Strategies to Enhance Diagnostic Capabilities for the New Drug-Resistant Tuberculosis (DR-TB) Drugs
Abstract
1. Introduction
2. Current Drugs for DR-TB
3. Current Drug Susceptibility Testing Methods
4. The Gaps and Challenges in the DST Implementation
5. Strategies for Achieving Equitable DST Access and Gap Closure
5.1. Strengthening the Health System
5.2. Sharing Infrastructure and Human Resources
5.3. Providing DST Machine and Reagents at Subsidized or Reduced Prices
5.4. Involvement of Drug Developers in the Development of DST Methods
5.5. Capacity-Building Initiatives
5.6. Maximizing the Utilization Efficiency of Sequencing Facilities
5.7. Encouraging Public–Private Partnerships
5.8. Recognizing Successful Case Studies or Initiatives
5.9. Promoting Research and Development
5.10. Initiatives for Technology Transfer
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Global Tuberculosis Report 2023; World Health Organization: Geneva, Switzerland, 2023; Available online: https://iris.who.int/bitstream/handle/10665/373828/9789240083851-eng.pdf?sequence=1 (accessed on 5 September 2024).
- Dean, A.S.; Zignol, M.; Cabibbe, A.M.; Falzon, D.; Glaziou, P.; Cirillo, D.M.; Köser, C.U.; Gonzalez-Angulo, L.Y.; Tosas-Auget, O.; Ismail, N.; et al. Prevalence and genetic profiles of isoniazid resistance in tuberculosis patients: A multicountry analysis of cross-sectional data. PLoS Med. 2020, 17, e1003008. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Consolidated Guidelines on Tuberculosis. Module 4: Treatment—Drug-Resistant Tuberculosis Treatment, 2022 Update; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Conradie, F.; Diacon, A.H.; Ngubane, N.; Howell, P.; Everitt, D.; Crook, A.M.; Mendel, C.M.; Egizi, E.; Moreira, J.; Timm, J.; et al. Bedaquiline, pretomanid and linezolid for treatment of extensively drug resistant, intolerant or non-responsive multidrug resistant pulmonary tuberculosis. N. Engl. J. Med. 2020, 382, 893. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Ahuja, S.D.; Akkerman, O.W.; Alffenaar, J.-W.C.; Anderson, L.F.; Baghaei, P.; Bang, D.; Barry, P.M.; Bastos, M.L.; Behera, D.; et al. Treatment correlates of successful outcomes in pulmonary multidrug-resistant tuberculosis: An individual patient data meta-analysis. Lancet 2018, 392, 821–834. [Google Scholar] [CrossRef] [PubMed]
- Conradie, F.; Diacon, A.H.; Ngubane, N.; Howell, P.; Everitt, D.; Crook, A.M.; Mendel, C.M.; Egizi, E.; Moreira, J.; Timm, J.; et al. Treatment of Highly Drug-Resistant Pulmonary Tuberculosis. N. Engl. J. Med. 2020, 382, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Conradie, F.; Bagdasaryan, T.R.; Borisov, S.; Howell, P.; Mikiashvili, L.; Ngubane, N.; Samoilova, A.; Skornykova, S.; Tudor, E.; Variava, E.; et al. Bedaquiline–Pretomanid–Linezolid Regimens for Drug-Resistant Tuberculosis. N. Engl. J. Med. 2022, 387, 810–823. [Google Scholar] [CrossRef]
- Derendinger, B.; Dippenaar, A.; de Vos, M.; Alberts, R.; Sirgel, F.; Dolby, T.; Spies, C.; Rigouts, L.; Metcalfe, J.; Engelthaler, D.; et al. High frequency of bedaquiline resistance in programmatically treated drug-resistant TB patients with sustained culture-positivity in Cape Town, South Africa. Int. J. Mycobacteriol. 2021, 4, e972–e982. [Google Scholar] [CrossRef]
- Millard, J.; Rimmer, S.; Nimmo, C.; O’Donnell, M. Therapeutic Failure and Acquired Bedaquiline and Delamanid Resistance in Treatment of Drug-Resistant TB. Emerg. Infect. Dis. 2023, 29, 1081. [Google Scholar] [CrossRef]
