Refinement of the rKLi8.3-Based Serodiagnostic ELISA Allows Detection of Canine Leishmaniosis in Dogs with Low Antibody Titers
Abstract
1. Introduction
2. Materials and Methods
2.1. Serum Samples
2.2. rKLi8.3 Recombinant Protein
2.3. Serodiagnostic Test Systems
3. Results
3.1. CanL Serodiagnosis Related to the Clinical Score—DPP, LFT and rKLi8.3-ELISA Show Similar Diagnostic Accuracy
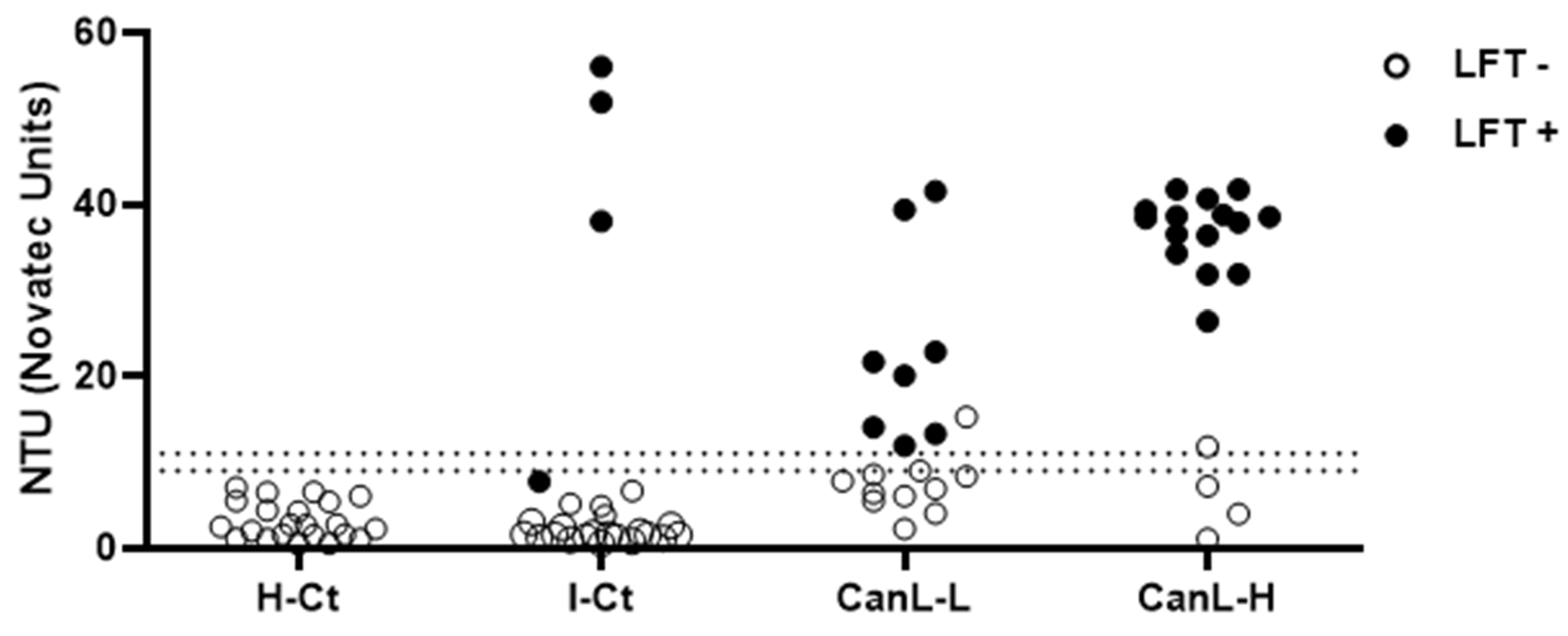
3.2. rKLi8.3-ELISA Shows Increased Sensitivity Compared to DPP and LFT—Antibody Titers Do Not Always Correlate with Parasite Load and Clinical Score
3.3. rKLi8.3-Based LFT and ELISA Showed High Specificity but Moderate Diagnostic Sensitivity in Dogs with Low Clinical Scores
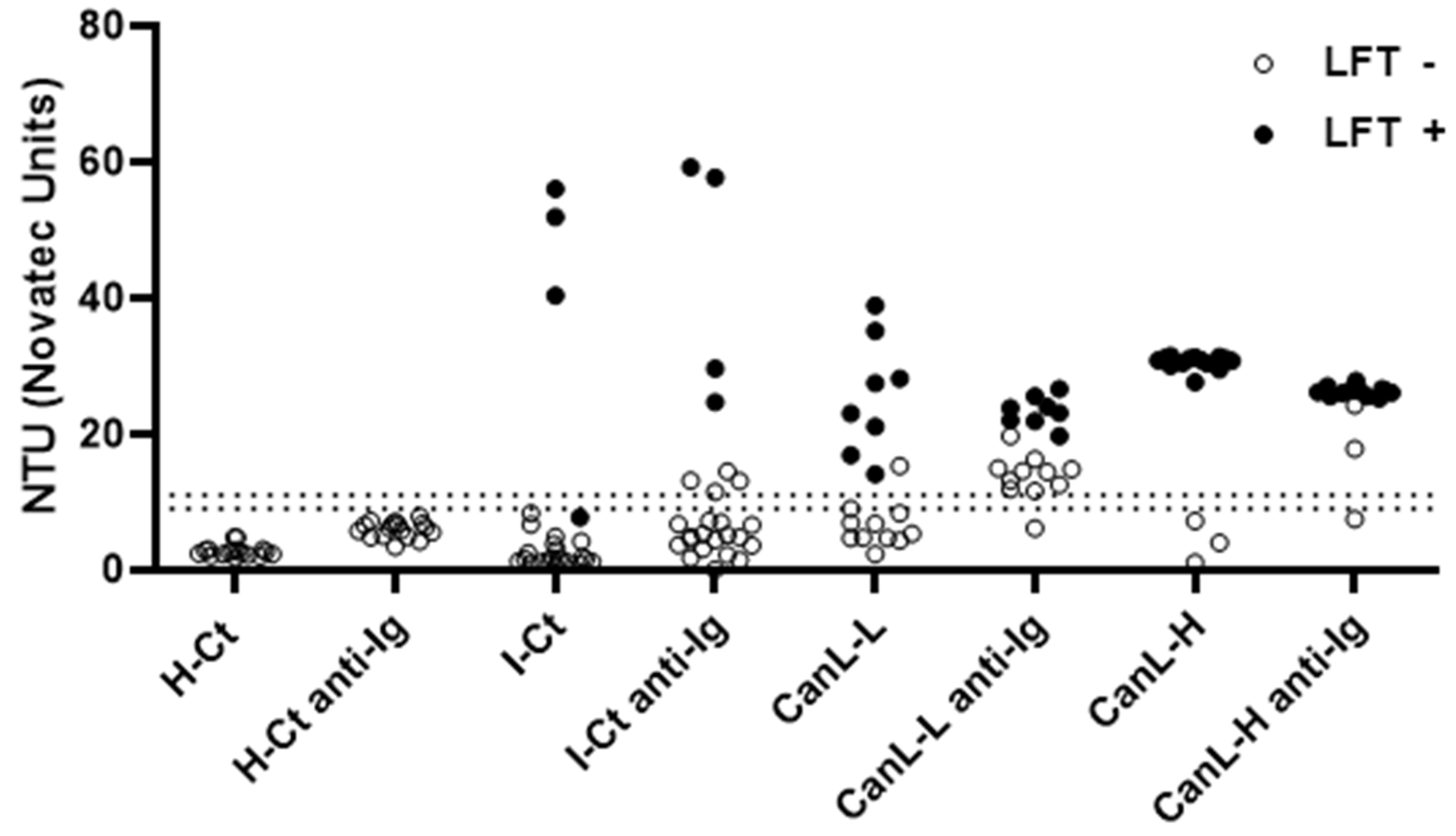
3.4. The Addition of a Secondary Anti-Dog IgG Antibody to the rKLi8.3-ELISA Improves the Diagnosis of CanL in Dogs with Low Antibody Titer at the Slight Expense of Specificity for Other Infections
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alemayehu, B.; Alemayehu, M. Leishmaniasis: A review on parasite, vector and reservoir host. Health Sci. J. 2017, 11. [Google Scholar] [CrossRef]
- Dos Santos, P.L.; de Oliveira, F.A.; Santos, M.L.B.; Cunha, L.C.S.; Lino, M.T.; de Oliveira, M.F.; Bomfim, M.O.; Silva, A.M.; de Moura, T.R.; de Jesus, A.R.; et al. The severity of visceral leishmaniasis correlates with elevated levels of serum IL-6, IL-27 and sCD14. PLoS Neglected Trop. Dis. 2016, 10, e0004375. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Leishmaniasis. Who.int. World Health Organization: WHO. 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis (accessed on 14 November 2023).
