1. Introduction
Escherichia coli is one of the most divergent and widespread species that can behave as a commensal in the human gastrointestinal tract and persists in water and soil regardless of the host. At the same time, it can behave as a pathogen causing different types of diseases both in and outside of a human or animal host’s digestive system [
1]. The remarkable and complex plasticity of the
E. coli genome contributes to the formation of the pathogenic potential. Thus, as a result of taking up and accumulating pathogenic factors, multidrug-resistant high-risk clones capable of causing a wide range of diseases in humans in a certain biotope/system can emerge [
2,
3].
According to the combination of special genetic markers,
E. coli can be subdivided into eight phylogenetic groups: A, B1, B2, C, D, E, F, and G. Such phylogroup classification of
E. coli is useful for comparative analysis of serogroup, virulence, and resistance traits, as well as diversity assessment of
E. coli populations within various hosts and environments [
4,
5].
The pathogenic potential of
E. coli is implemented by the presence of various virulence factors. There are different
E. coli pathotypes represented by a group of clones that share a certain set of specific virulence determinants [
2]. The most common
E. coli ST131 clone from phylogenetic group B2 is the predominant clone of high-risk worldwide, and recently another ST1193 clone was recognized as an emerging high-risk clone belonging to the same group. High-risk
E. coli clones are spreading very quickly, which leads to their existence in different niches, in human and animal intestinal tracts, and persistence in the environment [
3]. Moreover, phylogenomic approaches have shown that only four sequence types (STs) are responsible for extraintestinal infections (ST131, ST73, and ST95 of a phylogroup B2, and ST69 of a phylogroup D), which were always studied in epidemiological surveillance investigations and were therefore named “the big four ExPEC (extraintestinal pathogenic
E. coli) clones” [
6].
Most
E. coli strains are characterized by possessing either type I-E or I-F CRISPR/Cas systems. These findings can explain an evolutionary interrelation between CRISPR and pathogenicity in
E. coli [
7]. Taking into account the continuously accumulating data, it becomes apparent that adaptive immunity is not the only role of CRISPR/Cas systems. It was shown that the expression of many bacterial genes affecting the virulence and group behavior of pathogenic bacteria is regulated by these systems. Additionally, CRISPR/Cas participates in DNA repair and accelerates the evolution of the genomes [
8]. Other studies indicated an association between the presence of the CRISPR sequence and decreased antibiotic resistance, thus suggesting that the presence of CRISPR limits the adaptability of the microorganism [
7,
9].
It is noteworthy that most genome sequencing projects emphasize the analysis of multidrug-resistant clones, clinically relevant pathogens, or epidemiological outbreaks. However, there is a lack of genomic data on opportunistic
E. coli populations obtained from healthy patients, namely, puerperant women, who take a specific place in maternity care facilities. They are not patients with some kind of pathology, but, at the same time, they are “healthy patients” with an open system (maternal birth canal) or surgical suture (after caesarian section), which are susceptible to infections in hospital conditions. Women who give birth by cesarean section labor are estimated to have a 5-fold to 20-fold risk of bacterial infection in comparison to women who give birth vaginally [
10]. Regardless of the labor type, women in the early postpartum period face transient immunodeficiency, and decreased activity of local tissue immunity, which increases their sensitivity to bacterial infection [
11]. At the same time,
E. coli bacteria play a major role in the etiology of puerperal sepsis [
12].
As a part of our ongoing molecular surveillance program, here we provide a detailed investigation of E. coli isolates collected from maternal birth canal discharge of puerperant women in two perinatal centers of Ekaterinburg, Russia (referenced as ‘Crie-Pu’ isolates below). We used whole genome sequencing (WGS) to characterize E. coli isolates as a dominating bacteria collected. The entire collection of the strains was examined in terms of population structure, phenotypic and genotypic profiles of antimicrobial resistance, virulence factors, plasmid replicons, and analysis of CRISPR-elements patterns. Information on resistance and virulence genes and correlation analysis is an important tool for epidemiological studies in assessing the pathogenic potential and total pool of important determinants in a population of opportunistic E. coli and monitoring the emergence of new clinically and epidemically significant resistance and virulence phenotypes.
3. Results
3.1. Isolate Typing
The 53 isolates of
E. coli belonged to 31 different STs without a significant prevalence of any individual one. Most numerous were ST69 (n = 8), ST73 (n = 5), and ST131 (n = 6), while 29 different STs were represented only by less than four isolates or even by a single one. Phylogroup classification demonstrated that
E. coli isolates were distributed in six diverse phylogroups—A, B1, B2, C, D, and F, with the prevalence of B2 represented by 32 samples belonging to 18 STs (
Table S2). The phylogroup D combined nine isolates of ST69 and ST349 (single sample). A and B1 phylogroups consisted of four and five isolates, respectively, while C and F were represented by a single sample each (
Table S2). The exact phylogenetic group was not identified for three isolates (Crie-Pu1335, 1340, and 1370), the last of which carried boundary traits of B2 and F phylogroups. Additionally, twenty different O serogroups were distinguished, O50 (15%), O15 (13%), O16 (7.5%), and O75 (9%) were the most frequent among the isolates under investigation. O-serogroup diversity is described in more detail in the section “Virulence genes” as long as both O-serogroup antigen and virulence factors are essential for the pathogenicity assessment of
E. coli.
3.2. CRISPR Element Distribution of the Crie-Pu E. coli Isolates
Approximately 68% of the
E. coli genomes available in the CRISPRCasdb are predicted to harbor active CRISPR/Cas systems (
https://crisprcas.i2bc.paris-saclay.fr/MainDb/StrainList, accessed on 3 July 2024). High variability of CRISPR/Cas systems was observed along with previously known diversity and plasticity of
E. coli genomes. This is the first work that shows a thorough description of CRISPR/Cas elements in Russian
E. coli isolates of clinical origin (listed in
Table S3) collected from healthy patients, in comparison to the strains of the same species that were available from public databases (
https://crisprcas.i2bc.paris-saclay.fr/MainDb/StrainList, accessed on 3 July 2024).
A total of 26.4% (14 out of 53) of the studied
E. coli isolates harbored neither CRISPR array nor cas cassette. Six isolates (11.3%) from the set possessed confirmed CRISPR arrays but lacked cas cassettes, and thirty-three samples (62.3%) carried different putative CRISPR/Cas systems containing cas cassettes (
Table S3). It should be noted that a similar distribution of CRISPR arrays and CRISPR/Cas systems containing cas cassettes was observed for our
E. coli isolates and the 3210 reference
E. coli isolates available in the CRISPRCas database (
https://crisprcas.i2bc.paris-saclay.fr/MainDb/StrainList, accessed on 3 July 2024). Namely, 2470 and 2193 isolates were characterized as carrying CRISPR arrays and cas cassettes, respectively, while 740 strains did not have any CRISPR/Cas elements.
Among 33 Crie-Pu
E. coli isolates carrying putative CRISPR/Cas systems containing cas cassettes, 19 carried the Type I-E CRISPR/Cas system (57.5%) and 14 carried the Type I-F CRISPR/Cas system (42.4%). It should be noted that Type I-E CRISPR/Cas systems are found more frequently (
p ˂ 0.05) in the group of reference
E. coli isolates available in the CRISPRCas database (
https://crisprcas.i2bc.paris-saclay.fr/MainDb/StrainList, accessed on 3 July 2024), while Type I-F CRISPR/Cas systems occurred much more frequently (
p ˂ 0.00001) in the experimental group of Crie-Pu isolates.
