Causal Mediation Analysis of Foodborne Salmonella Outbreaks in the United States: Serotypes and Food Vehicles
Abstract
1. Introduction
2. Materials and Methods
2.1. Dataset
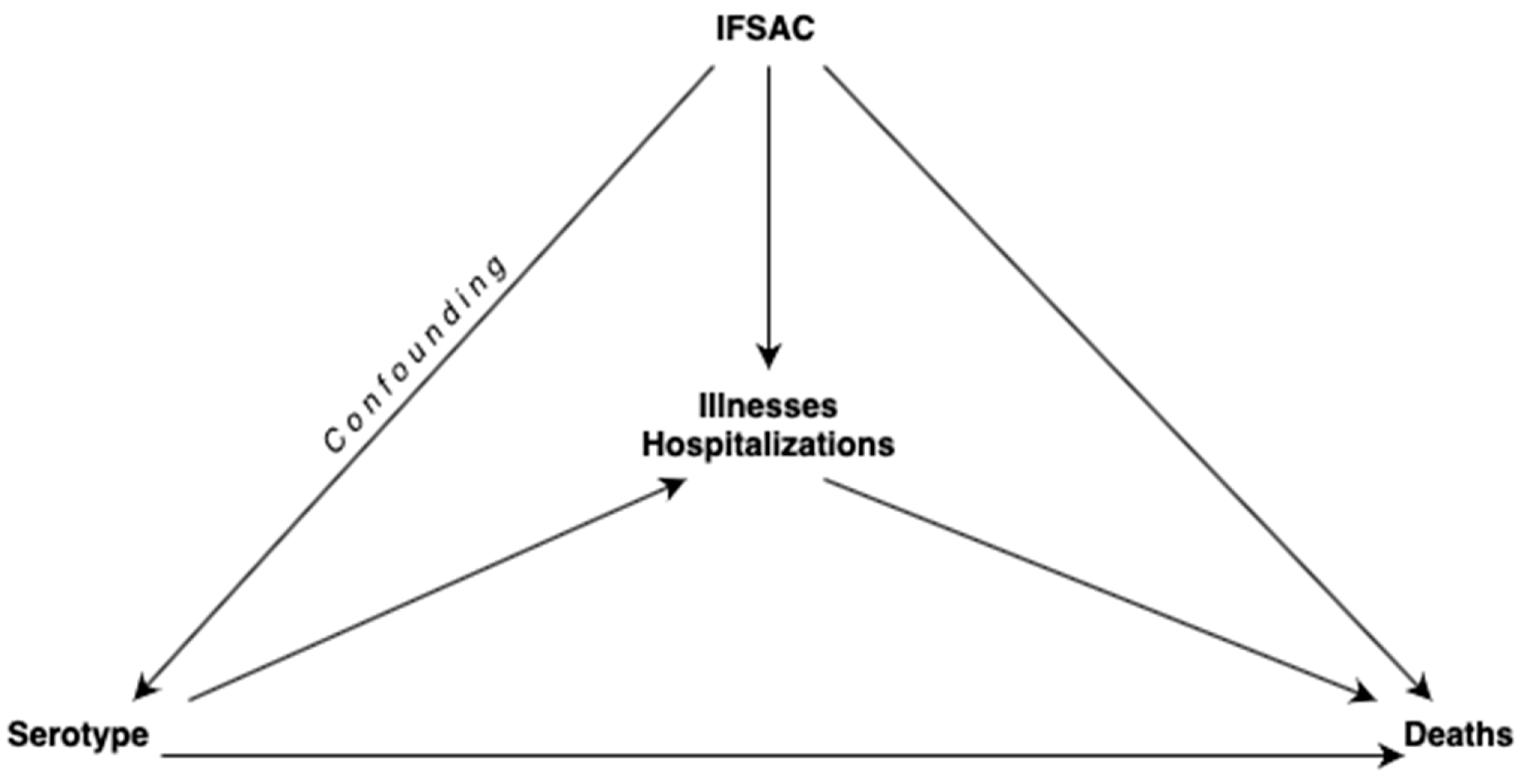
2.2. Causal Mediation Analysis with Two Mediators
2.2.1. Assignment of Mediators
2.2.2. Negative Binomial Regression Model
2.2.3. Mediation Analysis
- where and are the active and the reference values for exposure (), and is the value at which the mediators are controlled. represents the counterfactual outcome mediators that could have been seen had the exposure been set to be . indicates the counterfactual value of the outcome that could have been seen had exposure been set to be and the mediator to be .
