Porcine Monocyte DNA Traps Formed during Infection with Pathogenic Clostridioides difficile Strains
Abstract
1. Introduction
2. Materials and Methods
2.1. C. difficile Strains and Culture
2.2. Porcine Monocyte Isolation and Culture
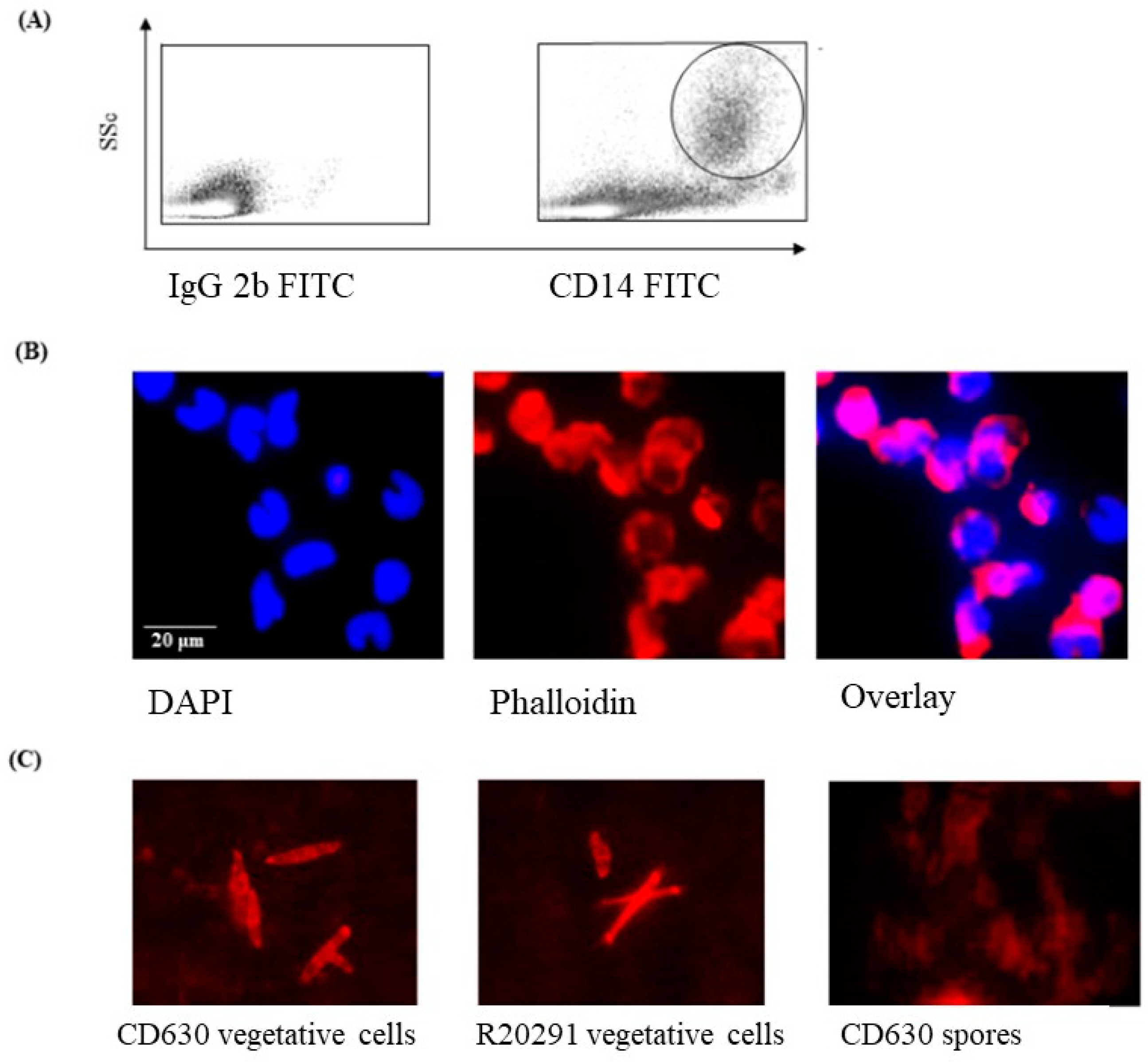
2.3. Fluorescence-Activated Cell Sorting (FACS)
2.4. Monocyte Infection Studies
2.5. Measurement of ROS in Monocytes
2.6. Measurement of RNS in Monocytes
2.7. Fluorescence Microscopy
2.8. Statistical Analysis
3. Results
3.1. Phenotype and Morphology of Uninfected (Steady-State) Porcine Monocytes
3.2. Staining of C. difficile Vegetative Cells
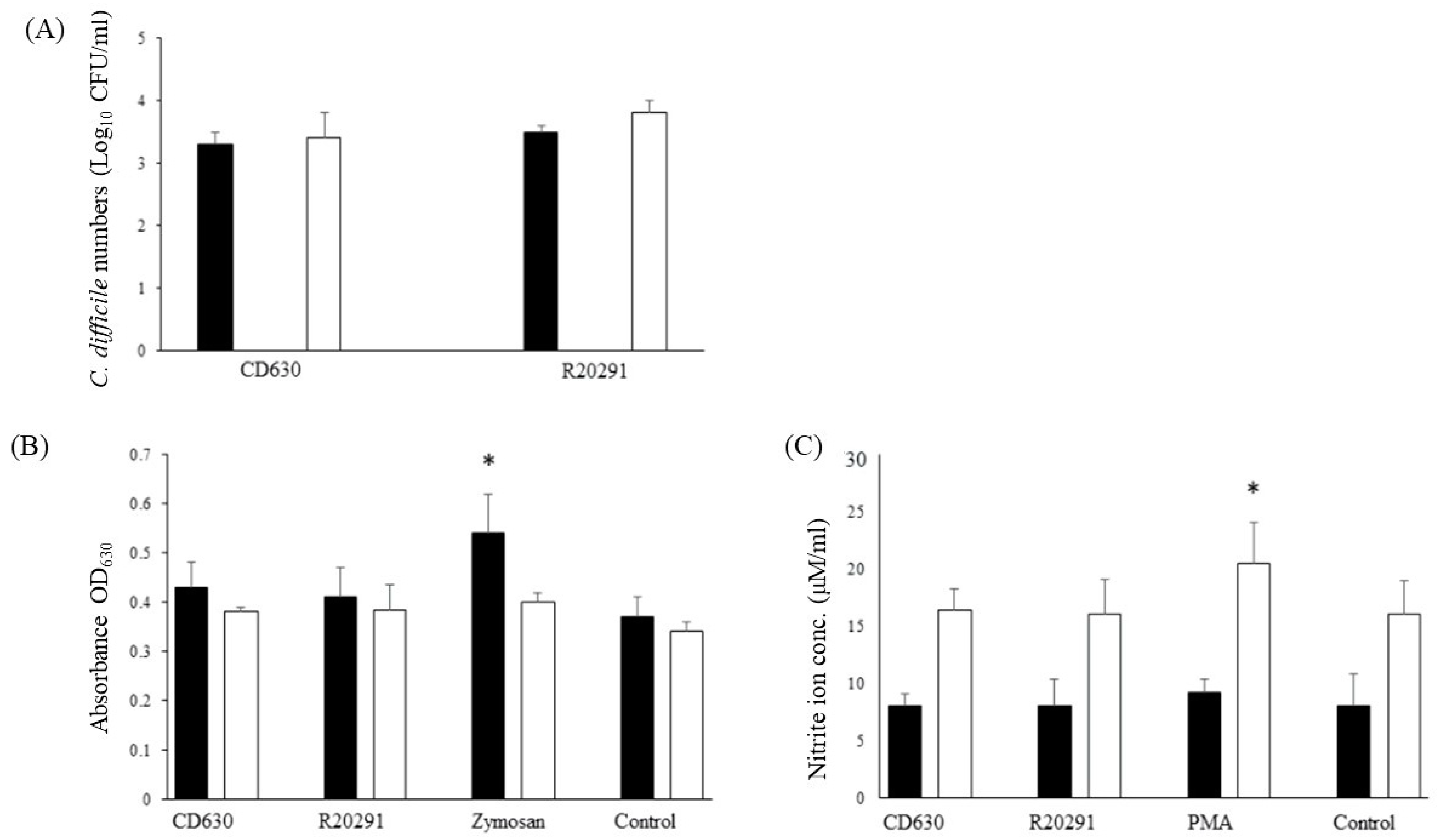
3.3. Survival Dynamics of C. difficile in Porcine Monocytes
3.4. Reactive Oxygen and Nitrogen Species Produced by Porcine Monocytes in Response to C. difficile Infection
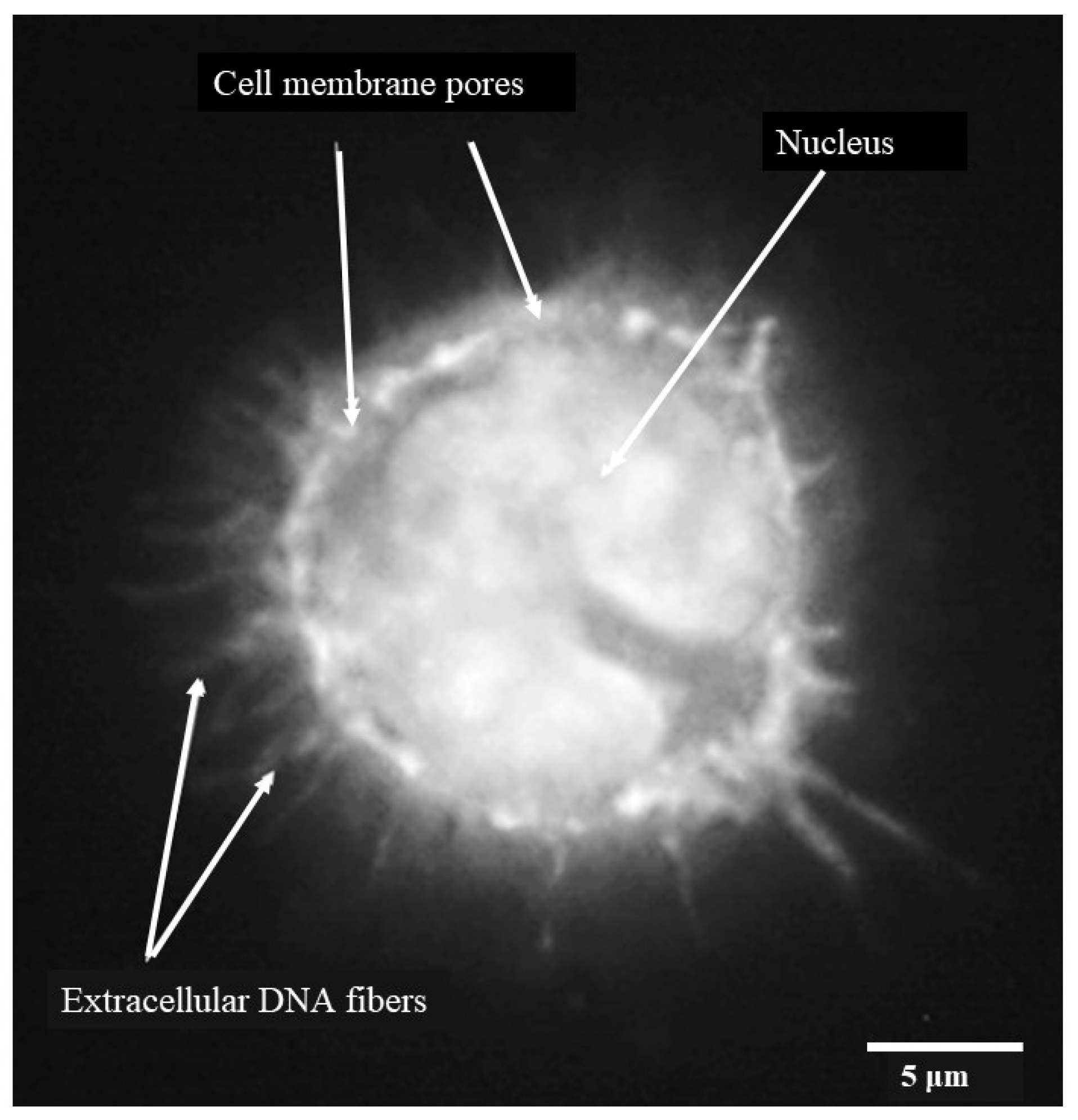
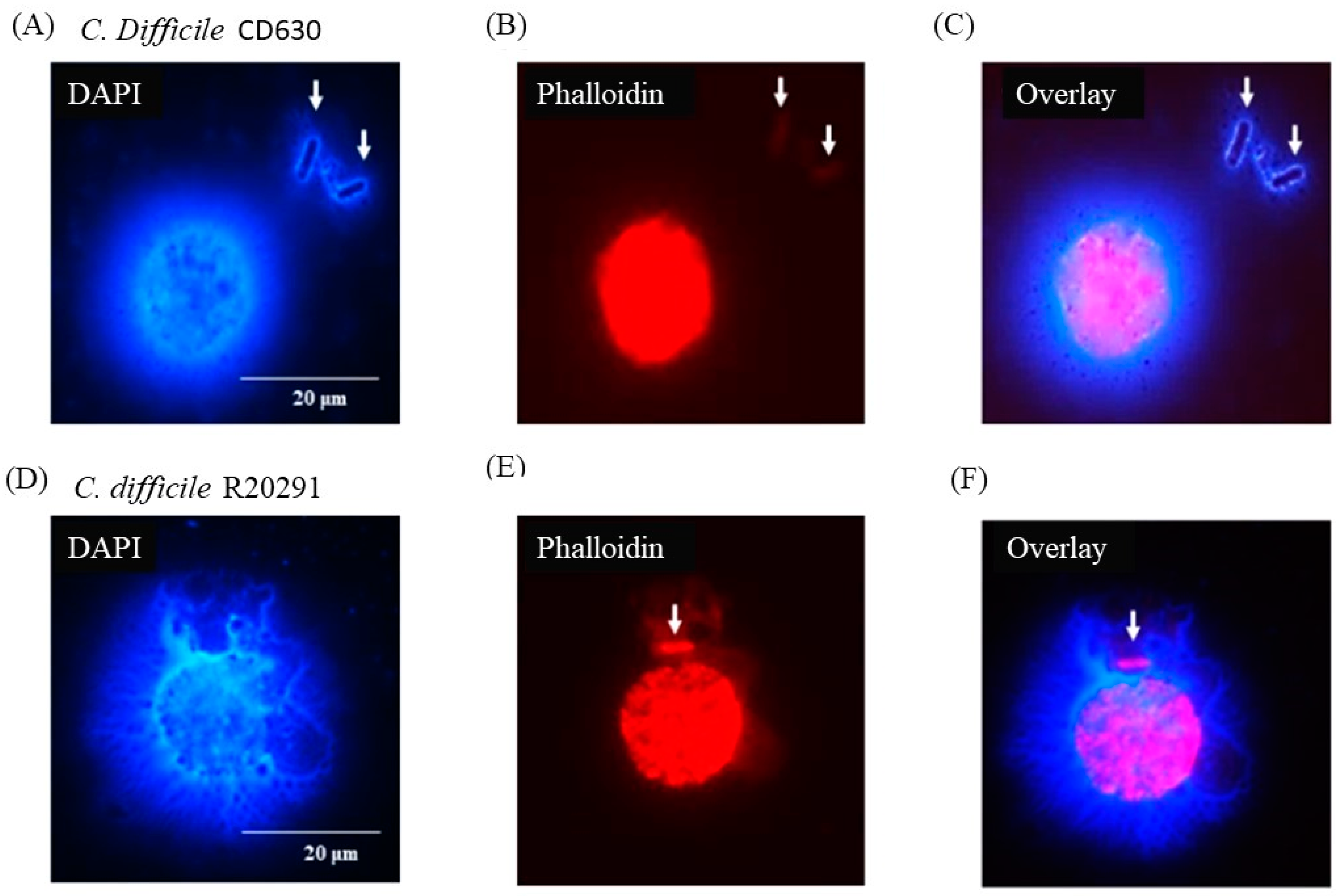
3.5. Porcine Monocyte form DNA Traps to Immobilize C. difficile
3.6. Following 24 h Culture of Porcine Monocytes with C. difficile, Complete Etosis Occurred but Extracellular DNA Was Still Able to Entrap CD630 or R20291
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Songer, J.G. The emergence of Clostridium difficile as a pathogen of food animals. Anim. Health Res. Rev. 2004, 5, 321–326. [Google Scholar] [CrossRef]
- Squire, M.M.; Riley, T.V. Clostridium difficile infection in humans and piglets: A ‘One Health’ opportunity. Curr. Top. Microbiol. Immunol. 2013, 365, 299–314. [Google Scholar]
- Moono, P.; Foster, N.F.; Hampson, D.J.; Knight, D.R.; Bloomfield, L.E.; Riley, T.V. Clostridium difficile Infection in Production Animals and Avian Species: A Review. Foodborne Pathog. Dis. 2016, 13, 647–655. [Google Scholar] [CrossRef]
