Tuberculous Pericarditis in Childhood: A Case Report and a Systematic Literature Review
Abstract
1. Introduction
2. Case Report
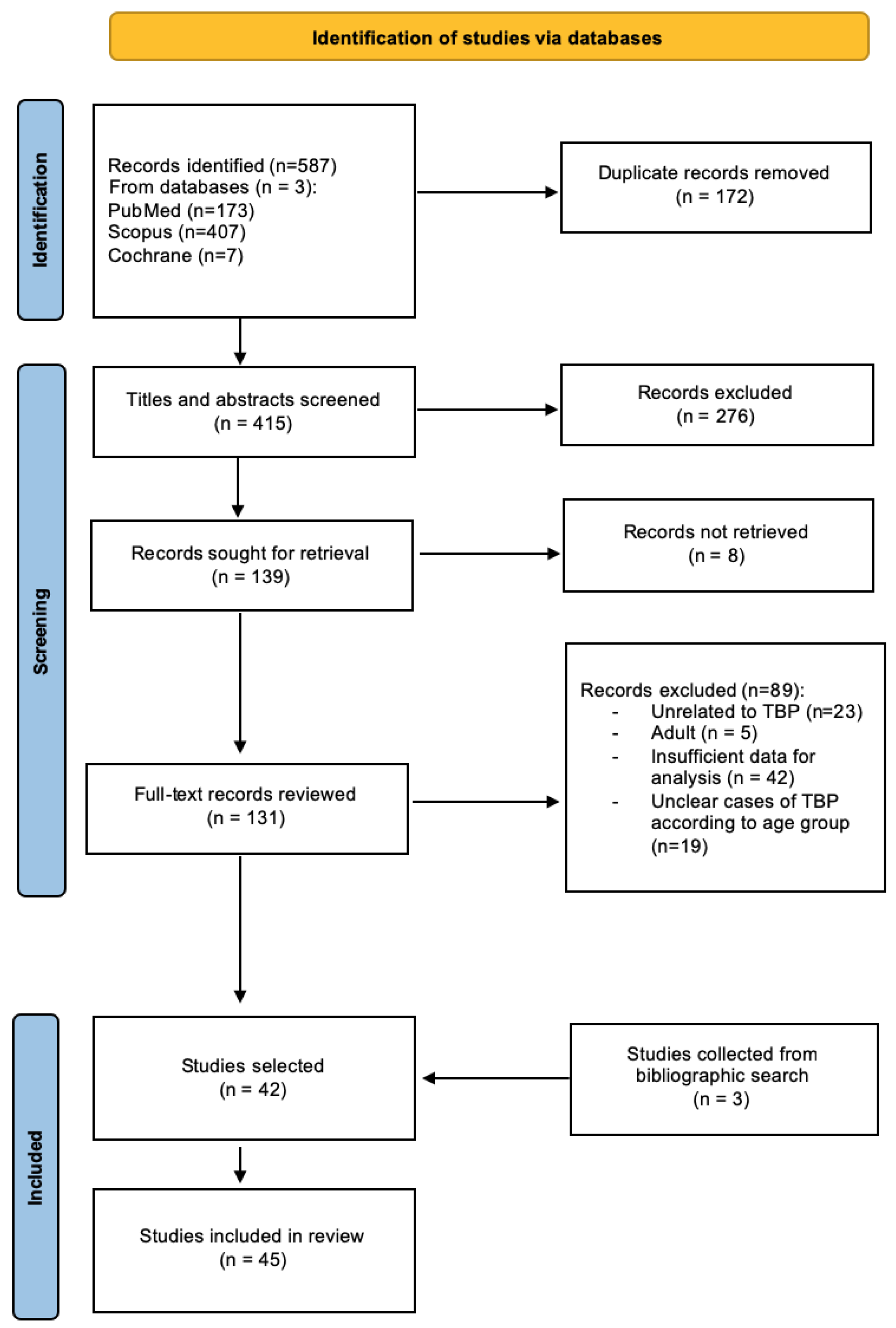
3. Materials and Methods
4. Results
5. Discussion
- -
- In the dry stage, when the bacillus reaches the pericardium via the retrograde lymphatic spread, hematogenous dissemination or contiguity from surrounding tissues and a poorly organized lymphomonocytic inflammatory infiltrate appear. This stage, characterized by clinical manifestations of acute pericarditis such as chest pain, pericardial friction rub, and ST elevation in the absence of effusion, is rarely observed in TBP in children.
- -
- An effusive stage, the most commonly observed, can manifest clinically with signs and symptoms of heart failure or cardiac tamponade due to a conspicuous effusion, or it can present in mixed form, with both a compressive effusion and a visceral constriction present at the same time.
- -
- An adsorptive stage is characterized by reabsorption of the pericardial effusion, organization of the infiltrate to form granulomas with caseous necrosis, and thickening of the pericardium due to fibrin and collagen deposition. In this stage, signs and symptoms are those of constrictive pericarditis, although a dense fluid can still be found through echocardiography or imaging.
- -
- A constrictive stage, when there is no residual fluid and fibrosis of the visceral and parietal pericardial layers, leads to the formation of a fibrocalcific stratum that encases the cardiac chambers, resulting in the classic presentation of constrictive pericarditis.
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AFB | Acid-fast bacilli |
| ADA | Adenosine deaminase |
| AF | Antitubercular treatment |
| ART | Antiretroviral therapy |
| ATT | Antitubercular treatment |
| CXR | Chest X-ray |
| ECG | Electrocardiogram |
| EPTB | Extrapulmonary tuberculosis |
| Genotype MTBDRplus | |
| HAART | Highly active antiretroviral therapy |
| HRZE | Isoniazid, rifampicin, pyrazinamide, ethambutol |
| IRIS | Immune Reconstitution Inflammatory Syndrome |
| IVC | Inferior vena cava |
| JIA | Juvenile Idiopathic Arthritis |
| JVP | Jugular venous pressure |
| LABD | Linear IgA Bullous Dermatitis |
| LA | Left atrium |
| LV | Left ventricle |
| MTB | Mycobacterium tuberculosis |
| NP | Not performed |
| NR | Not reported |
| PCR | Polymerase chain reaction |
| PAS | Para-aminosalicylic acid |
| RA | Right atrium |
| RV | Right ventricle |
| SVC | Superior vena cava |
| TB | Tuberculosis |
| TBP | Tuberculous pericarditis |
References
- WHO. Global Tuberculosis Report 2022. 2022. Available online: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2022 (accessed on 11 August 2023).
- Global Tuberculosis Report 2021. Available online: https://www.who.int/publications-detail-redirect/9789240037021 (accessed on 11 August 2023).