3. Results
3.1. Frequency of Serotypes Linked to Salmonella Outbreaks
3.2. Frequency of IFSAC Food Categories Linked to Salmonella Outbreaks
3.3. Data Summary for Frequency of Serotypes Linked to Salmonella Outbreaks
3.4. Illness, Hospitalization, and Death Counts by the Negative Binomial Regression Model
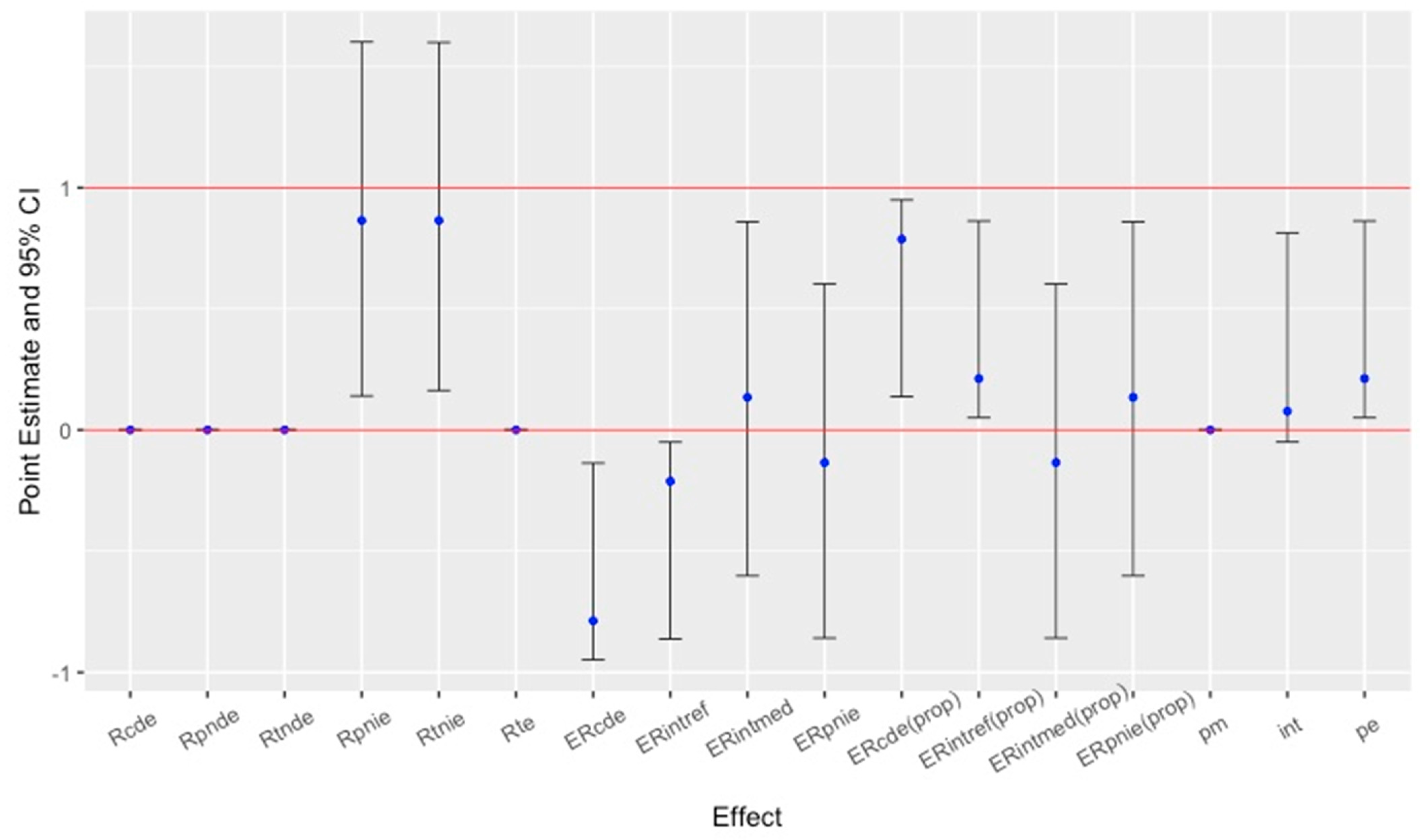
3.5. Results of Causal Role of Mediators
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.V.; Widdowson, M.-A.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne Illness Acquired in the United States—Major Pathogens. Emerg. Infect. Dis. 2011, 17, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Jackson, B.R.; Griffin, P.M.; Cole, D.; Walsh, K.A.; Chai, S.J. Outbreak-Associated Salmonella enterica Serotypes and Food Commodities, United States, 1998–2008. Emerg. Infect. Dis. 2013, 19, 1239–1244. [Google Scholar] [CrossRef] [PubMed]
- Canning, M.; Birhane, M.G.; Dewey-Mattia, D.; Lawinger, H.; Cote, A.; Gieraltowski, L.; Schwensohn, C.; Tagg, K.A.; Francois Watkins, L.K.; Park Robyn, M.; et al. Salmonella Outbreaks Linked to Beef, United States, 2012–2019. J. Food Prot. 2023, 86, 100071. [Google Scholar] [CrossRef] [PubMed]
- Crowe, S.J.; Mahon, B.E.; Vieira, A.R.; Gould, L.H. Vital Signs: Multistate Foodborne Outbreaks—United States, 2010–2014. MMWR Morb. Mortal. Wkly. Rep. 2015, 64, 1221–1225. [Google Scholar] [CrossRef] [PubMed]
- Gould, L.H.; Walsh, K.A.; Vieira, A.R.; Herman, K.; Williams, I.T.; Hall, A.J.; Cole, D.; Centers for Disease Control and Prevention. Surveillance for Foodborne Disease Outbreaks-United States, 1998–2008. Morb. Mortal. Wkly. Report. Surveill. Summ. 2013, 62, 1–34. [Google Scholar]
- Marus, J.R.; Magee, M.J.; Manikonda, K.; Nichols, M.C. Outbreaks of Salmonella enterica Infections Linked to Animal Contact: Demographic and Outbreak Characteristics and Comparison to Foodborne Outbreaks—United States, 2009–2014. Zoonoses Public Health 2019, 66, 370–376. [Google Scholar] [CrossRef]
- Snyder, T.R.; Boktor, S.W.; M’ikanatha, N.M. Salmonellosis Outbreaks by Food Vehicle, Serotype, Season, and Geographical Location, United States, 1998 to 2015. J. Food Prot. 2019, 82, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.A.; Eade, C.R.; Wiedmann, M. Embracing Diversity: Differences in Virulence Mechanisms, Disease Severity, and Host Adaptations Contribute to the Success of Nontyphoidal Salmonella as a Foodborne Pathogen. Front. Microbiol. 2019, 10, 1368. [Google Scholar] [CrossRef] [PubMed]
- Dewey-Mattia, D.; Manikonda, K.; Hall, A.J.; Wise, M.E.; Crowe, S.J. Surveillance for Foodborne Disease Outbreaks—United States, 2009–2015. MMWR Surveill. Summ. 2018, 67, 1–11. [Google Scholar] [CrossRef]
- Jones, T.F.; Ingram, L.A.; Cieslak, P.R.; Vugia, D.J.; Tobin-D’Angelo, M.; Hurd, S.; Medus, C.; Cronquist, A.; Angulo, F.J. Salmonellosis Outcomes Differ Substantially by Serotype. J. Infect. Dis. 2008, 198, 109–114. [Google Scholar] [CrossRef]