- Kuijper, E.J.; Coignard, B.; Tüll, P. Emergence of Clostridium difficile-associated disease in North America and Europe. Clin. Microbiol. Infect. 2006, 12 (Suppl. 6), 2–18. [Google Scholar] [CrossRef]
- Keessen, E.C.; Harmanus, C.; Dohmen, W.; Kuijper, E.J.; Lipman, L.J. Clostridium difficile infection associated with pig farms. Emerg. Infect. Dis. 2013, 19, 1032–1034. [Google Scholar] [CrossRef]
- Steele, J.; Feng, H.; Parry, N.; Tzipori, S. Piglet Models for Acute or Chronic Clostridium difficile Illness (CDI). J. Infect. Dis. 2010, 201, 428–434. [Google Scholar] [CrossRef]
- Lyerly, D.M.; E Saum, K.; MacDonald, D.K.; Wilkins, T.D. Effects of Clostridium difficile toxins given intragastrically to animals. Infect. Immun. 1985, 47, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Keel, M.K.; Songer, J.G. The comparative pathology of Clostridium difficile-associated disease. Vet. Pathol. 2006, 43, 225–240. [Google Scholar] [CrossRef] [PubMed]
- Cowardin, C.A.; Petri, W.A., Jr. Host recognition of Clostridium difficile and the innate immune response. Anaerobe 2014, 30, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Songer, J.G.; Uzal, F.A. Clostridial enteric infections in pigs. J. Vet. Diagn. Investig. 2005, 17, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Waddell, A.; Ahrens, R.; Steinbrecher, K.; Donovan, B.; Rothenberg, M.E.; Munitz, A.; Hogan, S.P. Colonic eosinophilic inflammation in experimental colitis is mediated by Ly6C(high) CCR2(+) inflammatory monocyte/macrophage-derived CCL11. J. Immunol. 2011, 186, 5993–6003. [Google Scholar] [CrossRef]