- He, W.; Liu, C.; Liu, D.; Ma, A.; Song, Y.; He, P.; Bao, J.; Li, Y.; Zhao, B.; Fan, J.; et al. Prevalence of Mycobacterium tuberculosis resistant to bedaquiline and delamanid in China. J. Glob. Antimicrob. Resist. 2021, 26, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Arora, G.; Bothra, A.; Prosser, G.; Arora, K.; Sajid, A. Role of post-translational modifications in the acquisition of drug resistance in Mycobacterium tuberculosis. FEBS J. 2021, 288, 3375–3393. [Google Scholar] [CrossRef]
- Van Rie, A.; Walker, T.; de Jong, B.; Rupasinghe, P.; Rivière, E.; Dartois, V.; Sonnenkalb, L.; Machado, D.; Gagneux, S.; Supply, P.; et al. Balancing access to BPaLM regimens and risk of resistance. Lancet Infect. Dis. 2022, 22, 1411–1412. [Google Scholar] [CrossRef] [PubMed]
- Kontsevaya, I.; Cabibbe, A.M.; Cirillo, D.M.; DiNardo, A.R.; Frahm, N.; Gillespie, S.H.; Holtzman, D.; Meiwes, L.; Petruccioli, E.; Reimann, M.; et al. Update on the diagnosis of tuberculosis. Clin. Microbiol. Infect. 2023, 30, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Georghiou, S.B.; Penn-Nicholson, A.; de Vos, M.; Macé, A.; Syrmis, M.W.; Jacob, K.; Mape, A.; Parmar, H.; Cao, Y.; Coulter, C.; et al. Analytical performance of the Xpert MTB/XDR® assay for tuberculosis and expanded resistance detection. Diagn. Microbiol. Infect. Dis. 2021, 101, 115397. [Google Scholar] [CrossRef]
- Vasiliu, A.; Saktiawati, A.M.I.; Duarte, R.; Lange, C.; Cirillo, D.M. Implementing molecular tuberculosis diagnostic methods in limited-resource and high-burden countries. Breathe 2022, 18, 220226. [Google Scholar] [CrossRef] [PubMed]
- Vogel, M.; Utpatel, C.; Corbett, C.; Kohl, T.A.; Iskakova, A.; Ahmedov, S.; Antonenka, U.; Dreyer, V.; Ibrahimova, A.; Kamarli, C.; et al. Implementation of whole genome sequencing for tuberculosis diagnostics in a low-middle income, high MDR-TB burden country. Sci. Rep. 2021, 11, 15333. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Treatment Guidelines for Drug-Resistant Tuberculosis, 2016 Update; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Bastos, M.L.; Lan, Z.; Menzies, D. An updated systematic review and meta-analysis for treatment of multidrug-resistant tuberculosis. Eur. Respir. J. 2017, 49, 1600803. [Google Scholar] [CrossRef]
- Diacon, A.H.; Pym, A.; Grobusch, M.; Patientia, R.; Rustomjee, R.; Page-Shipp, L.; Pistorius, C.; Krause, R.; Bogoshi, M.; Churchyard, G.; et al. The Diarylquinoline TMC207 for Multidrug-Resistant Tuberculosis. N. Engl. J. Med. 2009, 360, 2397–2405. [Google Scholar] [CrossRef] [PubMed]
- Diacon, A.H.; Donald, P.R.; Pym, A.; Grobusch, M.; Patientia, R.F.; Mahanyele, R.; Bantubani, N.; Narasimooloo, R.; De Marez, T.; Van Heeswijk, R.; et al. Randomized pilot trial of eight weeks of bedaquiline (TMC207) treatment for multidrug-resistant tuberculosis: Long-term outcome, tolerability, and effect on emergence of drug resistance. Antimicrob. Agents Chemother. 2012, 56, 3271–3276. [Google Scholar] [CrossRef]
- Pym, A.S.; Diacon, A.H.; Tang, S.J.; Conradie, F.; Danilovits, M.; Chuchottaworn, C.; Vasilyeva, I.; Andries, K.; Bakare, N.; De Marez, T.; et al. Bedaquiline in the treatment of multidrug- and extensively drugresistant tuberculosis. Eur. Respir. J. 2016, 47, 564–574. [Google Scholar] [CrossRef]
- Borisov, S.E.; Dheda, K.; Enwerem, M.; Leyet, R.R.; D’Ambrosio, L.; Centis, R.; Sotgiu, G.; Tiberi, S.; Alffenaar, J.W.; Maryandyshev, A.; et al. Effectiveness and safety of bedaquilinecontaining regimens in the treatment of MDR- and XDR-TB: A multicentre study. Eur. Respir. J. 2017, 49, 1700387. [Google Scholar] [CrossRef]
