HIV-Infected Hepatic Stellate Cells or HCV-Infected Hepatocytes Are Unable to Promote Latency Reversal among HIV-Infected Mononuclear Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Viral Stocks
2.3. Cellular Infection
2.4. Determination of HIV Latency Reversal
2.5. Statistical Analysis
3. Results
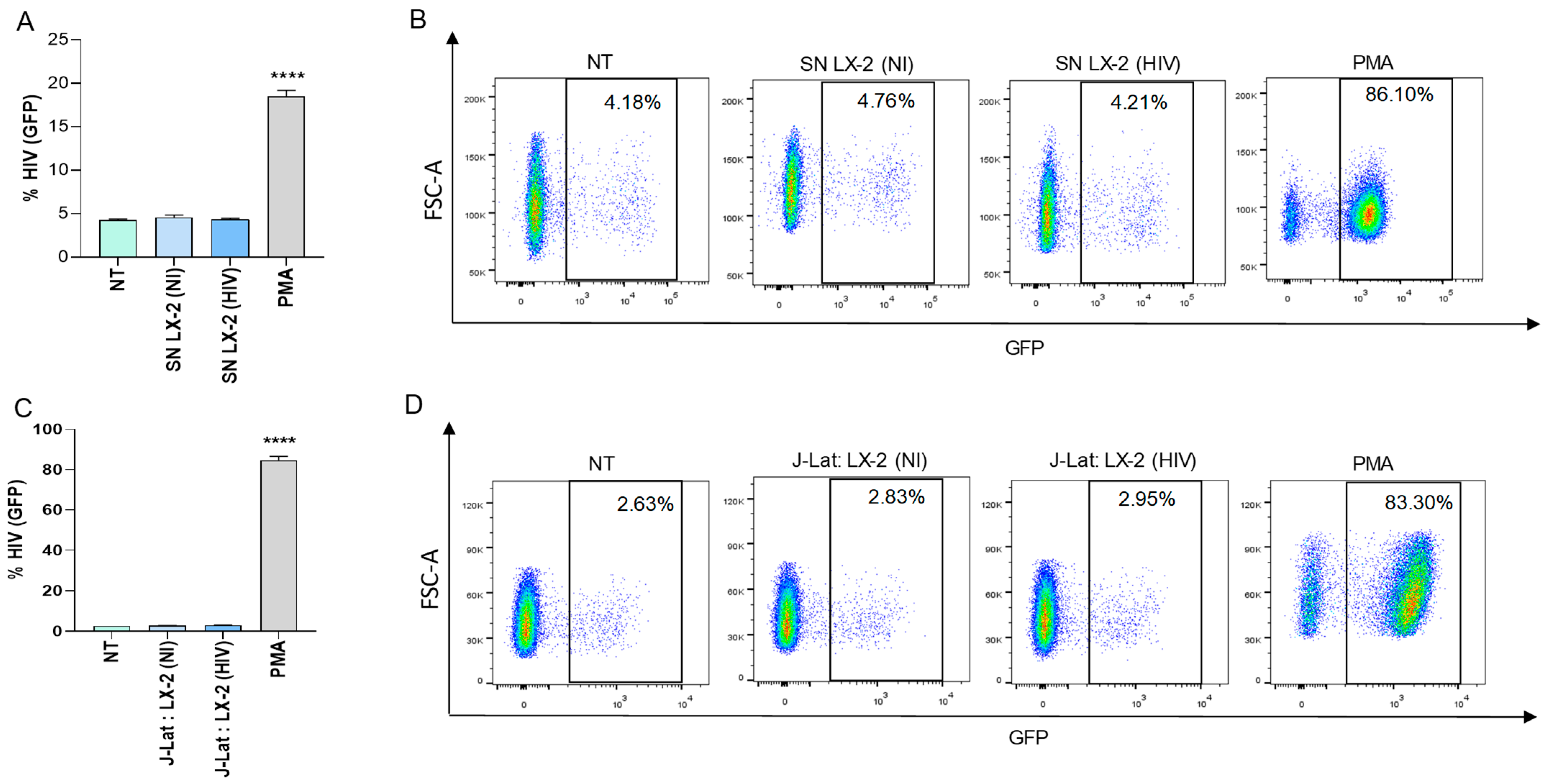
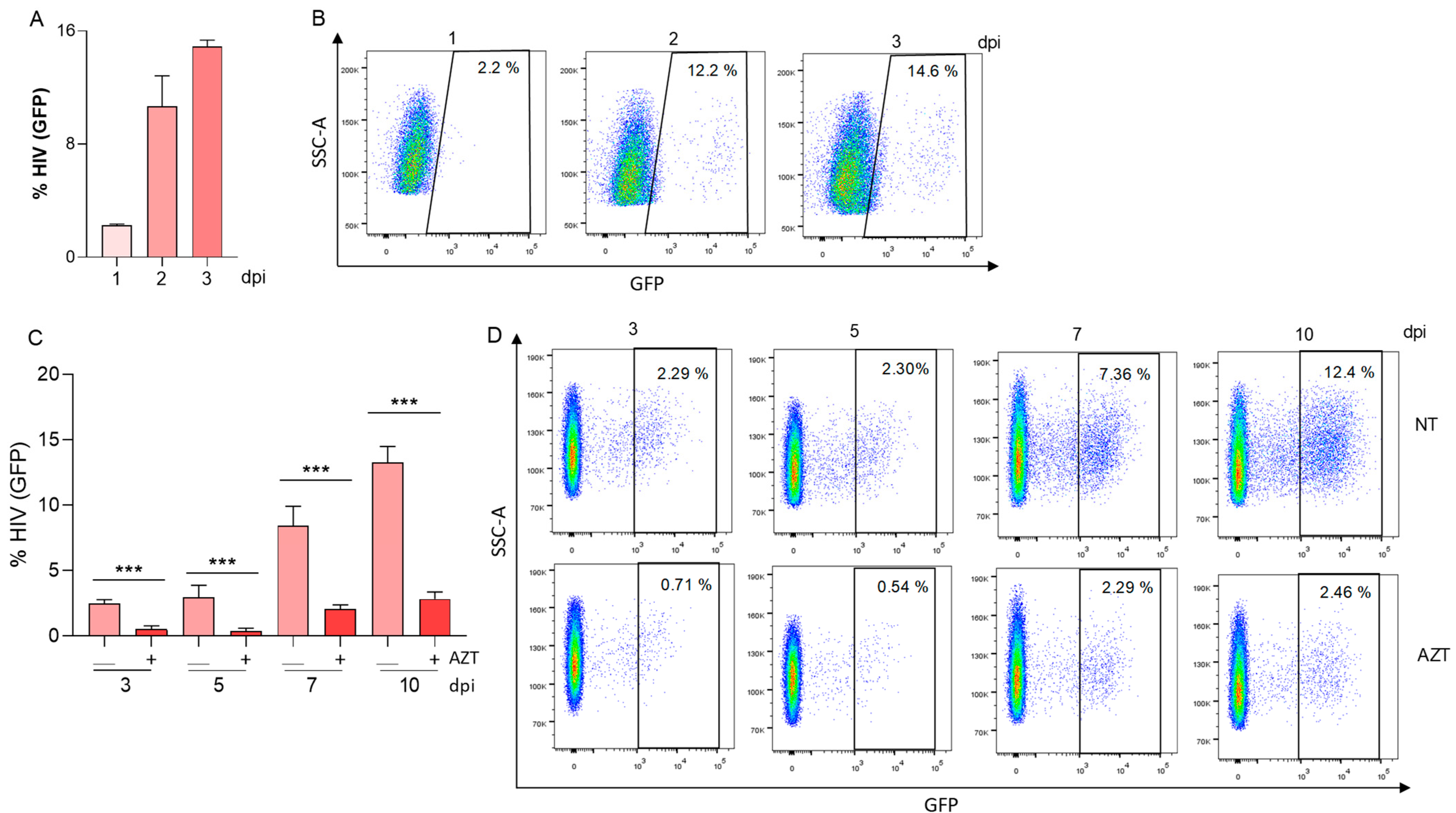
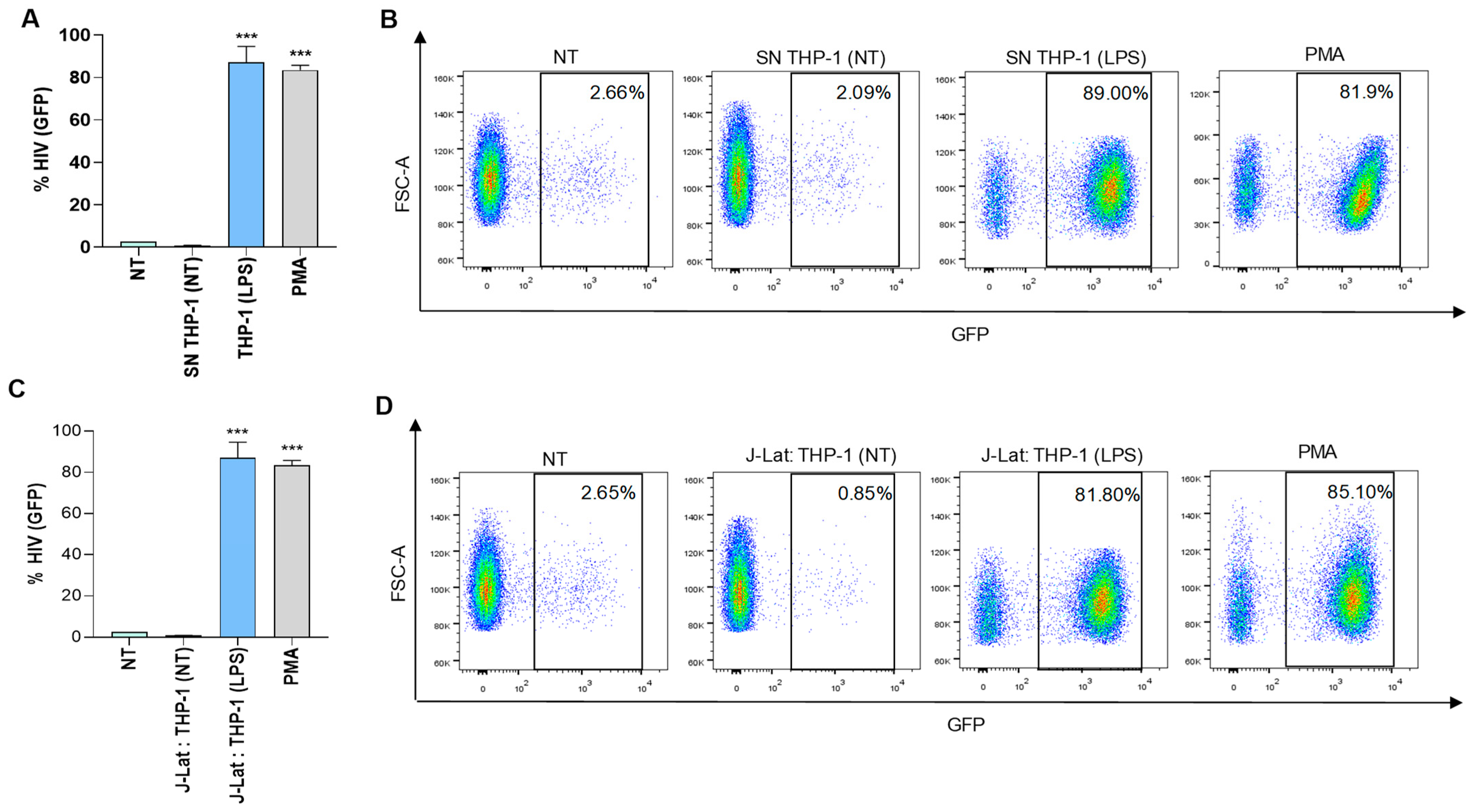
3.1. HIV-Infected Hepatic Stellate Cells (LX-2) Were Not Able to Reverse Viral Latency in J-Lat Cells
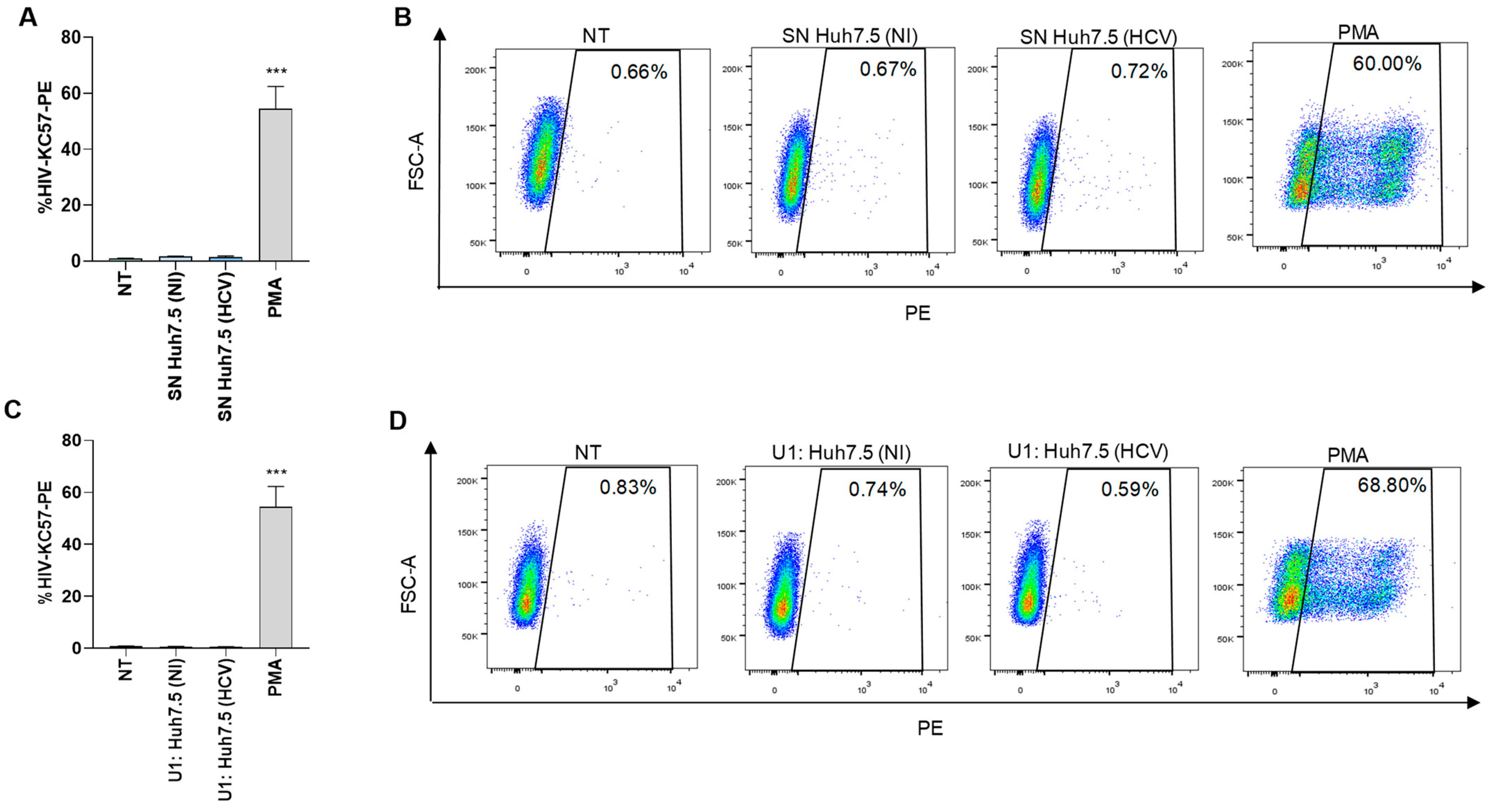
3.2. HCV-Infected Hepatocytes (Huh7.5 Cells) Were Not Able to Reverse Viral Latency in J-Lat Cells and U1 Cells
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- (WHO). W.H.O. Global HIV Programme. Available online: https://www.who.int/teams/global-hiv-hepatitis-and-stis-programmes/hiv/strategic-information/hiv-data-and-statistics (accessed on 29 July 2022).
- Back, D.; Marzolini, C. The challenge of HIV treatment in an era of polypharmacy. J. Int. AIDS Soc. 2020, 23, e25449. [Google Scholar] [CrossRef]
- Poorolajal, J.; Hooshmand, E.; Mahjub, H.; Esmailnasab, N.; Jenabi, E. Survival rate of AIDS disease and mortality in HIV-infected patients: A meta-analysis. Public Health 2016, 139, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Barnighausen, T.; Bloom, D.E.; Humair, S. Human Resources for Treating HIV/AIDS: Are the Preventive Effects of Antiretroviral Treatment a Game Changer? PLoS ONE 2016, 11, e0163960. [Google Scholar] [CrossRef] [PubMed]
- Barton, K.M.; Palmer, S.E. How to Define the Latent Reservoir: Tools of the Trade. Curr. HIV/AIDS Rep. 2016, 13, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Katlama, C.; Deeks, S.G.; Autran, B.; Martinez-Picado, J.; van Lunzen, J.; Rouzioux, C.; Miller, M.; Vella, S.; Schmitz, J.E.; Ahlers, J.; et al. Barriers to a cure for HIV: New ways to target and eradicate HIV-1 reservoirs. Lancet 2013, 381, 2109–2117. [Google Scholar] [CrossRef]
- Li, K.; Liu, B.; Ma, R.; Zhang, Q. HIV Tissue Reservoirs: Current Advances in Research. AIDS Patient Care STDs 2023, 37, 284–296. [Google Scholar] [CrossRef]
