Silver Nanoparticles from Duddingtonia flagrans: Evaluation of Potential Ovicidal Activity on Toxocara canis Eggs
Abstract
1. Introduction
2. Materials and Methods
2.1. Collecting T. canis Eggs
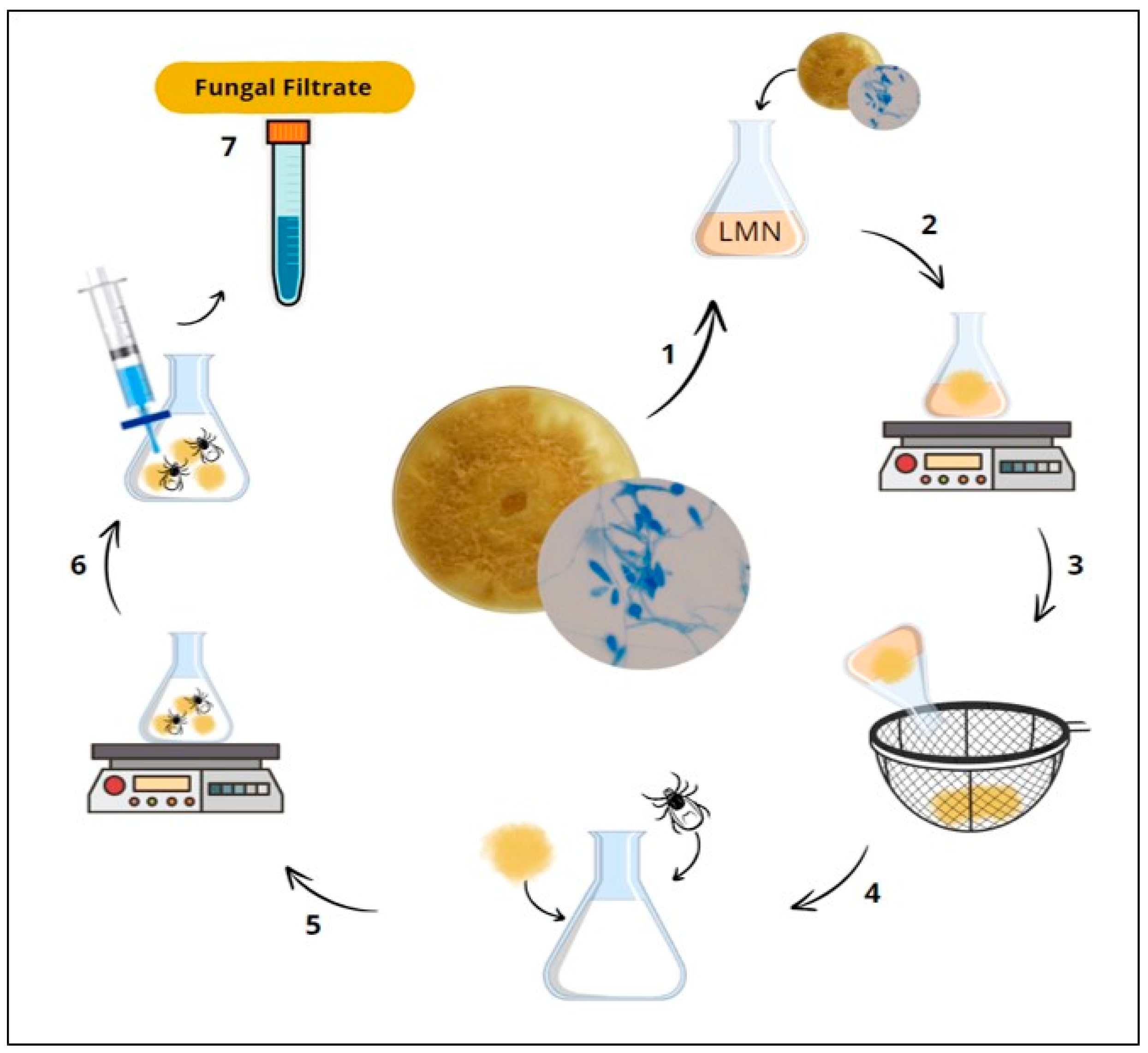
2.2. Producing D. flagrans Fungal Filtrate
2.3. Fungal Filtrate Protein Analysis
2.4. Biosynthesis of Silver Nanoparticles (AgNPs) Derived from Fungal Filtrate
2.5. AgNPs’ Biosynthesis Confirmation and Analysis
2.6. Assay A
2.7. Assay B
2.8. Statistical Analysis
3. Results and Discussion
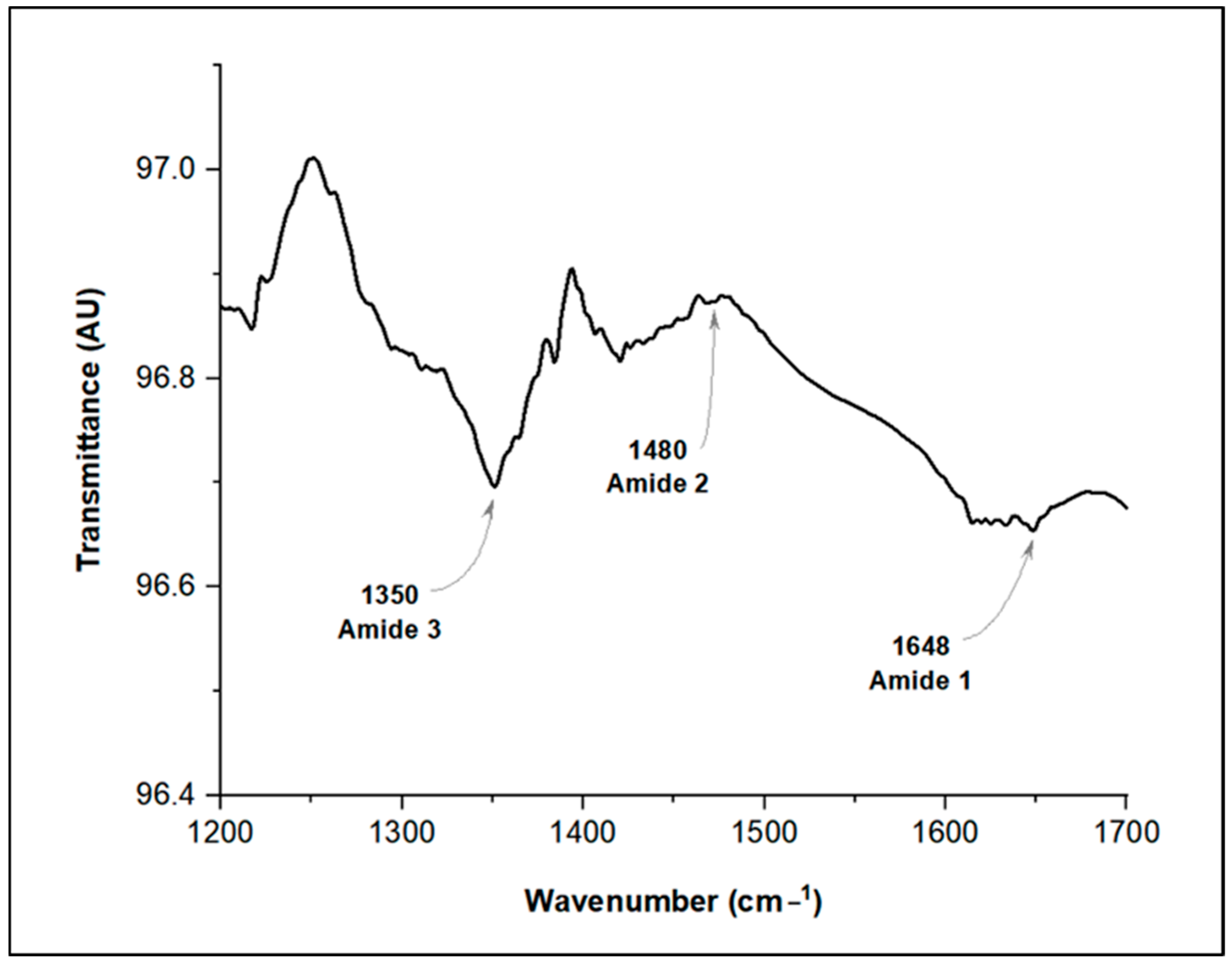
3.1. Fungal Filtrate Production from D. flagrans and Protein Analysis
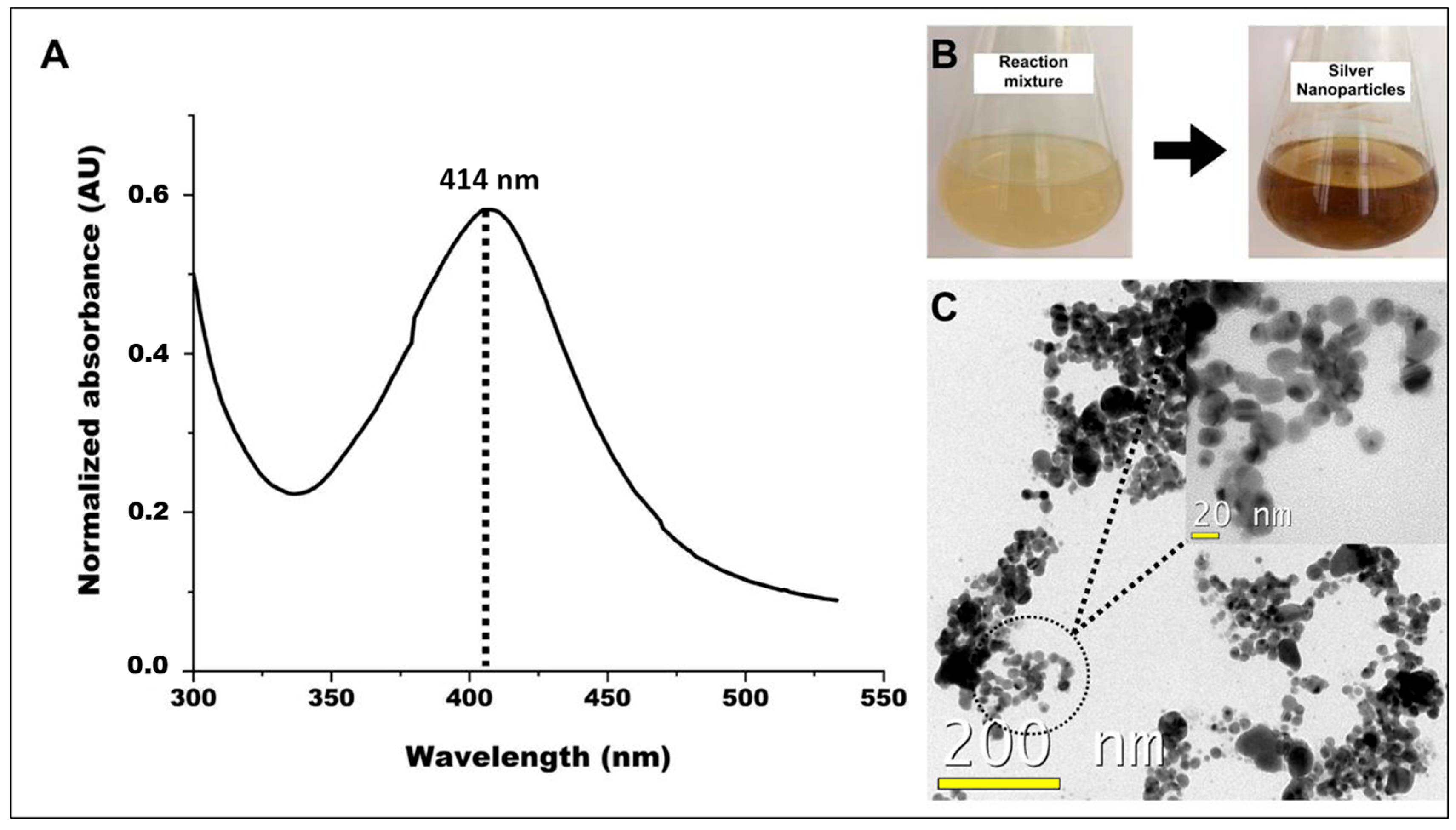
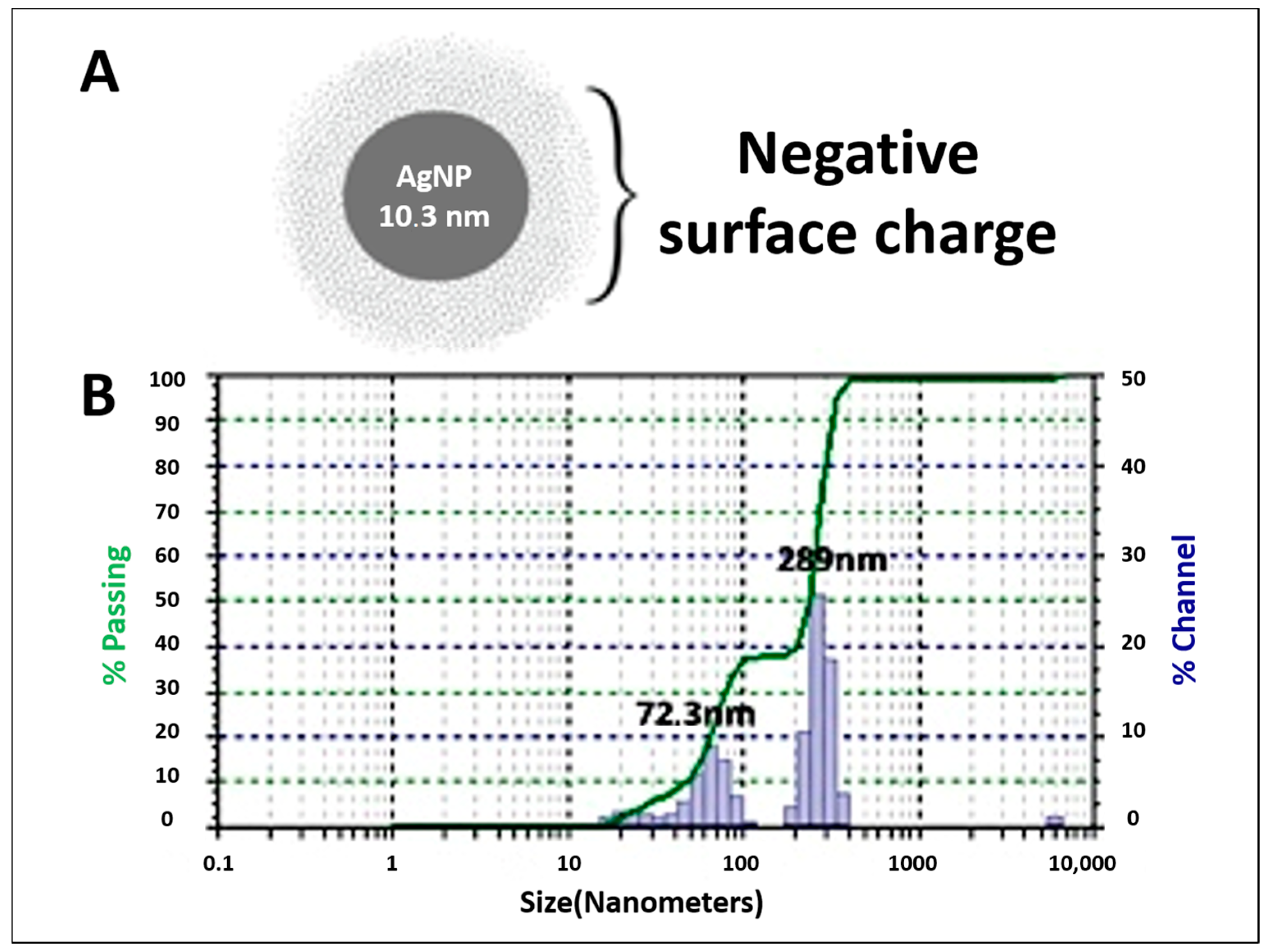
3.2. Silver Nanoparticles Biosynthesis
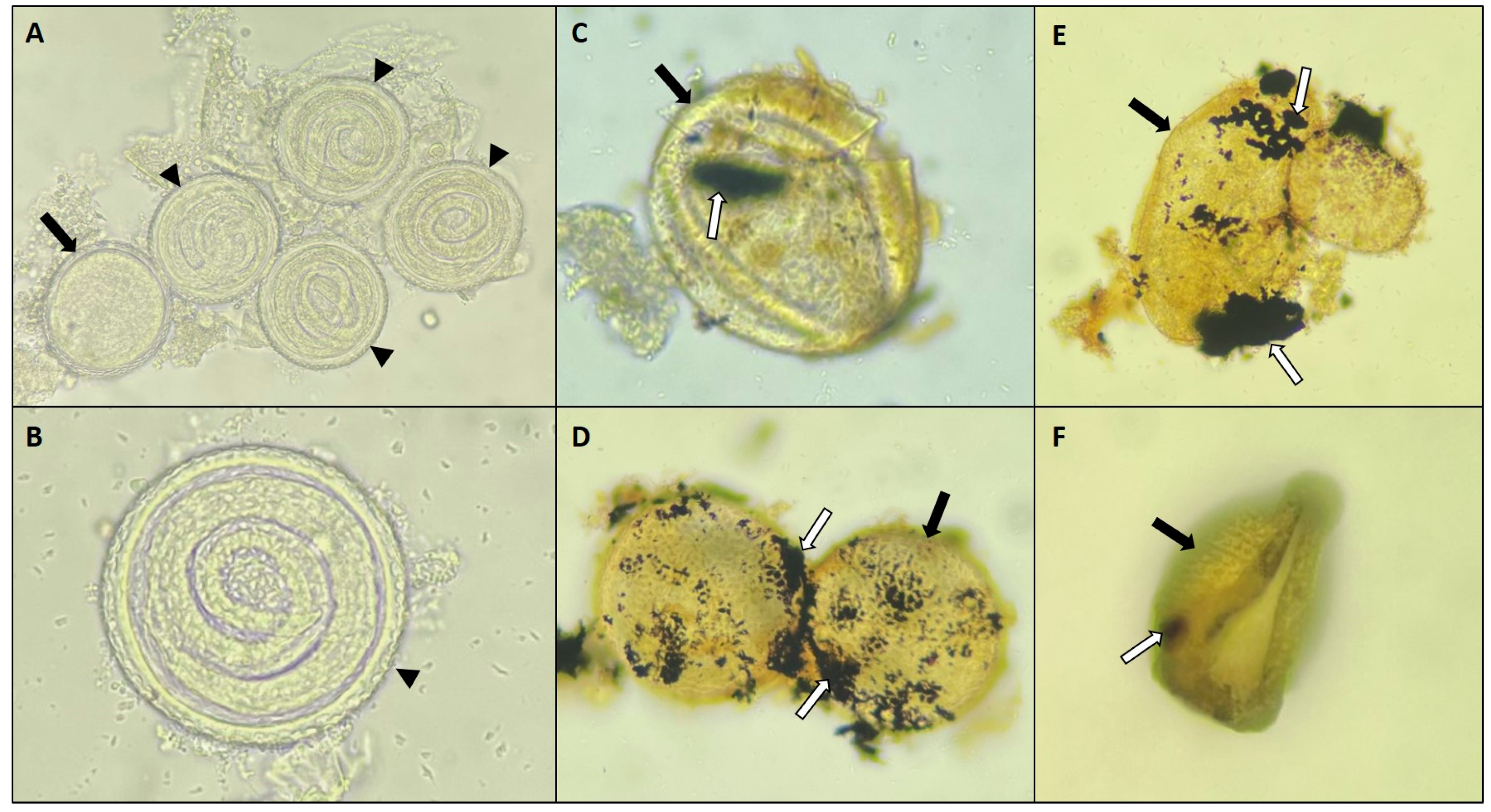
3.3. Nanoparticles Versus T. canis eggs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mazhar, T.; Shrivastava, V.; Tomar, R.S. Green synthesis of bimetallic nanoparticles and its applications: A review. J. Pharm. Sci. Res. 2017, 9, 102–110. [Google Scholar]
- Moghaddam, A.B.; Namvar, F.; Moniri, M.; Tahir, P.M.; Azizi, S.; Mohamad, R. Nanoparticles Biosynthesized by Fungi and Yeast: A Review of Their Preparation, Properties, and Medical Applications. Molecules 2015, 20, 16540–16565. [Google Scholar] [CrossRef] [PubMed]
- Mejía-Méndez, J.L.; López-Mena, E.R.; Sánchez-Arreola, E. Activities against Lung Cancer of Biosynthesized Silver Nanoparticles: A Review. Biomedicines 2023, 11, 389. [Google Scholar] [CrossRef]
- Alghuthaymi, M.A.; Almoammar, H.; Rai, M.; Said-Galiev, E.; Abd-Elsalam, K.A. Myconanoparticles: Synthesis and their role in phytopathogens management. Biotechnol. Biotechnol. Equip. 2015, 29, 221–236. [Google Scholar] [CrossRef]
- Shi, T.; Sun, X.; He, Q.Y. Cytotoxicity of Silver Nanoparticles Against Bacteria and Tumor Cells. Curr. Protein Pept. Sci. 2018, 19, 525–536. [Google Scholar] [CrossRef]
- Hawadak, J.; Kojom Foko, L.P.; Pande, V.; Singh, V. In vitro antiplasmodial activity, hemocompatibility and temporal stability of Azadirachta indica silver nanoparticles. Artif. Cells Nanomed. Biotechnol. 2022, 50, 286–300. [Google Scholar] [CrossRef]
- Abdullah, J.A.A.; Jiménez-Rosado, M.; Guerrero, A.; Romero, A. Effect of Calcination Temperature and Time on the Synthesis of Iron Oxide Nanoparticles: Green vs. Chemical Method. Materials 2023, 16, 1798. [Google Scholar] [CrossRef]
- Flores-Rábago, K.M.; Rivera-Mendoza, D.; Vilchis-Nestor, A.R.; Juarez-Moreno, K.; Castro-Longoria, E. Antibacterial Activity of Biosynthesized Copper Oxide Nanoparticles (CuONPs) Using Ganoderma sessile. Antibiotics 2023, 12, 1251. [Google Scholar] [CrossRef]
