Genetic and Biochemical Characterization of AXC-2 from Achromobacter ruhlandii
Abstract
1. Introduction
2. Materials and Methods
2.1. Genetic and Biochemical Characterization of AXC-2 from Ar38
2.1.1. Whole-Genome Sequencing of A. ruhlandii Ar38
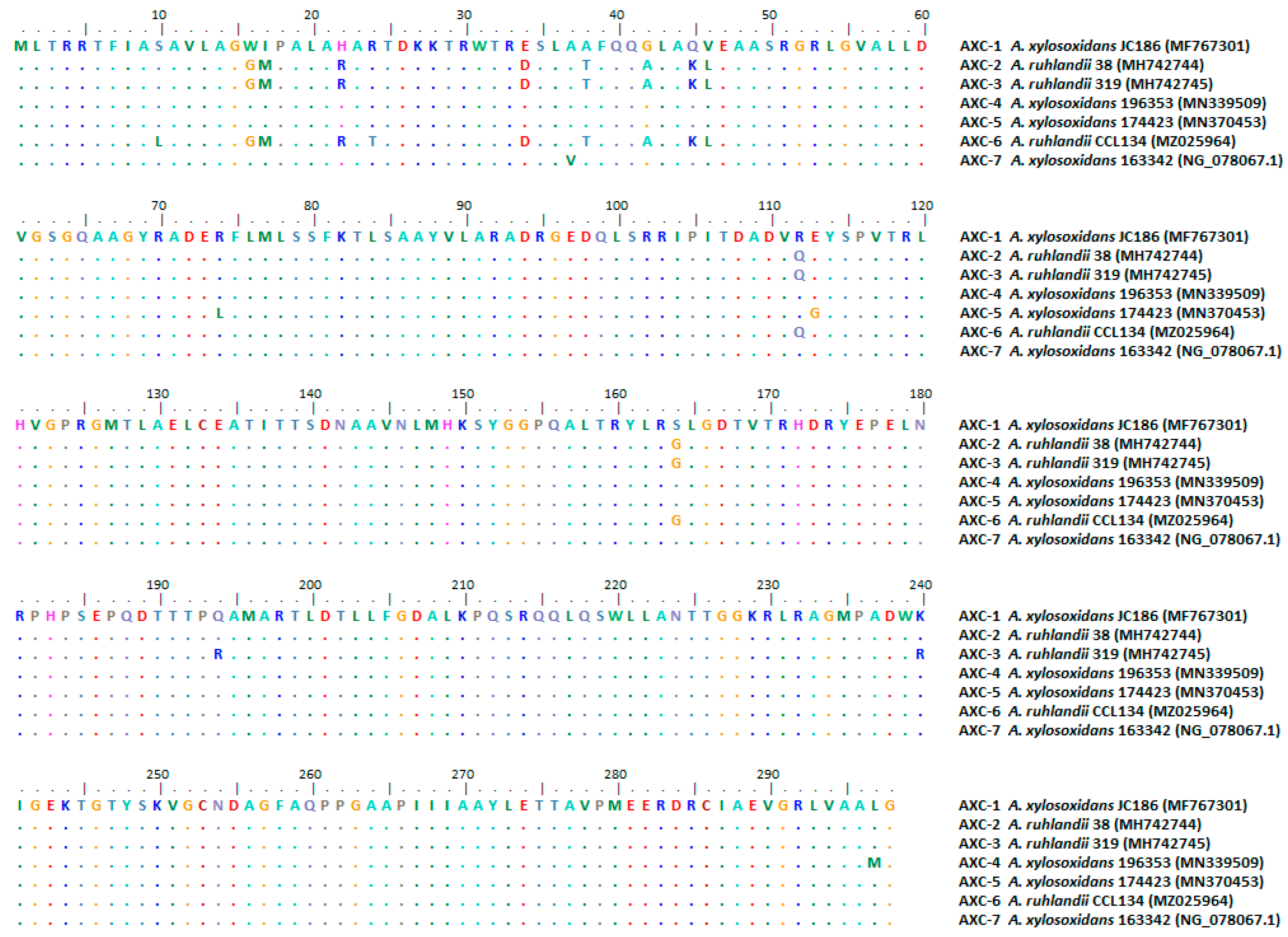
2.1.2. Comparative Analysis of AXC Sequences Deposited in the NCBI Database
2.1.3. β-Lactamase Production and Purification
2.1.4. Kinetic Studies
2.2. In Silico Analysis of AXC Variants from Achromobacter spp.
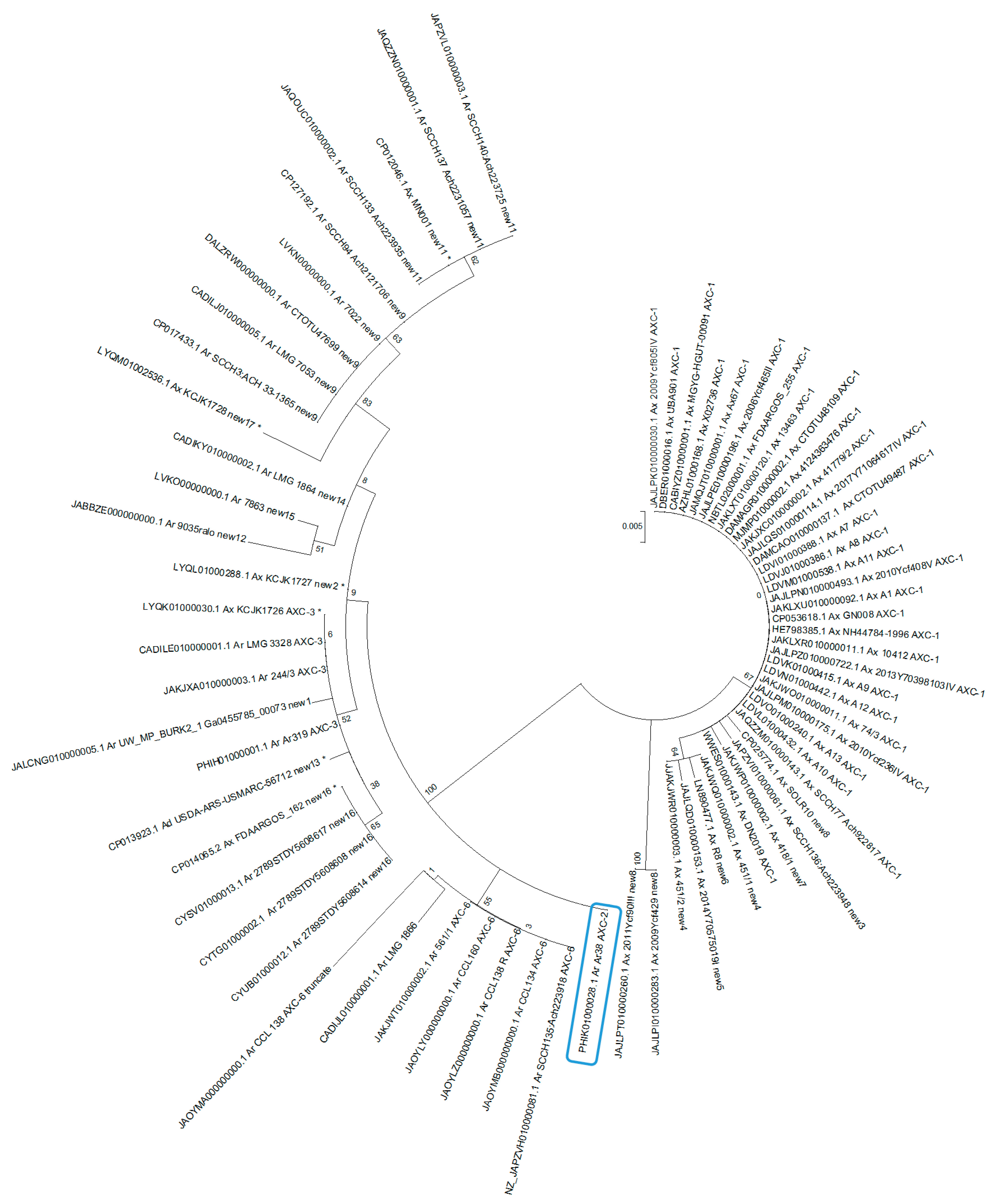
2.2.1. Phylogenetic Analysis of AXC Variants from Achromobacter Genomes Available in the Genome NCBI Database
2.2.2. Analysis of the Distribution of AXC in A. xylosoxidans Genomes
2.2.3. Analysis of the Genetic Context of blaAXC in A. xylosoxindans and A. ruhlandii Genomes
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Parte, A.C.; Sarda Carbasse, J.; Meier-Kolthoff, J.P.; Reimer, L.C.; Goker, M. List of Prokaryotic names with Standing in Nomenclature (LPSN) moves to the DSMZ. Int. J. Syst. Evol. Microbiol. 2020, 70, 5607–5612. [Google Scholar] [CrossRef]
- Yabuuchi, E.; Yano, I. Achromobacter gen. nov. and Achromobacter xylosoxidans (ex Yabuuchi and Ohyama 1971) nom. rev. Int. J. Syst. Bacteriol. 1981, 31, 477–478. [Google Scholar] [CrossRef]
- Yabuuchi, E.; Kawamura, Y.; Kosako, Y.; Ezaki, T. Emendation of genus Achromobacter and Achromobacter xylosoxidans (Yabuuchi and Yano) and proposal of Achromobacter ruhlandii (Packer and Vishniac) comb. nov., Achromobacter piechaudii (Kiredjian et al.) comb. nov., and Achromobacter xylosoxidans subsp. denitrificans (Ruger and Tan) comb. nov. Microbiol. Immunol. 1998, 42, 429–438. [Google Scholar] [CrossRef]
- Gomila, M.; Tvrzova, L.; Teshim, A.; Sedlacek, I.; Gonzalez-Escalona, N.; Zdrahal, Z.; Sedo, O.; Gonzalez, J.F.; Bennasar, A.; Moore, E.R.; et al. Achromobacter marplatensis sp. nov., isolated from a pentachlorophenol-contaminated soil. Int. J. Syst. Evol. Microbiol. 2011, 61, 2231–2237. [Google Scholar] [CrossRef]
- Dumolin, C.; Peeters, C.; Ehsani, E.; Tahon, G.; De Canck, E.; Cnockaert, M.; Boon, N.; Vandamme, P. Achromobacter veterisilvae sp. nov., from a mixed hydrogen-oxidizing bacteria enrichment reactor for microbial protein production. Int. J. Syst. Evol. Microbiol. 2019, 70, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Spilker, T.; Vandamme, P.; Lipuma, J.J. Identification and distribution of Achromobacter species in cystic fibrosis. J. Cyst. Fibros. 2013, 12, 298–301. [Google Scholar] [CrossRef] [PubMed]