- Sharma, S.K.; Mohan, A. Extrapulmonary tuberculosis. Indian J. Med. Res. 2004, 120, 316–353. [Google Scholar]
- Chiang, S.S.; Starke, J.R. Principles and Practice of Pediatric Infectious Diseases, 5th ed.; Elsevier: Philadelphia, PA, USA, 2018. [Google Scholar]
- Mayosi, B.M.; Burgess, L.J.; Doubell, A.F. Tuberculous Pericarditis. Circulation 2005, 112, 3608–3616. [Google Scholar] [CrossRef]
- Bizzi, E.; Picchi, C.; Mastrangelo, G.; Imazio, M.; Brucato, A. Recent advances in pericarditis. Eur. J. Intern. Med. 2022, 95, 24–31. [Google Scholar] [CrossRef]
- Lima, N.d.A.; Stancic, C.; Vos, D.; Insua, M.M.d.C.D.; Lima, C.C.d.V.; de Castro, R.L.; Maravelas, R.; Melgar, T. Hospital admissions for tuberculous pericarditis in the United States 2002–2014. Int. J. Mycobacteriology 2019, 8, 347–350. [Google Scholar] [CrossRef]
- López-López, J.P.; Posada-Martínez, E.L.; Saldarriaga, C.; Wyss, F.; Ponte-Negretti, C.I.; Alexander, B.; Miranda-Arboleda, A.F.; Martínez-Sellés, M.; Baranchuk, A. The Neglected Tropical Diseases, Other Infectious Diseases Affecting the Heart (the NET-Heart Project). Tuberculosis and the Heart. J. Am. Heart Assoc. 2021, 10, e019435. [Google Scholar] [CrossRef]
- Dybowska, M.; Błasińska, K.; Gątarek, J.; Klatt, M.; Augustynowicz-Kopeć, E.; Tomkowski, W.; Szturmowicz, M. Tuberculous Pericarditis—Own Experiences and Recent Recommendations. Diagnostics 2022, 12, 619. [Google Scholar] [CrossRef]
- Isiguzo, G.; Bruyn, E.D.; Howlett, P.; Ntsekhe, M. Diagnosis and Management of Tuberculous Pericarditis: What Is New? Curr. Cardiol. Rep. 2020, 22, 2. [Google Scholar] [CrossRef]
- Coulter, J.B.S.; Walsh, K.; King, S.J.; Shears, P. Tuberculous pericarditis in a child. J. Infect. 1996, 32, 157–160. [Google Scholar] [CrossRef]
- Blatt, M.L. Pericarditis as a Primary Clinical Manifestation of Tuberculosis in Childhood. Arch. Pediatr. Adolesc. Med. 1928, 35, 631. [Google Scholar] [CrossRef]
- Shanks, R.A. Tuberculous pericarditis in childhood: A report of four cases. Glasg. Med. J. 1952, 33, 399–403. [Google Scholar]
- Boyd, G.L. Tuberculous Pericarditis in Children. Arch. Pediatr. Adolesc. Med. 1953, 86, 293–300. [Google Scholar] [CrossRef]
- Miller, F.J.W.; Seal, R.M.E.; Taylor, M.D. Tuberculosis in Children: Evolution, Control, Treatment; J. & A. Churchill: London, UK, 1963. [Google Scholar]
- Keith, J.D.; Rowe, R.D.; Vlad, P. Heart Disaese in Infancy and Children, 3rd ed.; Macmillan: New York, NY, USA, 1978. [Google Scholar]
- Fowler, N.O. Tuberculous pericarditis. JAMA 1991, 266, 99–103. [Google Scholar] [CrossRef]
- Hugo-Hamman, C.T.; Scher, H.; De Moor, M.M.A. Tuberculous pericarditis in children: A review of 44 cases. Pediatr. Infect. Dis. J. 1994, 13, 13–17. [Google Scholar] [CrossRef]
- Obihara, N.J.; Walters, E.; Lawrenson, J.; Garcia-Prats, A.J.; Hesseling, A.C.; Schaaf, H.S. Tuberculous Pericardial Effusions in Children. J. Pediatr. Infect. Dis. Soc. 2018, 7, 346–349. [Google Scholar] [CrossRef]
- Paramitha, W.; Murni, I.K.; Arguni, E.; Setyowireni, D. Tuberculous pericarditis in adolescents: A case series. Paediatr. Indones. 2020, 60, 109–115. [Google Scholar] [CrossRef]
- Maltezou, H.C.; Spyridis, P.; Kafetzis, D.A. Extra-pulmonary tuberculosis in children. Arch. Dis. Child. 2000, 83, 342–346. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Gobir, A.; Ariyibi, S.O.; Ibraheem, R.M.; Johnson, A.R.; Abdulkadi, M.B.; Katibi, O.S.; Adeoye, P.O.; Udoh, E.A.; Ilesanmi, O.N.; Folaranmi, O.O. Disseminated Tuberculosis in a Nigerian Adolescent with Linear IgA Bullous Dermatosis: A Case Report and Review of Literature. West Afr. J. Med. 2022, 39, 646–650. [Google Scholar]
- Taxak, A.; Nandi, D.; Shaw, M.; Kumar, S. Coconut heart in a child. J. Cardiovasc. Comput. Tomogr. 2022, 16, e42–e44. [Google Scholar] [CrossRef]
- Mucheleng’Anga, L.A.; Himwaze, C.M.; Telendiy, V.; Simumba, S.; Soko, J.; Kayonde, N.; Mulenga, B.; Hamukale, A.; Shibemba, A.L.; Lungu, P.S.; et al. Incidental Tuberculosis in sudden, unexpected, and violent deaths in the community Lusaka, Zambia - A descriptive forensic post-mortem examination study. Int. J. Infect. Dis. 2022, 124, S75–S81. [Google Scholar] [CrossRef]
- Swaminathan, A.; Cros, P.D.; Achar, J.; Kliescikova, J.; Mirgayosieva, S.; Pirmahmadzoda, B. A case report of a child with probable drug resistant tuberculous pericarditis with a review of challenges involved in diagnosis, treatment and follow up of children with DR-TB pericarditis. BMC Infect. Dis. 2020, 20, 298. [Google Scholar] [CrossRef]
- Khera, S.; Kumar, V.; Ramamurthy, R.; Ramar, P. Favourable outcome in a child with presumed tubercular pancarditis treated with empirical antitubercular therapy. BMJ Case Rep. 2020, 13, e235125. [Google Scholar] [CrossRef]
- Kathwate, J. Mediastinal tubercular lymph nodes completely encasing aorta in a child—A rare complication. Pediatr. Oncall 2022, 19. [Google Scholar] [CrossRef]
- Noguera-Julian, A.; Calzada-Hernández, J.; Brinkmann, F.; Roy, R.B.; Bilogortseva, O.; Buettcher, M.; Carvalho, I.; Chechenyeva, V.; Falcón, L.; Goetzinger, F.; et al. Tuberculosis Disease in Children and Adolescents on Therapy With Antitumor Necrosis Factor-ɑ Agents: A Collaborative, Multicenter Paediatric Tuberculosis Network European Trials Group (ptbnet) Study. Clin. Infect. Dis. 2019, 71, 2561–2569. [Google Scholar] [CrossRef]