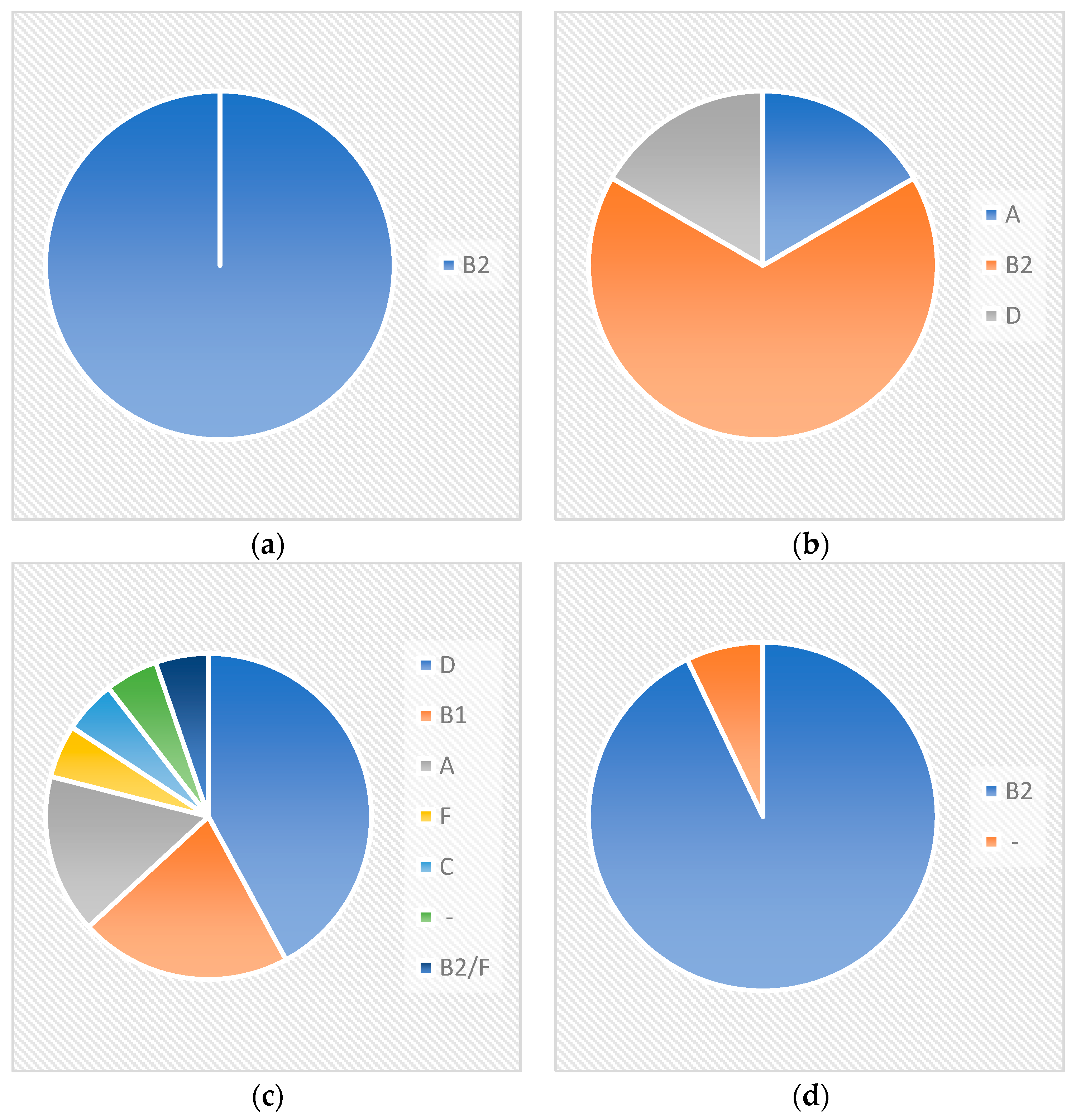
Crie-Pu isolates bearing neither a CRISPR array nor a cas cassette belonged to eight different sequence types, the predominant being ST131 and ST73—five and three Crie-Pu isolates without cas cassettes belonged to these STs, respectively (
Figure 1). Crie-Pu isolates with confirmed CRISPR arrays, but without cas cassettes, belonged to 5 different sequence types, the predominant being ST73 possessed by two Crie-Pu isolates (
Figure 1).
Crie-Pu isolates with Type I-E CRISPR/Cas systems belonged to 12 different sequence types with the dominance of ST69 (eight out of 19 Crie-Pu isolates) (
Figure 1). The isolates with Type I-F CRISPR/Cas systems belonged to nine different sequence types, the predominant being ST141 (four isolates), ST1993, and ST80 (two isolates each) (
Figure 1).
All Crie-Pu isolates bearing neither a CRISPR array nor cas cassette belonged to the phylogenetic group B2 (
Figure 2), while the isolates with confirmed CRISPR arrays, but without cas cassettes, belonged to three different phylogenetic groups—B2 (four isolates), A, and D (one isolate each) (
Figure 2).
Crie-Pu isolates with Type I-E CRISPR/Cas systems belonged to six different phylogenetic groups—D (eight isolates), B1 (4), A (3), F, C, and B2/F, while the isolate Crie-Pu1335 was not assigned to any known phylogroup (
Figure 2). Almost all isolates with Type I-F CRISPR/Cas systems belonged to the B2 phylogenetic group, and only Crie-Pu1340 was not assigned to any group (
Figure 2).
At the same time, almost all of the Type I-E CRISPR/Cas systems (18 out of 19) consisted of eight genes encoding Cas1, Cas2, Cas3, Cas5, Cas6, Cas7, Cse1, and Cse2. Crie-Pu1332 Type I-E isolate carried four cas genes—cas3, cse1, cas1, and cas2, and Crie-Pu1252 Type I-E isolate carried an additional cas3 gene.
