Tracing the Origin, Spread, and Molecular Evolution of Dengue Type 1 Cases That Occurred in Northern Italy in 2023
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Sequencing
2.2. Phylogenetic Analysis
2.3. Similarity Analysis
2.4. Phylodynamic and Phylogeographic Analysis
3. Results
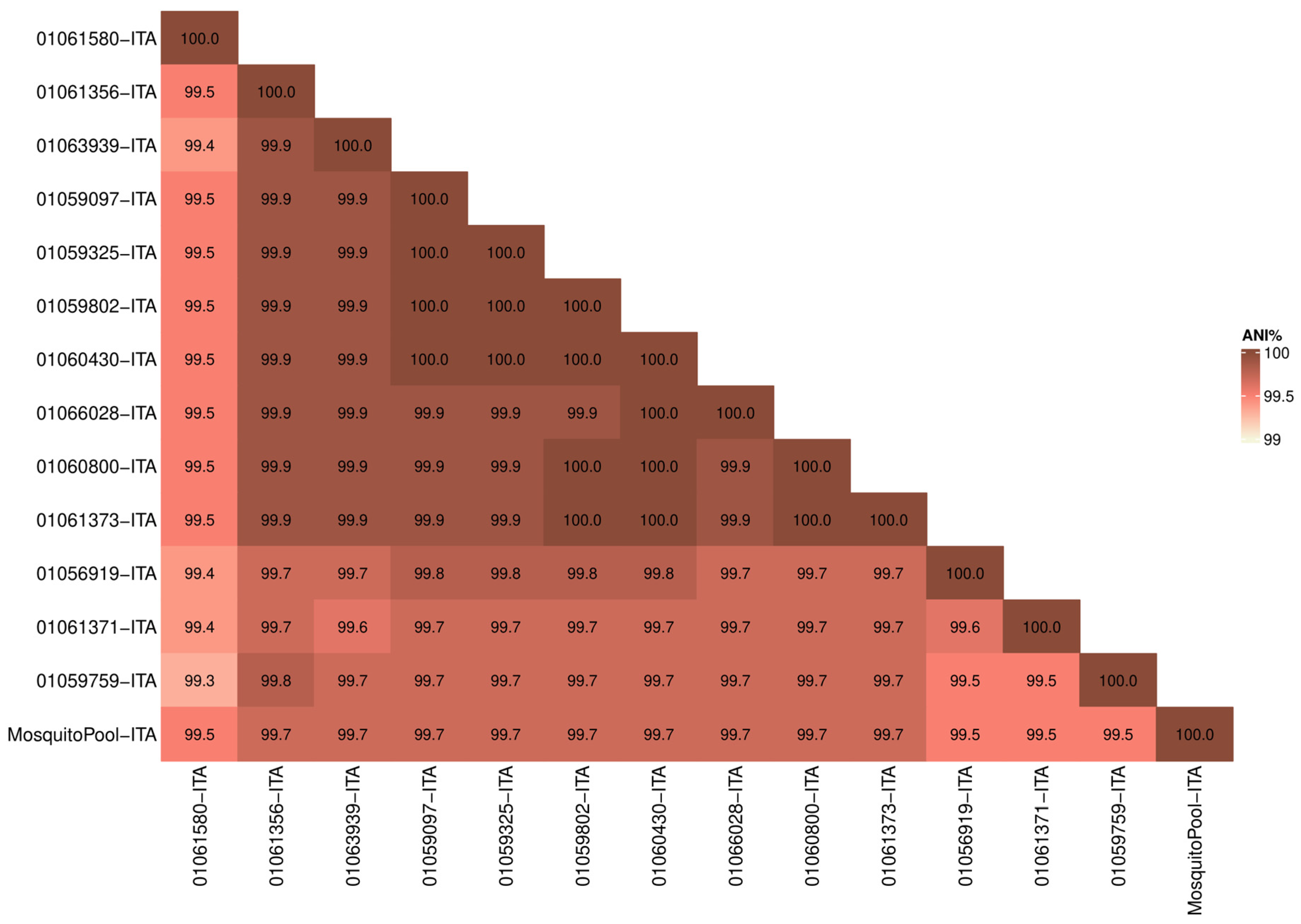
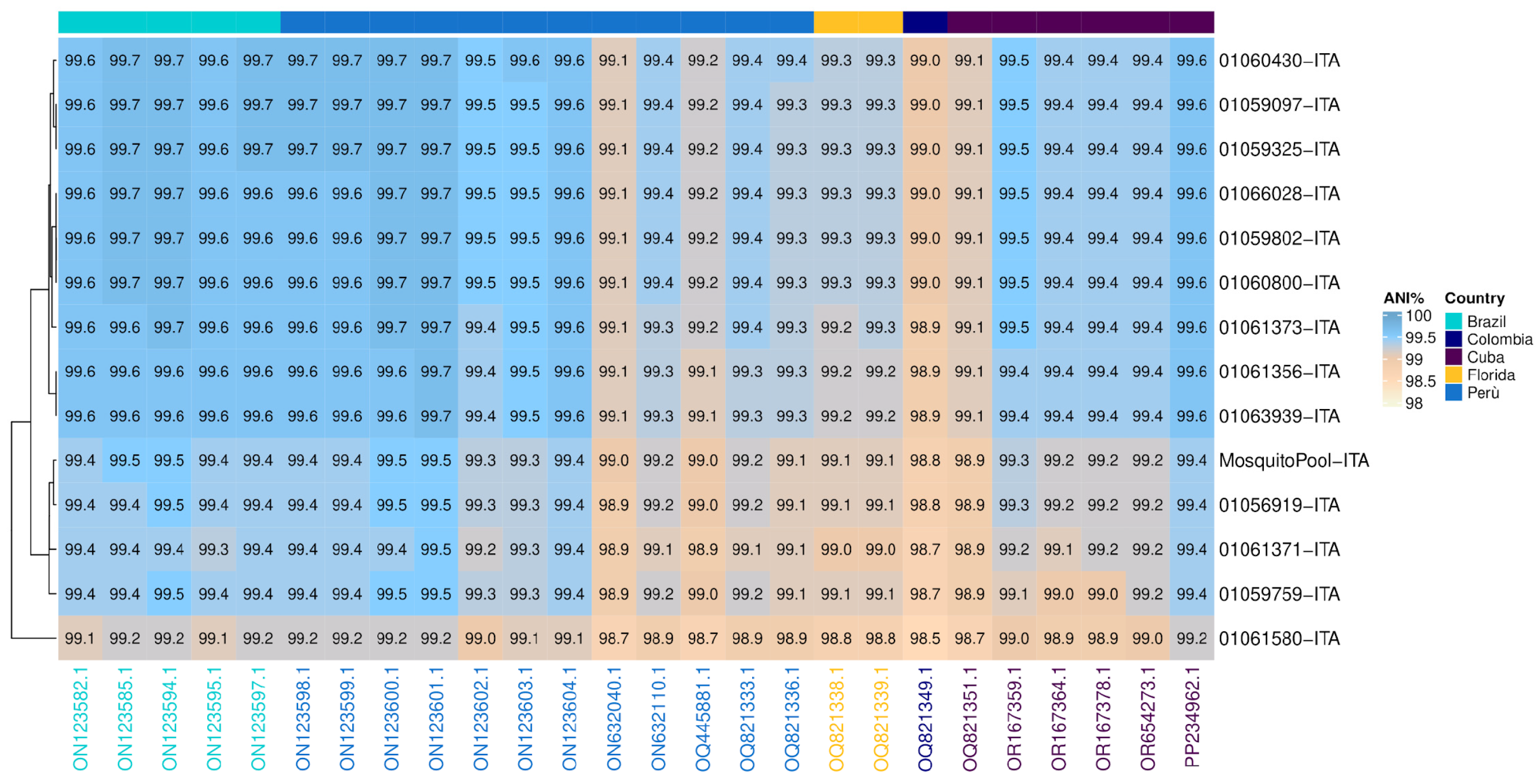
3.1. Similarity Analysis
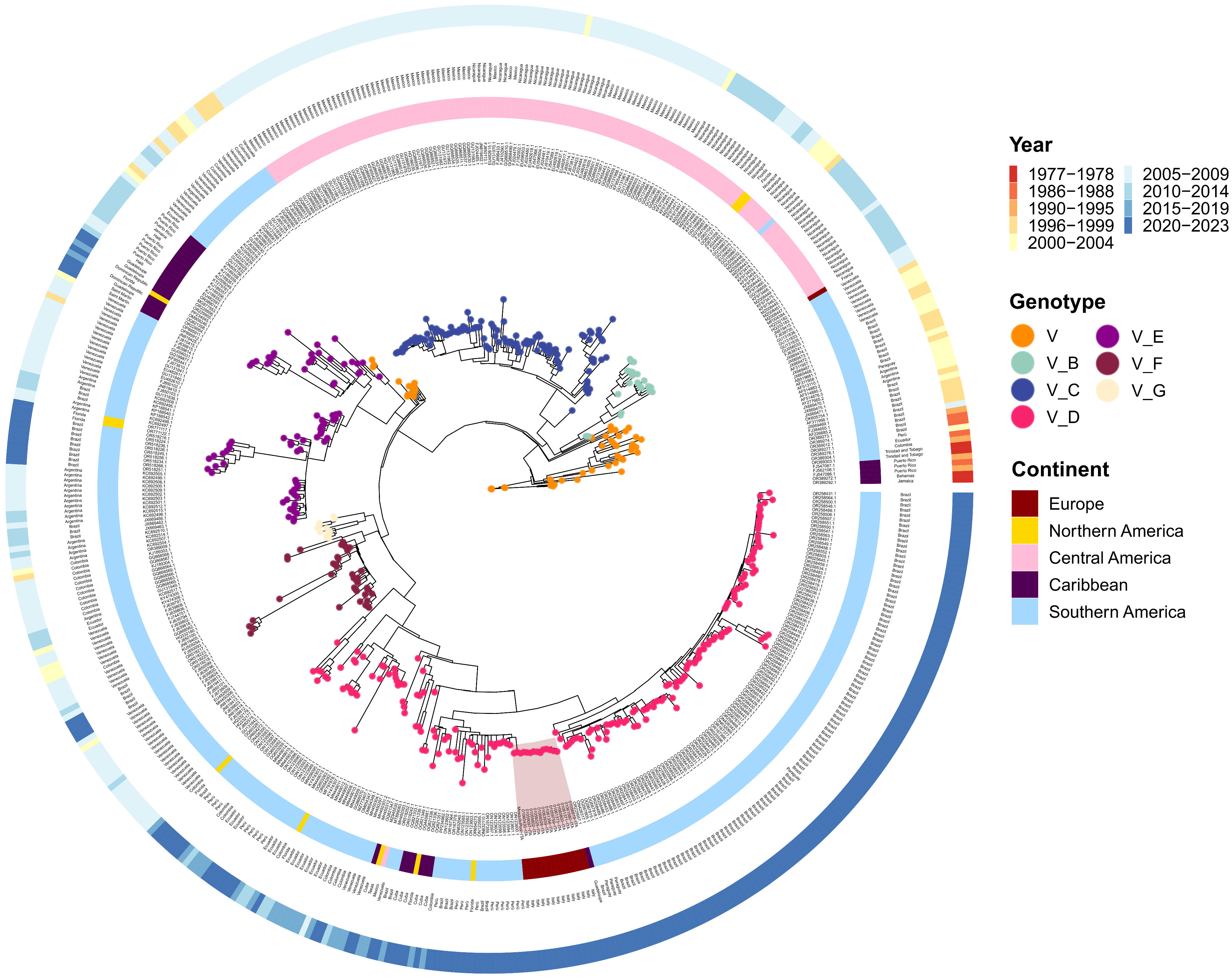
3.2. Phylogenetic Analysis
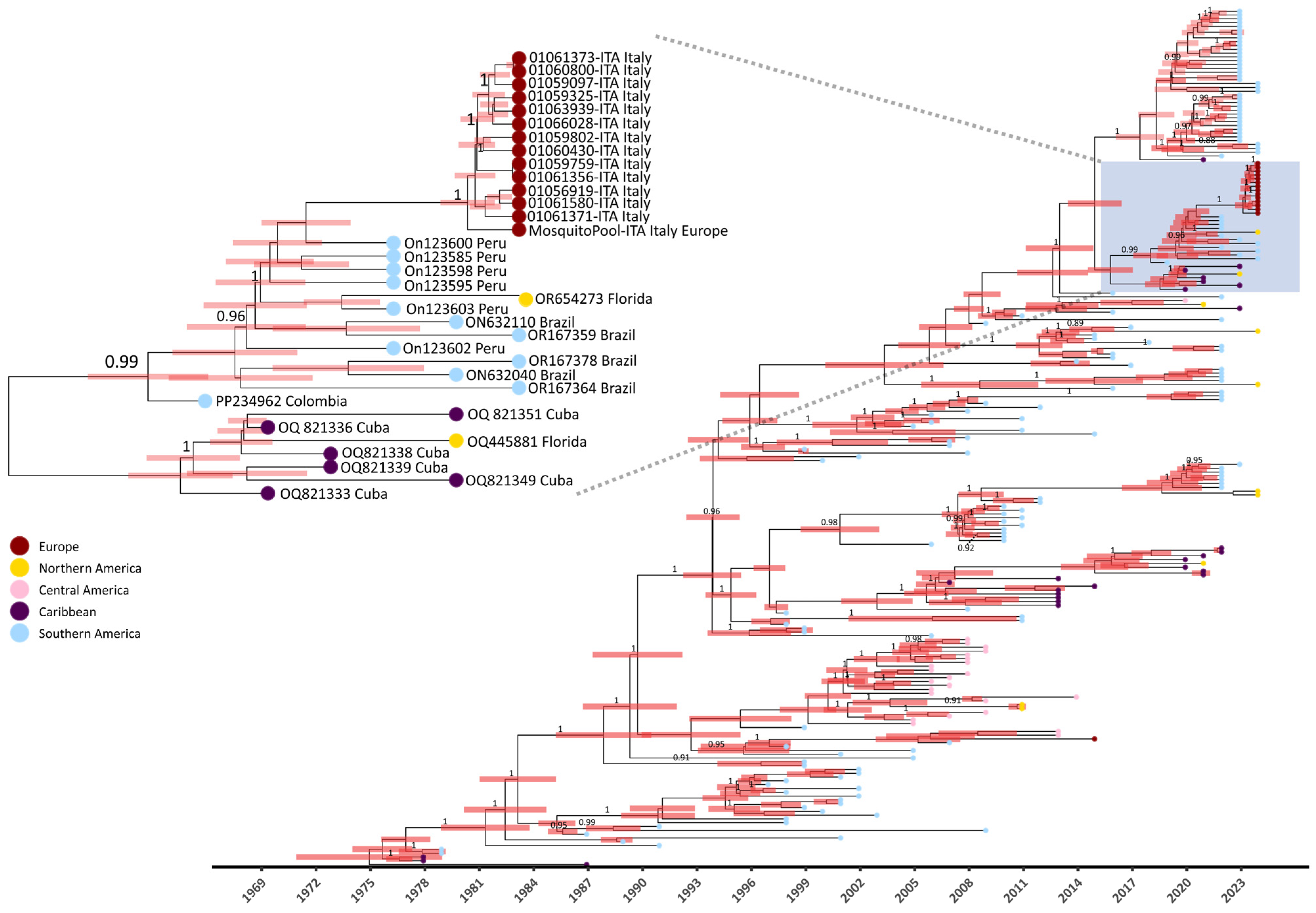
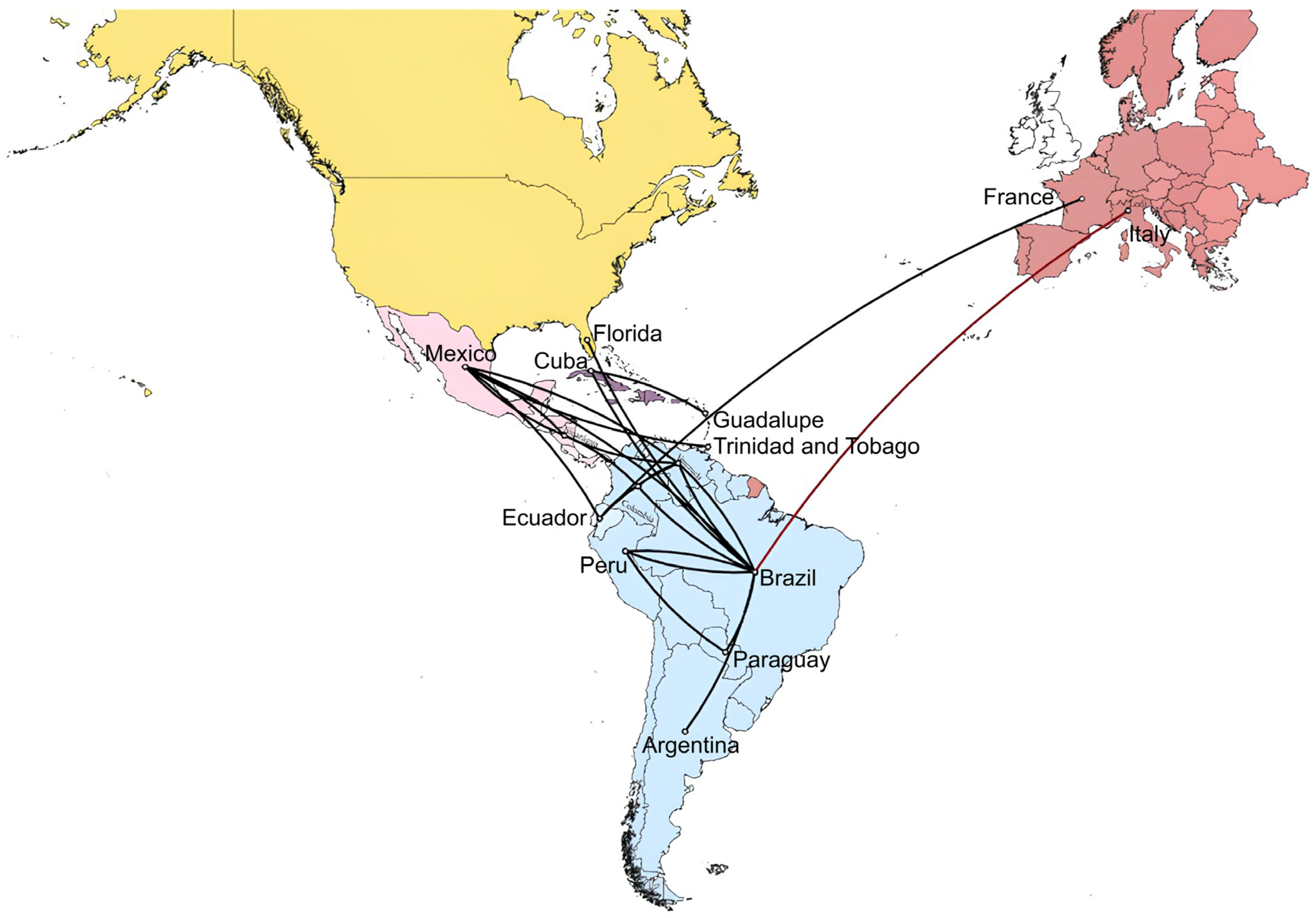
3.3. Phylodynamic and Phylogeographic Diffusion
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANI | Average Nucleotide Identity |
| BIC | Bayesian Information Criterion |
| CDS | Coding DNA Sequence |
| DENV-1 | Dengue Virus type 1 |
| ESS | Effective Sample Size |
| HPD | Highest Posterior Density |
| IZSLER | Istituto Zooprofilattico Sperimentale della Lombardia e Emilia-Romagna |
| ML | Maximum Likelihood |
| MCC | Maximum Clade Credibility MCM = Markov Chain Monte Carlo |
| ORF | Open Reading Frame |
| SH-aLRT | Shimodaira–Hasegawa approximate Likelihood-Ratio Test |
| tMRCA | time to the Most Recent Common Ancestor |
| UB | Ultrafast Bootstrap approximation test |
References