- Dell’Annunziata, F.; Mosidze, E.; Folliero, V.; Lamparelli, E.P.; Lopardo, V.; Pagliano, P.; Porta, G.D.; Galdiero, M.; Bakuridze, A.D.; Franci, G. Eco-friendly synthesis of silver nanoparticles from peel and juice C. limon and their antiviral efficacy against HSV-1 and SARS-CoV-2. Virus Res. 2024, 349, 199455. [Google Scholar] [CrossRef]
- Lima, A.K.O.; Souza, L.M.D.S.; Reis, G.F.; Junior, A.G.T.; Araújo, V.H.S.; Santos, L.C.D.; Silva, V.R.P.D.; Chorilli, M.; Braga, H.C.; Tada, D.B.; et al. Synthesis of Silver Nanoparticles Using Extracts from Different Parts of the Paullinia cupana Kunth Plant: Characterization and In Vitro Antimicrobial Activity. Pharmaceuticals 2024, 17, 869. [Google Scholar] [CrossRef]
- Dubey, R.K.; Shukla, S.; Hussain, Z. Green Synthesis of Silver Nanoparticles; A Sustainable Approach with Diverse Applications. Chin. J. Appl. Physiol. 2023, 39, e20230007. [Google Scholar] [CrossRef] [PubMed]
- Rolim, W.R.; Pelegrino, M.T.; Lima, B.A.; Ferraz, L.S.; Costa, F.N.; Bernardes, J.S.; Rodigues, T.; Brocchi, M.; Seabra, A.b. Green tea extract mediated biogenic synthesis of silver nanoparticles: Characterization, cytotoxicity evaluation and antibacterial activity. Appl. Surf. Sci. 2019, 463, 66. [Google Scholar] [CrossRef]
- Duran, N.; Seabra, A.B. Biogenic Synthesized Ag/Au Nanoparticles: Production, Characterization, and Applications. Curr. Nanosci. 2018, 14, 82–94. [Google Scholar] [CrossRef]
- Chowdhury, S.; Basu, A.; Kundu, S. Green synthesis of protein capped silver nanoparticles from phytopathogenic fungus Macrophomina phaseolina (Tassi) Goid with antimicrobial properties against multidrug-resistant bacteria. Nanoscale Res. Lett. 2014, 9, 365–376. [Google Scholar] [CrossRef]
- Costa Silva, L.P.; Oliveira, J.P.; Keijok, W.J.; da Silva, A.R.; Aguiar, A.R.; Guimarães, M.C.C.; Ferraz, C.M.; Araújo, J.V.; Tobias, F.L.; Braga, F.R. Extracellular biosynthesis of silver nanoparticles using the cell-free filtrate of nematophagous fungus Duddingtonia flagrans. Int. J. Nanomed. 2017, 12, 6373–6381. [Google Scholar] [CrossRef]
- Soares, F.E.F.; Ferreira, J.M.; Genier, H.L.A.; Al-Ani, L.K.T.; Aguilar-Marcelino, L. Biological control 2.0: Use of nematophagous fungi enzymes for nematode control. J. Pestic. Res. J. 2023, 4, 100025. [Google Scholar]