- Vanhamel, J.; Bruggemans, A.; Debyser, Z. Establishment of latent HIV-1 reservoirs: What do we really know? J. Virus Erad. 2019, 5, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Dufour, C.; Gantner, P.; Fromentin, R.; Chomont, N. The multifaceted nature of HIV latency. J. Clin. Investig. 2020, 130, 3381–3390. [Google Scholar] [CrossRef]
- Wolf, G.; Singh, N.J. Modular Approaches to Understand the Immunobiology of Human Immunodeficiency Virus Latency. Viral Immunol. 2021, 34, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Platt, L.; Easterbrook, P.; Gower, E.; McDonald, B.; Sabin, K.; McGowan, C.; Yanny, I.; Razavi, H.; Vickerman, P. Prevalence and burden of HCV co-infection in people living with HIV: A global systematic review and meta-analysis. Lancet. Infect. Dis. 2016, 16, 797–808. [Google Scholar] [CrossRef]
- Laskus, T.; Radkowski, M.; Piasek, A.; Nowicki, M.; Horban, A.; Cianciara, J.; Rakela, J. Hepatitis C virus in lymphoid cells of patients coinfected with human immunodeficiency virus type 1: Evidence of active replication in monocytes/macrophages and lymphocytes. J. Infect. Dis. 2000, 181, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Blackard, J.T.; Smeaton, L.; Hiasa, Y.; Horiike, N.; Onji, M.; Jamieson, D.J.; Rodriguez, I.; Mayer, K.H.; Chung, R.T. Detection of hepatitis C virus (HCV) in serum and peripheral-blood mononuclear cells from HCV-monoinfected and HIV/HCV-coinfected persons. J. Infect. Dis. 2005, 192, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Dobseu, R.; Nanfack, A.; Kowo, M.; Ambada, G.; Kamgaing, R.; Chenwi, C.; Fainguem, N.; Ka’e, A.; Ngangoum, E.; Sosso, S.; et al. Evaluation of hepatic fibrosis in HIV/HCV co-infected individuals in Yaounde, Cameroon: Usefulness of APRI score in resource-constrained settings. BMC Infect. Dis. 2020, 20, 758. [Google Scholar] [CrossRef] [PubMed]
- Joshi, D.; O’Grady, J.; Dieterich, D.; Gazzard, B.; Agarwal, K. Increasing burden of liver disease in patients with HIV infection. Lancet 2011, 377, 1198–1209. [Google Scholar] [CrossRef]