- Brathwaite Dick, O.; San Martín, J.L.; Montoya, R.H.; del Diego, J.; Zambrano, B.; Dayan, G.H. The history of dengue outbreaks in the Americas. Am. J. Trop. Med. Hyg. 2012, 87, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, J.S.; Gubler, D.J.; Petersen, L.R. Emerging flaviviruses: The spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nat. Med. 2004, 10, S98–S109. [Google Scholar] [CrossRef] [PubMed]
- Seligman, S.J. Constancy and diversity in the flavivirus fusion peptide. Virol. J. 2008, 5, 27. [Google Scholar] [CrossRef]
- Bell, S.M.; Katzelnick, L.; Bedford, T. Dengue genetic divergence generates within-serotype antigenic variation, but serotypes dominate evolutionary dynamics. eLife 2019, 8, e42496. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- ECDC. European Centre for Disease Prevention and Control. Available online: https://www.ecdc.europa.eu/en (accessed on 12 June 2024).
- La Ruche, G.; Souarès, Y.; Armengaud, A.; Peloux-Petiot, F.; Delaunay, P.; Desprès, P.; Lenglet, A.; Jourdain, F.; Leparc-Goffart, I.; Charlet, F.; et al. First two autochthonous dengue virus infections in metropolitan France, September 2010. Eurosurveillance 2010, 15, 19676. [Google Scholar] [CrossRef]