- Braga, F.R.; Soares, F.E.F.; Giuberti, T.Z.; Lopes, A.D.C.G.; Lacerda, T.; Ayupe, T.H.; Queiroz, P.V.; Gouveia, A.S.; Pinheiro, L.; Araújo, A.L.; et al. Nematocidal activity of extracellular enzymes produced by the nematophagous fungus Duddingtonia flagrans on cyathostomin infective larvae. Vet. Parasitol. 2015, 212, 214–218. [Google Scholar] [CrossRef]
- Rodrigues, J.A.; Feitosa, T.F.; Vilela, V.L.R. A systematic review on products derived from nematophagous fungi in the biological control of parasitic helminths of animals. World J. Microbiol. Biotechnol. 2024, 40, 224. [Google Scholar] [CrossRef]
- Araújo, J.V.d.; Fonseca, J.d.S.; Barbosa, B.B.; Valverde, H.A.; Santos, H.A.; Braga, F.R. The Role of Helminthophagous Fungi in the Biological Control of Human and Zoonotic Intestinal Helminths. Pathogens 2024, 13, 741. [Google Scholar] [CrossRef]
- Bampidis, V.; Azimonti, G.; Bastos, M.L.; Christensen, H.; Dusemund, B.; Durjava, M.K.; Kouba, M.; López-Alonso, M.; López Puente, S.; Marcon, F.; et al. Safety and efficacy of BioWorma® (Duddingtonia flagrans NCIMB 30336) as a feed additive for all grazing animals. EFSA J. 2020, 18, e06208. [Google Scholar]
- Braga, F.R.; Ferraz, C.M.; da Silva, E.N.; Araújo, J.V. Efficiency of the Bioverm® (Duddingtonia flagrans) fungal formulation to control in vivo and in vitro of Haemonchus contortus and Strongyloides papillosus in sheep. 3 Biotech 2020, 10, 62. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, A.C.M.S.; Silva, L.P.C.; Ferraz, C.M.; Tobias, F.L.; Araújo, J.V.; Loureiro, B.; Braga, G.M.A.M.; Veloso, F.B.R.; Soares, F.E.F.; Fronza, M.; et al. Nematicidal activity of silver nanoparticles from the fungus Duddingtonia flagrans. Int. J. Nanomed. 2019, 14, 2341–2348. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, C.M.; Silva, L.P.C.; Soares, F.E.F.; Souza, R.L.O.; Tobias, F.L.; Araújo, J.V.; Veloso, F.B.R.; Laviola, F.P.; Endringer, D.C.; de Gives, P.M.; et al. Effect of silver nanoparticles (AgNP’s) from Duddingtonia flagrans on cyathostomins larvae (subfamily: Cyathostominae). J. Invertebr. Pathol. 2020, 174, 107395. [Google Scholar] [CrossRef]
- Ferraz, C.M.; de Oliveira, M.L.C.; de Assis, J.P.B.; Silva, L.P.C.; Tobias, F.L.; Lima, T.F.; Soares, F.E.F.; Vilela, V.L.R.; Araújo, J.V.; Braga, F.R. In vitro evaluation of the nematicidal effect of Duddingtonia flagrans silver nanoparticles against strongylid larvae (L3). Biocontrol. Sci. Technol. 2021, 32, 905–909. [Google Scholar] [CrossRef]
- Król, G.; Fortunka, K.; Majchrzak, M.; Piktel, E.; Paprocka, P.; Mańkowska, A.; Lesiak, A.; Karasiński, M.; Strzelecka, A.; Durnaś, B.; et al. Metallic Nanoparticles and Core-Shell Nanosystems in the Treatment, Diagnosis, and Prevention of Parasitic Diseases. Pathogens 2023, 12, 838. [Google Scholar] [CrossRef]