- Brotherton, B.J.; Kimani-Mangoli, E.W.; Shirk, A.M. Water Bottle–Shaped Heart in a Five-Year-Old Boy. Am. J. Trop. Med. Hyg. 2019, 101, 3–4. [Google Scholar] [CrossRef]
- Martínez, M.D.R.O.; Dvorkin, J.; Sollitto, G.; Conejeros, W.; Garrido, M.; Cazalas, M. Hepatomegaly as a form of presentation in constrictive pericarditis. A pediatric clinical case. Arch. Argent. Pediatr. 2019, 117, E523–E526. [Google Scholar] [CrossRef]
- Kumar, R.; Raja, J.; Rawat, S.; Srivastava, A.; Thingnam, S.K.S. Chronic constrictive pericarditis complicated with huge right atrial thrombus in a child with abdominal tuberculosis: A rare life-threatening condition. J. Surg. Case Rep. 2019, 2019, rjz295. [Google Scholar] [CrossRef]
- Girit, S. Pericardial hydatid cyst and tuberculosis co-existence. Turk. J. Thorac. Cardiovasc. Surg. 2018, 26, 312–315. [Google Scholar] [CrossRef]
- Pilania, R.K.; Mahajan, S.; Das, A.; Vaishnavi, K.; Kumar, R.; Bhatia, A.; Dayal, D. Anasarca as the Initial Manifestation of Tuberculous Pericarditis in a Child. J. Pediatr. Infect. Dis. 2017, 13, 236–239. [Google Scholar] [CrossRef]
- Suárez, G.A. Tuberculosis or collagenosis: A diagnostic dilemma. Rev. Cuba. Pediatría. 2018, 90. [Google Scholar]
- Peter, I.D.; Shehu, A.U.; Ibrahim, U.; Asani, M.; Aliyu, I.; Sanusi, Y.; Ahmad, J. Pyopericardium with cardiac tamponade in a Nigerian child with acute osteomyelitis. J. Cardiovasc. Echogr. 2017, 27, 71. [Google Scholar] [CrossRef]
- Jakimów-Kostrzewa, A.; Małek, Ł.A.; Grabowski, K.; Werner, B.; Brzewski, M. A case of tuberculous pericarditis on cardiac magnetic resonance. Kardiol. Pol. 2017, 75, 1354. [Google Scholar] [CrossRef][Green Version]
- Melit, L.E.; Targu-Mures, P.C.; Marginean, C.O.; Rolea, G.; Sasaran, V.S.; Marginean, C.D. tuberculous pericarditis, a topical pathology in pediatrics—A case report and a review of the literature. Rom. J. Infect. Dis. 2017, 20, 80–83. [Google Scholar] [CrossRef]
- Chiu, N.-C.; Wu, S.-J.; Chen, M.-R.; Peng, C.-C.; Chang, L.; Chi, H.; Lin, C.-Y. A Mysterious Effusion: Tuberculous Pericarditis. J. Pediatr. 2016, 174, 271–271.e1. [Google Scholar] [CrossRef]
- Faustino, M.; Mendes, I.C.; Anjos, R. Constrictive Pericarditis: A Challenging Diagnosis in Paediatrics. Case Rep. Cardiol. 2015, 2015, 402740. [Google Scholar] [CrossRef]
- Yoon, S.-A.; Hahn, Y.-S.; Hong, J.M.; Lee, O.-J.; Han, H.-S. Tuberculous Pericarditis Presenting as Multiple Free Floating Masses in Pericardial Effusion. J. Korean Med. Sci. 2012, 27, 325–328. [Google Scholar] [CrossRef]
- Gupta, S.K.; Saxena, A.; Talwar, S. Chronic Constrictive Pericarditis: Unique Cause of Heart Failure in a Child With Tetralogy of Fallot. Pediatr. Cardiol. 2012, 33, 165–167. [Google Scholar] [CrossRef]
- Campagnucci, V.P.; Silva, A.M.R.P.E.; Catani, L.H.; Rivetti, L.A. Recurrent Giant Left Ventricular Aneurysm of Tuberculous Etiology in a Child: Case Report. Heart Surg. Forum 2012, 15, E318–E319. [Google Scholar] [CrossRef]
- Gulati, G.; Kothari, S. Diffuse infiltrative cardiac tuberculosis. Ann. Pediatr. Cardiol. 2011, 4, 87–89. [Google Scholar] [CrossRef]
- Rabie, H.; Lomp, A.; Goussard, P.; Nel, E.; Cotton, M. Paradoxical Tuberculosis associated Immune Reconstitution Inflammatory Syndrome Presenting with Chylous Ascites and Chylothorax in a HIV-1 Infected Child. J. Trop. Pediatr. 2010, 56, 355–358. [Google Scholar] [CrossRef]
- Takawira, F.F.; Joshi, J.A.; Plessis, D.J.D. Development of a Subaortic Aneurysm Secondary to Disseminated Tuberculosis in a Child. Ann. Thorac. Surg. 2010, 90, 644–647. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lee, S.E.; Choi, J.W.; Choi, S.I.; Chun, E.J.; Choi, J.Y. A case of transient left ventricular apical ballooning syndrome in a child: Clinical features and imaging findings. Int. J. Cardiovasc. Imaging 2010, 26, 345–351. [Google Scholar] [CrossRef]
- Samady, M.M.E.; Moukirish, A.A.; Hatle, L.K.; Arfi, A.M.; Galal, M.O. Doppler Echocardiographic Signs to Differentiate Between Chronic Lung Disease and Right Ventricular Dysfunction in a Child with Calcified Pericardium. J. Coll. Physicians Surg. Pak. 2009, 19, 518–519. [Google Scholar]
- Cetin, I.I.; Azak, E.; Saygili, A.; Demirhan, B.; Tokel, K. A case of arrhythmogenic right ventricular dysplasia presenting with symptoms of right-sided heart failure secondary to constrictive pericarditis. Pediatr. Int. 2005, 47, 115–118. [Google Scholar] [CrossRef]
- Sharifi-Mood, B.; Naini, R.A.; Eazadi, M. Cystic Tuberculous Pericarditis. J. Res. Med. Sci. 2005, 10, 236–238. [Google Scholar]
- Meyburg, J.; Schmidt, K.G.; Nützenadel, W.; Bettendorf, M. Tuberculous pericarditis in an infant evolving during triple chemotherapy. Eur. J. Pediatr. 2002, 161, 138–141. [Google Scholar] [CrossRef]
- Browne, G.J.; Hort, J.; Lau, K.C. Pericardial effusions in a pediatric emergency department. Pediatr. Emerg. Care 2002, 18, 285–289. [Google Scholar] [CrossRef]
- Tutar, H.E.; Yilmaz, E.; Atalay, S.; Ucar, T.; Uysalel, A.; Kiziltepe, U.; Gumus, H. The changing aetiological spectrum of pericarditis in children. Ann. Trop. Paediatr. 2002, 22, 251–256. [Google Scholar] [CrossRef]
- Equi, A.; Redington, A.; Rosenthal, M.; Taylor, G.M.; Jaswon, M.; Bush, A. Pulmonary artery occlusion from tuberculous lymphadenopathy in a child. Pediatr. Pulmonol. 2001, 31, 311–313. [Google Scholar] [CrossRef]