- Chen, X.; Katchar, K.; Goldsmith, J.D.; Nanthakumar, N.; Cheknis, A.; Gerding, D.N.; Kelly, C.P. A mouse model of Clostridium difficile-associated disease. Gastroenterology 2008, 135, 1984–1992. [Google Scholar] [CrossRef]
- Sadighi Akha, A.A.; Theriot, C.M.; Erb-Downward, J.R.; McDermott, A.J.; Falkowski, N.R.; Tyra, H.M.; Rutkowski, D.T.; Young, V.B.; Huffnagle, G.B. Acute infection of mice with Clostridium difficile leads to eIF2α phosphorylation and pro-survival signalling as part of the mucosal inflammatory response. Immunology 2013, 140, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Jarchum, I.; Liu, M.; Shi, C.; Equinda, M.; Pamer, E.G. Critical role for MyD88-mediated neutrophil recruitment during Clostridium difficile colitis. Infect. Immun. 2012, 80, 2989–2996. [Google Scholar] [CrossRef] [PubMed]
- McDermott, A.J.; Falkowski, N.R.; McDonald, R.A.; Frank, C.R.; Pandit, C.R.; Young, V.B.; Huffnagle, G.B. Role of interferon-γ and inflammatory monocytes in driving colonic inflammation during acute Clostridium difficile infection in mice. Immunology 2017, 150, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Brito, G.A.C.; Sullivan, G.W.; Ciesla, J.W.P.; Carper, H.T.; Mandell, G.L.; Guerrant, R.L. Clostridium difficile Toxin A alters in vitro-adherent neutrophil morphology and function. J. Infect. Dis. 2002, 185, 1297–1306. [Google Scholar] [CrossRef]
- Goorhuis, A.; Bakker, D.; Corver, J.; Debast, S.B.; Harmanus, C.; Notermans, D.W.; Bergwerff, A.A.; Dekker, F.W.; Kuijper, E.J. Emergence of Clostridium difficile infection due to a new hypervirulent strain, polymerase chain reaction ribotype 078. Clin. Infect. Dis. 2008, 47, 1162–1170. [Google Scholar] [CrossRef]