- O’Rourke, H.P.; Vazquez, E. Mediation Analysis with Zero-Inflated Substance Use Outcomes: Challenges and Recommendations. Addict. Behav. 2019, 94, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Cheng, N.F.; Guo, Z.; Gregorich, S.; Ismail, A.I.; Gansky, S.A. Mediation Analysis for Count and Zero-Inflated Count Data. Stat. Methods Med. Res. 2017, 27, 2756–2774. [Google Scholar] [CrossRef] [PubMed]
- Thomson, J.L.; Landry, A.S.; Walls, T.I. Direct and Indirect Effects of Food and Nutrition Security on Dietary Choice and Healthfulness of Food Choice: Causal Mediation Analysis. Curr. Dev. Nutr. 2024, 8, 102081. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Coffman, D.L.; Luta, G.; Niaura, R.S. Tutorial on Causal Mediation Analysis with Binary Variables: An Application to Health Psychology Research. Health Psychol. 2023, 42, 778–787. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zheng, C.; Kim, C.; Van Poucke, S.; Lin, S.; Lan, P. Causal Mediation Analysis in the Context of Clinical Research. Ann. Transl. Med. 2016, 4, 425. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.J.; Wikswo, M.E.; Manikonda, K.; Roberts, V.A.; Yoder, J.S.; Gould, L.H. Acute Gastroenteritis Surveillance through the National Outbreak Reporting System, United States. Emerg. Infect. Dis. 2013, 19, 1305–1309. [Google Scholar] [CrossRef]
- Dewey-Mattia, D.; Roberts, V.A.; Vieira, A.; Fullerton, K.E. Foodborne (1973–2013) and Waterborne (1971–2013) Disease Outbreaks—United States. MMWR Morb. Mortal. Wkly. Rep. 2016, 63, 79–84. [Google Scholar] [CrossRef]
- Nsoesie, E.O.; Kluberg, S.A.; Brownstein, J.S. Online Reports of Foodborne Illness Capture Foods Implicated in Official Foodborne Outbreak Reports. Prev. Med. 2014, 67, 264–269. [Google Scholar] [CrossRef]
- White, A.E.; Tillman, A.R.; Hedberg, C.; Bruce, B.B.; Batz, M.; Seys, S.A.; Dewey-Mattia, D.; Bazaco, M.C.; Walter, E.S. Foodborne Illness Outbreaks Reported to National Surveillance, United States, 2009–2018. Emerg. Infect. Dis. 2022, 28, 1117–1127. [Google Scholar] [CrossRef]
- Valeri, L.; Vanderweele, T.J. Mediation Analysis Allowing for Exposure-Mediator Interactions and Causal Interpretation: Theoretical Assumptions and Implementation with SAS and SPSS Macros. Psychol. Methods 2013, 18, 137–150. [Google Scholar] [CrossRef]
- VanderWeele, T.; Vansteelandt, S. Mediation Analysis with Multiple Mediators. Epidemiol. Methods 2014, 2, 95–115. [Google Scholar] [CrossRef]
- VanderWeele, T.J. Explanation in Causal Inference: Methods for Mediation and Interaction; Oxford University Press: Oxford, UK, 2015. [Google Scholar]
- Chin, H.C.; Quddus, M.A. Modeling Count Data with Excess Zeroes. Sociol. Methods Res. 2003, 32, 90–116. [Google Scholar] [CrossRef]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Springer: New York, NY, USA, 2002; ISBN 0-387-95457-0. [Google Scholar]
- BS1125. GitHub-BS1125/CMAverse: A Suite of Functions for Reproducible Causal Mediation Analyses; GitHub: San Francisco, CA, USA, 2021; Available online: https://github.com/BS1125/CMAverse (accessed on 17 December 2024).
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 17 December 2024).