- Lin, J.H.; Chen, S.J.; Wu, M.H.; Lee, P.I.; Chang, C.I. Fibrinofibrous pericarditis mimicking a pericardial tumor. J. Formos. Med. Assoc. Taiwan Yi Zhi 2000, 99, 59–61. [Google Scholar]
- Weber, S. Tuberculosis and Pericarditis in Children. Trop. Dr. 1999, 29, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.; Kelly, K.; Shaw, K.N. Chest pain and cardiomegaly without pulmonary involvement: An atypical presentation of pediatric mycobacterial disease. Pediatr. Emerg. Care 1995, 11, 35–36. [Google Scholar] [CrossRef] [PubMed]
- Nelson, C.T.; Taber, L.H. Diagnosis of tuberculous pericarditis with a fluorochrome stain. Pediatr. Infect. Dis. J. 1995, 14, 1004–1007. [Google Scholar] [PubMed]
- Kher, A.; Lahiri, K.; Jain, M. Congenital lipodystrophy with defective leucocyte function (a case report). J. Postgrad. Med. 1990, 36, 48–50. [Google Scholar] [PubMed]
- Bolt, R.J.; Rammeloo, L.A.; Van Furth, A.M.; Van Well, G.T.J. A 15-year-old girl with a large pericardial effusion. Eur. J. Pediatr. 2008, 167, 811–812. [Google Scholar] [CrossRef][Green Version]
- Massoure, P.-L.; Boddaert, G.; Caumes, J.-L.; Gaillard, P.-E.; Lions, C.; Grassin, F. Porridge-like tuberculous cardiac tamponade: Treatment difficulties in the Horn of Africa. Gen. Thorac. Cardiovasc. Surg. 2010, 58, 276–278. [Google Scholar] [CrossRef]
- Shodikin, M.A. Case Report: Tuberculosis with Pericardial Effusion in Children. Med. Health Sci. J. 2022, 6, 47–52. [Google Scholar] [CrossRef]
- World Health Organization. WHO Global Lists of High Burden Countries for Tuberculosis (TB), TB/HIV and Multidrug/Rifampicin-resistant TB (MDR/RR-TB), 2021–2025: Background Document; World Health Organization: Geneva, Switzerland, 2021; Available online: https://apps.who.int/iris/handle/10665/341980 (accessed on 11 August 2023).
- Bagri, N.K.; Yadav, D.K.; Agarwal, S.; Aier, T.; Gupta, V. Pericardial effusion in children: Experience from tertiary care center in Northern India. Indian Pediatr. 2014, 51, 211–213. [Google Scholar] [CrossRef]
- Buonsenso, D.; Lancella, L.; Valentini, P. A Twenty-year Retrospective Study of Pediatric Tuberculosis in Two Tertiary Hospitals in Rome. Arch. Dis. Child. 2012, 97, A11–A12. [Google Scholar] [CrossRef]
- Curry, C.; Zuhlke, L.; Mocumbi, A.; Kennedy, N. Acquired heart disease in low-income and middle-income countries. Arch. Dis. Child. 2017, 103, 73–77. [Google Scholar] [CrossRef]
- Ntsekhe, M.; Mayosi, B.M. Tuberculous pericarditis with and without HIV. Heart Fail. Rev. 2013, 18, 367–373. [Google Scholar] [CrossRef]
- Mishra, O.; Yusuf, S.; Ali, Z.; Nath, G. Lysozyme levels for the diagnosis of tuberculous effusions in children. J. Trop. Pediatr. 2000, 46, 296–300. [Google Scholar] [CrossRef]
- Mishra, O.P.; Kumar, R.; Ali, Z.; Prasad, R.; Nath, G. Evaluation of polymerase chain reaction and adenosine deaminase assay for the diagnosis of tuberculous effusions in children. Arch. Dis. Child. 2006, 91, 985–989. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Marais, B.J.; Gie, R.P.; Schaaf, H.S.; Starke, J.R.; Hesseling, A.C.; Donald, P.R.; Beyers, N. A proposed radiological classification of childhood intra-thoracic tuberculosis. Pediatr. Radiol. 2004, 34, 886–894. [Google Scholar] [CrossRef] [PubMed]
- Foster, K.; Alton, H. Chronic lung infection in children. Paediatr. Respir. Rev. 2003, 4, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.-Y.; Li, Y.-H.; Tsai, W.-C.; Tsai, L.-M.; Chao, T.-H.; Yung, Y.-J.; Chen, J.-H. Usefulness of echocardiographic intrapericardial abnormalities in the diagnosis of tuberculous pericardial effusion. Am. J. Cardiol. 2001, 87, 1133–1135. [Google Scholar] [CrossRef] [PubMed]
- Strang, J.I.G.; Nunn, A.J.; Johnson, D.A.; Casbard, A.; Gibson, D.G.; Girling, D.J. Management of tuberculous constrictive pericarditis and tuberculous pericardial effusion in Transkei: Results at 10 years follow-up. QJM 2004, 97, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Nahid, P.; Dorman, S.E.; Alipanah, N.; Barry, P.M.; Brozek, J.L.; Cattamanchi, A.; Chaisson, L.H.; Chaisson, R.E.; Daley, C.L.; Grzemska, M.; et al. Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America Clinical Practice Guidelines: Treatment of Drug-Susceptible Tuberculosis. Clin. Infect. Dis. 2016, 63, e147–e195. [Google Scholar] [CrossRef] [PubMed]
- Pasipanodya, J.G.; Mubanga, M.; Ntsekhe, M.; Pandie, S.; Magazi, B.T.; Gumedze, F.; Myer, L.; Gumbo, T.; Mayosi, B.M. Tuberculous Pericarditis is Multibacillary and Bacterial Burden Drives High Mortality. EBioMedicine 2015, 2, 1634–1639. [Google Scholar] [CrossRef][Green Version]

| Epidemiology | Clinical Manifestations | Diagnosis | Treatment And Outcome | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Author, Year, Country | Number of Patients | Sex and Age (years) | Signs and Symptoms | Cardiovascular Signs and Symptoms | Positive Microbiology and/or Elevated ADA | Histology Indicative of MTB | Echocardiographic/US and/or ECG Findings | Medical Treatment | Surgical Treatment | Outcome |