- Dawson, L.F.; Donahue, E.H.; Cartman, S.T.; Barton, R.H.; Bundy, J.; McNerney, R.; Minton, N.P.; Wren, B.W. The analysis of para-cresol production and tolerance in Clostridium difficile 027 and 012 strains. BMC Microbiol. 2011, 11, 86. [Google Scholar] [CrossRef]
- Burns, D.A.; Minton, N.P. Sporulation studies in Clostridium difficile. J. Microbiol. Methods 2011, 87, 133–138. [Google Scholar] [CrossRef]
- Ibrahim, H.; Askar, B.; Hulme, S.; Neilson, P.; Barrow, P.; Foster, N. Differential Immune Phenotypes in Human Monocytes Induced by Non-Host-Adapted Salmonella enterica Serovar Choleraesuis and Host-Adapted S. Typhimurium. Infect. Immun. 2018, 86, e00509-18. [Google Scholar] [CrossRef]
- Park, B.H.; Fikrig, S.M.; Smithwick, E.M. Infection and nitroblue-tetrazolium reduction by neutrophils. A diagnostic acid. Lancet 1968, 2, 532–534. [Google Scholar] [CrossRef]
- Mutamba, S.; Allison, A.; Mahida, Y.; Barrow, P.; Foster, N. Expression of IL-1Rrp2 by human myelomonocytic cells is unique to DCs and facilitates DC maturation by IL-1F8 and IL-1F9. Eur. J. Immunol. 2012, 42, 607–617. [Google Scholar] [CrossRef]
- Penha, R.; Higgins, J.; Mutamba, S.; Barrow, P.; Mahida, Y.; Foster, N. IL-36 receptor is expressed by human blood and intestinal T lymphocytes and is dose-dependently activated via IL-36β and induces CD4+ lymphocyte proliferation. Cytokine 2016, 85, 18–25. [Google Scholar] [CrossRef]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef] [PubMed]
- Yipp, B.G.; Kubes, P. NETosis: How vital is it? Blood 2013, 122, 2784–2794. [Google Scholar] [CrossRef] [PubMed]