- Gjenero-Margan, I.; Aleraj, B.; Krajcar, D.; Lesnikar, V.; Klobucar, A.; Pem-Novosel, I.; Kurečić-Filipović, S.; Komparak, S.; Martić, R.; Duričić, S.; et al. Autochthonous dengue fever in Croatia, August-September 2010. Eurosurveillance 2011, 16, 19805. [Google Scholar] [CrossRef] [PubMed]
- Alves, M.J.; Fernandes, P.L.; Amaro, F.; Osório, H.; Luz, T.; Parreira, P.; Andrade, G.; Zé-Zé, L.; Zeller, H. Clinical presentation and laboratory findings for the first autochthonous cases of dengue fever in Madeira island, Portugal, October 2012. Eurosurveillance 2013, 18, 20398. [Google Scholar] [CrossRef] [PubMed]
- Succo, T.; Leparc-Goffart, I.; Ferré, J.-B.; Roiz, D.; Broche, B.; Maquart, M.; Noel, H.; Catelinois, O.; Entezam, F.; Caire, D.; et al. Autochthonous dengue outbreak in Nîmes, South of France, July to September 2015. Eurosurveillance 2016, 21, 30240. [Google Scholar] [CrossRef]
- Cassaniti, I.; Ferrari, G.; Senatore, S.; Rossetti, E.; Defilippo, F.; Maffeo, M.; Vezzosi, L.; Campanini, G.; Sarasini, A.; Paolucci, S.; et al. Preliminary results on an autochthonous dengue outbreak in Lombardy Region, Italy, August 2023. Eurosurveillance 2023, 28, 2300471. [Google Scholar] [CrossRef]