- Pembroke, T.; Deschenes, M.; Lebouche, B.; Benmassaoud, A.; Sewitch, M.; Ghali, P.; Wong, P.; Halme, A.; Vuille-Lessard, E.; Pexos, C.; et al. Hepatic steatosis progresses faster in HIV mono-infected than HIV/HCV co-infected patients and is associated with liver fibrosis. J. Hepatol. 2017, 67, 801–808. [Google Scholar] [CrossRef]
- Darcis, G.; Bouchat, S.; Kula, A.; Van Driessche, B.; Delacourt, N.; Vanhulle, C.; Avettand-Fenoel, V.; De Wit, S.; Rohr, O.; Rouzioux, C.; et al. Reactivation capacity by latency-reversing agents ex vivo correlates with the size of the HIV-1 reservoir. Aids 2017, 31, 181–189. [Google Scholar] [CrossRef]
- Nabel, G.; Baltimore, D. An inducible transcription factor activates expression of human immunodeficiency virus in T cells. Nature 1987, 326, 711–713. [Google Scholar] [CrossRef] [PubMed]
- Stroud, J.C.; Oltman, A.; Han, A.; Bates, D.L.; Chen, L. Structural basis of HIV-1 activation by NF-kappaB—A higher-order complex of p50:RelA bound to the HIV-1 LTR. J. Mol. Biol. 2009, 393, 98–112. [Google Scholar] [CrossRef]
- Kushner, L.E.; Wendelboe, A.M.; Lazzeroni, L.C.; Chary, A.; Winters, M.A.; Osinusi, A.; Kottilil, S.; Polis, M.A.; Holodniy, M. Immune biomarker differences and changes comparing HCV mono-infected, HIV/HCV co-infected, and HCV spontaneously cleared patients. PLoS ONE 2013, 8, e60387. [Google Scholar] [CrossRef] [PubMed]
- Marquez, M.; Romero-Cores, P.; Montes-Oca, M.; Martin-Aspas, A.; Soto-Cardenas, M.J.; Guerrero, F.; Fernandez-Gutierrez, C.; Giron-Gonzalez, J.A. Immune activation response in chronic HIV-infected patients: Influence of Hepatitis C virus coinfection. PLoS ONE 2015, 10, e0119568. [Google Scholar] [CrossRef]
- Lopez-Huertas, M.R.; Palladino, C.; Garrido-Arquero, M.; Esteban-Cartelle, B.; Sanchez-Carrillo, M.; Martinez-Roman, P.; Martin-Carbonero, L.; Ryan, P.; Dominguez-Dominguez, L.; Santos, I.L.; et al. HCV-coinfection is related to an increased HIV-1 reservoir size in cART-treated HIV patients: A cross-sectional study. Sci. Rep. 2019, 9, 5606. [Google Scholar] [CrossRef]
- Ganesan, M.; Poluektova, L.Y.; Kharbanda, K.K.; Osna, N.A. Liver as a target of human immunodeficiency virus infection. World J. Gastroenterol. 2018, 24, 4728–4737. [Google Scholar] [CrossRef]