- Spilker, T.; Vandamme, P.; Lipuma, J.J. A multilocus sequence typing scheme implies population structure and reveals several putative novel achromobacter species. J. Clin. Microbiol. 2012, 50, 3010–3015. [Google Scholar] [CrossRef] [PubMed]
- Veschetti, L.; Sandri, A.; Patuzzo, C.; Melotti, P.; Malerba, G.; Lleo, M.M. Genomic characterization of Achromobacter species isolates from chronic and occasional lung infection in cystic fibrosis patients. Microb. Genom. 2021, 7, 606. [Google Scholar] [CrossRef] [PubMed]
- Garrigos, T.; Dollat, M.; Magallon, A.; Chapuis, A.; Varin, V.; Bador, J.; Makki, N.; Cremet, L.; Persyn, E.; Cardot-Martin, E.; et al. Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry for Rapid Detection of Isolates Belonging to the Epidemic Clones Achromobacter xylosoxidans ST137 and Achromobacter ruhlandii DES from Cystic Fibrosis Patients. J. Clin. Microbiol. 2021, 59, e0094621. [Google Scholar] [CrossRef] [PubMed]
- Papalia, M.; Figueroa-Espinosa, R.; Steffanowski, C.; Barberis, C.; Almuzara, M.; Barrios, R.; Vay, C.; Gutkind, G.; Di Conza, J.; Radice, M. Expansion and improvement of MALDI-TOF MS databases for accurate identification of Achromobacter species. J. Microbiol. Methods 2020, 172, 105889. [Google Scholar] [CrossRef]
- De Baets, F.; Schelstraete, P.; Van Daele, S.; Haerynck, F.; Vaneechoutte, M. Achromobacter xylosoxidans in cystic fibrosis: Prevalence and clinical relevance. J. Cyst. Fibros. 2007, 6, 75–78. [Google Scholar] [CrossRef]
- Turel, O.; Kavuncuoglu, S.; Hosaf, E.; Ozbek, S.; Aldemir, E.; Uygur, T.; Hatipoglu, N.; Siraneci, R. Bacteremia due to Achromobacter xylosoxidans in neonates: Clinical features and outcome. Braz. J. Infect. Dis. 2013, 17, 450–454. [Google Scholar] [CrossRef]
- Hansen, C.R.; Pressler, T.; Nielsen, K.G.; Jensen, P.O.; Bjarnsholt, T.; Hoiby, N. Inflammation in Achromobacter xylosoxidans infected cystic fibrosis patients. J. Cyst. Fibros. 2010, 9, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Firmida, M.C.; Pereira, R.H.; Silva, E.A.; Marques, E.A.; Lopes, A.J. Clinical impact of Achromobacter xylosoxidans colonization/infection in patients with cystic fibrosis. Braz. J. Med. Biol. Res. 2016, 49, e5097. [Google Scholar] [CrossRef] [PubMed]
