14, 1643. [Google Scholar] [CrossRef]
- Olsson, A.G.; Angelin, B.; Assmann, G.; Binder, C.J.; Björkhem, I.; Cedazo-Minguez, A.; Cohen, J.; von Eckardstein, A.; Farinaro, E.; Müller-Wieland, D.; et al. Can cholesterol be too low? Possible risks of extremely low levels. J. Intern. Med. 2017, 281, 534–553. [Google Scholar] [CrossRef]
- Wu, Z.; Huang, Z.; Lichtenstein, A.H.; Liu, Y.; Chen, S.; Jin, Y.; Na, M.; Bao, L.; Wu, S.; Gao, X. The risk of ischemic stroke and hemorrhagic stroke in Chinese adults with low-density lipoprotein cholesterol concentrations < 70 mg/dL. BMC Med. 2021, 19, 142. [Google Scholar]
- Hofmaenner, D.A.; Arina, P.; Kleyman, A.; Black, L.P.; Salomao, R.; Tanaka, S.; Guirgis, F.W.; Arulkumaran, N.; Singer, M.M. Association Between Hypocholesterolemia and Mortality in Critically Ill Patients with Sepsis: A Systematic Review and Meta-Analysis. Crit. Care Explor. 2023, 5, e0860. [Google Scholar] [CrossRef]
| Patient ID | Mean EC50 ¹ | Standard Error | No. of Experiments | No. of Previous VL Episodes |
|---|---|---|---|---|
| (µM) | (µM) | |||
| 5567 2 | 0.042 | 0.007 | 5 | 2 |
| 5768 2 | 0.037 | 0.006 | 5 | 2 |
| 6905 2 | 0.034 | 0.007 | 4 | 2 |
| 7053 2 | 0.024 | 0.008 | 5 | 2 |
| 5609 3 | 0.026 | 0.006 | 4 | 0 |
| MHOM/BR/2005/NLC 4 | 0.051 | 0.005 | 3 | - |
| Characteristics | First Episode n (%) | Relapsing n (%) | p-Value |
|---|---|---|---|
| Sex | |||
| Male | 6 (100.0) | 20 (87.0) | |
| Female | 0 (0.0) | 3 (13.0) | 0.350 |
| Age group | |||
| Up to 40 years old | 4 (66.7) | 8 (34.8) | |
| More than 40 years | 2 (33.3) | 15 (65.2) | 0.158 |
| Symptoms and signs | |||
| Weight loss | 6 (100.0) | 17 (73.4) | 0.160 |
| Pallor | 5 (83.3) | 17 (73.4) | 0.631 |
| Fever | 6 (100.0) | 13 (56.5) | 0.046 |
| Asthenia/weakness | 4 (66.7) | 14 (60.9) | 0.794 |
| Splenomegaly | 3 (50.0) | 13 (56.5) | 0.775 |
| Hepatomegaly | 2 (33.3) | 9 (39.1) | 0.794 |
| Jaundice | 2 (33.3) | 8 (34.8) | 0.947 |
| HAART * | 6/6 (100.0) | 15/23 (0.65) | 0.43 |
| Death | 0 (0.0) | 4 (17.4) | 0.55 |
| Laboratory Data | Course of VL | p-Value c | |||
|---|---|---|---|---|---|
| First Episode (n = 6) | Relapsing (n = 23) | ||||
| n a | Mean (95% CI b) | n a | Mean (95% CI) | ||
| Hemoglobin (g/dL) | 5 | 8.0 (3.6; 12.5) | 19 | 7.8 (7.2; 8.4) | 0.522 |
| Leukocytes (per mm3) | 5 | 4.643 (1060; 4; 8.223) | 21 | 2.397 (1713; 3.083) | 0.013 |
| Neutrophils (cells/mm3) | 5 | 2410 (−272; 5092.9) | 19 | 1409 (889; 1930) | 0.076 |
| Lymphocytes (cells/mm3) | 5 | 1654 (−88; 3396) | 19 | 660 (457; 864) | 0.005 |
| Platelets (number/mm3) | 6 | 151,952 (151,952;184,813) | 21 | 190,217 (89.298; 291,135) | 0.297 |
| Albumin (g/dL) | 1 | 1.8 | 10 | 2.7 (1.9; 3.6) | - |
| Globulin (g/dL) | 1 | 9.9 | 9 | 6.0 (4.8;7.2) | - |
| CD4+ (cells/mm3) | 5 | 152 (−32; 332) | 15 | 142 (89; 196) | 0.877 |
| CD8+ (cells/mm3) | 5 | 955 (−197.4; 2106.55) | 14 | 606 (423; 789) | 0.222 |
| Lipid Profile | Outcome | p-Value | |
|---|---|---|---|
| Survival (n a = 25) Mean (95% CI b) | Death (n = 4) Mean (95% CI) | ||
| TC c (mg/dL) | 68.3 (56.1; 80.5) | 60.7 (20.8; 100.7) | 0.633 |
| LDL d (mg/dL) | 20.4 (14.2; 26.7) | 3.6 (−3.8; 10.9) | 0.038 |
| HDL e (mg/dL) | 32.7 (25.3; 40.2) | 37.3 (8.6; 65.9) | 0.644 |
| VLDL f (mg/dL) | 15.2 (12.7; 17.6) | 20.0 (5.7; 34.2) | 0.170 |
| TG g (mg/dL) | 75.8 (63.7; 88.0) | 99.8 (28.4; 171.1) | 0.170 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, R.V.S.; Uliana, S.R.B.; Yasunaka, J.K.U.Y.; Veloso, C.S.; Sousa, E.; Ferreira, M.M.L.; Carvalho, V.S.; Ferreira, G.R.; Costa, D.L.; Costa, C.H.N. Low Plasma Lipids Are Associated with Relapsing and Lethal Visceral Leishmaniasis in HIV-Infected Patients. Pathogens 2024, 13, 450. https://doi.org/10.3390/pathogens13060450
Silva RVS, Uliana SRB, Yasunaka JKUY, Veloso CS, Sousa E, Ferreira MML, Carvalho VS, Ferreira GR, Costa DL, Costa CHN. Low Plasma Lipids Are Associated with Relapsing and Lethal Visceral Leishmaniasis in HIV-Infected Patients. Pathogens. 2024; 13(6):450. https://doi.org/10.3390/pathogens13060450
Chicago/Turabian StyleSilva, Renata V. S., Silvia R. B. Uliana, Jenicer K. U. Y. Yasunaka, Cláudio S. Veloso, Emille Sousa, Maria M. L. Ferreira, Vivianne S. Carvalho, Gabriel R. Ferreira, Dorcas L. Costa, and Carlos H. N. Costa. 2024. "Low Plasma Lipids Are Associated with Relapsing and Lethal Visceral Leishmaniasis in HIV-Infected Patients" Pathogens 13, no. 6: 450. https://doi.org/10.3390/pathogens13060450
APA StyleSilva, R. V. S., Uliana, S. R. B., Yasunaka, J. K. U. Y., Veloso, C. S., Sousa, E., Ferreira, M. M. L., Carvalho, V. S., Ferreira, G. R., Costa, D. L., & Costa, C. H. N. (2024). Low Plasma Lipids Are Associated with Relapsing and Lethal Visceral Leishmaniasis in HIV-Infected Patients. Pathogens, 13(6), 450. https://doi.org/10.3390/pathogens13060450