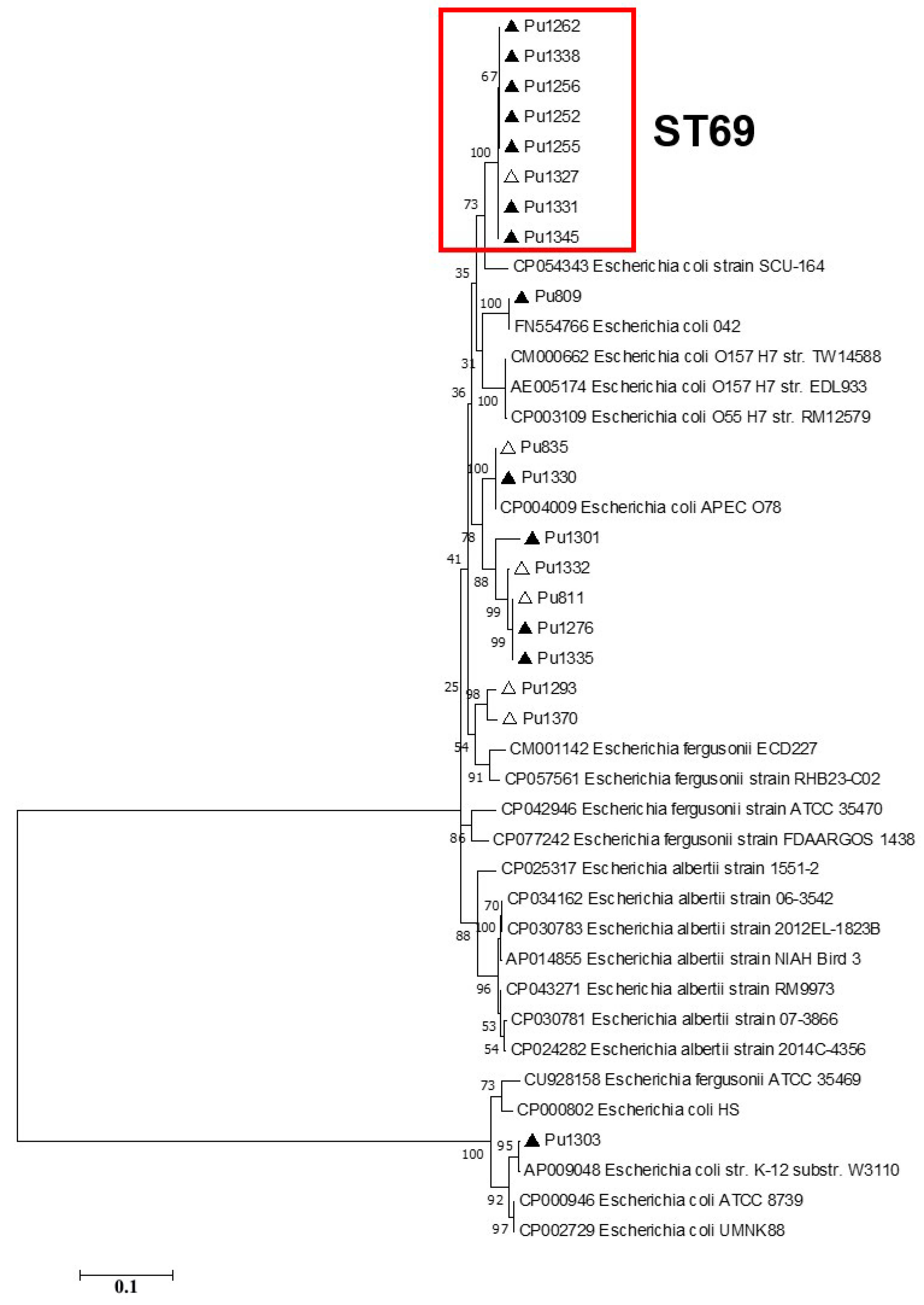
According to the phylogenetic analysis of the full-length Type I-E
cas gene sequences, ST69 isolates formed a separate clade on phylogenetic trees, while the topology of the Crie-Pu isolates belonging to the other STs differed slightly between the phylogenetic trees (
Figure 3).
The CRISPR/Cas loci of most Type I-F Crie-Pu isolates (13 out of 14) consisted of six genes encoding Cas6/Csy4 endoribonuclease, three Csy proteins (Csy3, Csy2, and Csy1), Cas3f helicase/RNase, and Cas1f endonuclease located in the vicinity of CRISPR arrays, except for the Crie-Pu1367 isolate, which carried only cas6, csy1, csy2, and csy3 genes.
Phylogenetic analysis of the Type I-F
cas gene sequences showed that separate clades on phylogenetic trees were formed by (i)
E. coli Crie-Pu ST141 and ST1993 isolates and (ii)
E. coli Crie-Pu ST80 isolates. It is worth noting that the isolates belonging to other genetic lines (STs) did not form separate clades and were dispersed throughout the phylogenetic trees (
Figure 4).
3.3. Susceptibility to Antibiotics
Phenotypic antimicrobial susceptibility testing showed that 28
E. coli isolates (53%) were susceptible to all antibiotics in the panel used (
Table S1). Phenotypic resistance to three antimicrobial compounds of different groups (aminoglycosides/penicillins, fluoroquinolones, and cephalosporins) was detected in two isolates (Crie-Pu 1299 and 829, correspondingly). Resistance to two antibiotics of different groups simultaneously (cephalosporins in combination with aminoglycosides, penicillins, or fluoroquinolones, as well as aminoglycosides and fluoroquinolones) was detected in 11 isolates. The remaining 12 isolates were resistant to only one of the antibiotics used in the panel. The identified phenotypes were not associated with the type of labor (cesarean section or vaginal birth), as well as with the prescribed antibiotic therapy. However, it should be noted that the multidrug-resistant isolates (Crie-Pu1299 and 829) were identified in two puerperant women with a cesarean section and one with a complicated vaginal birth. In these two cases, antibiotic therapy was prescribed (
Table S1).
3.4. Antimicrobial Resistance Genetic Determinants
In silico searching for AMR determinants revealed the genes and gene clusters conferring resistance to aminoglycosides, beta-lactams, chloramphenicol, fluoroquinolones, trimethoprim, fosfomycin, macrolides, sulfonamides, and tetracycline.
The genomes of seven isolates of 28 susceptible to all antimicrobial compounds were characterized by the presence of 1 to 7 AMR genes including carbapenemase-encoding
blaDHA-1 and extended-spectrum β-lactamase (ESBL)-encoding genes (
blaCTX-M-15 and
blaTEM-1C) (
Table S4).
The genomes of all remaining antibiotic-resistant isolates, except for four isolates belonging to phylogroup B2, contained ESBL- and other β-lactamase encoding genes:
blaTEM (n = 19),
blaCTX-M (n = 15),
blaDHA-1 (n = 2), and
blaOXA-1 (n = 1). Two isolates of the B2 phylogroup (Crie-Pu1235 and 1290) combined two β-lactamase genes of different types in their genomes (
blaCTX-M-27 +
blaTEM-1B and
blaCTX-M-15 +
blaOXA-1, respectively). It is noteworthy that both strains belonged to ST131, but had different serotypes O16:H5 and O25:H4 (
Table S4). The diversity of identified alleles of the epidemically significant ESBL gene
blaCTX-M-3,
-15, and
-27 draws attention since these genes were revealed in almost a third of the studied strains from a short period of research within the same perinatal center and in a small group of puerperant women.
Most of the isolates of phylogroup B2 (14 out of 22) lacked genes responsible for resistance to aminoglycosides, chloramphenicol, macrolides, and sulfonamides. Four isolates of that group carried only a single gene determining resistance to chloramphenicol (two isolates), tetracycline, or trimethoprim (
Table S4).
All isolates of the phylogroup D were characterized by the presence of 1–4 genes that determined resistance to aminoglycosides and one or two genes encoding resistance to trimethoprim. We also marked a combination of
aadA5 (aminoglycoside) and
dfrA17 (trimethoprim) resistance genes in
E. coli genomes simultaneously. The only isolate (Crie-Pu1256) from this phylogroup and the entire studied set was characterized by the presence of the
fosA providing resistance to fosfomycin (
Table S4).