| Gobir et al. [23] 2022 Nigeria | 1 | F 11 y | Fever, cough, weight loss, night sweats, lymphadenopathy | Dyspnea, orthopnea, lower limbs pitting edema, ascites and abdominal distention, bilateral neck swelling (bull neck appearance), tachycardia, tachypnea, muffled heart sounds, gallop rhythm | Gene Xpert (sputum) | Multiple caseating granulomas (lymph node biopsy); Exudative pericardial fluid | Massive pericardial effusion with reduced LV function (EF 30%) | HRZE, prednisolone; furosemide *, spironolactone *, hydrochlorothiazide *, dapsone * | Emergency pericardiostomy (2 L of serosanguineous fluid over six weeks) | Positive |
| Taxak et al. [24] 2022 India | 1 | F 11 y | NR | Ascites, splenomegaly, hepatomegaly, dilation of IVC, SVC, and main pulmonary artery | NR | NR | “Restricted filling of hypocontractile LV” | ATT | Pericardiectomy | Positive |
| Mucheleng’anga et al. [25] 2022 Zambia | 1 | M 14 y | Absent | Absent | NP | Autoptic findings of TB pancarditis | NP | NP | NP | Negative |
| Swaminathan [26] 2020 Tajikistan | 1 | M 2.3 y | Fever, cough, weight loss, decreased activity | Absent | GenoType MTBDRplus, H-resistant MBT (pleural fluid) | NP | “Thickened pericardium with no effusion or calcifications” | HRZE; capreomycin †, moxifloxacin †, cycloserine †, PAS †, protionamide†, linezolid †; prednisone | Pericardiectomy | Positive |
| Khera et al. [27] 2020 India | 1 | F 11 y | Fever, weight loss, loss of appetite, multiple mediastinal lymphadenopathy | Hepatomegaly, exertional dyspnea, lower limbs pitting edemas, facial edema, increased JVP, tachycardia, tachypnea, muffled heart sounds, gallop rhythm | Absent | NP | “Thickened pericardium; thickened myocardium with speckled calcifications, intracardiac mass on RA, biventricular dysfunction” | ATT; prednisolone; furosemide *, enalapril * | NP | Positive |
| Shah et al. [28] 2020 India | 1 | F 8 y | Fever, weight loss, pallor, calcified mediastinal lymph nodes encasing the aorta | Ascites | AFB (abscess sample smear) | NR | “Solidified collection in superior mediastinum encasing the aorta” | ATT, streptomycin †, ethambutol †, linezolid †, amikacin†, levofloxacin †, moxifloxacin †, PAS †, cyloserine †, prednisolone | Pericardiocentesis | Positive |
| Shodikin et al. [62] 2020 Indonesia | 1 | M 14 y | Fever, low body weight, conjunctival anemia, anemia | Dyspnea, chest pain, distended abdomen, JV distention, tachycardia, tachypnea, distant heart sounds | NR | Hemorrhagic fluid with histiocytes and no granulomas | Massive pericardial effusion with preserved EF; low voltage QRS (ECG) | HRZE, methylprednisolone, pyridoxine, furosemide * | Pericardiocentesis and catheterization (1.2 L) | Positive |
| Paramitha et al. [20] 2020 Indonesia | 1 | M 12 y | Fever, fatigue, pallor, malnutrition | Dyspnea | AFB (pericardial fluid) | NP | Pericardial effusion | HRZE, ciprofloxacin *, ampicillin * and cloxacillin *; prednisone | Pericardiocentesis (300 mL of fluid) | Positive |
| 1 | M 17 y | Cough, malnutrition, acute pharyngitis | Dyspnea | AFB (sputum) Gene Xpert (sputum) elevated ADA (pericardial fluid) | NP | “Massive pericardial effusion” | HRZE; corticosteroids | Emergency pericardiocentesis (1.15 L, repeated); Pericardial window | Positive | |
| 1 | F 17 y | Fever, weight loss, malnutrition, anemia, vomiting, abdominal pain | Dyspnea, hepatomegaly, ascites, tachycardia, tachypnea, muffled heart sounds | Elevated ADA (pericardial fluid) | NP | “Massive circumferential pericardial effusion, right ventricular and atrial collapse, swinging heart;” low voltage QRS (ECG) | ATT | Pericardiocentesis (460 mL xanthochromic fluid); Catheterization | Positive | |
| Noguera-Julian et al. [29] 2020 NR | 4 | F (1) 12.1 y | Fever, cough, fatigue, anorexia | NR | Culture and PCR (sputum) | NP | NR | HRZE, ofloxacin, amikacin; steroids * and methotrexate * | NP | Positive |
| M (1) 8.6 y | Fever, cough, headache, weight loss, joint pain | Ascites, abdominal distention | Culture and PCR (gastric aspirate) | NP | NR | HRZE; steroids | NP | Positive | ||
| M (1) 16.1 y | Fever, cough, weight loss, night sweats | Dyspnea, chest pain | PCR (lung biopsy) | NP | NR | HRZE; clarithromycin, amikacin | NP | Positive | ||
| F (1) 10.6 y | Fever, cough, weight loss, intestinal symptoms | NR | S-resistant MTB (gastric aspirate) | NP | NR | HRZE, quinolones †; steroids | NP | Positive | ||
| Brotherton et al. [30] 2019 Kenya | 1 | M 5 y | Fever, cough, fatigue, loss of appetite, anemia, severe malnutrition | Dyspnea, chest pain, tachypnea, muffled heart sounds | Absent | NP | “Moderate pericardial effusion with extensive fibrinous exudate from pericardium to epicardium” | ATT (4 drugs); prednisone | NP | Positive |
| Martínez et al. [31] 2019 Argentina | 1 | NS 16 y | Absent | Hepatomegaly, ascites and abdominal distension, increased IVC, abdominal pain, and weight gain (due to hepatomegaly) | Culture (NR) | “Severely thickened, rigid, and adhered pericardium with myocardial akinesia, fibrosis and lymphocytic infiltration” | “Biauricular dilation, mitral valve prolapse, mild to moderate mitral regurgitation, dyskinetic interventricular septum; increased pulmonary pressure” (cardiac catheterization) | HRZE; furosemide *, carvedilol *, enalapri l * | Pericardiectomy | Positive |
| Kumar et al. [32] 2019 India | 1 | M 7 y | fever, emaciation, pallor | dyspnea, engorged neck veins, ascites, tense abdomen, muffled heart sounds | Elevated ADA (ascitic fluid) | “Thick, whitish and glistening pericardium” | “Thickened pericardium, paradoxical movement of interatrial septum” | ATT | anterior pericardiectomy | Positive |