- Peixoto, H.M.; de Oliveira, M.R.F.; Romero, G.A.S. Serological diagnosis of canine visceral leishmaniasis in Brazil: Systematic review and meta-analysis. Trop. Med. Int. Health 2014, 20, 334–352. [Google Scholar] [CrossRef] [PubMed]
- Reis, A.B.; Martins, O.A.F.; Teixeira-Carvalho, A.; Giunchetti, R.C.; Carneiro, C.M.; Mayrink, W. Systemic and compartmentalized immune response in canine visceral leishmaniasis. Vet. Immunol. Immunopathol. 2009, 128, 87–95. [Google Scholar] [CrossRef] [PubMed]
- de Almeida Leal, G.G.; Roatt, B.M.; de Oliveira Aguiar-Soares, R.D.; Carneiro, C.M.; Giunchetti, R.C.; Teixeira-Carvalho, A.; Martins-Filho, O.A.; Francisco, A.F.; Cardoso, J.M.; Mathias, F.A.S.; et al. Immunological profile of resistance and susceptibility in naturally infected dogs by Leishmania infantum. Vet. Parasitol. 2014, 205, 472–482. [Google Scholar] [CrossRef]
- Courtenay, O.; Carson, C.; Calvo-Bado, L.; Garcez, L.M.; Quinnell, R.J. Heterogeneities in Leishmania infantum Infection: Using skin parasite burdens to identify highly infectious dogs. PLoS Neglected Trop. Dis. 2014, 8, e2583. [Google Scholar] [CrossRef]
- Figueiredo, F.B.; Madeira, M.F.; Nascimento, L.D.; Abrantes, T.R.; Mouta-Confort, E.; Passos, S.R.; Schubach, T.M. Canine visceral leishmaniasis: Study of methods for the detection of IgG in serum and eluate samples. Rev. Do Inst. De Med. Trop. De São Paulo 2010, 52, 193–196. [Google Scholar] [CrossRef][Green Version]
- Rosário, E.Y.; Genaro, O.; França-Silva, J.C.; da Costa, R.T.; Mayrink, W.; Reis, A.B.; Carneiro, M. Evaluation of enzyme-linked immunosorbent assay using crude Leishmania and recombinant antigens as a diagnostic marker for canine visceral leishmaniasis. Mem. Do Inst. Oswaldo Cruz 2005, 100, 197–203. [Google Scholar] [CrossRef]
- Iniesta, L.; Fernández-Barredo, S.; Bulle, B.; Gómez, M.T.; Piarroux, R.; Gállego, M.; Alunda, J.M.; Portús, M. Diagnostic techniques to detect cryptic leishmaniasis in dogs. Clin. Vaccine Immunol. 2002, 9, 1137–1141. [Google Scholar] [CrossRef][Green Version]
- Quinnell, R.J.; Courtenay, O.; Davidson, S.; Garcez, L.; Lambson, B.; Ramos, P.; Shaw, J.J.; Shaw, M.A.; Dye, C. Detection of Leishmania infantum by PCR, serology and cellular immune response in a cohort study of Brazilian dogs. Parasitology 2001, 122, 3. [Google Scholar] [CrossRef]
- Paz, G.F.; Rugani, J.M.N.; Marcelino, A.P.; Gontijo, C.M.F. Implications of the use of serological and molecular methods to detect infection by Leishmania spp. in urban pet dogs. Acta Trop. 2018, 182, 198–201. [Google Scholar] [CrossRef]
- Porrozzi, R.; Santos da Costa, M.V.; Teva, A.; Falqueto, A.; Ferreira, A.L.; dos Santos, C.D.; Fernandes, A.P.; Gazzinelli, R.T.; Campos-Neto, A.; Grimaldi, G., Jr. Comparative evaluation of enzyme-linked immunosorbent assays based on crude and recombinant leishmanial antigens for serodiagnosis of symptomatic and asymptomatic Leishmania infantum visceral infections in dogs. Clin. Vaccine Immunol. 2007, 14, 544–548. [Google Scholar] [CrossRef]
- Venturin, G.L.; Bragato, J.P.; Silva, K.L.O.; de Lima, V.M.F. Recombinant K28 antigen in ELISA in the diagnosis of canine visceral leishmaniosis. Parasite Immunol. 2015, 37, 670–673. [Google Scholar] [CrossRef]