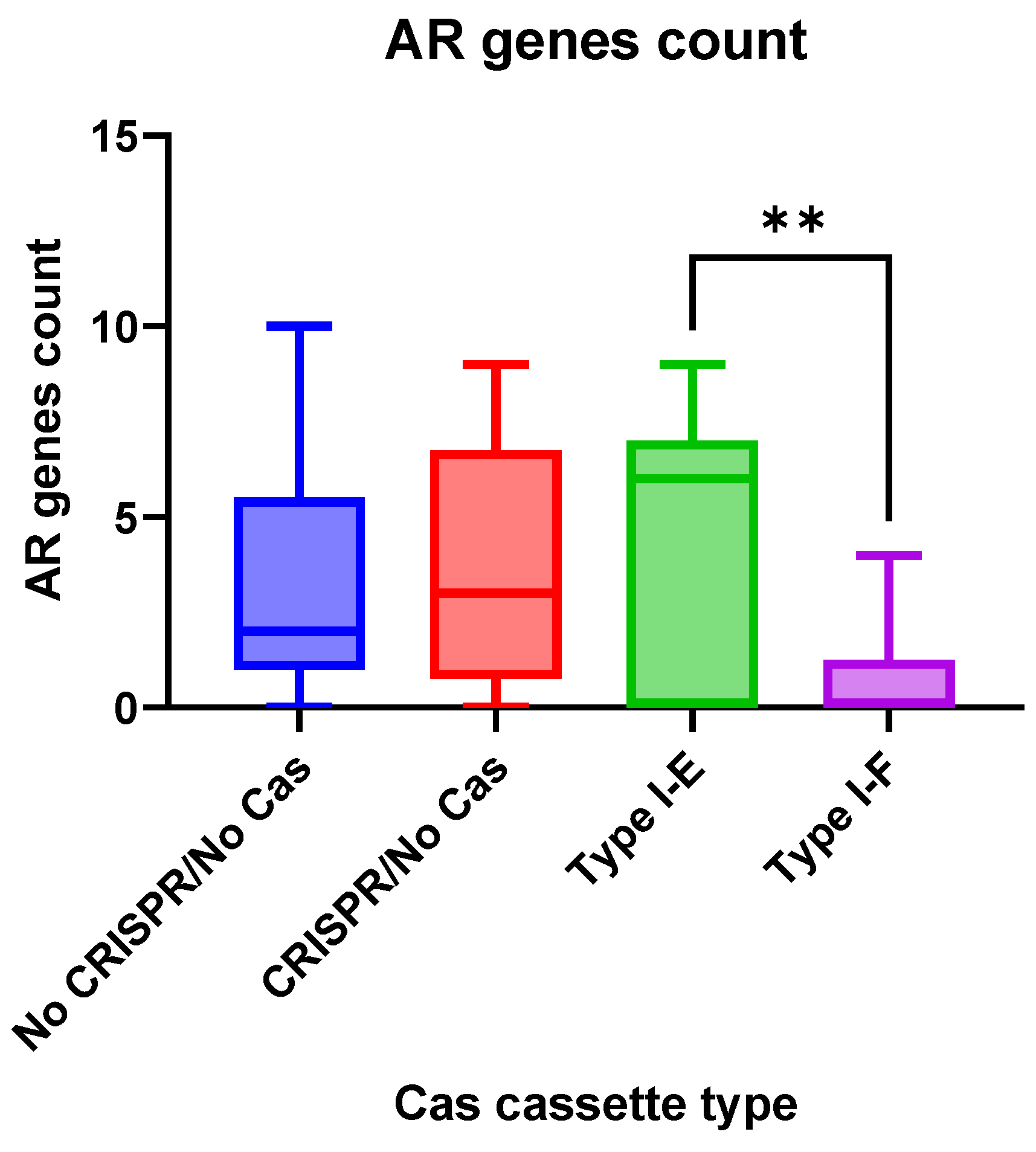
Antimicrobial resistance genes were observed more frequently (
p = 0.0058) in the group “Type I-E”
E. coli isolates when compared to the “Type I-F” isolates (
Figure 5).
3.5. Virulence Genes
E. coli isolates under investigation included a diverse repertoire of virulence-associated genes with 24 genes detected in each of the 53 isolates, namely, the multidrug efflux pump subunit gene acrB4; the allantoinase gene allB; the intimin-like adhesin gene fdeC; the outer membrane protein gene ompA; the enterobactin iron acquisition system genes entABCES; the ferric uptake genes fes and fur; the phosphogluconate dehydrogenase gene gndA; the invasion genes ibeB, C; the transcriptional regulatory protein genes (cgs operon, phoP, pmrA, rcsB, rpoS); and the fimbrial chaperone genes (yagV-Z/ecpA-E).
Additionally, more than 200 virulence genes were present variably, according to phylogroup and serotype of the isolates under investigation (
Table S2). Genes involved in adhesion, iron acquisition, immune evasion, and toxins were widespread. Since the virulence profiles of the isolates were very heterogenic, we will describe them by focusing on the main functional groups mentioned above.
Adhesins represent the molecules involved in signaling pathways between bacteria and host cells thereby stimulating tissue colonization and invasion [
22]. This is the most important pathogenicity-related factor in
E. coli. The most common adhesion determinants in our sample set were
fim genes encoding type 1 fimbriae. All isolates except one (Crie-Pu1338) harbored this gene cluster. The main part of the isolates of B2 and D phylogroups carried genes of P-fimbriae (
pap). The isolates of serogroups O25 and O50 had the most complete gene set composing this cluster. The genes associated with mannosoresistant pili (
sfa and
foc) were observed only in B2 isolates mainly belonging to serogroups O6, O18, and O50 (
Table S2).
Iron acquisition is a significant property facilitating bacterial survival during the infection process. It is also necessary for general growth, fitness, and electron transfer during cellular respiration [
23]. The main players of iron uptake in bacterial cells are different types of siderophores, namely, aero, entero-, yersiniabactins, and salmochelin. As to the
E. coli strains of our research, the full aerobactin operons
iucABCD and
iutA were present in 43.4% (n = 23) of the isolates. In the dominant phylogroup B2, these genes were associated with O16, O18, and O25 serotypes. A ferric enterobactin cluster (
fepA-G) was observed in all isolates except Crie-Pu1367 (ST141-B2-O50:H6). The ferric yersiniabactin uptake receptor
fyuA was not found in only six isolates. Four of them belonged to A (n = 2) and B1 (n = 2) phylogroups, and the rest—to C and D phylogroups. The iron-regulatory proteins
irp1 (n = 45, 85.0%) and
irp2 (n = 44, 83.7%) were present in the same fraction, and the complete yersiniabactin siderophore operon
ybtAEPQSTYX was additionally presented in these isolates. The salmochelin siderophore system encoded by
iroBCDEN was revealed in 90.0% (n = 48) of the isolates belonging to all phylogenetic groups except for Crie-Pu1239 (St59-O1:H7) of the F phylogroup. B2-ST131-O16:H5 isolates (n = 4) were characterized by the absence of salmochelin genes, and the same iron uptake genes profile was exhibited by ST131-O25:H4 isolates (n = 2), and one of each isolate from ST404 and ST1193 belonging to the same serotype (O75:H5). Most of the B2 isolates (n = 16) were characterized by the absence of aerobactin operone, predominantly, and these samples mostly referred to O6:H1, O50:H6, and O75:H7 serotypes (
Table S2).