- Chaillon, A.; Gianella, S.; Dellicour, S.; Rawlings, S.A.; Schlub, T.E.; De Oliveira, M.F.; Ignacio, C.; Porrachia, M.; Vrancken, B.; Smith, D.M. HIV persists throughout deep tissues with repopulation from multiple anatomical sources. J. Clin. Investig. 2020, 130, 1699–1712. [Google Scholar] [CrossRef] [PubMed]
- Jordan, A.; Bisgrove, D.; Verdin, E. HIV reproducibly establishes a latent infection after acute infection of T cells in vitro. EMBO J. 2003, 22, 1868–1877. [Google Scholar] [CrossRef] [PubMed]
- Bieniasz, P.D.; Cullen, B.R. Multiple blocks to human immunodeficiency virus type 1 replication in rodent cells. J. Virol. 2000, 74, 9868–9877. [Google Scholar] [CrossRef]
- Folks, T.M.; Justement, J.; Kinter, A.; Dinarello, C.A.; Fauci, A.S. Cytokine-induced expression of HIV-1 in a chronically infected promonocyte cell line. Science 1987, 238, 800–802. [Google Scholar] [CrossRef]
- Adachi, A.; Gendelman, H.E.; Koenig, S.; Folks, T.; Willey, R.; Rabson, A.; Martin, M.A. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J. Virol. 1986, 59, 284–291. [Google Scholar] [CrossRef]
- Freed, E.O.; Englund, G.; Martin, M.A. Role of the basic domain of human immunodeficiency virus type 1 matrix in macrophage infection. J. Virol. 1995, 69, 3949–3954. [Google Scholar] [CrossRef] [PubMed]
- Mateu, G.; Donis, R.O.; Wakita, T.; Bukh, J.; Grakoui, A. Intragenotypic JFH1 based recombinant hepatitis C virus produces high levels of infectious particles but causes increased cell death. Virology 2008, 376, 397–407. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hokello, J.; Sharma, A.L.; Dimri, M.; Tyagi, M. Insights into the HIV Latency and the Role of Cytokines. Pathogens 2019, 8, 137. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, N.E.; Lane, B.R.; Bock, P.J.; Markovitz, D.M. Protein phosphatase 2A enhances activation of human immunodeficiency virus type 1 by phorbol myristate acetate. J. Virol. 2003, 77, 2276–2281. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Poli, G.; Bressler, P.; Kinter, A.; Duh, E.; Timmer, W.C.; Rabson, A.; Justement, J.S.; Stanley, S.; Fauci, A.S. Interleukin 6 induces human immunodeficiency virus expression in infected monocytic cells alone and in synergy with tumor necrosis factor alpha by transcriptional and post-transcriptional mechanisms. J. Exp. Med. 1990, 172, 151–158. [Google Scholar] [CrossRef]