- Farmer, A.; Beltran, T.; Choi, Y.S. Prevalence of Toxocara species infection in the U.S.: Results from the National Health and Nutrition Examination Survey, 2011–2014. PLoS Negl. Trop. Dis. 2017, 11, e0005818. [Google Scholar] [CrossRef]
- Kopp, S.R.; Coleman, G.T.; Traub, R.J.; McCarthy, J.S.; Kotze, A.C. Acetylcholine receptor subunit genes from Ancylostoma caninum: Altered transcription patterns associated with pyrantel resistance. Int. J. Parasitol. 2009, 39, 435–441. [Google Scholar] [CrossRef]
- Ma, G.; Holland, C.V.; Wang, T.; Hofmann, A.; Fan, C.K.; Maizels, R.M.; Hotez, P.J.; Gasser, R.B. Human toxocariasis. Lancet Infect. Dis. 2018, 18, 14–24. [Google Scholar] [CrossRef]
- Dantas-Torres, F.; Otranto, D. Dogs, cats, parasites, and humans in Brazil: Opening the black box. Parasit. Vectors 2014, 7, 22. [Google Scholar] [CrossRef]
- Chen, J.; Liu, Q.; Liu, G.H.; Zheng, W.B.; Hong, S.J.; Sugiyama, H.; Zhu, X.Q.; Elsheikha, H.M. Toxocariasis: A silent threat with a progressive public health impact. Infect. Dis. Poverty 2018, 7, 59. [Google Scholar] [CrossRef]
- Wharton, D.A. Nematode egg-shells. Parasitology 1980, 81, 447–463. [Google Scholar] [CrossRef] [PubMed]
- Ayçiçek, H.; Yarsan, E.; Sarimehmetoğlu, H.O.; Tanyüksel, M.; Girginkardeşler, N.; Özyurt, M. Efficacy of Some Disinfectants on Embryonated Eggs of Toxocara canis. Turk. J. Med. Sci. 2001, 31, 35–39. [Google Scholar]
- Deutz, A.; Fuchs, K.; Auer, H.; Kerbl, U.; Aspöck, H.; Köfer, J. Toxocara-infestations in Austria: A study on the risk of infection of farmers, slaughterhouse staff, hunters and veterinarians. Parasitol. Res. 2005, 97, 390–394. [Google Scholar] [CrossRef]
- Ma, G.; Rostami, A.; Wang, T.; Hofmann, A.; Hotez, P.J.; Gasser, R.B. Global and regional seroprevalence estimates for human toxocariasis: A call for action. Adv. Parasitol. 2020, 109, 275–290. [Google Scholar]
- Ponce-Macotela, M.; Martínez-Gordillo, M.N. Toxocara: Seroprevalence in Mexico. Adv. Parasitol. 2020, 109, 341–355. [Google Scholar]
- Van Knapen, F.; Franchimont, J.H. Steam sterilisation of sandpits infected with Toxocara eggs. Brit. Med. J. 1979, 1, 1320. [Google Scholar] [CrossRef][Green Version]
- Kamiya, M.; Ooi, H.K.; Nomura, T. The effect of radiation on the viability and migratory ability of second-stage larvae of Toxocara canis in mice. Vet. Parasitol. 1987, 24, 87–92. [Google Scholar] [CrossRef]