- Ndjeka, N.; Schnippel, K.; Master, I.; Meintjes, G.; Maartens, G.; Romero, R.; Padanilam, X.; Enwerem, M.; Chotoo, S.; Singh, N.; et al. High treatment success rate for multidrug-resistant and extensively drug-resistant tuberculosis using a bedaquiline-containing treatment regimen. Eur. Respir. J. 2018, 52, 1801528. [Google Scholar] [CrossRef]
- Skripconoka, V.; Danilovits, M.; Pehme, L.; Tomson, T.; Skenders, G.; Kummik, T.; Cirule, A.; Leimane, V.; Kurve, A.; Levina, K.; et al. Delamanid improves outcomes and reduces mortality in multidrug-resistant tuberculosis. Eur. Respir. J. 2013, 41, 1393–1400. [Google Scholar] [CrossRef]
- Maryandyshev, A.; Pontali, E.; Tiberi, S.; Akkerman, O.; Ganatra, S.; Sadutshang, T.D.; Alffenaar, J.W.; Amale, R.; Mullerpattan, J.; Topgyal, S.; et al. Bedaquiline and delamanid combination treatment of 5 patients with pulmonary extensively drug-resistant tuberculosis. Emerg. Infect. Dis. 2017, 23, 1718–1721. [Google Scholar] [CrossRef] [PubMed]
- Ferlazzo, G.; Mohr, E.; Laxmeshwar, C.; Hewison, C.; Hughes, J.; Jonckheere, S.; Khachatryan, N.; De Avezedo, V.; Egazaryan, L.; Shroufi, A.; et al. Early safety and efficacy of the combination of bedaquiline and delamanid for the treatment of patients with drug-resistant tuberculosis in Armenia, India, and South Africa: A retrospective cohort study. Lancet Infect. Dis. 2018, 18, 536–544. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Consolidated Guidelines on Drug-Resistant Tuberculosis Treatment; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Gils, T.; Lynen, L.; de Jong, B.C.; Van Deun, A.; Decroo, T. Pretomanid for tuberculosis: A systematic review. Clin. Microbiol. Infect. 2022, 28, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Nyang’wa, B.-T.; Berry, C.; Kazounis, E.; Motta, I.; Parpieva, N.; Tigay, Z.; Solodovnikova, V.; Liverko, I.; Moodliar, R.; Dodd, M.; et al. A 24-Week, All-Oral Regimen for Rifampin-Resistant Tuberculosis. N. Engl. J. Med. 2022, 387, 2331–2343. [Google Scholar] [CrossRef] [PubMed]
- Vanino, E.; Granozzi, B.; Akkerman, O.W.; Munoz-Torrico, M.; Palmieri, F.; Seaworth, B.; Tiberi, S.; Tadolini, M. Update of drug-resistant tuberculosis treatment guidelines: A turning point. Int. J. Infect. Dis. 2023, 130, S12–S15. [Google Scholar] [CrossRef]
- Alffenaar, J.W.C.; Akkerman, O.W.; Tiberi, S.; Sotgiu, G.; Migliori, G.B.; Montaner, P.G.; Palmero, D.J.; Denholm, J.; Douglas, P.; Lau, J.S.; et al. Should we worry about bedaquiline exposure in the treatment of multidrug-resistant and extensively drug-resistant tuberculosis? Eur. Respir. J. 2020, 55, 1901908. [Google Scholar] [CrossRef]
- van Zyl-Smit, R.N.; Binder, A.; Meldau, R.; Mishra, H.; Semple, P.L.; Theron, G.; Peter, J.; Whitelaw, A.; Sharma, S.K.; Warren, R.; et al. Comparison of quantitative techniques including Xpert MTB/RIF to evaluate mycobacterial burden. PLoS ONE 2011, 6, e28815. [Google Scholar] [CrossRef]
- World Health Organization. WHO Consolidated Guidelines on Tuberculosis. Module 3: Diagnosis—Rapid Diagnostics for Tuberculosis Detection, 3rd ed.; World Health Organization: Geneva, Switzerland, 2024. [Google Scholar]
- Ngangue, Y.R.; Mbuli, C.; Neh, A.; Nshom, E.; Koudjou, A.; Palmer, D.; Ndi, N.N.; Qin, Z.Z.; Creswell, J.; Mbassa, V.; et al. Diagnostic Accuracy of the Truenat MTB Plus Assay and Comparison with the Xpert MTB/RIF Assay to Detect Tuberculosis among Hospital Outpatients in Cameroon. J. Clin. Microbiol. 2022, 60, e00155-22. [Google Scholar] [CrossRef]