- Popa, G.L.; Papa, M.I. Salmonella Spp. Infection–A Continuous Threat Worldwide. GERMS 2021, 11, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Li, F.; Dong, N.; Tian, S.; Zhang, H.; Du, X.; Zhou, X.; Xu, X.; Yang, H.; Xie, J.; et al. Investigation of a Salmonellosis Outbreak Caused by Multidrug Resistant Salmonella Typhimurium in China. Front. Microbiol. 2020, 11, 801. [Google Scholar] [CrossRef]
- Alt, K.; Simon, S.; Helmeke, C.; Kohlstock, C.; Prager, R.; Tietze, E.; Rabsch, W.; Karagiannis, I.; Werber, D.; Frank, C.; et al. Outbreak of Uncommon O4 Non-Agglutinating Salmonella Typhimurium Linked to Minced Pork, Saxony-Anhalt, Germany, January to April 2013. PLoS ONE 2015, 10, e0128349. [Google Scholar] [CrossRef]
- Folster, J.P.; Grass, J.E.; Bicknese, A.; Taylor, J.; Friedman, C.R.; Whichard, J.M. Characterization of Resistance Genes and Plasmids from Outbreaks and Illness Clusters Caused by Salmonella Resistant to Ceftriaxone in the United States, 2011–2012. Microb. Drug Resist. 2017, 23, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Nichols, M.; Gollarza, L.; Sockett, D.; Aulik, N.; Patton, E.; Francois Watkins, L.K.; Gambino-Shirley, K.J.; Folster, J.P.; Chen, J.C.; Tagg, K.A.; et al. Outbreak of Multidrug-Resistant Salmonella Heidelberg Infections Linked to Dairy Calf Exposure, United States, 2015–2018. Foodborne Pathog. Dis. 2022, 19, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Punchihewage-Don, A.J.; Hawkins, J.; Adnan, A.M.; Hashem, F.; Parveen, S. The Outbreaks and Prevalence of Antimicrobial Resistant Salmonella in Poultry in the United States: An Overview. Heliyon 2022, 8, e11571. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.C.; Grass, J.E.; Richardson, L.C.; Nisler, A.L.; Bicknese, A.S.; Gould, L.H. Antimicrobial Resistance in Salmonella That Caused Foodborne Disease Outbreaks: United States, 2003–2012. Epidemiol. Infect. 2016, 145, 766–774. [Google Scholar] [CrossRef]
- Barton Behravesh, C.; Mody, R.K.; Jungk, J.; Gaul, L.; Redd, J.T.; Chen, S.; Cosgrove, S.; Hedican, E.; Sweat, D.; Chávez-Hauser, L.; et al. 2008 Outbreak of Salmonella Saintpaul Infections Associated with Raw Produce. N. Engl. J. Med. 2011, 364, 918–927. [Google Scholar] [CrossRef] [PubMed]
- Taylor, E.; Kastner, J.; Renter, D. Challenges Involved in the Salmonella Saintpaul Outbreak and Lessons Learned. J. Public Health Manag. Pract. 2010, 16, 221–231. [Google Scholar] [CrossRef]
- Chen, R.; Cheng, R.A.; Wiedmann, M.; Orsi, R.H. Development of a Genomics-Based Approach to Identify Putative Hypervirulent Nontyphoidal Salmonella Isolates: Salmonella enterica Serovar Saintpaul as a Model. mSphere 2022, 7, e0073021. [Google Scholar] [CrossRef] [PubMed]
- McGeoch, L.J.; Hoban, A.; Sawyer, C.; Rabie, H.; Painset, A.; Browning, L.; Brown, D.; McCarthy, C.; Nelson, A.; Firme, A.; et al. Salmonella Saintpaul Outbreak Associated with Cantaloupe Consumption, the United Kingdom and Portugal, September to November 2023. Epidemiol. Infect. 2024, 152, e78. [Google Scholar] [CrossRef]