- Kruize, Z.; Kootstra, N.A. The Role of Macrophages in HIV-1 Persistence and Pathogenesis. Front. Microbiol. 2019, 10, 2828. [Google Scholar] [CrossRef]
- Mastroianni, C.M.; Lichtner, M.; Mascia, C.; Zuccala, P.; Vullo, V. Molecular mechanisms of liver fibrosis in HIV/HCV coinfection. Int. J. Mol. Sci. 2014, 15, 9184–9208. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Wu, G.; Li, S.; Weinberg, E.M.; Kumthip, K.; Peng, L.F.; Mendez-Navarro, J.; Chen, W.C.; Jilg, N.; Zhao, H.; et al. HIV and HCV cooperatively promote hepatic fibrogenesis via induction of reactive oxygen species and NFkappaB. J. Biol. Chem. 2011, 286, 2665–2674. [Google Scholar] [CrossRef]
- Semmo, N.; Klenerman, P. CD4+ T cell responses in hepatitis C virus infection. World J. Gastroenterol. 2007, 13, 4831–4838. [Google Scholar] [CrossRef]
- Gao, B.; Jeong, W.I.; Tian, Z. Liver: An organ with predominant innate immunity. Hepatology 2008, 47, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Szabo, G.; Mandrekar, P.; Dolganiuc, A. Innate immune response and hepatic inflammation. Semin. Liver Dis. 2007, 27, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Presser, L.D.; Haskett, A.; Waris, G. Hepatitis C virus-induced furin and thrombospondin-1 activate TGF-beta1: Role of TGF-beta1 in HCV replication. Virology 2011, 412, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, H.; Kato, N.; Otsuka, M.; Goto, T.; Yoshida, H.; Shiratori, Y.; Omata, M. Hepatitis C virus core protein upregulates transforming growth factor-beta 1 transcription. J. Med. Virol. 2004, 72, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Sandler, N.G.; Koh, C.; Roque, A.; Eccleston, J.L.; Siegel, R.B.; Demino, M.; Kleiner, D.E.; Deeks, S.G.; Liang, T.J.; Heller, T.; et al. Host response to translocated microbial products predicts outcomes of patients with HBV or HCV infection. Gastroenterology 2011, 141, 1220–1230, 1230 e1221–1223. [Google Scholar] [CrossRef] [PubMed]