| Obihara et al. [19] 2018 South Africa | 30 | F (12) M (18) 3.9 y (median) | Fever (13), cough (20), weight loss (25), night sweats (4), Abdominal pain (4), diarrhea (4), vomiting (4), lymphadenopathy (19), clubbing (4), rash (3), Peripheral lymphadenopathy (13) | Hepatomegaly (21), splenomegaly (6), dyspnea (8), chest pain (3), distended abdomen (10), malnutrition edemas (7), tachypnea (7), pericardial friction rub (3) | Culture (respiratory specimens) (19); Culture (lymph node aspirates) (6); Culture (pericardial fluid/tissue) (2); Culture (cerebrospinal fluid) (1) GenoType MTBDRplus (2 MDR, 1 RMR) | NR | Pericardial effusion (29) | ATT (30), second-line agents † (3); prednisone (24); diuretics * (3) | Pericardiocentesis (465 mL median) (5); Pericardiectomy (2) | Positive (20); Negative (1); NR (9) |
| Girit et al. [33] 2018 Turkey | 1 | F 15 y | Fever, cough | Severe exertional dyspnea, orthopnea, chest pain, JV distension, pulsus paradoxus, decreased heart sounds, tachycardia | PCR (pericardial fluid) elevated ADA (pericardial fluid) | Necrotic granulomatous reaction | Massive pericardial effusion and fibrin-coated mass adjacent to RV; low QRS voltage (ECG) | HRZE; corticosteroids | emergency pericardiocentesis (1 L of hemorrhagic fluid drained); partial pericardiectomy and abscess drainage (median sternotomy) | Positive |
| Dayal et al. [34] 2018 India | 1 | M 10 y | Absent | Anasarca (sole initial presentation), hepatomegaly, ascites (abdomen US), tachycardia, tachypnea | AFB (pericardial biopsy), elevated ADA (pericardial fluid) | “Dense adhesions between pericardium and pleural cavity, purulent fluid, multiple epithelioid granulomas” | Moderate pericardial effusion, organized pericardial collections | HRZE; steroids | Pericardiectomy; catheterization | Positive |
| Abreu Suárez et al. [35] 2018 Cuba | 1 | F 17 y | Fever, weight loss, subcarinal and mediastinal lymphadenopathy | Absent | Culture (biopsy of pre-sternal lesion) | NP | NR | ATT (5 drugs); steroids | Pericardial drainage | Positive |
| Igoche et al. [36] 2017 Nigeria | 1 | M 4 y | Fever, cough, weight loss, generalized lymphadenopathy | Hepatomegaly increased JVP, distended neck veins, tachypnea, tachycardia, distant heart sounds | Absent | NP | Massive circumferential pericardial effusion, heart swinging, RA and RV collapse | ATT | Pericardiocentesis (180 mL of creamy pus), Pericardiostomy | Positive |
| Jakimów-Kostrzewa [37] 2017 Poland | 1 | F 1.5 y | High fever, cough, mediastinal and hiatal lymphadenomegaly | Dyspnea | “Microbiological and genetic analysis” | NP | “Banana-like shaped encapsulated mass” | ATT | Surgical removal of the mass | Positive |
| Melit et al. [38] 2017 Romania | 1 | M 15 y | Fever, cough, malaise, rhinorrhea, saburral tongue, hyperemic pharynx, hypertrophic tonsils, dizziness, vomiting | Orthopnea, thoracic pain, bleared cardiac sounds, arterial hypotension | PCR (pericardial fluid) | Aspecific evidence of inflammatory cells | Pericardial effusion | ATT; furosemide *, spironolactone *; cephalosporin *, aminoglycoside *, meropenem *, vancomycin *, fluoroquinolone * | Pericardiocentesis (150 mL of serohemorrhagic fluid) | Positive |
| Chiu et al. [39] 2016 Taiwan | 1 | M 4 y | Cough | Dyspnea, hepatomegaly, JV engorgement, tachycardia, tachypnea, subcostal retractions | Culture (pericardial surgical specimen) | Caseous necrosis (surgical specimen) | Dip and plateau pattern confirmed with cardiac catheterization (constrictive pericarditis) | ATT; corticosteroids | Pericardiectomy | Positive |
| Faustino et al. [40] 2015 Portugal | 1 | M 14 y | Low body weight | Hepatomegaly, ascites, congestion of JV, tachypnea | Culture (sputum) (11 y) | Thickened, adherent, non-calcified pericardium with extensive fibrosis | Enlargement of RA, RV, IVC, and suprahepatic veins, septal bounce; “constrictive pericarditis suspicion confirmed with cardiac catheterization” | ATT; diuretics * | Pericardiectomy | Positive |
| Yoon et al. [41] 2012 South Korea | 1 | M 14 y | Fever, cough, malaise, night sweats, bilateral hilar lymphadenopathy | Tachycardia, tachypnea | AFB H-resistant (sputum) AFB (pericardial fluid) AFB (biopsy of intrapericardial masses) PCR (biopsy of intrapericardial masses) | “Soft yellowish discoid masses composed of pink, amorphous meshwork of threads”, RBC, WBC | “Free-floating multiple round discoid masses in a large amount of pericardial effusion” | HRZE, prednisone | Pericardiostomy (removal of masses) | Positive |
| Gupta et al. [42] 2012 India | 1 | M 1.5 y | Fever, pallor | Hepatomegaly, facial edema, ascites, tachycardia | Absent | “Organizing fibrinous pericarditis” (surgical specimen) | “Mild, organized pericardial effusion and thickened pericardium”; findings consistent with TOF | ATT | Pericardiectomy | Positive |
| Campagnucci et al. [43] 2012 Brazil | 1 | F 10 y | Joint pain | NR | Absent | Diffuse fibrous bands form tight adhesions. Anterior descending coronary artery compressed by fibrous bands | Pericardial effusion | ATT | Pericardiocentesis, surgical removal of mediastinal adhesions | Positive |
| Gulati et al. [44] 2011 India | 1 | M 12 y | Absent | Exertional fatigue, hepatomegaly, raised JVP, tachycardia | NR | NR | “Infiltrating mass lesion involving RV and LV; Incomplete right bundle branch block and prominent, diffuse ST-T changes, saddle-shaped ST elevation in V1” (ECG) | ATT | NP | Positive |