- Munnoch, S.A.; Ward, K.; Sheridan, S.; Fitzsimmons, G.J.; Shadbolt, C.T.; Piispanen, J.P.; Wang, Q.; Ward, T.J.; Worgan, T.L.M.; Oxenford, C.; et al. A Multi-State Outbreak of Salmonella Saintpaul in Australia Associated with Cantaloupe Consumption. Epidemiol. Infect. 2009, 137, 367. [Google Scholar] [CrossRef] [PubMed]
- Laughlin, M.; Bottichio, L.; Weiss, J.; Higa, J.; McDonald, E.; Sowadsky, R.; Fejes, D.; Saupe, A.; Provo, G.; Seelman, S.; et al. Multistate Outbreak of Salmonella Poona Infections Associated with Imported Cucumbers, 2015–2016. Epidemiol. Infect. 2019, 147, e270. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control; European Food Safety Authority. Multi-Country Outbreak of Salmonella Poona Infections Linked to Consumption of Infant Formula. EFSA Support. Publ. 2019, 16, 1594E. [Google Scholar] [CrossRef]
- Morasi, R.M.; Rall, V.L.M.; Dantas, S.T.A.; Alonso, V.P.P.; Silva, N.C.C. Salmonella spp. In Low Water Activity Food: Occurrence, Survival Mechanisms, and Thermoresistance. J. Food Sci. 2022, 87, 2310–2323. [Google Scholar] [CrossRef] [PubMed]
- Basler, C.; Nguyen, T.-A.; Anderson, T.C.; Hancock, T.; Behravesh, C.B. Outbreaks of Human Salmonella Infections Associated with Live Poultry, United States, 1990–2014. Emerg. Infect. Dis. 2016, 22, 1705–1711. [Google Scholar] [CrossRef] [PubMed]
- Moffatt, C.R.; Musto, J. Salmonella and Egg-Related Outbreaks. Microbiol. Aust. 2013, 34, 94. [Google Scholar] [CrossRef]
- Cardoso, M.J.; Nicolau, A.I.; Borda, D.; Nielsen, L.; Maia, R.L.; Møretrø, T.; Ferreira, V.; Knøchel, S.; Langsrud, S.; Teixeira, P. Salmonella in Eggs: From Shopping to Consumption—A Review Providing an Evidence-Based Analysis of Risk Factors. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2716–2741. [Google Scholar] [CrossRef] [PubMed]
- Chousalkar, K.; Gast, R.; Martelli, F.; Pande, V. Review of Egg-Related Salmonellosis and Reduction Strategies in United States, Australia, United Kingdom and New Zealand. Crit. Rev. Microbiol. 2017, 44, 290–303. [Google Scholar] [CrossRef] [PubMed]
- Hanning, I.B.; Nutt, J.D.; Ricke, S.C. Salmonellosis Outbreaks in the United States due to Fresh Produce: Sources and Potential Intervention Measures. Foodborne Pathog. Dis. 2009, 6, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Topalcengiz, Z.; Danyluk, M.D. Fate of Generic and Shiga Toxin-Producing Escherichia Coli (STEC) in Central Florida Surface Waters and Evaluation of EPA Worst Case Water as Standard Medium. Food Res. Int. 2019, 120, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Topalcengiz, Z.; Mcegan, R.; Danyluk, M.D. Fate of Salmonella in Central Florida Surface Waters and Evaluation of EPA Worst Case Water as a Standard Medium. J. Food Prot. 2019, 82, 916–925. [Google Scholar] [CrossRef]
- Topalcengiz, Z.; Jeamsripong, S.; Spanninger, P.; Persad, A.; Wang, F.; Buchanan, R.L.; LeJeune, J.; Kniel, K.E.; Jay-Russell, M.; Danyluk, M.D. Survival of Shiga Toxin–Producing Escherichia coli in Various Wild Animal Feces That May Contaminate Produce. J. Food Prot. 2020, 83, 1420–1429. [Google Scholar] [CrossRef] [PubMed]