- Gobran, S.T.; Ancuta, P.; Shoukry, N.H. A Tale of Two Viruses: Immunological Insights Into HCV/HIV Coinfection. Front. Immunol. 2021, 12, 726419. [Google Scholar] [CrossRef]
- Margolis, D.M.; Archin, N.M.; Cohen, M.S.; Eron, J.J.; Ferrari, G.; Garcia, J.V.; Gay, C.L.; Goonetilleke, N.; Joseph, S.B.; Swanstrom, R.; et al. Curing HIV: Seeking to Target and Clear Persistent Infection. Cell 2020, 181, 189–206. [Google Scholar] [CrossRef]
- Wen, Y.; Lambrecht, J.; Ju, C.; Tacke, F. Hepatic macrophages in liver homeostasis and diseases-diversity, plasticity and therapeutic opportunities. Cell Mol. Immunol. 2021, 18, 45–56. [Google Scholar] [CrossRef]
- Ficht, X.; Iannacone, M. Immune surveillance of the liver by T cells. Sci. Immunol. 2020, 5, eaba2351. [Google Scholar] [CrossRef]
- Friedman, S.L. Mechanisms of hepatic fibrogenesis. Gastroenterology 2008, 134, 1655–1669. [Google Scholar] [CrossRef]
- Chen, W.; Xu, Y.; Li, H.; Tao, W.; Xiang, Y.; Huang, B.; Niu, J.; Zhong, J.; Meng, G. HCV genomic RNA activates the NLRP3 inflammasome in human myeloid cells. PLoS ONE 2014, 9, e84953. [Google Scholar] [CrossRef] [PubMed]
- Salloum, S.; Holmes, J.A.; Jindal, R.; Bale, S.S.; Brisac, C.; Alatrakchi, N.; Lidofsky, A.; Kruger, A.J.; Fusco, D.N.; Luther, J.; et al. Exposure to human immunodeficiency virus/hepatitis C virus in hepatic and stellate cell lines reveals cooperative profibrotic transcriptional activation between viruses and cell types. Hepatology 2016, 64, 1951–1968. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, S.D.; Lu, H.K.; Chang, J.J.; Rhodes, A.; Lewin, S.R.; Cameron, P.U. The Pathway To Establishing HIV Latency Is Critical to How Latency Is Maintained and Reversed. J. Virol. 2018, 92, 10–1128. [Google Scholar] [CrossRef]
- Kandathil, A.J.; Sugawara, S.; Goyal, A.; Durand, C.M.; Quinn, J.; Sachithanandham, J.; Cameron, A.M.; Bailey, J.R.; Perelson, A.S.; Balagopal, A. No recovery of replication-competent HIV-1 from human liver macrophages. J. Clin. Investig. 2018, 128, 4501–4509. [Google Scholar] [CrossRef] [PubMed]