| Rabie et al. [45] 2010 South Africa | 1 | M 3 y | Fever, anemia | Abdominal distention due to ascites | AFB (bone marrow aspirate); Culture (bone marrow aspirate); Culture (blood) | Granulomas (bone marrow aspirate) | “Pericardial effusion with diastolic dysfunction” | HRZE, ethionamide, prednisone, methylprednisolone | NP | Positive |
| Takawira et al. [46] 2010 South Africa | 1 | M 3 y | Fever, cough, night sweats, underweight, generalized lymphadenopathy (axillary, supraclavicular, cervical, submandibular, paratracheal) | Hepatomegaly, splenomegaly, generalized body edemas, ascites, tachypnea, tachycardia, muffled heart sounds, gallop rhythm | AFB (lymph node biopsy); Culture (gastric aspirate) | Extensive fibrosis in the thoracic cavity and pericardial space (surgery); caseating granulomas (biopsy of supraclavicular lymph node) | “Loculated pericardial effusion” | HRZE; prednisone; cardiac failure treatment * | Surgery for the subaortic aneurysm | Positive |
| Lee et al. [47] 2010 South Korea | 1 | M 14 y | Fever, cough, weakness, pallor | Exertional dyspnea, hepatomegaly, tachycardia, tachypnea, systolic murmur, friction rub (after pericardiocentesis), weak peripheral pulses | Elevated ADA (pericardial fluid) | NR | “Large pericardial effusion and diastolic collapse of RV wall”; low QRS voltage and flat T waves (ECG) | NR | Emergency pericardiocentesis | Positive |
| Massoure et al. [61] 2010 Djibouti | 1 | M 16 y | NR | Cardiac tamponade | Culture (pericardial fluid), after treatment initiation | Pericardial fibrinous pockets, fibrinous strands | Thick echogenic porridge-like pericardial effusion, compression of RV and RA, IVC 20 mm; ST elevations (ECG) | HRZE | Pericardiocentesis (serosanguineous fluid), pericardiotomy and catheterization | Positive |
| El Samady et al. [48] 2009 Saudi Arabia | 1 | F 7 y | Fever, cough, weight loss | Absent | NR | NP | Pericardial calcification | ATT | NP | NR |
| Bolt et al. [60] 2007 Netherlands | 1 | F 15 y | Fever, cough, weight loss, decreased appetite, fatigue, anemia | Dyspnea, chest pain, hepatomegaly, soft cardiac tones, friction rub, tachypnea, hypotension | PCR (pericardial biopsy) | NR | “Large pericardial effusion with exudative debris and fibrin strands” | HRZE; prednisolone | Pericardiocentesis | Positive |
| Çetin et al. [49] 2005 Turkey | 1 | M 8 y | Absent, abdominal pain, arthralgia | Massive hepatosplenomegaly, anasarca, ascites, distended neck veins, dilated IVC, pulsus paradoxus, tachycardia, gallop rhythm | Absent | Fibrinous pericarditis; pericardium infiltrated with lymphoid aggregates, fibrin, and capillary proliferation (autoptic finding) | “Four-sided pericardial effusion, massively thickened pericardial wall, massive dilatation of RA and RV” | ATT (2 drugs); diuretic and positive inotropic therapy * | Pericardiectomy | Negative |
| Sharifi-Mood et al. [50] 2005 Iran | 1 | F 6 y | Fever, loss of appetite | Exertional dyspnea, tachycardia, tachypnea | Absent | “Cyst in the mediastinal area extending to pericardium; necrosis with caseating granuloma” | Nonspecific ST segment and T-wave changes (ECG) | HRZE; steroids | Surgical partial removal of the cyst | Positive |
| Meyburg et al. [51] 2002 Germany | 1 | F 2.5 y | Fever, cough | Hepatomegaly, dilation of IVC, tachypnea, tachycardia | AFB (pericardial fluid); PCR (pericardial fluid); Culture (pericardial fluid) PCR (gastric aspirate) | NP | Large pericardial effusion and dilation of IVC | HRZS; prednisone | Emergency pericardiocentesis (220 mL of thick amber fluid, repeated) | Positive |
| Browne et al. [52] 2002 Australia | 1 | M 14 y | Fever | Respiratory distress, chest pain, enlarged JV, pericardial rub | AFB (sputum and pericardial fluid) | Fibrosis | “Large effusion, persistent with some adherence” | HRZ; prednisone | Pericardiocentesis (hemoserous fluid 250 mL); catheterization; pericardiectomy | Positive |
| Tutar et al. [53] 2002 Turkey | 1 | NR 4 y | NR | “Signs and symptoms of constrictive pericarditis” | NR | Histological diagnosis of TBP | Findings indicative of constrictive pericarditis | ATT | Surgical drainage of effusion, pericardiectomy | NR |
| Equi et al. [54] 2001 UK | 1 | F 10 y | Cough, lethargy, weight loss, extensive mediastinal lymphadenopathy with some calcification | Chest pain | PCR (lymph node biopsy) INNO-LiPA Rif.TB (lymph node biopsy) | Granuloma with necrosis and calcification (lymph node biopsy) | “Global pericardial effusion” | HRZ, pyridoxine | Pericardiocentesis (700 mL bloodstained fluid); Pulmonary endarterectomy and autologous pericardial patch repair | Positive |
| Maltezou et al. [21] 2000 Greece | 1 | NR 9 y | Fever, weight loss | Dyspnea, respiratory distress, chest pain, ascites | Culture (pericardial fluid) Culture (gastric aspirate) | NP | NR | HRZS; corticosteroids | Pericardiocentesis | Positive |
| Lin et al. [55] 2000 Taiwan | 1 | F 0.8 y | Fever, poor appetite | Hepatomegaly, edema | NR | “Tuberculous fibrinofibrous pericarditis” | “Solid mass originating from the thickened pericardium, compressing the heart” | ATT | Pericardiectomy | Positive |