- Loures, F.V.; Röhm, M.; Lee, C.K.; Santos, E.; Wang, J.P.; Specht, C.A.; Calich, V.L.G.; Urban, C.F.; Levitz, S.M. Recognition of Aspergillus fumigatus hyphae by human plasmacytoid dendritic cells is mediated by dectin-2 and results in formation of extracellular traps. PLoS Pathog. 2015, 11, e1004643. [Google Scholar] [CrossRef] [PubMed]
- Boe, D.M.; Curtis, B.J.; Chen, M.M.; A Ippolito, J.; Kovacs, E.J. Extracellular traps and macrophages: New roles for the versatile phagocyte. J. Leukoc. Biol. 2015, 97, 1023–1035. [Google Scholar] [CrossRef]
- Steinberg, B.E.; Grinstein, S. Unconventional roles of the NADPH oxidase: Signaling, ion homeostasis, and cell death. Sci. STKE 2007, 379, pe11. [Google Scholar] [CrossRef]
- Staerck, C.; Gastebois, A.; Vandeputte, P.; Calenda, A.; Larcher, G.; Gillmann, L.; Papon, N.; Bouchara, J.-P.; Fleury, M.J. Microbial antioxidant defense enzymes. Microb. Pathog. 2017, 110, 56–65. [Google Scholar] [CrossRef]
- Dryden, M.; Cooke, J.; Salib, R.; Holding, R.; Pender, S.L.; Brooks, J. Hot topics in reactive oxygen therapy: Antimicrobial and immunological mechanisms, safety and clinical applications. J. Glob. Antimicrob. Resist. 2017, 8, 194–198. [Google Scholar] [CrossRef]
- Dryden, M. Reactive oxygen species: A novel antimicrobial. Int. J. Antimicrob. Agents 2018, 51, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Webster, S.J.; Daigneault, M.; Bewley, M.A.; Preston, J.A.; Marriott, H.M.; Walmsley, S.R.; Read, R.C.; Whyte, M.K.B.; Dockrell, D.H. Distinct cell death programs in monocytes regulate innate responses following challenge with common causes of invasive bacterial disease. J. Immunol. 2010, 185, 2968–2979. [Google Scholar] [CrossRef] [PubMed]