- Edwards, B.D.; Greysson-Wong, J.; Somayaji, R.; Waddell, B.; Whelan, F.J.; Storey, D.G.; Rabin, H.R.; Surette, M.G.; Parkins, M.D. Prevalence and Outcomes of Achromobacter Species Infections in Adults with Cystic Fibrosis: A North American Cohort Study. J. Clin. Microbiol. 2017, 55, 2074–2085. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.H.; Leao, R.S.; Carvalho-Assef, A.P.; Albano, R.M.; Rodrigues, E.R.; Firmida, M.C.; Folescu, T.W.; Plotkowski, M.C.; Bernardo, V.G.; Marques, E.A. Patterns of virulence factor expression and antimicrobial resistance in Achromobacter xylosoxidans and Achromobacter ruhlandii isolates from patients with cystic fibrosis. Epidemiol. Infect. 2017, 145, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Gade, S.S.; Norskov-Lauritsen, N.; Ridderberg, W. Prevalence and species distribution of Achromobacter sp. cultured from cystic fibrosis patients attending the Aarhus centre in Denmark. J. Med. Microbiol. 2017, 66, 686–689. [Google Scholar] [CrossRef]
- Amoureux, L.; Bador, J.; Bounoua Zouak, F.; Chapuis, A.; de Curraize, C.; Neuwirth, C. Distribution of the species of Achromobacter in a French Cystic Fibrosis Centre and multilocus sequence typing analysis reveal the predominance of A. xylosoxidans and clonal relationships between some clinical and environmental isolates. J. Cyst. Fibros. 2016, 15, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Isler, B.; Kidd, T.J.; Stewart, A.G.; Harris, P.; Paterson, D.L. Achromobacter Infections and Treatment Options. Antimicrob. Agents Chemother. 2020, 64, e01025-20. [Google Scholar] [CrossRef]
- Wang, M.; Ridderberg, W.; Hansen, C.R.; Hoiby, N.; Jensen-Fangel, S.; Olesen, H.V.; Skov, M.; Lemming, L.E.; Pressler, T.; Johansen, H.K.; et al. Early treatment with inhaled antibiotics postpones next occurrence of Achromobacter in cystic fibrosis. J. Cyst. Fibros. 2013, 12, 638–643. [Google Scholar] [CrossRef]
- Bador, J.; Amoureux, L.; Duez, J.M.; Drabowicz, A.; Siebor, E.; Llanes, C.; Neuwirth, C. First description of an RND-type multidrug efflux pump in Achromobacter xylosoxidans, AxyABM. Antimicrob. Agents Chemother. 2011, 55, 4912–4914. [Google Scholar] [CrossRef] [PubMed]