Toxins play an important role in infections of different localizations since they contribute to the spread of bacteria in tissues, increasing cytotoxicity, resistance to neutrophils, as well as damage and disruption of host cell metabolism, leading to a biotope environment more favorable for
E. coli [
24]. Toxin genes were not revealed in two isolates with unidentified phylogroups (Crie-Pu1335 and 1340); in two samples of A and all isolates of B1 phylogroups (n = 4); in a single strain of C1 (Crie-Pu1330); and in separate isolates of B2 and D phylogroups (n = 5 and n = 2, correspondingly). The most frequently identified toxin genes were
cnf1 (cytotoxic necrotizing factor) and
hlyA-D (hemolysin). The combination of these genes was more common for B2 isolates, especially, for serogroups O50 and O6 (
Table S2). Several isolates belonging to A, B2, D, and F phylogroups harbored serine protease autotransporters genes (
pic and
sat) and the enterotoxin determinant
senB. The combination with maximal numbers of toxin determinants was identified in three isolates of the B2 phylogroup, with two of them (Crie-Pu1235 and 1290, both ST131) belonging to O16:H5 and O25:H4 serotypes, correspondingly, and the isolate Crie-Pu1304-ST73-O18ac:H1 (
Table S2).
Protectins defend bacteria from the host immune system and from various unfavorable conditions. In particular, protectins include bacterial capsules, outer membrane proteins, and lipopolysaccharide components. Most of the
E. coli isolates under investigation (n = 38, mainly the isolates from the B2 and D phylogroups) carried the
kps cluster, determining the synthesis of a polysialic acid capsule. Some of these isolates additionally harbored the
neu cluster responsible for polysialic acid production. Only ten isolates of the B2 phylogroup (mainly belonging to O50 and O6 serotypes) carried the
tcpC gene involved in the suppression of innate immunity (
Table S2).
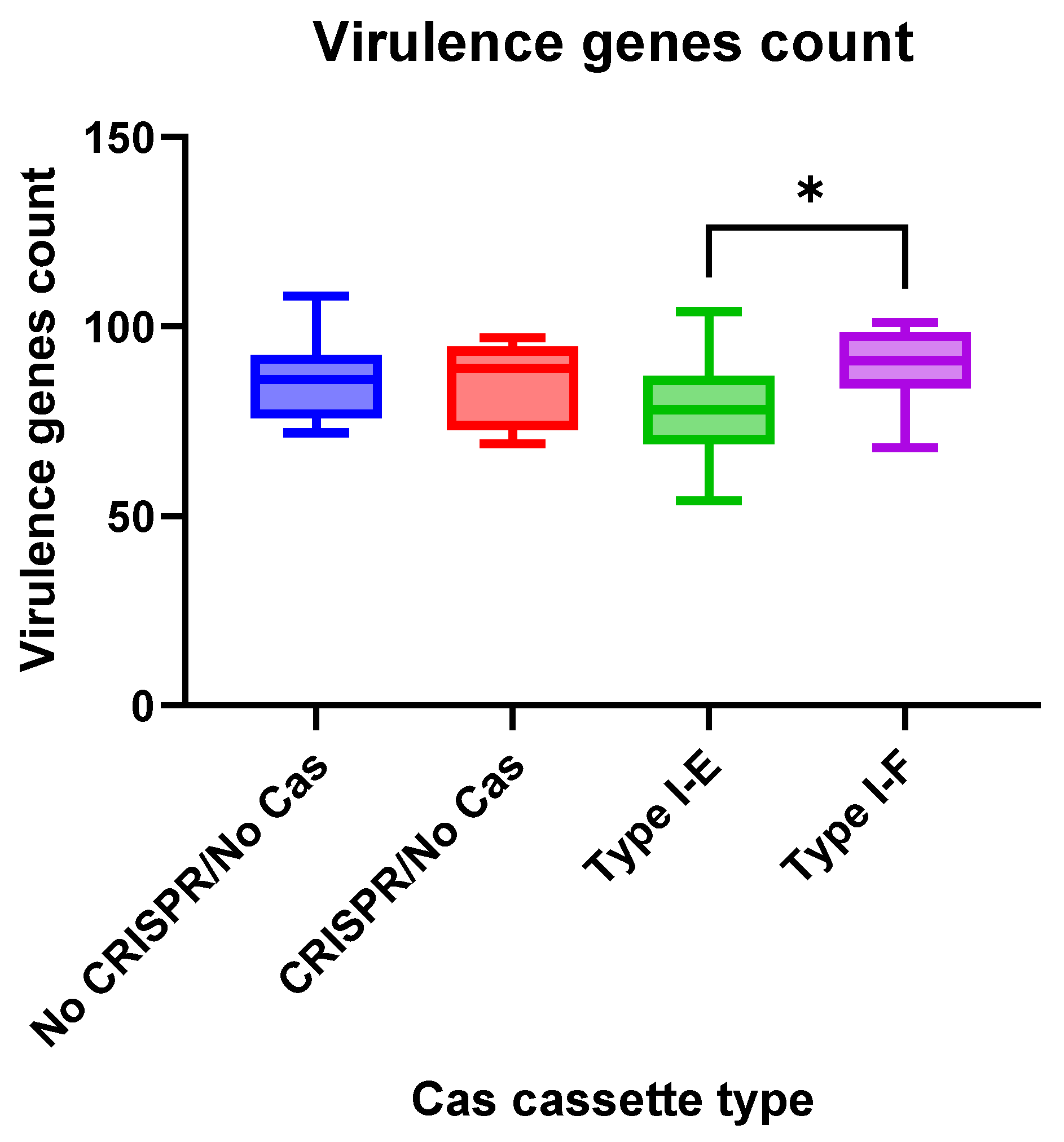
We observed insignificant differences in virulence gene numbers for the
E. coli isolates vs. CRISPR-element patterns. Meanwhile, virulence factor genes were found more frequently (
p = 0.0412) in the group “Type I-F”
E. coli isolates when compared to the “Type I-E” isolates (
Figure 6).
3.6. Plasmid Replicons of Crie-Pu E. coli Isolates
Since plasmids are very important vehicles of antibiotic resistance genes [
25], we performed a search of plasmid sequences in the WGS data obtained. The list of plasmid replicons identified in the isolates is shown in
Table S5. Plasmid sequences were revealed in 50/53 (94%) isolates analyzed. In total, 24 different types of plasmid replicons belonging to 15 varieties of Inc-type plasmids and nine varieties of Col-type plasmids were found (
Table S5). IncFIB and Col156 plasmids were the most frequently carried replicons in our collection, revealed in 27 and 18 Crie-Pu isolates, respectively. Col- or Inc-type plasmid replicons were separately carried by nine and seven
E. coli isolates, respectively, while thirty-seven isolates under investigation included the replicons of both types. The total replicon number usually was between 1 and 4, while the maximum number of plasmid replicons belonging to different types in one
E. coli genome reached eight (Crie-Pu1331) (
Table S5).
However, in order to obtain reliable plasmid sequences, long-read sequencing is usually required, which we plan to perform in the future.