- Bassøe, C.F.; Smith, I.; Sørnes, S.; Halstensen, A.; Lehmann, A.K. Concurrent measurement of antigen- and antibody-dependent oxidative burst and phagocytosis in monocytes and neutrophils. Methods 2000, 21, 203–220. [Google Scholar] [CrossRef] [PubMed]
- Seuna, E.; Nurmi, E. Therapeutical trials with antimicrobial agengts and culture cecal microflora in Salmonella infantis infections in chickens. Poult. Sci. 1979, 58, 1171–1174. [Google Scholar] [CrossRef] [PubMed]
- Lo Vecchio, A.; Cohen, M.B. Fecal microbiota transplantation for Clostridium difficile infection: Benefits and barriers. Curr. Opin. Gastroenterol. 2014, 30, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Kociolek, L.K.; Gerding, D.N. Breakthroughs in the treatment and prevention of Clostridium difficile infection. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 150–160. [Google Scholar] [CrossRef]
- Walter, J.; Shanahan, F. Fecal microbiota-based treatment for recurrent Clostridioides difficile infection. Cell 2023, 186, 1087. [Google Scholar] [CrossRef]
- Berchieri, A.; Barrow, P.A. Further studies on the inhibition of colonization of the chicken alimentary tract with Salmonella typhimurium by pre-colonization with an avirulent mutant strain. Epidemiol. Infect. 1990, 104, 427–441. [Google Scholar] [CrossRef]
- Lovell, M.A.; Barrow, P.A. Intestinal colonisation of gnotobiotic pigs by Salmonella organisms; interaction between isogenic and unrelated strains. J. Med. Microbiol. 1999, 48, 907–916. [Google Scholar] [CrossRef]