- Georghiou, S.B.; Gomathi, N.S.; Rajendran, P.; Nagalakshmi, V.; Prabakaran, L.; Prem Kumar, M.M.; Macé, A.; Tripathy, S.; Ruhwald, M.; Schumacher, S.G.; et al. Accuracy of the Truenat MTB-RIF Dx assay for detection of rifampicin resistance-associated mutations. Tuberculosis 2021, 127, 102064. [Google Scholar] [CrossRef]
- Gegia, M.; Winters, N.; Benedetti, A.; van Soolingen, D.; Menzies, D. Treatment of isoniazid-resistant tuberculosis with first-line drugs: A systematic review and meta-analysis. Lancet Infect. Dis. 2017, 17, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Vogensen, V.B.; Anthony, R.M.; Kerstjens, H.A.M.; Tiberi, S.; de Steenwinkel, J.E.M.; Akkerman, O.W. The case for expanding worldwide access to point of care molecular drug susceptibility testing for isoniazid. Clin. Microbiol. Infect. 2022, 28, 1047–1049. [Google Scholar] [CrossRef]
- Liu, C.F.; Song, Y.M.; He, P.; Liu, D.X.; He, W.C.; Li, Y.M.; Zhao, Y.L. Evaluation of Multidrug Resistant Loop-mediated Isothermal Amplification Assay for Detecting the Drug Resistance of Mycobacterium tuberculosis. Biomed. Environ. Sci. 2021, 34, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Alagna, R.; Cabibbe, A.M.; Miotto, P.; Saluzzo, F.; Köser, C.U.; Niemann, S.; Gagneux, S.; Rodrigues, C.; Rancoita, P.V.M.; Cirillo, D.M. Is the new WHO definition of extensively drug-resistant tuberculosis easy to apply in practice? Eur. Respir. J. 2021, 58, 2100959. [Google Scholar] [CrossRef]
- WHO. Rapid Communication: Key Changes to the Treatment of DRUG-Resistant Tuberculosis; WHO: Geneva, Switzerland, 2022. [Google Scholar]
- Tagliani, E.; Saluzzo, F.; Cirillo, D.M. Microbiological tests and laboratory tests: The value of point-of-care testing. In The Challenge of Tuberculosis in the 21st Century; European Respiratory Society: Sheffield, UK, 2023. [Google Scholar]
- World Health Organization. Use of Targeted Next-Generation Sequencing to Detect Drug-Resistant Tuberculosis: Rapid Communication; World Health Organization: Geneva, Switzerland, 2023; Available online: https://www.who.int/publications/i/item/9789240076372 (accessed on 15 March 2024).
- Jouet, A.; Gaudin, C.; Badalato, N.; Allix-Béguec, C.; Duthoy, S.; Ferré, A.; Diels, M.; Laurent, Y.; Contreras, S.; Feuerriegel, S.; et al. Deep amplicon sequencing for culture-free prediction of susceptibility or resistance to 13 anti-tuberculous drugs. Eur. Respir. J. 2021, 57, 2002338. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Catalogue of Mutations in Mycobacterium Tuberculosis Complex and Their Association with Drug Resistance, 2nd ed.; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- Farooq, H.Z.; Cirillo, D.M.; Hillemann, D.; Wyllie, D.; Van der Werf, M.J.; Ködmön, C.; Nikolayevskyy, V. Limited capability for testing mycobacterium tuberculosis for susceptibility to new drugs. Emerg. Infect. Dis. 2021, 27, 985–987. [Google Scholar] [CrossRef]
- de Araujo, L.; Cabibbe, A.M.; Mhuulu, L.; Ruswa, N.; Dreyer, V.; Diergaardt, A.; Günther, G.; Claassens, M.; Gerlach, C.; Utpatel, C.; et al. Implementation of targeted next-generation sequencing for the diagnosis of drug-resistant tuberculosis in low-resource settings: A programmatic model, challenges, and initial outcomes. Front. Public Health 2023, 11, 1204064. [Google Scholar] [CrossRef]
- Rivière, E.; Heupink, T.H.; Ismail, N.; Dippenaar, A.; Clarke, C.; Abebe, G.; Heusden, P.; Warren, R.; Meehan, C.J.; Van Rie, A. Capacity building for whole genome sequencing of Mycobacterium tuberculosis and bioinformatics in high TB burden countries. Brief. Bioinform. 2021, 22, bbaa246. [Google Scholar] [CrossRef]