- Lauricella, M.A.; Maidana, C.G.; Frias, V.F.; Romagosa, C.M.; Negri, V.; Benedetti, R.; Sinagra, A.J.; Luna, C.; Tartaglino, L.; Laucella, S.; et al. An rK28-Based immunoenzymatic assay for the diagnosis of canine visceral leishmaniasis in Latin America. Am. J. Trop. Med. Hyg. 2016, 95, 92–98. [Google Scholar] [CrossRef]
- Dantas-Torres, F.; Sales, K.G.S.; da Silva, L.G.; Otranto, D.; Figueredo, L.A. Level of agreement between two commercially available rapid serological tests and the official screening test used to detect Leishmania seropositive dogs in Brazil. Vet. J. 2018, 234, 102–104. [Google Scholar] [CrossRef]
- Grimaldi, G., Jr.; Teva, A.; Ferreira, A.L.; dos Santos, C.B.; Pinto, I.D.; de-Azevedo, C.T.; Falqueto, A. Evaluation of a novel chromatographic immunoassay based on Dual-Path Platform technology (DPP® CANL rapid test) for the serodiagnosis of canine visceral leishmaniasis. Trans. R. Soc. Trop. Med. Hyg. 2012, 106, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, R.; Shams-Eldin, H.; Witt, S.; Latz, A.; Heinz, D.; Fresco-Taboada, A.; Aira, C.; Hübner, M.P.; Sukyte, D.; Visekruna, A.; et al. Development of a novel enzyme-linked immunosorbent assay and lateral flow test system for improved serodiagnosis of visceral leishmaniasis in different areas of endemicity. Microbiol. Spectr. 2023, 11, 3. [Google Scholar] [CrossRef]
- Mahdavi, R.; Martinkovic, F.; Shams-Eldin, H.; Pereira, I.E.; Reis, A.B.; Latz, A.; Heinz, D.; Aira, C.; Fresco-Taboada, A.; Abass, E.; et al. Comparative study of a novel lateral flow rapid test with conventional serological test-systems for the diagnosis of Canine Leishmaniosis in Croatia and Brazil. Pathogens 2024, 13, 109. [Google Scholar] [CrossRef]
- Lindmark, R.; Thorén-Tolling, K.; Sjöquist, J. Binding of immunoglobulins to protein A and immunoglobulin levels in mammalian sera. J. Immunol. Methods 1983, 62, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Dahlbom, I.; Agardh, D.; Hansson, T. Protein A and protein G ELISA for the detection of IgG autoantibodies against tissue transglutaminase in childhood celiac disease. Clin. Chim. Acta 2008, 395, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Prina, E.; Roux, E.; Mattei, D.; Milon, G. Leishmania DNA is rapidly degraded following parasite death: An analysis by microscopy and real-time PCR. Microbes Infect. 2007, 9, 1307–1315. [Google Scholar] [CrossRef]
- Cavalcanti, A.S.; Ribeiro-Alves, M.; Pereira, L.D.O.; Mestre, G.L.; Ferreira, A.B.R.; Morgado, F.N.; Boité, M.C.; Cupolillo, E.; Moraes, M.O.; Porrozzi, R. Parasite load induces progressive spleen architecture breakage and impairs cytokine mRNA expression in Leishmania infantum naturally infected dogs. PLoS ONE 2015, 10, e0123009. [Google Scholar] [CrossRef]
- Solano-Gallego, L.; Koutinas, A.; Miro, G.; Cardoso, L.; Pennisi, M.G.; Ferrer, L.; Bourdeau, P.; Oliva, G.; Baneth, G. Directions for the diagnosis, clinical staging, treatment and prevention of canine leishmaniosis. Vet. Parasitol. 2009, 165, 22. [Google Scholar] [CrossRef]
- Leite, B.M.; Solcà, M.D.; Santos, L.C.; Coelho, L.B.; Amorim, L.D.; Donato, L.E.; Passos, S.M.; Almeida, A.O.; Veras, P.S.; Fraga, D.B. The mass use of deltamethrin collars to control and prevent canine visceral leishmaniasis: A field effectiveness study in a highly endemic area. PLoS Neglected Trop. Dis. 2018, 12, e0006496. [Google Scholar] [CrossRef]