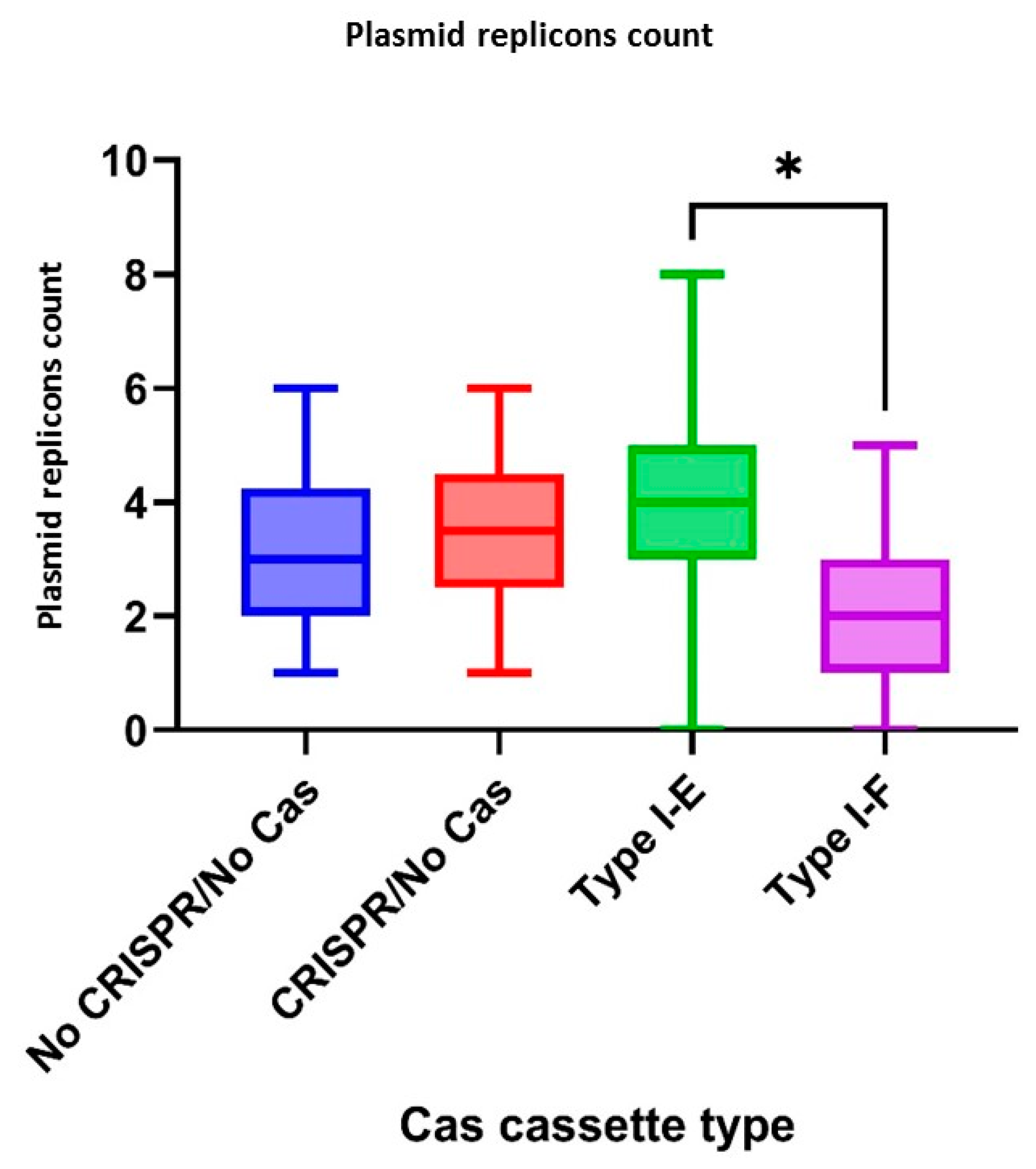
According to CRISPR-element sets of the studied isolates, plasmid replicons were observed more frequently (
p = 0.0333) in the group “Type I-E”
E. coli isolates when compared to the “Type I-F” isolates (
Figure 7).
3.7. CRISPR Arrays of the E. coli Isolates
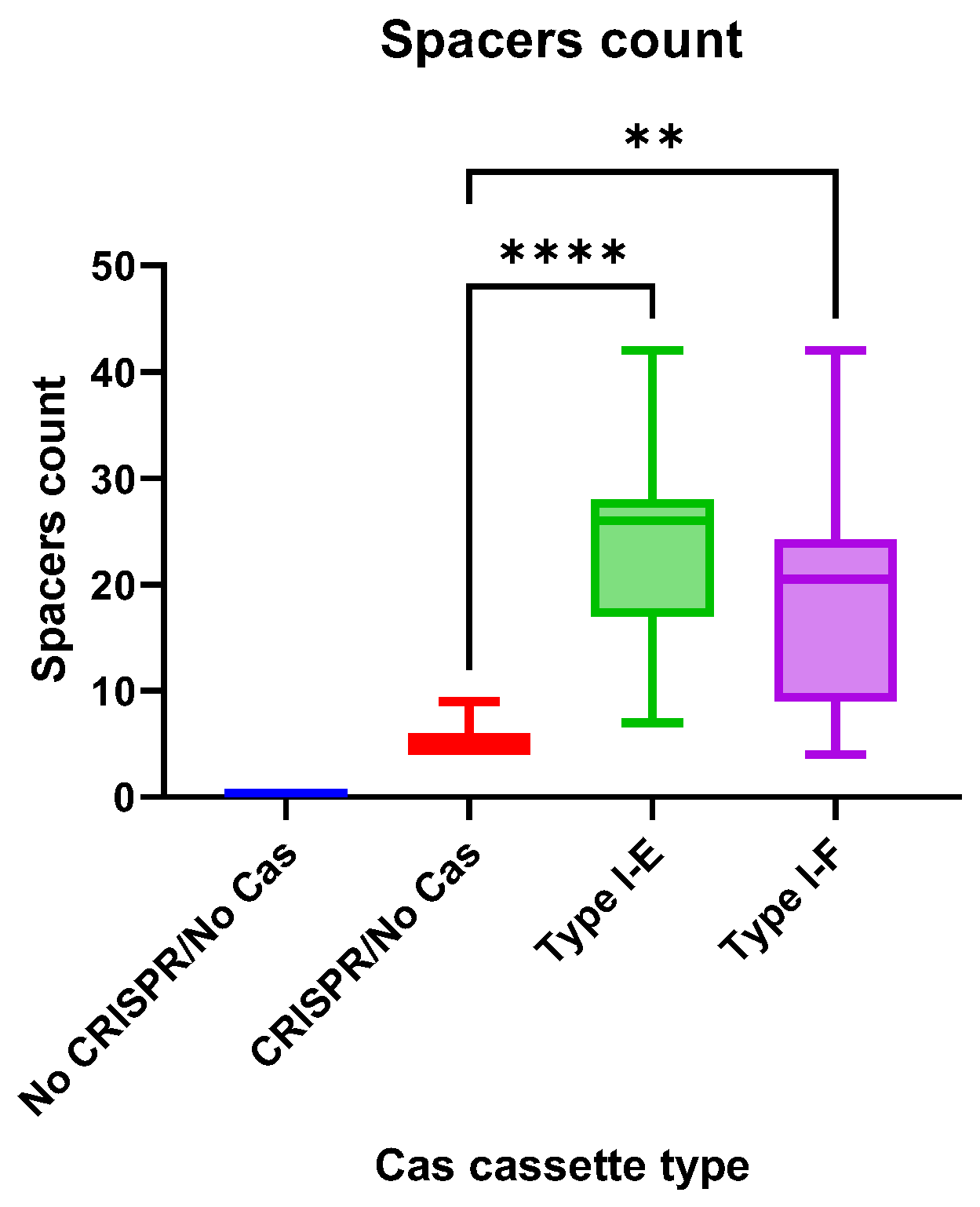
The CRISPR array spacers number did not differ significantly between “Type I-E” and “Type I-F”
E. coli isolates, but the number of spacers in both groups was significantly higher than in “CRISPR/No Cas” (
Figure 8).