- Madhuri, K.; Deshpande, S.; Dharmashale, S.; Bharadwaj, R. Utility of line probe assay for the early detection of multidrug-resistant pulmonary tuberculosis. J. Glob. Infect. Dis. 2015, 7, 60–65. [Google Scholar] [CrossRef]
- Martinez, E.; Bustamante, A.; Menon, R.; Wang, Q.; Jelfs, P.; Marais, B.; Chen, S.C.A.; Sintchenko, V. Whole-genome sequencing of Mycobacterium tuberculosis for rapid diagnostics: Feasibility of a decentralised model. Lancet Respir. Med. 2016, 4, e13–e14. [Google Scholar] [CrossRef]
- Olaru, I.D.; Patel, H.; Kranzer, K.; Perera, N. Turnaround time of whole genome sequencing for mycobacterial identification and drug susceptibility testing in routine practice. Clin. Microbiol. Infect. 2018, 24, 659-e5. [Google Scholar] [CrossRef]
- Uddin, M.K.M.; Cabibbe, A.M.; Nasrin, R.; Ghodousi, A.; Nobel, F.A.; Rahman, S.M.M.; Ahmed, S.; Ather, M.F.; Razzaque, S.M.A.; Raihan, M.A.; et al. Targeted next-generation sequencing of Mycobacterium tuberculosis from patient samples: Lessons learned from high drug-resistant burden clinical settings in Bangladesh. Emerg. Microbes Infect. 2024, 13, 2392656. [Google Scholar] [CrossRef] [PubMed]
- Maningi, N.E.; Malinga, L.A.; Antiabong, J.F.; Lekalakala, R.M.; Mbelle, N.M. Comparison of line probe assay to BACTEC MGIT 960 system for susceptibility testing of first and second-line anti-tuberculosis drugs in a referral laboratory in South Africa. BMC Infect. Dis. 2017, 17, 795. [Google Scholar] [CrossRef]
- MacLean, E.; Kohli, M.; Weber, S.F.; Suresh, A.; Schumacher, S.G.; Denkinger, C.M.; Pai, M. Advances in molecular diagnosis of tuberculosis. J. Clin. Microbiol. 2020, 58, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Campelo, T.A.; Cardoso de Sousa, P.R.; Nogueira, L.D.L.; Frota, C.C.; Zuquim Antas, P.R. Revisiting the methods for detecting Mycobacterium tuberculosis: What has the new millennium brought thus far? Access Microbiol. 2021, 3, 000245. [Google Scholar] [CrossRef] [PubMed]
- Ninan, M.M.; Gowri, M.; Christopher, D.J.; Rupali, P.; Michael, J.S. The diagnostic utility of line probe assays for multidrug-resistant tuberculosis. Pathog. Glob. Health 2016, 110, 194–199. [Google Scholar] [CrossRef]
- Yadav, R.N.; Verma, A.K.; Kaushik, G. Laboratory cost analysis of conventional and newer molecular tests for diagnosis of presumptive multidrug-resistant tuberculosis patients. J. Glob. Infect. Dis. 2022, 14, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Iyer, A.; Ndlovu, Z.; Sharma, J.; Mansoor, H.; Bharati, M.; Kolan, S.; Morales, M.; Das, M.; Issakidis, P.; Ferlazzo, G.; et al. Operationalising targeted next-generation sequencing for routine diagnosis of drug-resistant TB. Public Health Action 2023, 13, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.G.; Smith, C.; Lapierre, P.; Shea, J.; Patel, K.; Halse, T.A.; Dickinson, M.; Escuyer, V.; Rowlinson, M.C.; Musser, K.A. Direct detection of drug-resistant Mycobacterium tuberculosis using targeted next generation sequencing. Front. Public Health 2023, 11, 1206056. [Google Scholar] [CrossRef]
- Ministry of Health Republic of Indonesia. Pemeriksaan Line Probe Assay (LPA) Lini Dua; Ministry of Health Republic of Indonesia: Batam, Indonesia, 2020; pp. 1–128.
- Ministry of Health Republic of Indonesia. Petunjuk Teknis Pemeriksaan Tuberkulosis Menggunakan Tes Cepat Molekuler GeneXpert; Ministry of Health Republic of Indonesia: Batam, Indonesia, 2023; 241p. Available online: https://tbindonesia.or.id/wp-content/uploads/2023/12/2023_Buku-Petunjuk-Teknis-Pemeriksaan-TBC-Menggunakan-Alat-TCM-GeneXpert_2023.pdf (accessed on 14 August 2024).