- O’Lorcain, P. The effect of freezing on the viability of Toxocara canis and T. cati embryonated eggs. J. Helminthol. 1995, 69, 169–171. [Google Scholar] [CrossRef]
- Ooi, H.K.; Lin, C.L.; Wang, J.S. Effect of ozone treatment on Toxocara canis eggs. J. Vet. Med. Sci. 1998, 60, 169–173. [Google Scholar] [CrossRef][Green Version]
- Romero, C.; Heredia, R.; Bolio, M.; Miranda, L.; Reyes, L.; Arredondo, M.; Flores, A. Comparison of In Vitro Efficacy of Six Disinfectants on the Hatching of Larval Eggs of Toxocara canis. Iran J. Parasitol. 2020, 15, 315–320. [Google Scholar] [CrossRef]
- Ciarmela, M.L.; Minvielle, M.C.; Lori, G.; Basualdo, J.A. Biological interaction between soil fungi and Toxocara canis eggs. Vet. Parasitol. 2002, 103, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Gortari, C.; Cazau, C.; Hours, R. Hongos nematófagos de huevos de Toxocara canis en un paseo público de La Plata, Argentina [Nematophagous fungi of Toxocara canis eggs in a public park of La Plata, Argentina]. Rev. Iberoam. Micol. 2007, 24, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Maia Filho, F.S.; Fonseca, A.O.S.; Persici, B.M.; Silveira, J.S.; Braga, C.Q.; Pötter, L.; Botton, S.A.; Pereira, D.I.B. Trichoderma virens as a biocontrol of Toxocara canis: In vivo evaluation. Rev. Iberoam. Micol. 2017, 34, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Meroni, G.; Laterza, G.; Tsikopoulos, A.; Tsikopoulos, K.; Vitalini, S.; Scaglia, B.; Iriti, M.; Onizzi, L.; Martino, P.A.; Soggiu, A. Antibacterial Potential of Essential Oils and Silver Nanoparticles against Multidrug-Resistant Staphylococcus pseudintermedius Isolates. Pathogens 2024, 13, 156. [Google Scholar] [CrossRef]
- Verocai, C.G.; Tavares, P.V.; Ribeiro, F.A.; Correia, T.R.; Scott, F.B. Effects of disinfectants on Toxocara canis embryogenesis and larval establishment in mice tissues. Zoonoses Public Health 2010, 57, 213–216. [Google Scholar] [CrossRef]
- Cruz, L.M.; Allanson, M.; Kwa, B.; Azizan, A.; Izurieta, R. Morphological changes of Ascaris spp. eggs during their development outside the host. J. Parasitol. 2012, 98, 63–68. [Google Scholar] [CrossRef]
- Zhang, S.; Angel, C.; Gu, X.; Liu, Y.; Li, Y.; Wang, L.; Zhou, X.; He, R.; Peng, X.; Yang, G.; et al. Efficacy of a chlorocresol-based disinfectant product on Toxocara canis eggs. Parasitol. Res. 2020, 119, 3369–3376. [Google Scholar] [CrossRef]
- Ayres, M.; Ayres, J.R.; Ayres, D.L.; Santos, A.S. BioEstat 3.0: Aplicações Estatísticas nas Áreas das Ciências Biológicas e Médicas; Sociedade Civil Mamirauá, CNPq: Belém, Brazil, 2003. [Google Scholar]