- Magallon, A.; Amoureux, L.; Garrigos, T.; Sonois, M.; Varin, V.; Neuwirth, C.; Bador, J. Role of AxyABM overexpression in acquired resistance in Achromobacter xylosoxidans. J. Antimicrob. Chemother. 2022, 77, 926–929. [Google Scholar] [CrossRef] [PubMed]
- Bador, J.; Neuwirth, C.; Liszczynski, P.; Mezier, M.C.; Chretiennot, M.; Grenot, E.; Chapuis, A.; de Curraize, C.; Amoureux, L. Distribution of innate efflux-mediated aminoglycoside resistance among different Achromobacter species. New Microbes New Infect. 2016, 10, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Bador, J.; Neuwirth, C.; Grangier, N.; Muniz, M.; Germe, L.; Bonnet, J.; Pillay, V.G.; Llanes, C.; de Curraize, C.; Amoureux, L. Role of AxyZ Transcriptional Regulator in Overproduction of AxyXY-OprZ Multidrug Efflux System in Achromobacter Species Mutants Selected by Tobramycin. Antimicrob. Agents Chemother. 2017, 61, e00290-17. [Google Scholar] [CrossRef] [PubMed]
- Magallon, A.; Roussel, M.; Neuwirth, C.; Tetu, J.; Cheiakh, A.C.; Boulet, B.; Varin, V.; Urbain, V.; Bador, J.; Amoureux, L. Fluoroquinolone resistance in Achromobacter spp.: Substitutions in QRDRs of GyrA, GyrB, ParC and ParE and implication of the RND efflux system AxyEF-OprN. J. Antimicrob. Chemother. 2021, 76, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Doi, Y.; Poirel, L.; Paterson, D.L.; Nordmann, P. Characterization of a naturally occurring class D beta-lactamase from Achromobacter xylosoxidans. Antimicrob. Agents Chemother. 2008, 52, 1952–1956. [Google Scholar] [CrossRef] [PubMed]
- Papalia, M.; Traglia, G.; Ruggiero, M.; Almuzara, M.; Vay, C.; Gutkind, G.; Ramirez, S.M.; Radice, M. Characterization of OXA-258 enzymes and AxyABM efflux pump from Achromobacter ruhlandii. J. Glob. Antimicrob. Resist. 2018, 14, 233–237. [Google Scholar] [CrossRef]
- Traglia, G.; Papalia, M.; Almuzara, M.; Gutkind, G.; Centron, D.; Vay, C.; Radice, M.; Ramirez, M.S. Presence of OXA-type enzymes in Achromobacter insuavis and A. dolens. Curr. Microbiol. 2014, 69, 501–506. [Google Scholar] [CrossRef]
- Neuwirth, C.; Freby, C.; Ogier-Desserrey, A.; Perez-Martin, S.; Houzel, A.; Pechinot, A.; Duez, J.M.; Huet, F.; Siebor, E. VEB-1 in Achromobacter xylosoxidans from cystic fibrosis patient, France. Emerg. Infect. Dis. 2006, 12, 1737–1739. [Google Scholar] [CrossRef]