CRISPR arrays of Type I-E Crie-Pu
E. coli isolates consisted of 450 spacers (189 unique spacers and 261 repeating spacers with 48 unique spacers among them). 71.1% of the analyzed spacers were identified as
E. coli CRISPR spacers (e.g., ‘
Escherichia coli strain C2-7 CRISPR repeat region’) using the MegaBLAST algorithm of Basic Local Alignment Search Tool (BLAST
®, National Library of Medicine). Only 4.2% of spacers were identified as phage sequences (e.g., ‘Bacteriophage sp. isolate 4198_46168, partial genome’), and 49.3% of spacers targeted plasmids (e.g., ‘
Escherichia coli strain KE47 plasmid unnamed1, complete sequence’) (
Table S6). The rest (around 14.2%) were self-targeting spacers (
Table S6).
In total, 224 spacers were found in Type I-F Crie-Pu
E. coli CRISPR arrays, with 75 of them being unique and 149 being repeating (with 39 unique spacers among the repeating ones). In addition, 61.6% (138 out of 224) were identified as spacers from known
E. coli CRISPR arrays (e.g., ‘
Escherichia coli strain 718 CRISPR1 repeat region’). Only five spacers (out of 224) targeted phage sequences (e.g., ‘
Salmonella phage SW3, complete genome’), and ten spacers targeted plasmids (e.g., ‘
Escherichia coli strain SCU-204 plasmid pSCU-204-5, complete sequence’) according to the analysis conducted using MegaBLAST algorithm of BLAST
® (National Library of Medicine) (
Table S7). The rest spacers (32.6%) targeted
E. coli genomes (
Table S7).
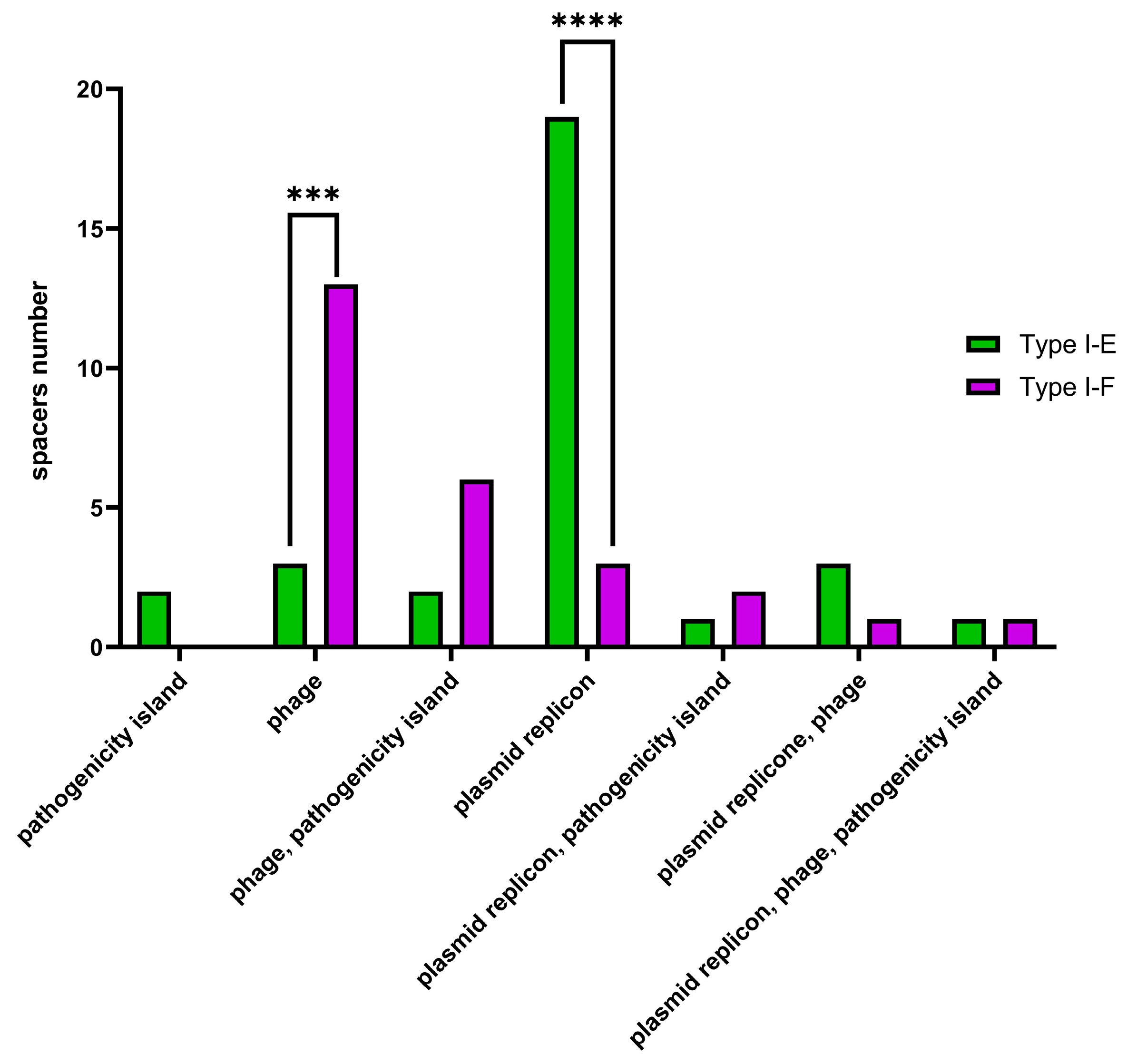
Additionally, Type I-E and Type I-F CRISPR spacers were analyzed using the CRISPRTarget web service (
http://crispr.otago.ac.nz/CRISPRDetect/predict_crispr_array.html accessed on 10 July 2024 [
17];
http://crispr.otago.ac.nz/CRISPRTarget/crispr_analysis.html accessed on 10 July 2024 [
18,
19]). CRISPRTarget revealed specific targets (namely, ‘plasmids’, ‘phages’, ‘pathogenicity islands’ or combinations thereof) for 31 Type I-E and 26 Type I-F
E. coli CRISPR spacers with 61.3% (19 out of 31) Type I-E spacers identified as targeting plasmids, while 50% (13 out of 26) Type I-F spacers were identified as targeting phages. It was shown that plasmid-targeting spacers were observed more frequently (
p ≤ 0.0001) within Type I-E spacers, whereas phage-targeting spacers (
p ≤ 0.001) were observed more frequently within Type I-F spacers (
Figure 9).
3.8. Correlation Analysis
In order to find out the interrelation between pathoadaptability factors and CRISPR-element patterns, a correlation analysis was performed. First of all, the datasets “Antibiotic resistance genes count”, “Virulence factors genes count” “Plasmids count” and “Spacers count” for “No CRISPR/No Cas”, “CRISPR/No Cas”, “Type I-E”, and “Type I-F” groups of Crie-Pu
E. coli isolates were normalized. Afterward, the nonparametric Spearman correlation was calculated, and correlation matrices were constructed. The resulting correlation matrices are presented in
Figure S1.