- Topalcengiz, Z.; Spanninger, P.; Jeamsripong, S.; Persad, A.; Buchanan, R.L.; Saha, J.; LeJeune, J.; Jay-Russell, M.; Kniel, K.E.; Danyluk, M.D. Survival of Salmonella in Various Wild Animal Feces That May Contaminate Produce. J. Food Prot. 2020, 83, 651–660. [Google Scholar] [CrossRef]
- Dyda, A.; Nguyen, P.Y.; Chughtai, A.A.; MacIntyre, C.R. Changing Epidemiology of Salmonella Outbreaks Associated with Cucumbers and Other Fruits and Vegetables. Glob. Biosecurity 2020, 2. [Google Scholar]
- Callejón, R.M.; Rodríguez-Naranjo, M.I.; Ubeda, C.; Hornedo-Ortega, R.; Garcia-Parrilla, M.C.; Troncoso, A.M. Reported Foodborne Outbreaks due to Fresh Produce in the United States and European Union: Trends and Causes. Foodborne Pathog. Dis. 2015, 12, 32–38. [Google Scholar] [CrossRef]
- Krishnasamy, V.P.; Marshall, K.; Dewey-Mattia, D.; Wise, M. Outbreak Characteristics and Epidemic Curves for Multistate Outbreaks of Salmonella Infections Associated with Produce: United States, 2009–2015. Foodborne Pathog. Dis. 2019, 17, 15–22. [Google Scholar] [CrossRef]
- Laufer, A.S.; Grass, J.; Holt, K.; Whichard, J.M.; Griffin, P.M.; Gould, L.H. Outbreaks of Salmonella Infections Attributed to Beef–United States, 1973–2011. Epidemiol. Infect. 2014, 143, 2003–2013. [Google Scholar] [CrossRef]
- Strickland, A.J.; Sampedro, F.; Hedberg, C.W. Quantitative Risk Assessment of Salmonella in Ground Beef Products and the Resulting Impact of Risk Mitigation Strategies on Public Health. J. Food Prot. 2023, 86, 100093. [Google Scholar] [CrossRef] [PubMed]
- Hilbe, J.M. Negative Binomial Regression; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar] [CrossRef]
- Benjamin, L.; Atwill, E.R.; Jay-Russell, M.; Cooley, M.; Carychao, D.; Gorski, L.; Mandrell, R.E. Occurrence of Generic Escherichia coli, E. coli O157 and Salmonella Spp. In Water and Sediment from Leafy Green Produce Farms and Streams on the Central California Coast. Int. J. Food Microbiol. 2013, 165, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Buyrukoğlu, G.; Buyrukoğlu, S.; Topalcengiz, Z. Comparing Regression Models with Count Da-ta to Artificial Neural Network and Ensemble Models for Prediction of Generic Escherichia coli Population in Agricultural Ponds Based on Weather Station Measurements. Microb. Risk Anal. 2021, 19, 100171. [Google Scholar] [CrossRef]
| Scale | Parameter * | Formula |
|---|---|---|
| Rate | ||
| Excess relative rate | ||
| Proportion excess relative rate | ||
| Overall | ||
| Serotype | Number of Outbreaks (%) | Illnesses (%) | Hospitalization (%) | Death (%) |
|---|---|---|---|---|
| Enteritidis | 815 (28.42) | 20,599 (25.12) | 1981 (17.98) | 18 (15.65) |
| Typhimuirum | 359 (12.52) | 9202 (11.22) | 1232 (11.18) | 20 (17.39) |
| Newport | 220 (7.67) | 8036 (9.80) | 1258 (11.42) | 14 (12.17) |
| * Other | 203 (7.08) | 5036 (6.14) | 739 (6.71) | 10 (8.70) |
| Heidelberg. | 165 (5.75) | 5653 (6.89) | 863 (7.83) | 10 (8.70) |
| Unknown | 139 (4.85) | 1943 (2.37) | 176 (1.60) | 2 (1.74) |
| Javiana | 95 (3.31) | 3923 (4.78) | 533 (4.84) | 5 (4.35) |
| I 4,[5],12:i:- | 88 (3.07) | 2078 (2.53) | 405 (3.68) | 5 (4.35) |