- Chen, Z.; Fang, H.; Wang, L.; Sun, F.; Wang, Y.; Yin, Z.; Yang, H.; Yang, W.; Wang, J.; Xia, P.; et al. IMP-1 encoded by a novel Tn402-like class 1 integron in clinical Achromobacter xylosoxidans, China. Sci. Rep. 2014, 4, 7212. [Google Scholar] [CrossRef]
- Yamamoto, M.; Nagao, M.; Hotta, G.; Matsumura, Y.; Matsushima, A.; Ito, Y.; Takakura, S.; Ichiyama, S. Molecular characterization of IMP-type metallo-beta-lactamases among multidrug-resistant Achromobacter xylosoxidans. J. Antimicrob. Chemother. 2012, 67, 2110–2113. [Google Scholar] [CrossRef] [PubMed]
- Sofianou, D.; Markogiannakis, A.; Metzidie, E.; Pournaras, S.; Tsakris, A. VIM-2 metallo-beta-lactamase in Achromobacter xylosoxidans in Europe. Eur. J. Clin. Microbiol. Infect. Dis. 2005, 24, 854–855. [Google Scholar] [CrossRef] [PubMed]
- Di Pilato, V.; Pollini, S.; Rossolini, G.M. Characterization of plasmid pAX22, encoding VIM-1 metallo-beta-lactamase, reveals a new putative mechanism of In70 integron mobilization. J. Antimicrob. Chemother. 2014, 69, 67–71. [Google Scholar] [CrossRef]
- Fleurbaaij, F.; Henneman, A.A.; Corver, J.; Knetsch, C.W.; Smits, W.K.; Nauta, S.T.; Giera, M.; Dragan, I.; Kumar, N.; Lawley, T.D.; et al. Proteomic identification of Axc, a novel beta-lactamase with carbapenemase activity in a meropenem-resistant clinical isolate of Achromobacter xylosoxidans. Sci. Rep. 2018, 8, 8181. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, J.; Zhang, G.; Lin, N.; Sha, Y.; Lu, J.; Zhu, T.; Zhang, X.; Li, Q.; Zhang, H.; et al. Identification and characterization of a novel beta-lactamase gene, bla(AMZ-1), from Achromobacter mucicolens. Front. Microbiol. 2023, 14, 1252427. [Google Scholar] [CrossRef]
- Ronne Hansen, C.; Pressler, T.; Hoiby, N.; Gormsen, M. Chronic infection with Achromobacter xylosoxidans in cystic fibrosis patients; a retrospective case control study. J. Cyst. Fibros. 2006, 5, 245–251. [Google Scholar] [CrossRef]
- Ridderberg, W.; Bendstrup, K.E.; Olesen, H.V.; Jensen-Fangel, S.; Norskov-Lauritsen, N. Marked increase in incidence of Achromobacter xylosoxidans infections caused by sporadic acquisition from the environment. J. Cyst. Fibros. 2011, 10, 466–469. [Google Scholar] [CrossRef]