In the “No CRISPR/No Cas” group of
E. coli isolates, a moderate negative correlation (
p = 0.025) was observed between the number of virulence factor genes and plasmids count (
Table 1), while the number of AMR genes positively correlated with the number of plasmids in the “Type I-E” and “Type I-F” groups (
Table 1).
4. Discussion
The work presented here focuses on whole genome analysis of
E. coli strains isolated from the maternal birth canal discharge of puerperant women on the 3rd or 4th day after labor. We described our results through the prism of CRISPR-elements patterns to assess the pathogenic potential of the isolates under study. Our previous investigations showed specific features of multidrug-resistant bacteria in terms of their pathoadaptability, and genomic and phenotypic adaptations that promote bacterial survival under hospital conditions [
21,
26]. It is worth noting that there is a lack of data regarding the phenotypic and genotypic characterization of
E. coli strains colonizing healthy women several days after labor.
The analysis of the population structure of the isolates showed a notable heterogeneity of the strains at genotype and phylogroup levels. The 53 studied samples belonged to 31 MLST-based sequence types and 20 O-serotypes. Phylogroup classification demonstrated that
E. coli isolates under investigation were distributed in six phylogroups—A, B1, B2, C, D, and F, and more than half of the isolates (32, 60%) belonged to phylogroup B2. It was reported that most ExPEC strains derived from this group [
27,
28], and common ExPEC types were revealed in our sample set. These types included ST73 (three isolates), ST95 (one isolate), ST131 (six isolates), ST1193 (one isolate), and ST69 (phylogroup D, eight isolates). Interestingly, similar heterogeneity of
E. coli isolates with the prevalence of the B2 phylogroup was described earlier for vaginal and endocervical strains collected from pregnant women [
29,
30] and cervix isolates of women in preterm labor [
31]. It is also worth mentioning that four ST141 isolates were revealed in our study since their prevalence has recently increased in both extraintestinal and intestinal diseases [
6]. In general, such a diversity of isolates collected from one department during a rather short period practically eliminates the possibility of nosocomial infection in this case.
In total, 62.3% of Crie-Pu
E. coli isolates were characterized by the presence of the CRISPR/Cas systems. Commonly, such a high percentage of CRISPR/Cas systems is found in the microorganisms used in fermentation processes (e.g., starter cultures, probiotic cultures, and so on) [
32,
33]. CRISPR/Cas systems provide protection to these microorganisms from phage invasions that dramatically affect the quality of products [
34] and represent one of the evolutionary mechanisms of adaptation and survival.
CRISPR/Cas systems Type I-E and I-F were found in 35.8% and 26.4% of Crie-Pu
E. coli isolates, respectively. On the one hand, Type I-E CRISPR/Cas distribution is consistent with the one published earlier [
35,
36]. On the other hand, we found that Type I-E CRISPR/Cas systems are found more frequently (
p ˂ 0.05) in the group of reference
E. coli isolates available in the CRISPRCas database. Type I-F CRISPR/Cas systems were overrepresented (
p ˂ 0.00001) in our collection of
E. coli isolates compared to the data published earlier [
35] and the fraction revealed in the reference group. This is due to the predominance of B2 isolates in our sample set since the Type I-F CRISPR/Cas system is a characteristic of B2
E. coli strains [
37]. It is worth noting that the Type I-F CRISPR/Cas system is more active than Type I-E, so it possesses a higher potential association with pathogenicity due to its presumable affinity to genetic elements and low prevalence [
7,
38]. Interestingly, according to phylogenetic analysis of Type I-E and I-F
cas genes, Crie-Pu
E. coli isolates belonging to ST69, 141, and 80 formed separate clades on maximum-likelihood trees, thus indicating their greater divergence compared to reference isolates from the database.
Apparently, there are several factors contributing to bacterial pathoadaptability, such as the number of AMR genes, virulence factors, plasmids, and the presence of apparently functional CRISPR/Cas systems. In the present study, we demonstrated that the number of AMR genes and plasmid replicons was higher in
E. coli isolates bearing Type I-E CRISPR/Cas system than in isolates with Type I-F systems, and this fact is consistent with the observation published earlier [
39]. In 2019, Long et al. demonstrated the role of the Type I-F system in limiting the acquisition of AMR [
37]. Interestingly, the majority of our
E. coli isolates carrying the I-F system included fewer antimicrobial resistance genes compared to other isolates under investigation, but were characterized by a lower number of plasmid-targeting spacers than Type I-E Crie-Pu
E. coli isolates. This fact is in contrast to the work of Aydin S. et al., who reported a much larger proportion of plasmid-specific spacers in
E. coli isolates susceptible to antimicrobial drugs [
39]. This observation suggests a more complex role of CRISPR/Cas systems in bacterial pathoadaptability and needs further investigation.
At the same time, in Type I-F
E. coli isolates the number of genes encoding virulence factors was higher. Several virulence factors such as
sfa (S fimbriae),
cnf1 (cytotoxic necrotizing factor 1),
focF and
focH (Fimbriae of serotype 1C),
iroB, and
hlyABCD (hemolysins) were seen more frequently in the group of Type I-F vs. Type I-E isolates. These virulence factors are frequently found in Uropathogenic
Escherichia coli (UPEC), which represents the primary cause of UTIs globally [
40] and is also associated with cystitis and pyelonephritis [
41,
42].
It is worth noting that the CRISPR/No Cas group of our Crie-Pu sample set was represented by six isolates only and they were characterized by an interrelation pattern of pathoadaptability factors similar to Type I-E E. coli isolates.
Furthermore, remarkable correlations were found between the pathoadaptability factors, such as the number of AMR genes, the number of virulence factors, and the number of plasmid replicons in the groups of Crie-Pu
E. coli isolates with different types of CRISPR/Cas systems. Thus, for Crie-Pu
E. coli isolates without CRISPR/Cas systems, we revealed the pattern of “more virulence genes-less plasmids” and vice versa. Another correlation pattern “more plasmids-more antibiotic resistance genes” and vice versa was observed in the “Type I-E” and “Type I-F” groups. This fact confers the potential association of Type I-E and Type I-F with
E. coli pathogenicity [
7] and may contribute to the pathoadaptability of
E. coli with different CRISPR/Cas systems. Moreover, the presence of spacers with identities to phage and plasmid sequences in Crie-Pu
E.coli strains indicates the defense role of CRISPR/Cas systems.
In our study, 14.2% of Type I-E spacers and 32.6% of Type I-F spacers were identified as self-targeting. It is known that
E. coli CRISPR spacers have a statistically significant tendency to target hosts compared to phage genomes [
43]. It is known that self-targeting spacers in CRISPR/Cas systems acquired from the host chromosome are involved in autoimmunity and cell death [
44]. Moreover, such spacers play an important role in the mRNA degradation process making it possible to overcome host immune reactions [
45]. In addition, self-targeting spacers may contribute to bacterial gene regulation and evolution [
8,
46]. It is assumed that CRISPR/Cas systems containing self-targeting spacers should be tightly regulated to maintain a balance between the risk of developing autoimmune reactions leading to cell death and the ability to resist phage invasions. These CRISPR/Cas systems still require further investigation [
47].