- Crispe, I.N. Immune tolerance in liver disease. Hepatology 2014, 60, 2109–2117. [Google Scholar] [CrossRef]
- Cao, Y.Z.; Dieterich, D.; Thomas, P.A.; Huang, Y.X.; Mirabile, M.; Ho, D.D. Identification and quantitation of HIV-1 in the liver of patients with AIDS. Aids 1992, 6, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Veenhuis, R.T.; Abreu, C.M.; Costa, P.A.G.; Ferreira, E.A.; Ratliff, J.; Pohlenz, L.; Shirk, E.N.; Rubin, L.H.; Blankson, J.N.; Gama, L.; et al. Monocyte-derived macrophages contain persistent latent HIV reservoirs. Nat. Microbiol. 2023, 8, 833–844. [Google Scholar] [CrossRef] [PubMed]
- Fulcher, J.A.; Hwangbo, Y.; Zioni, R.; Nickle, D.; Lin, X.; Heath, L.; Mullins, J.I.; Corey, L.; Zhu, T. Compartmentalization of human immunodeficiency virus type 1 between blood monocytes and CD4+ T cells during infection. J. Virol. 2004, 78, 7883–7893. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Sharron, M.; Montaner, L.J.; Weissman, D.; Doms, R.W. Quantification of CD4, CCR5, and CXCR4 levels on lymphocyte subsets, dendritic cells, and differentially conditioned monocyte-derived macrophages. Proc. Natl. Acad. Sci. USA 1999, 96, 5215–5220. [Google Scholar] [CrossRef]
- Koppensteiner, H.; Brack-Werner, R.; Schindler, M. Macrophages and their relevance in Human Immunodeficiency Virus Type I infection. Retrovirology 2012, 9, 82. [Google Scholar] [CrossRef] [PubMed]
- Sattentau, Q.J.; Stevenson, M. Macrophages and HIV-1: An Unhealthy Constellation. Cell Host Microbe 2016, 19, 304–310. [Google Scholar] [CrossRef]
- Crowe, S.; Zhu, T.; Muller, W.A. The contribution of monocyte infection and trafficking to viral persistence, and maintenance of the viral reservoir in HIV infection. J. Leukoc. Biol. 2003, 74, 635–641. [Google Scholar] [CrossRef]
- Chandra, P.K.; Gerlach, S.L.; Wu, C.; Khurana, N.; Swientoniewski, L.T.; Abdel-Mageed, A.B.; Li, J.; Braun, S.E.; Mondal, D. Mesenchymal stem cells are attracted to latent HIV-1-infected cells and enable virus reactivation via a non-canonical PI3K-NFkappaB signaling pathway. Sci. Rep. 2018, 8, 14702. [Google Scholar] [CrossRef]
- Pallikkuth, S.; Mohan, M. Adipose Tissue: Sanctuary for HIV/SIV Persistence and Replication. Trends Microbiol. 2015, 23, 748–750. [Google Scholar] [CrossRef]