- Lorusso, A.; Calistri, P.; Mercante, M.T.; Monaco, F.; Portanti, O.; Marcacci, M.; Cammà, C.; Rinaldi, A.; Mangone, I.; Di Pasquale, A.; et al. A “One-Health” approach for diagnosis and molecular characterization of SARS-CoV-2 in Italy. One Health 2020, 10, 100135. [Google Scholar] [CrossRef] [PubMed]
- Piralla, A.; Borghesi, A.; Di Comite, A.; Giardina, F.; Ferrari, G.; Zanette, S.; Figar, T.A.; Angelini, M.; Pisoni, C.; Pitrolo, A.M.G.; et al. Fulminant echovirus 11 hepatitis in male non-identical twins in northern Italy, April 2023. Eurosurveillance 2023, 28, 2300289. [Google Scholar] [CrossRef]
- Kalantar, K.L.; Carvalho, T.; de Bourcy, C.F.; Dimitrov, B.; Dingle, G.; Egger, R.; Han, J.; Holmes, O.B.; Juan, Y.-F.; King, R.; et al. IDseq—An open source cloud-based pipeline and analysis service for metagenomic pathogen detection and monitoring. GigaScience 2020, 9, giaa111. [Google Scholar] [CrossRef] [PubMed]
- Sayers, E.W.; Bolton, E.E.; Brister, J.R.; Canese, K.; Chan, J.; Comeau, D.C.; Connor, R.; Funk, K.; Kelly, C.; Kim, S.; et al. Database resources of the national center for biotechnology information. Nucleic Acids Res. 2022, 50, D20–D26. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Vilsker, M.; Moosa, Y.; Nooij, S.; Fonseca, V.; Ghysens, Y.; Dumon, K.; Pauwels, R.; Alcantara, L.C.; Vanden Eynden, E.; Vandamme, A.