| Braenderup | 73 (2.55) | 1377 (1.68) | 226 (2.05) | 1 (0.87) |
| Multiple | 73 (2.55) | 5288 (6.45) | 678 (6.15) | 6 (5.22) |
| Infantis | 67 (2.34) | 1697 (2.07) | 230 (2.09) | 1 (0.87) |
| Montevideo | 61 (2.13) | 1913 (2.33) | 224 (2.03) | 1 (0.87) |
| Thompson | 56 (1.95) | 1256 (1.53) | 114 (1.03) | 1 (0.87) |
| Saintpaul | 53 (1.85) | 3086 (3.76) | 616 (5.59) | 2 (1.74) |
| Muenchen | 42 (1.46) | 1164 (1.42) | 96 (0.87) | 2 (1.74) |
| Oranienburg | 39 (1.36) | 1733 (2.11) | 388 (3.52) | 1 (0.87) |
| Hadar | 25 (0.87) | 565 (0.69) | 87 (0.79) | 0 |
| Berta | 25 (0.87) | 583 (0.71) | 59 (0.54) | 1 (0.87) |
| Group B | 23 (0.80) | 413 (0.50) | 48 (0.44) | 0 |
| Paratyphi B | 22 (0.77) | 447 (0.55) | 41 (0.37) | 0 |
| Agona | 22 (0.77) | 598 (0.73) | 102 (0.93) | 0 |
| Anatum | 18 (0.63) | 465 (0.57) | 41 (0.37) | 1 (0.87) |
| Uganda | 18 (0.63) | 362 (0.44) | 64 (0.58) | 0 |
| Hartford | 15 (0.52) | 250 (0.30) | 23 (0.21) | 0 |
| Schwarzengrund | 15 (0.52) | 223 (0.27) | 37 (0.34) | 1 (0.87) |
| Stanley | 15 (0.52) | 237 (0.29) | 28 (0.25) | 0 |
| Weltevreden | 15 (0.52) | 184 (0.22) | 22 (0.20) | 0 |
| Miami | 14 (0.49) | 342 (0.42) | 76 (0.69) | 1 (0.87) |
| Bareilly | 13 (0.45) | 183 (0.22) | 20 (0.18) | 0 |
| Brandenburg | 13 (0.45) | 137 (0.17) | 30 (0.27) | 0 |
| Mbandaka | 13 (0.45) | 549 (0.67) | 59 (0.54) | 0 |
| Reading | 13 (0.45) | 537 (0.65) | 142 (1.29) | 1 (0.87) |
| Wirchow | 11 (0.38) | 220 (0.27) | 21 (0.19) | 0 |
| Baildon | 10 (0.35) | 505 (0.62) | 52 (0.47) | 3 (2.61) |
| Poona | 10 (0.35) | 1097 (1.34) | 244 (2.21) | 8 (6.96) |
| Typhi | 10 (0.35) | 115 (0.14) | 63 (0.57) | 0 |
| All | 2868 (100.00) | 81,996 (100.00) | 11,018 (100.00) | 115 (100.00) |
| IFSAC Category | Number of Outbreaks (%) | Illnesses (%) | Hospitalization (%) | Death (%) |
|---|---|---|---|---|
| Uncategorized | 1308 (45.61) | 22,338 (27.24) | 3000 (27.23) | 38 (33.04) |
| Multiple | 617 (21.51) | 18,613 (22.70) | 2029 (18.42) | 13 (11.30) |
| Chicken | 177 (6.17) | 5250 (6.40) | 809 (7.34) | 6 (5.22) |
| Eggs | 160 (5.58) | 5870 (7.16) | 400 (3.63) | 3 (2.61) |
| Pork | 99 (3.45) | 3004 (3.66) | 401 (3.64) | 4 (3.48) |
| Fruits | 83 (2.89) | 4279 (5.22) | 798 (7.24) | 18 (15.65) |
| Beef | 81 (2.82) | 2703 (3.30) | 448 4.07) | 3 (2.61) |
| Turkey | 65 (2.27) | 2685 (3.27) | 360 (3.27) | 3 (2.61) |
| Seeded vegetables | 59 (2.06) | 6099 (7.44) | 1113 (10.10) | 13 (11.30) |
| Dairy | 43 (1.50) | 1206 (1.47) | 204 (1.85) | 1 (0.87) |
| Sprouts | 39 (1.36) | 1667 (2.03) | 159 (1.44) | 2 (1.74) |
| * Other | 29 (1.01) | 846 (1.03) | 156 (1.42) | 0 |
| Nuts-seeds | 22 (0.77) | 1954 (2.38) | 347 (3.15) | 11 (9.57) |
| Vegetable row crops | 22 (0.77) | 962 (1.17) | 69 (0.63) | 0 |
| Fish | 21 (0.73) | 959 (1.17) | 91 (0.83) | 0 |
| Crustaceans | 11 (0.38) | 149 (0.18) | 30 (0.27) | 0 |
| Herbs | 11 (0.38) | 571 (0.70) | 83 (0.75) | 0 |
| Other meat | 11 (0.38) | 196 (0.24) | 21 (0.19) | 0 |
| Root/Underground | 10 (0.35) | 2645 (3.23) | 500 (4.54) | 0 |
| All | 2868 (100.00) | 81,996 (100.00) | 11,018 (100.00) | 115 (100.00) |