- Mendoza De Gives, P.; Vazquez-Prats, V.M. Reduction of Haemonchus contortus infective larvae by three nematophagous fungi in sheep faecal cultures. Vet. Parasitol. 1994, 5, 197–203. [Google Scholar] [CrossRef]
- Kumar, C.G.; Takagi, H. Microbial alcaline preteases: From a industrial view poit. Biotechnol. Adv. 1999, 17, 561–594. [Google Scholar] [CrossRef]
- Kaushik, P.; Malik, A. Fungal dye decolourization: Recent advances and future potential. Environ. Int. 2009, 35, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Garg, R.; Rani, P.; Garg, R.; Khan, M.A.; Khan, N.A.; Khan, A.H.; Américo-Pinheiro, J.H.P. Biomedical and catalytic applications of agri-based biosynthesized silver nanoparticles. Environ. Pollut. 2022, 310, 119830. [Google Scholar] [CrossRef] [PubMed]
- Ninganagouda, S.; Rathod, V.; Jyoti, H.; Singh, D.; Prema, K.; Haq, M.U. Extracellular biosynthesis of silver nanoparticles using Aspergillus flavus and their antimicrobial activity against gram negative MDR strains. Int. J. Pharma. Bio Sci. 2013, 4, 222–229. [Google Scholar]
- Bhattacharjee, S. DLS and zeta potential—What they are and what they are not? J. Control Release 2016, 235, 337–351. [Google Scholar] [CrossRef]
- Patel, V.R.; Agrawal, Y.K. Nanosuspension: An approach to enhance solubility of drugs. J. Adv. Pharm. Technol. Res. 2011, 2, 81–87. [Google Scholar]
- Lima, B.G.A.; Silva, R.R.; Meira, H.M.; Durval, I.J.B.; Macedo Bezerra Filho, C.; Silva, T.A.L.; Sarubbo, L.A.; Luna, J.M. Synthesis and Characterization of Silver Nanoparticles Stabilized with Biosurfactant and Application as an Antimicrobial Agent. Microorganisms 2024, 12, 1849. [Google Scholar] [CrossRef]
- Filippov, S.K.; Khusnutdinov, R.; Murmiliuk, A.; Inam, W.; Zakharova, L.Y.; Zhang, H.; Khutoryanskiy, V.V. Dynamic light scattering and transmission electron microscopy in drug delivery: A roadmap for correct characterization of nanoparticles and interpretation of results. Mater. Horiz. 2023, 10, 5354–5370. [Google Scholar] [CrossRef]
- Ji, Y.; Yang, X.; Ji, Z.; Zhu, L.; Ma, N.; Chen, D.; Jia, X.; Tang, J.; Cao, Y. DFT-Calculated IR Spectrum Amide I, II, and III Band Contributions of N-Methylacetamide Fine Components. ACS Omega 2020, 5, 8572–8578. [Google Scholar] [CrossRef]
- Hou, F.; Ma, X.; Fan, L.; Wang, D.; Wang, W.; Ding, T.; Ye, X.; Liu, D. Activation and conformational changes of chitinase induced by ultrasound. Food Chem. 2019, 1, 355–362. [Google Scholar] [CrossRef]