-M.; et al. Genome Detective: An automated system for virus identification from high-throughput sequencing data. Bioinformatics 2019, 35, 871–873. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Misawa, K.; Kuma, K.; Miyata, T. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; von Haeseler, A.; Lanfear, R. IQ-TREE 2: New Models and Efficient Methods for Phylogenetic Inference in the Genomic Era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef] [PubMed]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; Von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef] [PubMed]
- Shimodaira, H.; Hasegawa, M. Multiple Comparisons of Log-Likelihoods with Applications to Phylogenetic Inference. Mol. Biol. Evol. 1999, 16, 1114–1116. [Google Scholar] [CrossRef]
- Hoang, D.T.; Chernomor, O.; Von Haeseler, A.; Minh, B.Q.; Vinh, L.S. UFBoot2: Improving the Ultrafast Bootstrap Approximation. Mol. Biol. Evol. 2017, 35, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Smith, D.K.; Zhu, H.; Guan, Y.; Lam, T.T.Y. GGTREE: An r package for visualization and annotation of phylogenetic trees with their covariates and other associated data. Methods Ecol. Evol. 2017, 8, 28–36. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: http://www.R-project.org (accessed on 12 May 2024).
- Aboyoun, P.; Gentleman, R. Pwalign: Perform Pairwise Sequence Alignments, 2024. Available online: https://bioconductor.org/packages/pwalign (accessed on 12 May 2024).
- Gu, Z.; Eils, R.; Schlesner, M. Complex heatmaps reveal patterns and correlations in multidi-mensional genomic data. Bioinformatics 2016, 32, 2847–2849. [Google Scholar] [CrossRef] [PubMed]
- Clarke, T.H.; Brinkac, L.M.; Sutton, G.; E Fouts, D. GGRaSP: A R-package for selecting representative genomes using Gaussian mixture models. Bioinformatics 2018, 34, 3032–3034. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A.; Lam, T.T.; Max Carvalho, L.; Pybus, O.G. Exploring the temporal structure of heterochronous sequences using TempEst (formerly Path-O-Gen). Virus Evol. 2016, 2, vew007. [Google Scholar] [CrossRef] [PubMed]
- Suchard, M.A.; Lemey, P.; Baele, G.; Ayres, D.L.; Drummond, A.J.; Rambaut, A. Bayesian phylogenetic and phylodynamic data integration using BEAST 1.10. Virus Evol. 2018, 4, vey016. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior Summarization in Bayesian Phylogenetics Using Tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A. Figtree v.1.4.4, 2006. Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 24 May 2024).