- Griffin, A.M.J.; Caviedes, L.; Gilman, R.; Coronel, J.; Delgado, F.; Quispe, M.L.; Moore, D.A.J. Field and laboratory preparedness: Challenges to rolling out new multidrugresistant tuberculosis diagnostics. Rev. Panam. Salud Publica/Pan Am. J. Public Health 2009, 26, 120–127. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Leite, J.A.; Vicari, A.; Perez, E.; Siqueira, M.; Resende, P.; Motta, F.C.; Freitas, L.; Fernandez, J.; Parra, B.; Castillo, A.; et al. Implementation of a COVID-19 Genomic Surveillance Regional Network for Latin America and Caribbean region. PLoS ONE 2022, 17, e0252526. [Google Scholar] [CrossRef] [PubMed]
- Akoniyon, O.P.; Adewumi, T.S.; Maharaj, L.; Oyegoke, O.O.; Roux, A.; Adeleke, M.A.; Maharaj, R.; Okpeku, M. Whole Genome Sequencing Contributions and Challenges in Disease Reduction Focused on Malaria. Biology 2022, 11, 587. [Google Scholar] [CrossRef] [PubMed]
- Argimón, S.; Masim, M.A.L.; Gayeta, J.M.; Lagrada, M.L.; Macaranas, P.K.V.; Cohen, V.; Limas, M.T.; Espiritu, H.O.; Palarca, J.C.; Chilam, J.; et al. Integrating whole-genome sequencing within the National Antimicrobial Resistance Surveillance Program in the Philippines. Nat. Commun. 2020, 11, 2719. [Google Scholar] [CrossRef] [PubMed]
- Stop TB Partnership. Global Drug Facility Update on Access to Bedaquiline [Internet]. Available online: https://www.stoptb.org/news/global-drug-facility-update-access-to-bedaquiline (accessed on 3 October 2023).
- Dlamini, M.T.; Lessells, R.; Iketleng, T.; de Oliveira, T. Whole genome sequencing for drug-resistant tuberculosis management in South Africa: What gaps would this address and what are the challenges to implementation? J. Clin. Tuberc. Other Mycobact. Dis. 2019, 16, 100115. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Science Council Meeting, Geneva, Switzerland, 11–12 July 2022: Report; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Ness, T.; Van, L.H.; Petermane, I.; Duarte, R.; Lange, C.; Menzies, D.; Cirillo, D.M. Rolling out new anti-tuberculosis drugs without diagnostic capacity. Breathe 2023, 19, 230084. [Google Scholar] [CrossRef]
- Park, P.H.; Magut, C.; Gardner, A.; O’yiengo, D.O.; Kamle, L.; Langat, B.K.; Buziba, N.G.; Carter, E.J. Increasing access to the MDR-TB surveillance programme through a collaborative model in western Kenya. Trop. Med. Int. Health 2012, 17, 374–379. [Google Scholar] [CrossRef]
- Bishop, Ö.T.; Adebiyi, E.F.; Alzohairy, A.M.; Everett, D.; Ghedira, K.; Ghouila, A.; Kumuthini, J.; Mulder, N.J.; Panji, S.; Patterton, H.G. Bioinformatics education-perspectives and challenges out of Africa. Brief. Bioinform. 2015, 16, 355–364. [Google Scholar] [CrossRef]