- Yang, H.; Yang, S.; Kong, J. Obtaining information about protein secondary structures in aqueous solution using Fourier transform IR spectroscopy. Nat. Protoc. 2015, 10, 382–396. [Google Scholar] [CrossRef]
- Kong, J.; Yu, S. Fourier transform infrared spectroscopic analysis of protein secondary structures. Acta Biochim. Biophys. Sin. 2007, 39, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Morgan-Jones, G.; White, J.F.; Rodriguez-Kábana, R. Phytonematode patology: Ultrastructural studies. I parasitismo of Meloidogyne arenaria eggs by Verticillium chlamydosporium. Nematropica 1993, 13, 245–260. [Google Scholar]
- Schwartz, R.; Bidaisee, S.; Fields, P.J.; Macpherson, M.L.A.; Macpherson, C.N.L. The epidemiology and control of Toxocara canis in puppies. Parasite Epidemiol. Control 2021, 8, e00232. [Google Scholar] [CrossRef] [PubMed]
- Khademvatan, S.; Rahim, F.; Tavalla, M.; Abdizadeh, R.; Hashemitabar, M. PCR-based molecular characterization of Toxocara spp. using feces of stray cats: A study from Southwest Iran. PLoS ONE 2013, 8, e65293. [Google Scholar] [CrossRef]
- Nijsse, R.; Overgaauw, P.; Ploeger, H.; Mughini-Gras, L. Sources of environmental contamination with Toxocara spp.: An omnipresent parasite. Adv. Parasitol. 2020, 109, 585–614. [Google Scholar]
- Chieffi, P.P.; Lescano, S.A.Z.; Fonseca, G.R.; Santos, S.V. Human Toxocariasis: 2010 to 2020 Contributions from Brazilian Researchers. Res. Rep. Trop. Med. 2021, 12, 81–91. [Google Scholar] [CrossRef]
- Ibragimova, Z.H.B.; Anan’ko, G.G.; Kostina, N.E.; Teplyakova, T.V.; Mazurkova, N.A. Toxicity and Antiviral Activity of the Extracts of Submerged Mycelium of Nematophagous Duddingtonia flagrans Fungus in Vero Cell Culture. Bull. Exp. Biol. Med. 2015, 160, 246–248. [Google Scholar] [CrossRef]
- Voinot, M.; Bonilla, R.; Sousa, S.; Sanchís, J.; Canhão-Dias, M.; Delgado, J.R.; Lozano, J.; Sánchez-Andrade, R.; Arias, M.S.; de Carvalho, L.M. Control of Strongyles in First-Season Grazing Ewe Lambs by Integrating Deworming and Thrice-Weekly Administration of Parasiticidal Fungal Spores. Pathogens 2021, 10, 1338. [Google Scholar] [CrossRef]
- Viña, C.; Salmo, R.; Pena, M.V.; Palomero, A.M.; Hernández, J.A.; Cazapal-Monteiro, C.; Arias, M.S.; Sánchez-Andrade, R.; Paz-Silva, A. A New Comestible Formulation of Parasiticide Fungi to Reduce the Risk of Soil-Transmitted Helminth Infections in a Canine Shelter. Pathogens 2022, 11, 1391. [Google Scholar] [CrossRef]