- Bielejec, F.; Baele, G.; Vrancken, B.; Suchard, M.A.; Rambaut, A.; Lemey, P. SpreaD3: Interactive Visualization of Spatiotemporal History and Trait Evolutionary Processes. Mol. Biol. Evol. 2016, 33, 2167–2169. [Google Scholar] [CrossRef] [PubMed]
- Geoapify. Geoapify Website, 2020. Available online: https://www.geoapify.com/ (accessed on 12 May 2024).
- Italian Ministry of Health. National Plan for Prevention, Surveillance and Response to Arboviruses (2020–2025); Italian Ministry of Health: Rome, Italy, 2019. Available online: https://www.epicentro.iss.it/en/arboviral-diseases/surveillance (accessed on 24 May 2024).
- Caracciolo, I.; Mora-Cardenas, E.; Aloise, C.; Carletti, T.; Segat, L.; Burali, M.S.; Chiarvesio, A.; Totis, V.; Avšič–Županc, T.; Mastrangelo, E.; et al. Comprehensive response to Usutu virus following first isolation in blood donors in the Friuli Venezia Giulia region of Italy: Development of recombinant NS1-based serology and sensitivity to antiviral drugs. PLoS Negl. Trop. Dis. 2020, 14, e0008156. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Adelino, T.E.R.; Giovanetti, M.; Fonseca, V.; Xavier, J.; de Abreu, A.S.; Nascimento, V.A.D.; Demarchi, L.H.F.; Oliveira, M.A.A.; da Silva, V.L.; Mello, A.L.e.S.d.; et al. Field and classroom initiatives for portable sequence-based monitoring of dengue virus in Brazil. Nat. Commun. 2021, 12, 2296. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.M.; Mohammed, A.T.; Vu, T.T.; Khattab, M.; Doheim, M.F.; Mohamed, A.A.; Abdelhamed, M.M.; Shamandy, B.E.; Dawod, M.T.; Alesaei, W.A.; et al. Prevalence and burden of dengue infection in Europe: A systematic review and meta-analysis. Rev. Med. Virol. 2020, 30, e2093. [Google Scholar] [CrossRef] [PubMed]
- Vavassori, L.; Saddler, A.; Müller, P. Active dispersal of Aedes albopictus: A mark-release-recapture study using self-marking units. Parasites Vectors 2019, 12, 583. [Google Scholar] [CrossRef] [PubMed]
- Elbe, S.; Buckland-Merrett, G. Data, disease and diplomacy: GISAID’s innovative contribution to global health. Glob. Chall. 2017, 1, 33–46. [Google Scholar] [CrossRef]
| Strain ID | GenBank/GISAID Accession Numbers | Collection Date | Specimen Source | Gender | Age |
|---|---|---|---|---|---|
| 01056919-ITA | OR512929 [10] | 9 August 2023 | Urine | Male | 72 |
| 01059097-ITA | OR512927 [10] | 22 August 2023 | Plasma | Male | 88 |
| 01059325-ITA | OR512928 [10] | 23 August 2023 | Plasma | Female | 3 |
| 01059759-ITA | OR512925 [10] | 25 August 2023 | Plasma | Female | 82 |
| 01059802-ITA | OR512926 [10] | 25 August 2023 | Plasma | Male | 21 |
| Mosquitoes pool [10] | - | 30 August 2023 | Aedes albopictus | Female | - |
| 01060430-ITA | EPI ISL 19216783 | 29 August 2023 | Plasma | Male | 16 |
| 01060800-ITA | EPI ISL 19216784 | 31 August 2023 | Plasma | Female | 41 |
| 01061356-ITA | EPI ISL 19216785 | 4 September 2023 | Plasma | Male | 42 |
| 01061371-ITA | EPI ISL 19216786 | 4 September 2023 | Plasma | Female | 53 |
| 01061373-ITA | EPI ISL 19216787 | 4 September 2023 | Plasma | Female | 62 |
| 01061580-ITA | EPI ISL 19216788 | 5 September 2023 | Plasma | Female | 38 |
| 01063939-ITA | EPI ISL 19216789 | 15 September 2023 | Plasma | Male | 68 |
| 01066028-ITA | EPI ISL 19216790 | 25 September 2023 | Plasma | Male | 48 |
| Number of Strains | Continent | Country | Sampling Date |
|---|---|---|---|