- Vaish, M.; Bhatia, A.; Reed, S.G.; Chakravarty, J.; Sundar, S. Evaluation of rK28 antigen for serodiagnosis of visceral Leishmaniasis in India. Clin. Microbiol. Infect. 2012, 18, 81–85. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, F.L.N.; de Oliveira Riboldi, E.; Bello, G.L.; Ramos, R.R.; Barcellos, R.B.; Gehlen, M.; Halon, M.L.; Romão, P.R.T.; Dallegrave, E.; Rossetti, M.L.R. Canine visceral leishmaniasis diagnosis: A comparative performance of serological and molecular tests in symptomatic and asymptomatic dogs. Epidemiol. Infect. 2018, 146, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Di Pietro, S.; Crinò, C.; Falcone, A.; Crupi, R.; Francaviglia, F.; Vitale, F.; Giudice, E. Parasitemia and its daily variation in canine leishmaniasis. Parasitol. Res. 2020, 119, 3541–3548. [Google Scholar] [CrossRef] [PubMed]
- Bhullar, S.S.; Kashyap, R.S.; Chandak, N.H.; Purohit, H.J.; Taori, G.M.; Daginawala, H.F. Protein A-based ELISA: Its evaluation in the diagnosis of herpes simplex encephalitis. Viral Immunol. 2011, 24, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, M.F.; Xavier, C.C.; Almeida, A.M.P.; Reis, C.R.S. Evaluation of a multi-species Protein A-ELISA assay for plague serologic diagnosis in humans and other mammal hosts. PLoS Neglected Trop. Dis. 2022, 16, e0009805. [Google Scholar] [CrossRef] [PubMed]
- Otranto, D.; Paradies, P.; de Caprariis, D.; Stanneck, D.; Testini, G.; Grimm, F.; Deplazes, P.; Capelli, G. Toward diagnosing Leishmania infantum infection in asymptomatic dogs in an area where leishmaniasis is endemic. Clin. Vaccine Immunol. 2009, 16, 337–343. [Google Scholar] [CrossRef]
- Zijlstra, E.E.; Nur, Y.; Desjeux, P.; Khalil, E.A.G.; El-hassan, A.M.; Groen, J. Diagnosing visceral leishmaniasis with the recombinant K39 strip test: Experience from the Sudan. Trop. Med. Int. Health 2001, 6, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, W.F.; Cardoso, M.S.; de Carvalho Clímaco, M.; Silva, A.L.; Heidt, B.; Eersels, K.; van Grinsven, B.; Bartholomeu, D.C.; Bueno, L.L.; Cleij, T.; et al. Serodiagnosis of leishmaniasis in asymptomatic and symptomatic dogs by use of the recombinant dynamin-1-like protein from Leishmania infantum: A preliminary study. Acta Trop. 2023, 239, 106827. [Google Scholar] [CrossRef] [PubMed]
| Serum | Clinical Score | Parasite Number | DPP | LFT | ELISA |
|---|---|---|---|---|---|
| CanL-L 222 | 0 | 3.09 × 101 | − | − | − |
| CanL-L 286 | 1 | 5.97 × 101 | − | − | − |
| CanL-L 102 | 0 | 5.33 × 102 | − | − | − |
| CanL-L 284 | 0 | 9.10 × 102 | − | − | − |
| CanL-L 293 | 0 | 9.80 × 102 | − | − | − |
| CanL-L 103 | 0 | 8.17 × 103 | − | − | − |
| CanL-L 263 | 5 | 1.35 × 104 | − | − | − |
| CanL-L 101 | 0 | 1.64 × 104 | − | − | − |
| CanL-L 250 | 0 | 1.84 × 104 | − | − | − |
| CanL-L 229 | 2 | 4.83 × 105 | − | − | − |
| CanL-L 268 | 0 | 2.88 × 103 | + | + | + |
| CanL-L 238 | 0 | 6.11 × 103 | + | + | + |
| CanL-L 237 | 3 | 4.25 × 104 | + | + | + |
| CanL-L 116 | 0 | 5.62 × 104 | + | + | + |
| CanL-L 233 | 0 | 1.82 × 105 | + | + | + |
| CanL-L 108 | 0 | 2.97 × 105 | + | + | + |