- Papalia, M.; Steffanowski, C.; Traglia, G.; Almuzara, M.; Martina, P.; Galanternik, L.; Vay, C.; Gutkind, G.; Ramirez, M.S.; Radice, M. Diversity of Achromobacter species recovered from patients with cystic fibrosis, in Argentina. Rev. Argent. De Microbiol. 2019, 52, 13–18. [Google Scholar] [CrossRef]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. A J. Comput. Mol. Cell Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef]
- Aziz, R.K.; Bartels, D.; Best, A.A.; DeJongh, M.; Disz, T.; Edwards, R.A.; Formsma, K.; Gerdes, S.; Glass, E.M.; Kubal, M.; et al. The RAST Server: Rapid annotations using subsystems technology. BMC Genom. 2008, 9, 75. [Google Scholar] [CrossRef]
- Ghiglione, B.; Rodriguez, M.M.; Brunetti, F.; Papp-Wallace, K.M.; Yoshizumi, A.; Ishii, Y.; Bonomo, R.A.; Gutkind, G.; Klinke, S.; Power, P. Structural and Biochemical Characterization of the Novel CTX-M-151 Extended-Spectrum beta-Lactamase and Its Inhibition by Avibactam. Antimicrob. Agents Chemother. 2021, 65, e01757-20. [Google Scholar] [CrossRef] [PubMed]
- Papalia, M.; Almuzara, M.; Cejas, D.; Traglia, G.; Ramirez, M.S.; Galanternik, L.; Vay, C.; Gutkind, G.; Radice, M. OXA-258 from Achromobacter ruhlandii: A species-specific marker. J. Clin. Microbiol. 2013, 51, 1602–1605. [Google Scholar] [CrossRef] [PubMed]
- Jacoby, G.A. AmpC beta-lactamases. Clin. Microbiol. Rev. 2009, 22, 161–182. [Google Scholar] [CrossRef] [PubMed]
- Chalhoub, H.; Kampmeier, S.; Kahl, B.C.; Van Bambeke, F. Role of Efflux in Antibiotic Resistance of Achromobacter xylosoxidans and Achromobacter insuavis Isolates from Patients with Cystic Fibrosis. Front. Microbiol. 2022, 13, 762307. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Jinadatha, C.; Chatterjee, P.; Allton, Y.; Navarathna, D.H. Draft Genome Sequence of an Unusually Multidrug-Resistant Strain of Achromobacter xylosoxidans from a Blood Isolate. Microbiol. Resour. Announc. 2020, 9, e00194-20. [Google Scholar] [CrossRef]
- Amoureux, L.; Sauge, J.; Sarret, B.; Lhoumeau, M.; Bajard, A.; Tetu, J.; Bador, J.; Neuwirth, C. Study of 109 Achromobacter spp. isolates from 9 French CF centres reveals the circulation of a multiresistant clone of A. xylosoxidans belonging to ST 137. J. Cyst. Fibros. 2019, 18, 804–807. [Google Scholar] [CrossRef]