- Foster, N.; Lovell, M.A.; Marston, K.L.; Hulme, S.D.; Frost, A.J.; Bland, P.; Barrow, P.A. Rapid protection of gnotobiotic pigs against experimental salmonellosis following induction of polymorphonuclear leucocytes (PMN) by avirulent Salmonella. Infect. Immun. 2003, 71, 2182–2191. [Google Scholar] [CrossRef]
- Foster, N.; Hulme, S.; Lovell, M.; Reed, K.; Barrow, P. Stimulation of gp91 phagocytic oxidase and reactive oxygen species in neutrophils by avirulent Salmonella protects gnotobiotic piglets from lethal challenge with Salmonella Typhimurium F98 without inducing intestinal pathology. Infect. Immun. 2005, 133, 959–978. [Google Scholar]
- Borriello, S.P.; Barclay, F.E. Protection of hamsters against Clostridium difficile ileocaecitis by prior colonisation with non-pathogenic strains. J. Med. Microbiol. 1985, 19, 339–350. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lawrence, J.; Barrow, P.; Foster, N. Porcine Monocyte DNA Traps Formed during Infection with Pathogenic Clostridioides difficile Strains. Pathogens 2024, 13, 228. https://doi.org/10.3390/pathogens13030228
Lawrence J, Barrow P, Foster N. Porcine Monocyte DNA Traps Formed during Infection with Pathogenic Clostridioides difficile Strains. Pathogens. 2024; 13(3):228. https://doi.org/10.3390/pathogens13030228
Chicago/Turabian StyleLawrence, Jade, Paul Barrow, and Neil Foster. 2024. "Porcine Monocyte DNA Traps Formed during Infection with Pathogenic Clostridioides difficile Strains" Pathogens 13, no. 3: 228. https://doi.org/10.3390/pathogens13030228
APA StyleLawrence, J., Barrow, P., & Foster, N. (2024). Porcine Monocyte DNA Traps Formed during Infection with Pathogenic Clostridioides difficile Strains. Pathogens, 13(3), 228. https://doi.org/10.3390/pathogens13030228