- Wang, B.B.; Li, Y.L.; Tian, S.Y.; Wang, H.Z.; Li, X.; Wang, F.H.; Cai, K.Z. Chlamydospore dormancy and predatory activity of nematophagous fungus Duddingtonia flagrans. J. Basic. Microbiol. 2024, 64, 2300365. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, L.; Yi, S.; Huang, Y.; Lenaghan, S.C.; Zhang, M. Naturally occurring nanoparticles from Arthrobotrys oligospora as a potential immunostimulatory and antitumor agente. Adv. Funct. Mater. 2013, 23, 2175–2184. [Google Scholar] [CrossRef]
- Duddington, C.L. A new predacious species of Trichotecium. Trans. Br. Mycol. Soc. 1949, 32, 284–287. [Google Scholar] [CrossRef]
- Faedo, M.; Larsen, M.; Dimander, S.O.; Yeates, G.W.; Hoglund, J.; Waller, P.J. Growth of the Fungus Duddingtonia flagrans in soil surrounding feces deposited by cattle or sheep fed the fungus to control nematode parasites. Biol. Control 2002, 23, 64–70. [Google Scholar] [CrossRef]

| Days | G1 | G2 | %R (Ovicidal) |
|---|---|---|---|
| 15 | 131.4 a ± 46.2 | 249.1 b ± 45.8 | 47% |
| 30 | 116.6 a ± 45.3 | 185.1 b ± 59.2 | 37% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferraz, C.M.; Comério, L.C.; Segantine, V.B.S.; de Assis, J.P.B.; Costa Silva, L.P.; Bezerra, L.D.N.R.; de Araújo, J.V.; Vilela, V.L.R.; Soares, F.E.d.F.; Rossi, G.A.M.; et al. Silver Nanoparticles from Duddingtonia flagrans: Evaluation of Potential Ovicidal Activity on Toxocara canis Eggs. Pathogens 2024, 13, 1043. https://doi.org/10.3390/pathogens13121043
Ferraz CM, Comério LC, Segantine VBS, de Assis JPB, Costa Silva LP, Bezerra LDNR, de Araújo JV, Vilela VLR, Soares FEdF, Rossi GAM, et al. Silver Nanoparticles from Duddingtonia flagrans: Evaluation of Potential Ovicidal Activity on Toxocara canis Eggs. Pathogens. 2024; 13(12):1043. https://doi.org/10.3390/pathogens13121043
Chicago/Turabian StyleFerraz, Carolina Magri, Lara Coslop Comério, Vinícius Bastos Salles Segantine, João Pedro Barbosa de Assis, Laryssa Pinheiro Costa Silva, Lara De Nadai Rodrigues Bezerra, Jackson Victor de Araújo, Vinícius Longo Ribeiro Vilela, Filippe Elias de Freitas Soares, Gabriel Augusto Marques Rossi, and et al. 2024. "Silver Nanoparticles from Duddingtonia flagrans: Evaluation of Potential Ovicidal Activity on Toxocara canis Eggs" Pathogens 13, no. 12: 1043. https://doi.org/10.3390/pathogens13121043
APA StyleFerraz, C. M., Comério, L. C., Segantine, V. B. S., de Assis, J. P. B., Costa Silva, L. P., Bezerra, L. D. N. R., de Araújo, J. V., Vilela, V. L. R., Soares, F. E. d. F., Rossi, G. A. M., Tobias, F. L., Langoni, H., & Braga, F. R. (2024). Silver Nanoparticles from Duddingtonia flagrans: Evaluation of Potential Ovicidal Activity on Toxocara canis Eggs. Pathogens, 13(12), 1043. https://doi.org/10.3390/pathogens13121043