- Pallikkuth, S.; Sharkey, M.; Babic, D.Z.; Gupta, S.; Stone, G.W.; Fischl, M.A.; Stevenson, M.; Pahwa, S. Peripheral T Follicular Helper Cells Are the Major HIV Reservoir within Central Memory CD4 T Cells in Peripheral Blood from Chronically HIV-Infected Individuals on Combination Antiretroviral Therapy. J. Virol. 2015, 90, 2718–2728. [Google Scholar] [CrossRef] [PubMed]
- Fuller, K.; Owens, J.M.; Jagger, C.J.; Wilson, A.; Moss, R.; Chambers, T.J. Macrophage colony-stimulating factor stimulates survival and chemotactic behavior in isolated osteoclasts. J. Exp. Med. 1993, 178, 1733–1744. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, T.A.; Lewin, S.R. Shocking HIV out of hiding: Where are we with clinical trials of latency reversing agents? Curr. Opin. HIV AIDS 2016, 11, 394–401. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López, C.A.M.; Freiberger, R.N.; Sviercz, F.A.; Quarleri, J.; Delpino, M.V. HIV-Infected Hepatic Stellate Cells or HCV-Infected Hepatocytes Are Unable to Promote Latency Reversal among HIV-Infected Mononuclear Cells. Pathogens 2024, 13, 134. https://doi.org/10.3390/pathogens13020134
López CAM, Freiberger RN, Sviercz FA, Quarleri J, Delpino MV. HIV-Infected Hepatic Stellate Cells or HCV-Infected Hepatocytes Are Unable to Promote Latency Reversal among HIV-Infected Mononuclear Cells. Pathogens. 2024; 13(2):134. https://doi.org/10.3390/pathogens13020134
Chicago/Turabian StyleLópez, Cinthya Alicia Marcela, Rosa Nicole Freiberger, Franco Agustín Sviercz, Jorge Quarleri, and María Victoria Delpino. 2024. "HIV-Infected Hepatic Stellate Cells or HCV-Infected Hepatocytes Are Unable to Promote Latency Reversal among HIV-Infected Mononuclear Cells" Pathogens 13, no. 2: 134. https://doi.org/10.3390/pathogens13020134
APA StyleLópez, C. A. M., Freiberger, R. N., Sviercz, F. A., Quarleri, J., & Delpino, M. V. (2024). HIV-Infected Hepatic Stellate Cells or HCV-Infected Hepatocytes Are Unable to Promote Latency Reversal among HIV-Infected Mononuclear Cells. Pathogens, 13(2), 134. https://doi.org/10.3390/pathogens13020134