| 15 | Europe | Italy (14) | 2020–2023 (14) |
| France (1) | 2010–2014 (1) | ||
| 10 | North America | Florida (9) | 2010–2014 (2) 2020–2023 (7) |
| Texas (1) | 2020–2023 (1) | ||
| 2000–2004 (6) | |||
| Nicaragua (62) | 2005–2009 (30) | ||
| 131 | Central America | 2010–2014 (26) | |
| Mexico (69) | 2005–2009 (68) | ||
| 2015–2019 (1) | |||
| 1986–1988 (1) | |||
| Puerto Rico (11) | 1990–1995 (2) 2005–2009 (2) | ||
| 2010–2014 (6) | |||
| Haiti (2) | 2010–2014 (2) | ||
| Jamaica (2) | 1977–1978 (1) | ||
| 31 | Caribbean | 2010–2014 (1) | |
| Saint Martin (2) | 2020–2023 (2) | ||
| Cuba (7) | 2015–2019 (2) | ||
| 2020–2023 (5) | |||
| Dominican Republic (2) | 2015–2019 (2) | ||
| Guadeloupe (4) | 2020–2023 (4) | ||
| Bahamas (1) | 1977–1978 (1) | ||
| 1986–1988 (2) | |||
| 1990–1995 (1) | |||
| 1996–1999 (9) | |||
| Brazil (148) | 2000–2004 (6) | ||
| 2005–2009 (2) | |||
| 2010–2014 (6) | |||
| 2020–2023 (122) | |||
| 2000–2004 (3) | |||
| Argentina (24) | 2005–2009 (16) | ||
| 2010–2014 (5) | |||
| Paraguay (5) | 2000–2004 (1) | ||
| 2020–2023 (4) | |||
| 1996–1999 (9) | |||
| 2000–2004 (11) | |||
| Venezuela (75) | 2005–2009 (47) | ||
| 322 | South America | 2010–2014 (4) | |
| 2015–2019 (4) | |||
| 1990–1995 (1) | |||
| 1996–1999 (5) | |||
| 2000–2004 (2) | |||
| Colombia (27) | 2005–2009 (11) | ||
| 2010–2014 (1) | |||
| 2015–2019 (5) | |||
| 2020–2023 (2) | |||
| 1986–1988 (1) | |||
| Ecuador (18) | 2005–2009 (1) 2010–2014 (6) | ||
| 2015–2019 (10) | |||
| Peru (23) | 2000–2004 (1) | ||
| 2020–2023 (22) | |||
| Trinidad and Tobago (2) | 1977–1978 (2) |
| Continent | Sampling Date | Number of Strains |
|---|---|---|
| Europe (15) | 2020–2023 | 14 |
| 2010–2014 | 1 | |
| North America (9) | 2010–2014 | 2 |
| 2020–2023 | 7 | |
| Central America (24) | 2000–2004 | 2 |
| 2005–2009 | 18 | |
| 2010–2014 | 3 | |
| 2015–2019 | 1 | |
| Caribbean (26) | 1977–1978 | 2 |
| 1986–1988 | 1 | |
| 2005–2009 | 1 | |
| 2010–2014 | 7 | |
| 2015–2019 | 4 | |
| 2020–2023 | 11 | |
| South America (150) | 1977–1978 | 2 |
| 1986–1988 | 2 | |
| 1990–1995 | 2 | |
| 1996–1999 | 17 | |
| 2000–2004 | 14 | |
| 2005–2009 | 21 | |
| 2010–2014 | 15 | |
| 2015–2019 | 9 | |
| 2020–2023 | 68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romano, G.; Ferrari, G.; Pitrolo, A.M.G.; Rovida, F.; Piralla, A.; Baldanti, F. Tracing the Origin, Spread, and Molecular Evolution of Dengue Type 1 Cases That Occurred in Northern Italy in 2023. Pathogens 2024, 13, 1124. https://doi.org/10.3390/pathogens13121124
Romano G, Ferrari G, Pitrolo AMG, Rovida F, Piralla A, Baldanti F. Tracing the Origin, Spread, and Molecular Evolution of Dengue Type 1 Cases That Occurred in Northern Italy in 2023. Pathogens. 2024; 13(12):1124. https://doi.org/10.3390/pathogens13121124
Chicago/Turabian StyleRomano, Greta, Guglielmo Ferrari, Antonino Maria Guglielmo Pitrolo, Francesca Rovida, Antonio Piralla, and Fausto Baldanti. 2024. "Tracing the Origin, Spread, and Molecular Evolution of Dengue Type 1 Cases That Occurred in Northern Italy in 2023" Pathogens 13, no. 12: 1124. https://doi.org/10.3390/pathogens13121124
APA StyleRomano, G., Ferrari, G., Pitrolo, A. M. G., Rovida, F., Piralla, A., & Baldanti, F. (2024). Tracing the Origin, Spread, and Molecular Evolution of Dengue Type 1 Cases That Occurred in Northern Italy in 2023. Pathogens, 13(12), 1124. https://doi.org/10.3390/pathogens13121124