- Ali, T.; Singh, U.; Ohikhuai, C.; Panwal, T.; Adetiba, T.; Agbaje, A.; Olusola Faleye, B.; Shyam Klinton, J.; Oga-Omenka, C.; Tseja-Akinrin, A.; et al. Partnering with the private laboratories to strengthen TB diagnostics in Nigeria. J. Clin. Tuberc. Other Mycobact. Dis. 2023, 31, 100369. [Google Scholar] [CrossRef]
- Ntoumi, F.; Petersen, E.; Mwaba, P.; Aklillu, E.; Mfinanga, S.; Yeboah-Manu, D.; Maeurer, M.; Zumla, A. Blue Skies research is essential for ending the Tuberculosis pandemic and advancing a personalized medicine approach for holistic management of Respiratory Tract infections. Int. J. Infect. Dis. 2022, 124, S69–S74. [Google Scholar] [CrossRef]
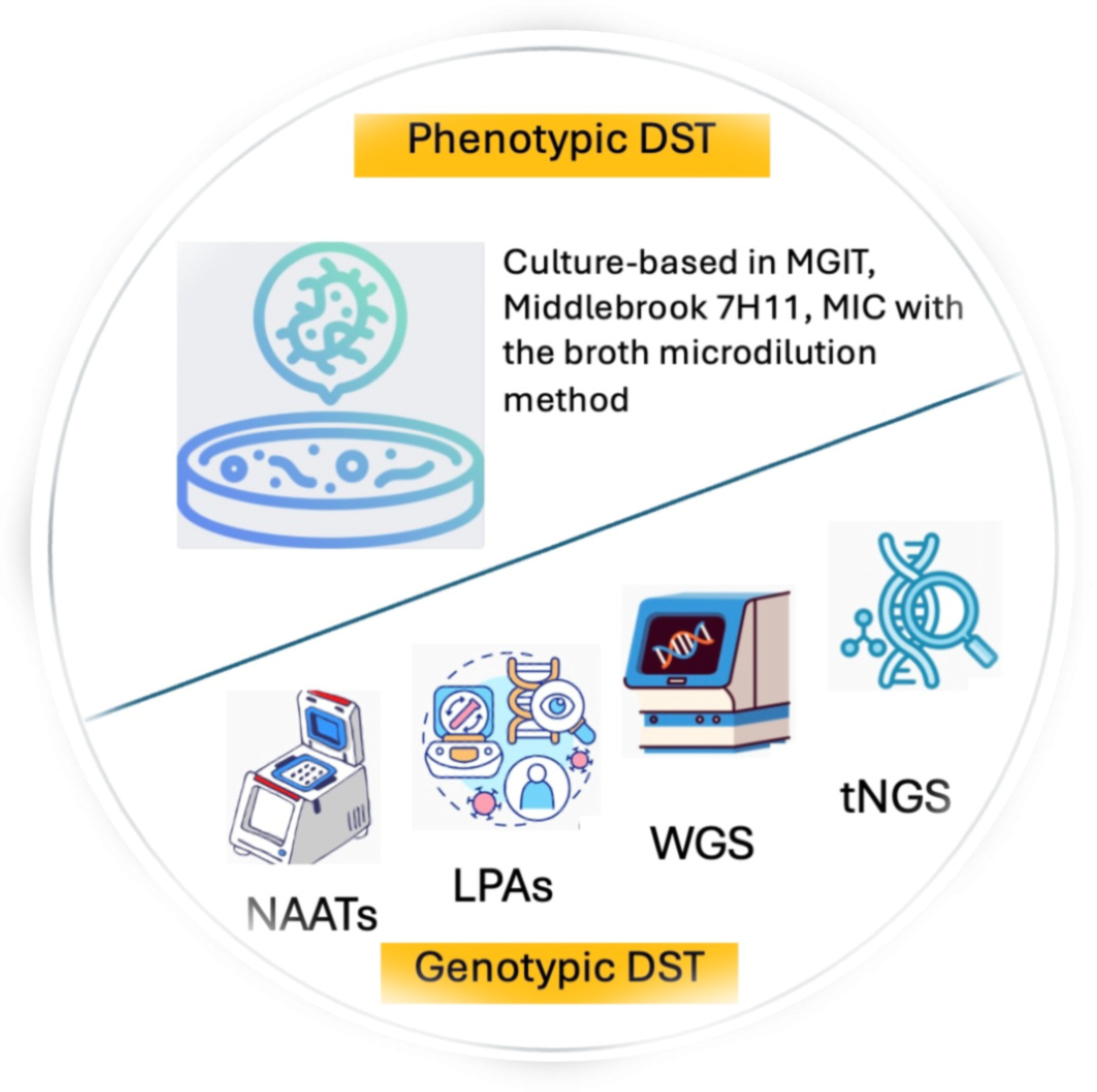
| Turnaround Time [48,49,50,51,52] | Sensitivity and Specificity [32,53,54,55] | Complexity of Procedure [15,33] | Complexity of Infrastructure [15,42] | Cost [16,56,57,58] | The Use in LMICs, Including the Setting [33,59,60] | Examples of Platforms [15,38] | |
|---|---|---|---|---|---|---|---|
| Phenotypic DST | |||||||
| Culture-based | Long (1–3 weeks for liquid culture, 4–6 weeks for solid culture) | Highly sensitive and specific (limit of detection: 10 CFU/mL) | Moderate, need suitable training | Moderate, need biosafety conditions | Moderate to high (21.5–119 USD) | Used as a reference test, usually in tertiary health centers/referral laboratories | Culture-based in MGIT, Middlebrook 7H11, MIC with the broth microdilution method |
| Genotypic DST | |||||||
| NAATs | Short (1–3 h, can extend up to 2 days if there are delays in sample shipment or result delivery) | Highly sensitive and specific (limit of detection: 15–150 CFU/mL) | Low to moderate | Low | Low (13.8 USD) | Used as an initial or confirmation test, usually in secondary health centers/laboratories | Xpert MTB/RIF and Xpert MTB/RIF Ultra (Cepheid, Sunnyvale, CA, USA); Truenat (Molbio, Verna, Goa, India); Abbott RealTime MTB and Abbott RealTime MTB RIF/INH (Abbott, Des Plaines, IL, USA); BD MAX MDR-TB (Becton Dickinson, Franklin Lakes, NJ, USA); cobas MTB and cobas MTB-RIF/INH (Roche, Basel, Switzerland); FluoroType MTBDR and FluoroType MTB (Hain Lifescience/Bruker, Tübingen, Germany); MDR-LAMP (Eiken, Tokyo, Japan) |
| LPAs | Short (5 h, can extend up to 2 days if there are delays in sample shipment or result delivery) | Highly sensitive and specific (limit of detection: 10,000 CFU/mL) | Moderate to high, need multiple steps | Moderate to high, need separate rooms for different steps | Low (18.6 USD) | Used as an initial or confirmation test, usually in secondary health centers/laboratories | GenoType MTBDRplus v1 and v2, and GenoType MTBDRsl (Hain Lifescience/Bruker, Tübingen, Germany); Genoscholar NTM + MDRTB II, and Genoscholar PZA-TB II (Nipro, Mechelen, Belgium) |