| Frequency of Outbreaks (%) | |||||
|---|---|---|---|---|---|
| Variable | [0, 5) | [5, 10) | [10, 100) | [100, 1000) | (≥1000) |
| Illness | 620 (21.62%) | 719 (25.07%) | 1386 (48.33%) | 139 (4.85%) | 4 (0.14%) |
| Hospitalization | 2340 (81.59%) | 308 (10.74%) | 209 (7.29%) | 11 (0.38%) | |
| Death | 2866 (99.93%) | 2 (0.07%) | |||
| Variables | Dispersion | Test Statistics | p-Value |
|---|---|---|---|
| Illness | 134.5764 | 3.4620 | 0.000 *** |
| Hospitalization | 15.7473 | 5.8027 | 0.000 *** |
| Death | 1.2177 | 2.3156 | 0.010 * |
| Variable (p-Value) | |||
|---|---|---|---|
| Illness | Hospitalization | Death | |
| Serotype | Baildon (0.061.) | Heidelberg (0.000 ***) | Baildon (0.026 *) |
| Braenderup (0.018 *) | I 4,[5],12:i:- (0.001 **) | Heidelberg (0.063.) | |
| Heidelberg (0.000 ***) | Infantis (0.056.) | Saintpaul (0.000 ***) | |
| Javiana (0.000 ***) | Javiana (0.001 **) | ||
| Montevideo (0.029 *) | Miami (0.077.) | ||
| Multiple (0.000 ***) | Multiple (0.000 ***) | ||
| Saintpaul (0.010 *) | Newport (0.000 ***) | ||
| Unknown (0.000 ***) | Oranienburg (0.022 *) | ||
| Weltevreden (0.091.) | Poona (0.012 *) | ||
| Reading (0.003 **) | |||
| Saintpaul (0.000 ***) | |||
| Typhimurium (0.028 *) | |||
| Unknown (0.000 ***) | |||
| IFSAC food category | Beef (0.000 ***) | Beef (0.000 ***) | Fruits (0.001 **) |
| Chicken (0.000 ***) | Chicken (0.000 ***) | Multiple (0.048 *) | |
| Dairy (0.007 **) | Dairy (0.011 *) | ||
| Eggs (0.000 ***) | Eggs (0.076.) | ||
| Fish (0.002 **) | Fruits (0.000 ***) | ||
| Fruits (0.000 ***) | Herbs (0.007 **) | ||
| Herbs (0.002 **) | Multiple (0.000 ***) | ||
| Multiple (0.000 ***) | Nuts-seeds (0.000 ***) | ||
| Nuts-seeds (0.000 ***) | Other (0.015 *) | ||
| Other (0.022 *) | Pork (0.002 **) | ||
| Pork (0.000 ***) | Root/underground (0.000 ***) | ||
| Root/underground (0.000 ***) | Seeded vegetables (0.000 ***) | ||
| Seeded vegetables (0.000 ***) | Sprouts (0.035) | ||
| Sprouts (0.000 ***) | Turkey (0.000 ***) | ||
| Turkey (0.000 ***) | |||
| Vegetable row crops (0.000 ***) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buyrukoğlu, G.; Moreira, J.; Topalcengiz, Z. Causal Mediation Analysis of Foodborne Salmonella Outbreaks in the United States: Serotypes and Food Vehicles. Pathogens 2024, 13, 1134. https://doi.org/10.3390/pathogens13121134
Buyrukoğlu G, Moreira J, Topalcengiz Z. Causal Mediation Analysis of Foodborne Salmonella Outbreaks in the United States: Serotypes and Food Vehicles. Pathogens. 2024; 13(12):1134. https://doi.org/10.3390/pathogens13121134
Chicago/Turabian StyleBuyrukoğlu, Gonca, Juan Moreira, and Zeynal Topalcengiz. 2024. "Causal Mediation Analysis of Foodborne Salmonella Outbreaks in the United States: Serotypes and Food Vehicles" Pathogens 13, no. 12: 1134. https://doi.org/10.3390/pathogens13121134
APA StyleBuyrukoğlu, G., Moreira, J., & Topalcengiz, Z. (2024). Causal Mediation Analysis of Foodborne Salmonella Outbreaks in the United States: Serotypes and Food Vehicles. Pathogens, 13(12), 1134. https://doi.org/10.3390/pathogens13121134