- Hansen, C.R.; Pressler, T.; Ridderberg, W.; Johansen, H.K.; Skov, M. Achromobacter species in cystic fibrosis: Cross-infection caused by indirect patient-to-patient contact. J. Cyst. Fibros. 2013, 12, 609–615. [Google Scholar] [CrossRef]

| MIC (μg/mL) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| AMP | PIP | CEX | FOX | CAZ | CRO | FEP | IPM | MEM | |
| E. coli TOP 10 F´ | 8 | 1 | 4 | 2 | 0.25 | ≤0.125 | ≤0.125 | 0.125 | 0.125 |
| E. coli TOP 10 F´ + pK19 | 8 | 1 | 4 | 2 | 0.25 | ≤0.125 | ≤0.125 | 0.125 | 0.125 |
| E. coli TOP 10 F´ + pK19 + AXC-2 | 32 | 2 | 64 | 2 | 0.5 | ≤0.125 | ≤0.125 | 0.5 | 0.5 |
| A. ruhlandii Ar38 | 512 | 16 | 512 | 256 | 16 | 512 | 64 | 16 | 8 |
| Antibiotic | Km (μM) | kcat (s−1) | kcat/Km (μM−1·s−1) |
|---|---|---|---|
| Nitrocefin | 202 ± 24 | 40 ± 3 | 0.20 ± 0.04 |
| Ampicillin | 344 ± 14 | 62 ± 5 | 0.18 ± 0.02 |
| Benzyl-penicillin | 1297 ± 409 | 125 ± 28 | 0.10 ± 0.05 |
| Cephalexin | 74 ± 14 | 16 ± 2 | 0.22 ± 0.07 |
| Cefoxitin | >1000 | NH | ND |
| Ceftazidime | >1000 | NH | ND |
| Ceftriaxone | 59 ± 6 | 5.5 ± 0.3 | 0.09 ± 0.01 |
| Cefepime | 872 ± 254 | 0.29 ± 0.07 | 0.0003 ± 0.0001 |
| Imipenem | 6.3 ± 0.8 | 0.0364 ± 0.0009 | 0.0058 ± 0.0009 |
| Meropenem | 8 ± 1 | 0.051 ± 0.002 | 0.007 ± 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papalia, M.; González-Espinosa, F.; Castedo, F.Q.; Gutkind, G.; Ramírez, M.S.; Power, P.; Radice, M. Genetic and Biochemical Characterization of AXC-2 from Achromobacter ruhlandii. Pathogens 2024, 13, 115. https://doi.org/10.3390/pathogens13020115
Papalia M, González-Espinosa F, Castedo FQ, Gutkind G, Ramírez MS, Power P, Radice M. Genetic and Biochemical Characterization of AXC-2 from Achromobacter ruhlandii. Pathogens. 2024; 13(2):115. https://doi.org/10.3390/pathogens13020115
Chicago/Turabian StylePapalia, Mariana, Francisco González-Espinosa, Fátima Quiroga Castedo, Gabriel Gutkind, María Soledad Ramírez, Pablo Power, and Marcela Radice. 2024. "Genetic and Biochemical Characterization of AXC-2 from Achromobacter ruhlandii" Pathogens 13, no. 2: 115. https://doi.org/10.3390/pathogens13020115
APA StylePapalia, M., González-Espinosa, F., Castedo, F. Q., Gutkind, G., Ramírez, M. S., Power, P., & Radice, M. (2024). Genetic and Biochemical Characterization of AXC-2 from Achromobacter ruhlandii. Pathogens, 13(2), 115. https://doi.org/10.3390/pathogens13020115