| WGS | Long (6–11 days, can extend up to 25 days if there are delays in sample shipment or result delivery) | Highly sensitive and specific | High, need culture prior to WGS and expertise of skilled human resources to process and analyze sequencing outputs | High, need appropriate installation, procurement, and supply chains, as well as reliable internet connectivity | High (141–277 USD) | Used as a confirmation test, in tertiary health centers/referral laboratories. WGS is also useful for surveillance and source investigation | Miseq, MiniSeq, NextSeq, HiSeq (Illumina, San Diego, CA, USA); Ion Personal Genome Machine Sequencer (Thermo Fisher Scientific, Waltham, MA, USA); PacBio RS II (Pacific Biosciences, Menlo Park, CA, USA); MinION (Oxford Nanopore Technologies, Oxford, UK) |
| (t)NGS | Short (2–3 days, can extend up to 10 days if there are delays in sample shipment or result delivery)) | Highly sensitive and specific (limit of detection: 100 CFU/mL) | High, need skilled human personnel, but can be used directly on clinical specimens | High, similar to WGS | High, but less than WGS (78.3–230 USD) | Used as a confirmation test, in tertiary health centers/referral laboratories | Same as WGS |
| Challenges | Proposed Solutions |
|---|---|
| Health system | |
| Limited tools and infrastructure, supply chain challenges, sustainability | |
| High cost (capital investment, running, data storage, and overhead expenses) | |
| Lack of expertise (bioinformatics, clinical interpretation) | |
| Inefficient use of the sequencing capacity, limited coverage |
|
| DST methods | |
| Lack of clear-cut values for new TB drugs (pDST), some resistance mechanisms cannot be explored, difficulty interpreting whole-genome variation data when a significant number of rare variants are present (gDST) | |
| Inappropriate use of DST |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saktiawati, A.M.I.; Vasiliu, A.; Saluzzo, F.; Akkerman, O.W. Strategies to Enhance Diagnostic Capabilities for the New Drug-Resistant Tuberculosis (DR-TB) Drugs. Pathogens 2024, 13, 1045. https://doi.org/10.3390/pathogens13121045
Saktiawati AMI, Vasiliu A, Saluzzo F, Akkerman OW. Strategies to Enhance Diagnostic Capabilities for the New Drug-Resistant Tuberculosis (DR-TB) Drugs. Pathogens. 2024; 13(12):1045. https://doi.org/10.3390/pathogens13121045
Chicago/Turabian StyleSaktiawati, Antonia Morita Iswari, Anca Vasiliu, Francesca Saluzzo, and Onno W. Akkerman. 2024. "Strategies to Enhance Diagnostic Capabilities for the New Drug-Resistant Tuberculosis (DR-TB) Drugs" Pathogens 13, no. 12: 1045. https://doi.org/10.3390/pathogens13121045
APA StyleSaktiawati, A. M. I., Vasiliu, A., Saluzzo, F., & Akkerman, O. W. (2024). Strategies to Enhance Diagnostic Capabilities for the New Drug-Resistant Tuberculosis (DR-TB) Drugs. Pathogens, 13(12), 1045. https://doi.org/10.3390/pathogens13121045