| CanL-L 282 | 1 | 4.52 × 105 | + | + | + |
| CanL-L 246 | 2 | 1.14 × 106 | + | − | + |
| CanL-L 251 | 0 | 5.26 × 106 | + | + | + |
| CanL-H 220 | 6 | 1.58 × 101 | − | − | − |
| CanL-H 230 | 7 | 9.54 × 104 | − | − | − |
| CanL-H 100 | 6 | 1.46 × 103 | − | − | + |
| CanL-H 285 | 12 | 2.56 × 103 | + | + | + |
| CanL-H 224 | 6 | 6.97 × 103 | + | + | + |
| CanL-H 234 | 6 | 1.21 × 104 | + | − | − |
| CanL-H 283 | 7 | 5.26 × 104 | + | + | + |
| CanL-H 259 | 14 | 9.67 × 104 | + | + | + |
| CanL-H 107 | 6 | 1.77 × 106 | + | + | + |
| CanL-H 256 | 7 | 4.85 × 106 | + | + | + |
| CanL-H 253 | 11 | 7.17 × 106 | + | + | + |
| CanL-H 241 | 10 | 7.54 × 106 | + | + | + |
| CanL-H 231 | 6 | 7.84 × 106 | + | + | + |
| CanL-H 257 | 6 | 8.07 × 106 | + | + | + |
| CanL-H 248 | 7 | 1.66 × 107 | + | + | + |
| CanL-H 255 | 12 | 3.16 × 107 | + | + | + |
| CanL-H 243 | 8 | 2.58 × 108 | + | + | + |
| CanL-H 239 | 15 | 2.92 × 108 | + | + | + |
| CanL-H 242 | 6 | 4.95 × 108 | + | + | + |
| Serum Dilution | CanL-L 251 | CanL-H 285 | CanL-H 256 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DPP | LFT | ELISA | ELISA + α-Ig | DPP | LFT | ELISA | ELISA + α-Ig | DPP | LFT | ELISA | ELISA + α-Ig | |
| 1/1 | + | + | ND | ND | + | + | ND | ND | ND | ND | ND | ND |
| 1/4 | + | + | ND | ND | + | + | ND | ND | ND | ND | ND | ND |
| 1/16 | + | + | ND | ND | + | + | ND | ND | + | + | ND | ND |
| 1/64 | − | − | + | + | + | + | + | + | + | + | ND | ND |
| 1/256 | ND | ND | + | + | − | − | + | + | + | + | + | + |
| 1/1024 | ND | ND | − | + | ND | ND | + | + | + | + | + | + |
| 1/4096 | ND | ND | − | − | ND | ND | − | + | + | + | + | + |
| 1/16,384 | ND | ND | ND | ND | ND | ND | ND | ND | − | − | − | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teixeira, H.C.; Valle, G.P.C.; Mahdavi, R.; Dias, P.S.M.; de Oliveira, E.E.; Aira, C.P.; Heinz, D.; Latz, A.; Lana, M.d.; Morgado, F.N.; et al. Refinement of the rKLi8.3-Based Serodiagnostic ELISA Allows Detection of Canine Leishmaniosis in Dogs with Low Antibody Titers. Pathogens 2024, 13, 246. https://doi.org/10.3390/pathogens13030246
Teixeira HC, Valle GPC, Mahdavi R, Dias PSM, de Oliveira EE, Aira CP, Heinz D, Latz A, Lana Md, Morgado FN, et al. Refinement of the rKLi8.3-Based Serodiagnostic ELISA Allows Detection of Canine Leishmaniosis in Dogs with Low Antibody Titers. Pathogens. 2024; 13(3):246. https://doi.org/10.3390/pathogens13030246
Chicago/Turabian StyleTeixeira, Henrique C., Giulia P. C. Valle, Rouzbeh Mahdavi, Priscila S. M. Dias, Erick E. de Oliveira, Cristina P. Aira, Daniela Heinz, Andreas Latz, Marta de Lana, Fernanda N. Morgado, and et al. 2024. "Refinement of the rKLi8.3-Based Serodiagnostic ELISA Allows Detection of Canine Leishmaniosis in Dogs with Low Antibody Titers" Pathogens 13, no. 3: 246. https://doi.org/10.3390/pathogens13030246
APA StyleTeixeira, H. C., Valle, G. P. C., Mahdavi, R., Dias, P. S. M., de Oliveira, E. E., Aira, C. P., Heinz, D., Latz, A., Lana, M. d., Morgado, F. N., Porrozzi, R., & Steinhoff, U. (2024). Refinement of the rKLi8.3-Based Serodiagnostic ELISA Allows Detection of Canine Leishmaniosis in Dogs with Low Antibody Titers. Pathogens, 13(3), 246. https://doi.org/10.3390/pathogens13030246