| Weber et al. [56] 1999 Zimbabwe | 4 | F (1) 6 y | Cough and dysentery | Absent | Culture (gastric washing) | NP | Pericardial effusion | ATT | NP | Positive |
| M (1) 11 y | Unspecific complaints | Absent | Culture (gastric washing) | NP | Pericardial effusion | ATT | NP | Positive | ||
| M (1) 0.2 y | Meningitis | Absent | Culture (gastric washing) | NP | Pericardial effusion | ATT | NP | Negative | ||
| M (1) 0.9 y | Failure to thrive intestinal obstruction | Absent | Culture (gastric washing) | NP | Pericardial effusion | NP | NP | Negative | ||
| Coulter et al. [11] 1996 UK | 1 | M 14 y | Fever, anorexia, weight loss, weakness, anemia, lymphadenomegaly (mediastinal and pretracheal) | Chest pain, hepatomegaly, tachypnea, decreased exercise tolerance, pulsus paradoxus, increased JVP, quiet heart sounds, tachycardia | Culture (pericardial aspirate, sputum); AFB (sputum) | NP | Large pericardial effusion with atrial compression, pericardial thickening, poor LV function, and mild tricuspid regurgitation; widespread T-wave inversion (ECG) | HRZ; prednisolone; blood transfusions | Pericardiocentesis (1 L of serosanguineous fluid, repeated with 1.15 L) | Positive |
| Cohen et al. [57] 1995 US | 1 | F 12 y | Fever, anemia | Chest pain | Absent | Caseating granulomas (mediastinal mass biopsy) | Pericardial effusion | HRZ | Pericardiocentesis (180 mL of serosanguineous fluid) | Positive |
| Nelson et al. [58] 1995 US | 1 | M 16 y | Fever, lymphadenopathy, sore throat | Dyspnea, chest pain, hepatomegaly | Fluorescence microscopy (pericardial biopsy); AFB kinyoun (pericardial biopsy); PCR (pericardial fluid) | Thickened pericardium with fibrinous exudate | Large pericardial effusion with fibrinous bands; concave ST elevation, decreased QRS (ECG) | HRZS; methylprednisolone | Pericardiocentesis (400 mL), pericardial window | Positive |
| Hugo-Hamman et al. [18] 1994 South Africa | 44 | F (24), M (20) 3.75 y (median) | Fever (28), cough (31), weight loss (16), Abdominal pain (5), vomiting (3), anemia (32) | Dyspnea (34), chest pain (13), hepatomegaly (34), elevated JVP (34), pulmonary edema (9), edema (7), pulsus paradoxus (18), friction rub (8) | Culture (pericardial fluid) (1); AFB (pericardial fluid) (1); Culture (pericardial biopsy) (1); AFB (pericardial biopsy) (1); AFB (gastric aspirate) (2); AFB (lymph node biopsy) (2) elevated ADA (pericardial fluid) (14) | Histologic features of MTB on pericardial biopsy (1) | Indicative signs of pericarditis (44), variable size effusion (37), thickened echo-bright pericardium (4), small effusion with a thick caseous coating (3); Complete ECG (28), low voltage QRS (7), diffuse T wave inversion (8), ST changes (3) | HRZ (44), E (39), prednisolone (18) | Pericardiectomy (5), pericardiocentesis (20), pericardial window (14) | Positive (43), Negative (1) |
| Kher et al. [59] 1990 India | 1 | F 6.5 y | Fever | Dyspnea, hepatomegaly, splenomegaly, lower limbs edemas, ascites, increased JVP, tachycardia, decreased heart sounds | ‘TB antigen’ (pericardial fluid) | Chronic inflammation (biopsy) | Low voltage QRS and elevated ST segments (ECG) | ATT | Pericardiocentesis (hemorrhagic fluid) | Positive |
| Relevant Reported Signs and Symptoms | Number of Patients | Frequency |
|---|---|---|
| fever | 76 | 62% |
| hepatomegaly | 74 | 60% |
| cough | 72 | 59% |
| dyspnea | 64 | 52% |
| weight loss or low body weight | 60 | 49% |
| increased JVP or JV turgor | 46 | 37% |
| chest pain | 28 | 23% |
| lymphadenopathy | 24 | 20% |
| tachypnea | 23 | 19% |
| edema | 22 | 18% |
| pulsus paradoxus | 21 | 17% |
| tachycardia | 17 | 14% |
| abdominal distention | 16 | 13% |
| ascites | 15 | 12% |
| muffled heart sounds | 13 | 11% |
| friction rub | 13 | 11% |
| abdominal pain | 12 | 10% |
| splenomegaly | 10 | 8% |
| pulmonary edema or orthopnea | 10 | 8% |
| vomiting | 9 | 7% |
| night sweats | 8 | 7% |
| fatigue | 9 | 7% |
| gallop rhythm | 4 | 3% |
| Other TB Manifestations | Number of Cases | Frequency |
|---|---|---|
| Pulmonary TB | 53 | 42% |
| Pleural effusion | 46 | 37% |
| Lymphadenopathy | 37 | 30% |
| Disseminated or miliary TB | 19 | 15% |
| Abdominal TB | 18 | 14% |
| TB Meningitis | 4 | 3% |
| Cardiac TB | 4 | 3% |
| TB Abscesses | 3 | 2% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venuti, L.; Condemi, A.; Albano, C.; Boncori, G.; Garbo, V.; Bagarello, S.; Cascio, A.; Colomba, C. Tuberculous Pericarditis in Childhood: A Case Report and a Systematic Literature Review. Pathogens 2024, 13, 110. https://doi.org/10.3390/pathogens13020110
Venuti L, Condemi A, Albano C, Boncori G, Garbo V, Bagarello S, Cascio A, Colomba C. Tuberculous Pericarditis in Childhood: A Case Report and a Systematic Literature Review. Pathogens. 2024; 13(2):110. https://doi.org/10.3390/pathogens13020110
Chicago/Turabian StyleVenuti, Laura, Anna Condemi, Chiara Albano, Giovanni Boncori, Valeria Garbo, Sara Bagarello, Antonio Cascio, and Claudia Colomba. 2024. "Tuberculous Pericarditis in Childhood: A Case Report and a Systematic Literature Review" Pathogens 13, no. 2: 110. https://doi.org/10.3390/pathogens13020110
APA StyleVenuti, L., Condemi, A., Albano, C., Boncori, G., Garbo, V., Bagarello, S., Cascio, A., & Colomba, C. (2024). Tuberculous Pericarditis in Childhood: A Case Report and a Systematic Literature Review. Pathogens, 13(2), 110. https://doi.org/10.3390/pathogens13020110








